Abstract
Purpose of Review:
Mental health disorders, especially depression, are prevalent among people living with HIV (PLWH) and are associated with cognitive impairment (CI) among HIV-uninfected (HIV) individuals. We conducted a comprehensive review of the link between depression and cognition among PLWH.
Recent Findings:
Studies examining depression and cognition in PLWH report high rates of current (median=24%) and lifetime depression (42%). There is reliable evidence that depression is associated with overall CI among PLWH, and in the cognitive domains of processing speed, executive function, learning and memory, and motor function. Although few studies have examined the interaction between HIV serostatus and depression on CI, there is no evidence of a stronger association between CI and depression in PLWH compared to HIV− controls.
Summary:
Depression is prevalent and reliably associated with CI in PLWH, with an overall pattern of domain-specific associations similar to that of HIV− individuals.
Keywords: HIV, Depression, Depressive Symptoms, Cognitive Impairment, HAND
Introduction
By 2030, the top two leading causes of burden of disease globally are projected to be HIV and depressive disorders[1]. The two disorders commonly co-occur; depression is the most common neuropsychiatric complication in persons living with HIV (PLWH) next to substance abuse[2, 3]. Depression is two- to three-times more common in PLWH versus the general population [2–5]. Revised estimates from the only nationally representative study among PLWH in the United States (U.S.) found an 18.5% prevalence of major depressive disorder (MDD) over the preceding 12 months[2, 3], which was two- to three-times higher than the general U.S. population. U.S. cohort studies yield similarly high prevalence estimates[5, 6]. The Women’s Interagency HIV study (WIHS), for instance, found that among women living with HIV the rate of current MDD based on diagnostic interview was 20% [4] versus 10% nationally, and the rate of lifetime MDD was 32.4% versus 22.9% nationally, respectively. Notably, the level of severity of depression was “serious” in 80% of women with MDD. Such studies indicate that depression is not only highly prevalent but also severe in PLWH.
Multiple factors contribute to the high prevalence of depression in PLWH[7]. These include the sadness and grief that come with initial diagnosis, the stress of living with a chronic illness, challenges of getting needed social support, and internalized stigma[8]. Additionally, PLWH have a high prevalence of psychosocial risk factors for depression including early life trauma, negative life events, violence exposure, financial instability, limited healthcare access, low education, and underemployment[9]. Behavioral risk factors such as substance and alcohol abuse and limited physical activity are also prevalent[4]. HIV-related clinical factors such as poor antiretroviral (ARV) adherence are linked to future depressive episodes, as are key risk factors observed in the general population, such as female sex, pre-existing mood disorders, and a family history of mood disorders[10].
The most serious complication of depression in PLWH is the two-fold increased risk of mortality[11], but cognitive impairment (CI) is also an important complication, one that is associated with decreased daily function[12–20] and quality of life[21–24]. Meta-analyses indicates that depression is associated with ARV non-adherence (r=0.19)[25–27]. Although depression is associated with medication non-adherence and decreased CD4 count, those clinical factors do not explain the relationship between depression and mortality[28]. Meta-analytic studies of HIV-uninfected (HIV−) individuals find that depression severity is most reliably associated with deficits in episodic memory, executive function, processing speed, and attention[29]. Importantly, these cognitive deficits persist in patients with remitted MDD[29]. Similarly, studies in PLWH show that depression is associated with deficits in those same domains[30–34], though that literature has not been comprehensively reviewed.
Prominent neurobiological features of depression contributing to cognitive symptoms include decreases in glucose metabolism in the prefrontal cortex (PFC)[35], and functional alterations of the anterior cingulate cortex (PFC subregion) and amygdala during performance of cognitive tasks[36–39]. In current models, functional alterations of the medial PFC and altered connectivity between medial PFC and limbic structures (i.e., amygdala, hippocampus, parahippocampal cortex), contribute to CI in MDD, and also disrupt cognition through effects on sympathetic arousal and glucocorticoid release[40, 41]. These functional brain alterations overlap in part with the HIV-associated alterations in brain circuitry[42]. Multiple neurobiological features of HIV infection, including chronic neuroinflammation, reduction of trophic factors, and alterations in dopamine and other neurotransmitters can contribute to depression in PLWH[43]. Mechanistically, neuroinflammation and impaired neurogenesis are key features of depression and HIV, and are central contributors to CI[43–45]. Similarly, alterations in hypothalamic pituitary adrenal axis function can contribute to CI in depression and HIV[40, 41]. Depression can influence cognition function in PLWH indirectly through decreased adherence to medication.
Given the high prevalence of depression among PLWH and the robust link between depression, cognition, and brain health in HIV− individuals, we conducted a comprehensive review of the link between depression and cognition among PLWH. Specifically, we addressed the following questions: 1) In cross-sectional analyses, what is the relationship between depression and cognition among PLWH? 2) In longitudinal analyses, what is the relationship between depression and cognition among PLWH? 3) Does the association between depression and cognition differ between PLWH versus HIV− individuals?
Methods
Data for determining depression and cognition associations among PLWH were identified by searches in PubMED (June 2018) for titles/abstracts containing MeSH terms “depression,” “depressed,” “mood,” or “anxiety” combined with “cognition,” “cognitive,” “HIV-associated Neurocognitive Disorders (HAND),” “neurocognitive,” “neurocognition,” or “neuropsych,” combined with “HIV,” or “HIV-infected” with additional limits of “English Language,” “Humans,” and after 2000. Our search yielded 563 abstracts which were reviewed for the following inclusion criteria: 1) HIV sample size ≥95, 2) standard depression inventory with validated cutoff score indicating depression or use of a structured clinical interview to indicate MDD”, 3) cognition determined based on two or more validated neuropsychological tests, and 4) associations provided between depression and cognition. Based on abstract and article review, 41 articles met criteria. Publications in some cases reflect different analyses from the same cohort, but the individual papers from a given study report on varying cross-sectional and longitudinal subsets over different lengths of follow-up. Therefore, in some cases, we have included findings from one study reported in more than one paper (e.g., WIHS, Multicenter AIDS Cohort Study-MACS, CNS HIV Anti-Retroviral therapy Effects Research-CHARTER).
Study Selection
Tables 1 and 2 provides characteristics of the 41 selected articles meeting inclusion criteria. Of the 41 articles, 29 studies produced 35 cross-sectional and 12 longitudinal analyses. Samples sizes ranged from 95 to 2099 PLWH (median=254) and 49% were based on U.S.samples. An HIV− control group was included in 17 of 41 studies (41%), with control group sample sizes ranging from 18 to 1793 (median=74). The articles span 22 different countries and include 18,737 (14,362 PLWH), mostly male (70%) with an average age in most studies in the 40s. Of studies reporting on ARV use and/or undetectable HIV RNA, 71% of participants analyzed were on ARVs and 61% had undetectable HIV RNA.
Table 1.
Cross-sectional studies included in the comprehensive review of the literature on the association between depression and cognitive function among people living with HIV (PLWH) in the era of effective antiretrovirals (ARVs).
| Study | PLWH n |
HIV− n |
Country | Male (%) | Age range or mean/SD | On ARVs (%) | UDVL (%) | Depression Measure |
Relationship between current depression & CI in PLWH |
|---|---|---|---|---|---|---|---|---|---|
| Blackstone (2012)[69] | 1574 | - | US | 76 | 44±8 | 86 | 59 | BDI-II | ** |
| CHARTER | |||||||||
| Rubin (2014)[30] | 708 | 278 | US | 0 | 44±7 | 65 | 51 | CES-D ≥16 | |
| WIHS | |||||||||
| Wright (2008)[46] | 658 | 161 | CN, FJ, GN, HH, KK, ID, MY, TH | 59 | 35±9 | 65 | - | CES-D ≥16 | ⊺ |
| Becker (2009)[47] | 428 | 207 | US | 100 | 50±7 | - | - | CES-D ≥16 | |
| MACS | |||||||||
| Haddow (2018)[48] | 448 | - | DK, GB, IT | 84 | 46 | 89 | 91 | PHQ-9 ≥10 | * |
| CIPHER | |||||||||
| Yasuf (2017)[70] | 418 | - | NG | 22 | 37±9 | 100 | 84 | CES-D ≥16 | ** |
| Gascón (2018)[49]† | 412 | - | BR | 68 | 45±11 | 100 | 84 | BDI 13-19 | *** |
| Bonnet (2013)[50] | 400 | - | FR | 79 | 42-53 | 89 | 85 | CES-D≥17♀; ≥23♂ | *** |
| ANRS C03 Aquitaine | |||||||||
| Starace (2000)[51] | 395 | - | IT | 68 | 35±8 | 74 | - | MADRS >19 | |
| ICONA | |||||||||
| Pinheiro (2016)[52] | 392 | - | BR | 45 | 43±12 | 89 | 65 | MINI-Plus | * |
| Judd (2016)[71] | 296 | 97 | US | 42 | 15-18 | 86 | 76 | HADS | |
| AALPHI | |||||||||
| Bloch (2016)[54] | 254 | 72 | AU | 99 | 49±10 | 92 | 79 | DASS-21>13 | ⊺ |
| Shimizu (2011)[33]†‖ | 285 | - | US | 84 | Y: 35±5 O: 54±5 |
72 | 49 | BDI | * |
| Hawaii Aging with HIV | |||||||||
| Tymchuk(2018)[55] | 265 | - | CA | 89 | 47±10 | 93 | - | PHQ-9 ≥10 | * |
| Castellon (2006)[56] | 247 | - | US | - | 37±8 | - | - | BDI | |
| Richardson (2005)[73] | 145 | 75 | US | 0 | 36±8 | 53 | - | CES-D ≥16 | |
| WIHS | |||||||||
| Akolo (2014)[74] | 133 | 77 | NG | 44 | 33±7 | 0 | 2 | BDI | ns |
| Kelly (2014)[58] | 106 | 103 | MW | 27 | 18-71 | 100 | - | SRQ-20 ≥8 | * |
| Fellows (2014)[32] | 186 | - | US | 65 | 44±7 | 77 | 29 | PRISM | |
| MHBB | |||||||||
| Schouten(2016)[59]† | 103 | 74 | NL | 100 | 49-62 | 100 | 100 | BDI 14-28 | ⊺ |
| AGEhIV | |||||||||
| Harrison (2017)[79]† | 103 | 70 | US | 69 | 45±10 | 100 | - | HADS | |
| Janssen (2015)[60] | 95 | 55 | NL | 85 | 49±10 | 100 | 100 | HADS | |
| Art-NeCo | |||||||||
| Rosenthal (2013)[75] † | 114 | 38 | US | 69 | 45±8 | 100 | 59 | BDI >16 | *** |
| NEAD/Oxidative Stress cohort | |||||||||
| Ammassari (2004)[61] | 135 | - | IT | 64 | 19-64 | 100 | - | MADRS | |
| ICONA | |||||||||
| Braganca (2011)[34] | 130 | - | PT | 82 | 18-50 | 100 | 100 | HAM-D≥10 | *** |
| Bryant (2015)[78] | 120 | - | US | 64 | 45±9 | 82 | 71 | CES-D | ** |
| Lawler (2010)[64] | 120 | - | BW | 50 | 37±6 | 98 | 80 | PRIME-MD | |
| Cysique (2011)[76] † | 116 | - | AU | 100 | 49±9 | 100 | 51 | DASS | ns |
| Simioni (2010)[66]† | 100 | - | CH | 72 | 47±9 | 100 | 100 | HAD-D≥10 | ** |
ns=not significant
p<0.05
p<0.01
p<0.001
p=0.06 except Wright et al. p=0.11.
Country Codes: AU=Australia, BR=Brazil, BW=Botswana, CA=Canada, CH=Switzerland, CN=China, DK=Denmark, FJ=Fiji, FR=France, GB=Great Brittan, GN=Guinea, ID=Indonesia, IT=Italy, KH=Cambodia, MW=Malawi, MY=Malaysia, NG=Nigeria, NL=Netherlands, PT=Portugal, TH=Thailand, US=United States
Depression Measures: BDI=Beck Depression Inventory, CES-D=Center for Epidemiological Studies-Depression, CIDI=Composite International Diagnostic Interview, DASS=Depression, anxiety, stress scale, e-M.I.N.I=Electronic Mini International Neuropsychiatric Interview, HADS=Hospital Depression and Anxiety Scale, HAM-D=Hamilton Rating Scale for Depression, MADRS=Montgomery–Asberg Depression Rating Scale, MINI-Plus=MINI-International Neuropsychiatric Interview, PRIME-MD= Primary Care Evaluation of Mental Disorders, mood module, PRISM=Psychiatric Research Interview for Substance and Mental Disorders, SCID=Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders; SRQ-20=self-reporting questionnaire
Study Cohorts: AALPHI= Adolescents and Adults Living with Perinatal HIV Cohort, ANRS=French Agency of AIDS and Hepatitis Research, Art-NeCo=NeuroCognition in HIV-infected Patients on long term effective combination antiretroviral therapy; CHARTER=CNS HIV Anti-Retroviral Therapy Effects Research cohort, CIPHER=Cognitive Impairment in People with HIV in the European Region, ICONA=Italian Cohort Naïve Antiretrovirals research programme, MACS=Multicenter AIDS Cohort Study; MHBB=Manhattan HIV Brain Bank; NEAD=Northeast AIDS Dementia Cohort; WIHS=Women’s Interagency HIV Study
Other: SD=standard deviation, UD VL=undetectable HIV RNA, CI=cognitive impairment
studies excluded individuals either endorsing severe depression via questionnaire (e.g., BDI >19)(Gascón et al.), meeting criteria for major depression according to the Diagnostic and Statistical Manual of Mental Disorders (Simioni et al.), or had a self-reported history of severe affective disorders (Rosenthal et al.)
Significant association in younger group only.
Table 2.
Longitudinal studies included in the comprehensive review of the literature on the association between depression and cognitive function among people living with HIV (PLWH) the era of effective antiretrovirals (ARVs).
| Study | PLWH n |
HIV− n |
Country | Male (%) | Age range or mean/SD | On ARVs (%) | UDVL (%) | Depression Measure |
Relationship between current depression & CI in PLWH |
|---|---|---|---|---|---|---|---|---|---|
| Molsberry [83] | 2099 | 1793 | US | 100 | 40±9 | 21 | - | CES-D≥16 | |
| MACS | |||||||||
| Armstrong [82] | 669 | 942 | US | 100 | 51±3 | - | - | CES-D≥16 | |
| MACS | |||||||||
| Rubin (2017)[31] | 646 | 300 | US | 0 | 45±9 | 77 | 52 | CES-D≥16 | **Avg |
| WIHS | |||||||||
| Heaton (2015)[77] | 436 | - | US | 80 | 44±8 | 70 | 41 | CIDI, BDI | CIDI⊺/BDI** |
| CHARTER | |||||||||
| Vo (2013) [53] | 362 | - | US | 100 | 35±8 | - | - | CES-D ≥16 | |
| MACS | |||||||||
| Grant (2014)[68] | 347 | - | US | 82 | 44±8 | 68 | 41 | CIDI | CIDI** |
| CHARTER | |||||||||
| Robinson-Papp (2008)[72] | 260 | 18 | US | 91 | 50±7 | 68 | - | BDI | ***B |
| MHBB | |||||||||
| Cysique (2007)[57] | 227 | - | US | 100 | 32±7 | 35 | - | SCID | nsB |
| CHARTER | |||||||||
| Gandhi (2010)[65]† | 104 | - | US | 72 | 47±6 | 100 | - | BDI ≥16 | nsB |
| NEAD/Oxidative Stress cohort | |||||||||
| Gibbie (2006)[63] | 129 | - | AU | 95 | 48 | 93 | 58 | BDI≥14; SCID | |
| Nakasujja(2010)[62] | 102 | 25 | UG | 27 | 30±6 | 0 | 0 | CES-D≥16 | |
| 3 & 6 months | 100 | - | |||||||
| Cysique(2016)[67] | 95 | - | AU | 98 | 56±8 | 100 | 98 | e-M.I.N.I | ***B |
ns=not significant
p<0.05
p<0.01
p<0.001
p=0.06
Country Codes: AU=Australia, UG=Uganda; US=United States
Depression Measures: BDI=Beck Depression Inventory, CES-D=Center for Epidemiological Studies-Depression, CIDI=Composite International Diagnostic Interview, e-M.I.N.I=Electronic Mini International Neuropsychiatric Interview, HDS=Hospital Depression Scale, MINI-Plus=MINI-International Neuropsychiatric Interview, SCID=Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders
Study Cohorts: CHARTER=CNS HIV Anti-Retroviral Therapy Effects Research cohort, MACS=Multicenter AIDS Cohort Study, MHBB=Manhattan HIV Brain Bank; NEAD=Northeast AIDS Dementia Cohort; WIHS=Women’s Interagency HIV Study
Other: SD=standard deviation, UD VL=undetectable HIV RNA, CI=cognitive impairment
individuals self-reporting a history of severe affective disorders were excluded from study participation
longitudinal studies that included a cross-sectional analysis at baseline;
longitudinal studies that included a cross-sectional analysis collapsed over time
Of the 41 studies assessing both depression and cognition in PLWH, 26 [30–32, 34, 46–67] included prevalence estimates of current depression (Figure 1A). Of the 26 studies, 22 (85%) measured depression via self-report questionnaires and the prevalence of current depression ranged from 6% to 41% (median=24.6%)[30, 31, 34, 46–51, 53–56, 58–63, 65–67]. Of the five studies measuring current MDD via structural clinical interviews, the prevalence of current MDD ranged between 20% and 27% (median= 24.2%)[32, 52, 57, 63, 64] and lifetime MDD ranged from 26% to 50% (median=42%)[57, 63, 67, 68]. Nine of these 26 studies included controls and two of those studies reported and compared rates of depression between serostatus groups [47, 58].
Figure 1.
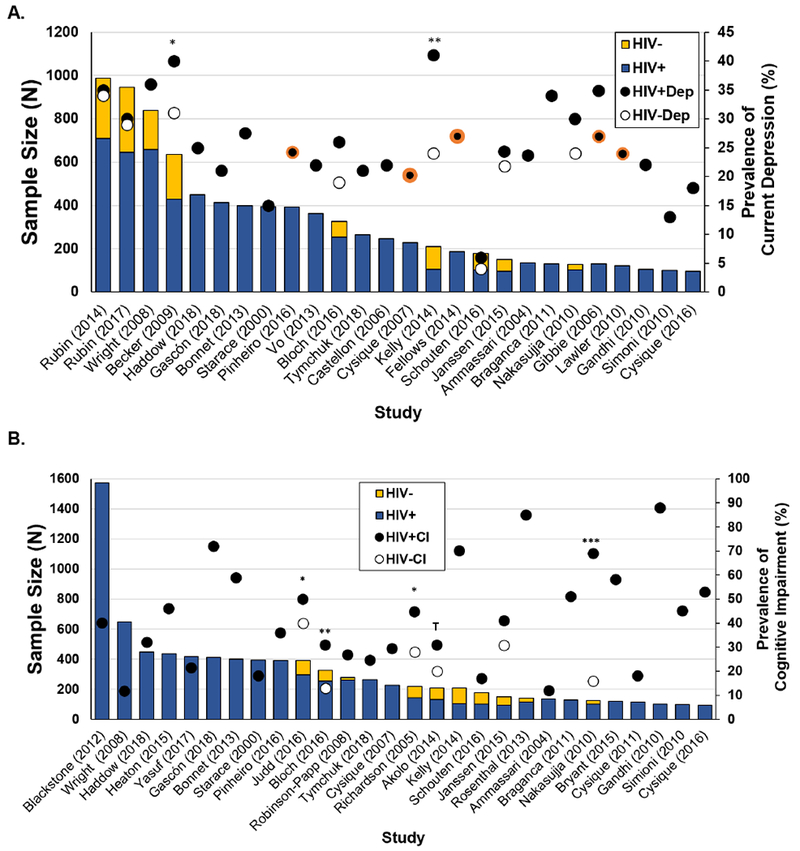
In the context of studies assessing depression and/or cognitive impairment, (A) prevalence of elevated depressive (Dep) symptoms and (B) cognitive impairment among HIV-infected (HIV+) and/or HIV-uninfected (HIV−) individuals.
Note. *p<0.05; **p<0.01; Tp<0.10; circles bordered in orange reflect depression being assessed using structural clinical diagnostic interviews whereas the remaining were assessed using self-report questionnaires
Among the 41 studies measuring both depression and cognition in PLWH, 28 [34, 46, 48–52, 54, 55, 57–62, 65–67, 69–78] included the prevalence of CI (Figure 1B). Of the 28 studies, the prevalence of CI ranged from 11.7% to 88% (median=40.5%). Ten of these 28 studies included the prevalence of HAND stage[48–50, 54, 58, 60, 65, 67, 70, 75]. Asymptomatic neurocognitive impairment (ANI) ranged from 10% to 55% (median=30.5%), mild neurocognitive disorder (MND) from 5% to 31% (median=11%), and HIV-associated dementia (HAD) from 0% to 31% (median=3%).
In cross-sectional analyses, what is the relationship between depression and cognition among PLWH?
Thirty-one cross-sectional analyses examined the association between depression and cognition among PLWH[30–34, 46, 48–52, 54–59, 61, 63–67, 69, 70, 72, 74–76, 78, 79]. Seven of the 31 analyses excluded current depression from study participation[33, 49, 59, 65, 66, 75, 76]. All studies used similar statistical approaches (analysis of variance, regression).
Twenty of 31 cross-sectional analyses measured depression via questionnaires and CI. Of the 20 studies, 14 (70%) reported a significant association between depression and CI[31, 33, 34, 48–50, 55, 58, 66, 69, 70, 72, 75, 78], three studies (15%) missed statistical significance two at p=0.06 [54, 59] and one at p=0.11 [46], and three found no significant association[65, 74, 76](Table 1). One reported the prevalence of depression by each HAND category [49] using Frascati criteria[80]. After excluding individuals with severe depression, a depression rate of 17% was reported for those with normal cognitive function, 15% for ANI, 40% for MND, and 35% for HAD. Studies not excluding individuals with severe depression report higher rates of depression among those with HAD (58%)[75]. Similar findings are reported in studies comparing rates of depression across combined categories of MND and HAD versus ANI and normal[49, 50, 58]. Among the MND/HAD group 39% and 45% had depression versus 15% to 19% of those with ANI or no CI. In these studies, depression increased the odds of MND/HAD versus ANI and/or being unimpaired. Specifically, in adjusted analyses, PLWH with depression versus PLWH without depression, had a 1.4- to 3.1- fold increased odds of MND/HAD versus ANI/being unimpaired[49, 50, 58]. Although rates of depression are lower among PLWH on ARVs and/or virally suppressed, rates of depression remain higher among those with (16% to 35%) versus without CI (4% to 22%)[55, 66]. One study provided an adjusted estimate whereby depression was associated with a 1.5 fold increased odds of having CI versus no CI [48]. Of the three studies noting non-significant trends[46, 54, 59], one had a smaller sample size (n=103) and excluded participants with severe depression[59] thus possibly attenuating the true association. In contrast to most, but not all studies[48], the second study[54] showing a trend assessed CI with a computerized battery, CogState. ARV use in the third study[46] was lower (65%) than other studies, raising the possibility that the effects of active viremia overwhelmed any effect of depression. The three studies finding no association had smaller sample sizes[65, 74, 76] and ARV use in one was 0% [74].
Of the three cross-sectional analyses[52, 57, 67] assessing depression via a structured diagnostic interview in relation to CI, two reported that MDD was a significant predictor of CI[52, 67]. These two studies used the MINI-International Neuropsychiatric Interview[81] whereas the third study used the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders[57]. The two studies with estimates suggest that 28% to 46% of PLWH with CI have MDD compared to 19% to 32% with no CI[52, 57]. The one study providing an adjusted odds ratio indicated that PLWH with MDD have a 1.9 fold increased odds of CI versus non-depressed PLWH[52]. The other study demonstrating a significant association between depression and CI found almost a linear relationship[67]. Cognition was best in PLWH with no history of MDD, followed by those treated for one major depressive episode (MDE), then those with chronic effectively treated MDD, those with chronic MDD that was treated but recurrent, and then those with an active MDE who were not treated[67].
Across the 31 cross-sectional analyses in PLWH, 17 examined associations between depression and specific cognitive domains[30–34, 48, 51, 55, 56, 59, 61, 63, 64, 74, 76, 78, 79](Table 3). For processing speed, six [31–34, 48, 78] of twelve [51, 56, 61, 64, 74, 79] studies (50%) demonstrated an association with depression. For executive function, five [31, 32, 34, 56, 63] of eleven [30, 48, 51, 61, 74, 78](45%) demonstrated an association; for motor function, four [34, 55, 63, 78] of nine [31, 51, 56, 61, 74](44%); for learning and memory, five [30–32, 34, 61] of twelve [33, 48, 51, 61, 64, 74, 78](42%), for attention and working memory, three [34, 63, 79] of eight [30, 31, 48, 74, 78](38%). No study demonstrated links with fluency [31, 48, 51, 56, 61, 74] or visuospatial/visuoconstructive functions[56]. Different neuropsychological tests often went to each cognitive domain and not all studies categorized all tests similarly. For example, one study[33] included grooved pegboard, digit symbol, and Trail Making Test Part B as processing speed measures. While this domain was associated with depression, other studies typically categorized grooved pegboard as a test of motor function and Trail Making Test Part B as a test of executive function. Additionally, some treated cognition continuously whereas others examined CI.
Table 3.
Relationship between current depression and domain specific cognitive performance in people living with HIV in the era of effective antiretrovirals (ARVs): Cross-sectional and longitudinal analyses.
| On ARVs (%) |
Cognitive Domains |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | N | EF | FLU | ATT/WM | PS | LRN/MEM | VS | Motor | |
| Cross-sectional | |||||||||
| Questionnaires | |||||||||
| Rubin (2014)[30] | 708 | 65 | ⊺ | ⊺ | * | ||||
| WIHS | |||||||||
| Rubin (2017)[31] | 646 | 77 | * | ⊺ | ⊺ | * | * | ⊺ | |
| WIHS | |||||||||
| Haddow (2018)[48] | 448 | 89 | ns | ns | ns | ** | ns | ||
| CIPHER | |||||||||
| Starace (2000)[51] | 395 | 74 | ns | ns | ns | ns | ns | ||
| ICONA | |||||||||
| Shimizu (2011)[33]†‖ | 285 | 72 | * | ns | |||||
| Hawaii Aging with HIV | |||||||||
| Tymchuk (2018)[55] | 265 | 93 | * | ||||||
| Castellon (2006)[56] | 247 | - | * | ns | ns | * | ns | ns | |
| Ammassari (2004)[61] | 135 | 100 | ns | ns | ns | ns | ns | ||
| ICONA | |||||||||
| Akolo (2014)[74] | 133 | 0 | ns | ns | ns | ns | ns | ⊺ | |
| Braganca (2011)[34] | 130 | 78 | * | ** | ** | * | ** | ||
| Bryant (2015)[78] | 120 | 82 | ns | ns | ** | ns | * | ||
| Harrison (2017)[79]† | 103 | 100 | ** | ns | |||||
| Diagnostic Interview | |||||||||
| Fellows (2014)[32] | 186 | 77 | ** | * | * | ||||
| MHBB | |||||||||
| Gibbie (2006)[63]B | 129 | 93 | * | * | * | ||||
| Lawler (2010)[64] | 120 | 98 | ns | ns | |||||
| Longitudinal | |||||||||
| Questionnaires | |||||||||
| Vo [53] | 362 | - | * | ns | *** | ||||
| MACS | |||||||||
| Diagnostic Interview | |||||||||
| Cysique (2007)[57] | 227 | 35 | ns | ns | ns | ns | ns | ns | |
| CHARTER | |||||||||
ns=not significant
p<0.05
p<0.01
p<0.001, p’s<0.08
Cognition: EF=executive function; FLU=Fluency; ATT/WM=attention and working memory; PS=processing speed; LRN/MEM=learning and memory; VS= visuospatial & visuoconstructive functions
studies excluded individuals either meeting criteria for major depression according to the Diagnostic and Statistical Manual of Mental Disorders
Significant association in younger group only.
=baseline assessment
Study Cohorts: CHARTER=CNS HIV Anti-Retroviral Therapy Effects Research cohort, ICONA=Italian Cohort Naïve Antiretrovirals research programme, MACS=Multicenter AIDS Cohort Study; MHBB=Manhattan HIV Brain Bank; WIHS=Women’s Interagency HIV Study
In longitudinal analyses, what is the relationship between depression and cognition among PLWH?
Four studies[57, 67, 68, 77], all in either US or Australia, examined the relationship between depression and CI over time among PLWH (Table 2). Three were from CHARTER[57, 68, 77]; although it is unclear whether the participants from each study are independent. Two studies demonstrated an association between depression and CI[68, 77]. One study demonstrated that current MDD predicted decline to symptomatic HAND[68]. Specifically, current MDD was associated with a 3-fold increased risk of decline to symptomatic HAND (95%CI 1.56-5.77, p=0.001). A second study also demonstrated an association between lifetime MDD and CI [77]. Specifically, lifetime MDD was associated with almost a two-fold increase risk for cognitive decline (RR=1.7, p=0.01). For current depression measured continuously with the BID, there was a significant association with cognitive decline (p=0.005). However, for recent MDD (last 30 days) with a diagnostic interview, there was only a non-significant association with cognitive decline (RR=1.7, p=0.06). Of the two studies[53, 57] examining longitudinal associations between changes in depression and changes in domain specific cognitive decline, one study found a significant association between depression and executive function and processing speed[53]. ARV use in the other study[57] was considerably lower (35%) than in other studies, again, raising the possibility that the effects of active viremia overwhelmed any effect of depression.
Does the association between depression and cognition differ between PLWH and HIV− individuals?
Cross-sectional analyses.
Seven studies examined the association between depression and cognition in samples of PLWH and HIV− controls[30, 47, 60, 62, 71, 73, 74]. Three of these studies did so in the combined sample[62, 71, 73] and demonstrated an association between depression and CI. Three studies examined depression and domain-specific cognitive function, and all studies examined these associations in the combined sample [30, 47, 60]. In WIHS, depression was associated with lower executive function, attention, learning, and memory[30]. In MACS, depression was associated with slower psychomotor speed; with a trend for memory[47]. In another study, depression was associated with slower psychomotor speed and motor function[60]. One study examined associations stratified by HIV− serostatus and found no significant associations but trends on motor in PLWH and fluency in HIV− controls[74]. One study examined the prevalence of CI and depression among PLWH versus HIV− individuals before and six months after initiating ARVs[62]. At both time points, the odds of having both CI and depression was significantly higher in PLWH (39% and 30%, respective time point) versus HIV− individuals (4% and 9%).
Longitudinal analyses.
Three, large-scale, multi-center, longitudinal analyses, including two in the MACS[82, 83] and one in WIHS[31], focused on the association between depression (CES-D ≥16) and cognition in combined samples of PLWH and controls. The largest MACS study included 2099 HIV+ and 1793 HIV− individuals, of who 21% were on ARVs and the standardized log10 viral load was on average 3.5 (SD=1.4)[83]. Participants performed tests of executive function, processing speed, attention and working memory, learning, memory, and motor function. A novel approach, mixed membership trajectory models, was used to characterize a few typical cognitive trajectories over time and to determine the probability of membership of each individual to those profiles. In this model, each individual did not necessarily belong exclusively to a membership category (profile) but rather had a weighted membership to each of the extreme profiles identified. Three distinct trajectories were identified including a “normal aging” profile (low probability of mild impairment until age 60), a “premature aging” profile (mild impairment starting at age 45-50), and an “unhealthy aging” profile (mild impairment in 20s and 30s). Notably, depression was a strong predictor of each participant’s closeness to each of the three trajectories. Specifically, depression increased closeness to the “unhealthy aging” profile (81% depressed) compared to both the “premature aging” (72% depressed) and “normal aging” (71% depressed) profiles (p’s<0.05). These estimates were not stratified by HIV-serostatus so it is unknown whether the combination of HIV-serostatus and depression increased closeness to the detrimental cognitive trajectories.
The second MACS study included 669 HIV+ and 942 HIV− men, with a mean age of 51.5 (SD=3.1) years, and only measured attention and executive function[82]. Depression and cognitive profiles were examined using group-based dual trajectory modeling, an approach enabling the identification of distinctive trajectories for each outcome (depression and cognition) and then allowing the examination of the interrelationship of those two outcomes across the trajectory groups over time. Three patterns were identified for depression, those who rarely or never have depression (HIV+ 50%; HIV− 61%), those with periodic depression (30% HIV+; 25% HIV−), and those with chronic depression (HIV+ 21%, 15% HIV−). For cognition, three profiles were identified - worst performing (HIV+ 47%; 45%), average performing (HIV+ 42%, HIV− 47%), and best-performing (HIV+11%; HIV− 8%). Among both PLWH and HIV− individuals, the chronic high depressed group had the highest percent membership in the worst-performing attention/executive function group (52%, 60%, respectively) and the rare/never depressed group had the highest percent membership in the best-performing attention/executive function group (13%, 8%, respectively). In adjusted analyses, the association between depression and cognition was only significant among HIV− individuals (p’s<0.05).
The WIHS study included 646 HIV+ (77% on ARVs; 52% HIV RNA undetectable) and 300 HIV− women, with a mean age of 45 (SD=9) years[31]. At the initial time point, 2, and 4 years later, seven cognitive domains were assessed including learning, memory, attention and working memory, processing speed, fluency, executive function, and motor function. Among PLWH, 32% had depression at one or two visits; 12% had depression at all visits (same percentages among HIV−). There was no cognitive domain in which the magnitude of change in performance over time depended on the combined influence of HIV-serostatus and depression. Rather, regardless of time, performance on attention and working memory depended on the combined influence of HIV-serostatus and depression (p=0.01). Similar to MACS[82], the interaction was driven by the HIV− and not the HIV+ individuals such that depression was associated with lower performance on this domain among HIV− (p<0.001) but not HIV+ women (p=0.12). Additionally, depression was associated with lower performance on global function (p<0.0001), memory (p=0.001), executive function (p<0.0001), psychomotor speed (p<0.0001), fluency (p=0.002), and motor function (p<0.0001) across time points.
Conclusion
Numerous studies have examined the association between cognition and depression in PLWH. The importance of considering this association is evident in the high rates of current and lifetime depression in these studies. Although rates of depression vary, the median rate for current depression was 24% in studies using self-report questionnaires[30, 31, 34, 46–51, 53–56, 58–63, 65–67] as well as in studies using structured diagnostic interviews[32, 52, 57, 63, 64]. Lifetime prevalence was on average 42%[57, 63, 67, 68]. The level of severity of MDD was examined in one study using structured diagnostic interviews and found to be “serious” in 80% of cases[4]. Thus, depression is an important and serious psychiatric comorbidity in PLWH.
There is reliable evidence that depression is associated with CI. Fourteen of 20 cross-sectional studies found a link between depression and CI[31, 33, 34, 48–50, 55, 58, 66, 69, 70, 72, 75, 78] and three studies (15%) noted trends in associations (p < .11)[46, 54, 59]. Studies using structured diagnostic interviews [52, 57, 67] also generally showed an association, except for one[57] where 35% of participants were on ARVs compared to other studies where more than 89% were on ARVs [52, 67]. The cognitive domains most reliably associated with depression across cross-sectional studies[30–34, 48, 51, 55, 56, 59, 61, 63, 64, 74, 76, 78, 79] were processing speed, executive function, learning and memory, and motor function, with many of the studies assessing those domains finding associations. Attention and working memory were associated with depression in 38% of cross-sectional studies.
Among the four longitudinal studies of the association between depression and cognition that did not include HIV− controls[57, 67, 68, 77] three were in CHARTER[57, 68, 77], one was in an Australian cohort[67], and of these two CHARTER studies[68, 77] found an association with depression. Although the Australian study found no overall association, certain aspects of depression including remission status, stability of MDD treatment, and severity of depression, were related to cognition[67]. The one CHARTER study that did not find a relationship excluded individuals with untreated MDD[76], whereas both of the CHARTER studies reporting a significant relationship did not indicate any exclusions based on MDD treatment. This raises the possibility that excluding for untreated MDD led to an underestimation of the general relationship between depression and CI. Indeed, CHARTER found evidence both that lifetime MDD predicted time to cognitive decline and conversely that the absence of lifetime MDD predicted cognitive improvement[77]. The other CHARTER study similarly demonstrated that current MDD was associated with a risk of decline to symptomatic HAND[68].
In longitudinal studies involving both PLWH and HIV− individuals, including all-male[82] and all-female cohorts[31], depression was associated with CI in PLWH and HIV− groups. In both cohorts, there were interactions between HIV serostatus and depression in select cognitive domains including attention/executive function, such that the associations were stronger or only significant in HIV− versus PLWH individuals. Thus, there is no evidence that PLWH are differentially susceptible to the negative associations between depression and CI. This appears true for women and men, because findings in WIHS and MACS are in agreement with the crosssectional studies which showed reliable associations with global function for PLWH and HIV− individuals. Additional studies of HIV+ women are needed, given that versus HIV+ men, HIV+ women have higher rates [84] of depression and more severe depressive symptoms[85–88].
Are depression-related cognitive deficits reversible in PLWH?
The observed association between depression and poor cognitive performance in PLWH may be (1) due to common biological pathways (e.g., neuroinflammation, dopaminergic alterations), (2) common psychosocial determinants (e.g., poverty, trauma, chronic stress); (4) common behavioral disorders (e.g., substance use, ARV nonadherence); (5) the causal influence of CI and related functional limitations on mood; (6) the causal influence of depression on CI and/or (7) the overlap between the signs and symptoms of CI and depression (e.g., psychomotor slowing, sleep disruption, poor nutrition) (see [7, 27, 29] for reviews). It is likely that all of these factors come into play, and that there is heterogeneity among individuals in the factors leading to these associations. Nevertheless, given the significant mental health burden experienced by PLWH, it is worthwhile to consider how treating depression might influence cognition in PLWH.
A variety of interventions are reported to be effective in treating depression in PLWH. Psychotherapeutic interventions are particularly effective, especially those involving cognitive behavioral therapy (CBT)[89]. CBT is efficacious in improving depressive symptoms in depressed PLWH[90], including those with substance use disorders[91, 92]. These interventions can be delivered via phone with equivalent efficacy with face-to-face interventions[93, 94]. Antidepressants can also be efficacious in improving depressive symptoms among PLWH[95–97], though its effects in women, minorities, and low- to middle-income countries are unknown. Additionally, less is known about the effects of antidepressants on HIV outcomes (e.g., CD4 count, HIV RNA). Some studies report increased viral suppression and CD4 T cells among PLWH initiating antidepressants[98]. Most studies on the effects of antidepressants on depression were conducted before 2000, so our understanding of antidepressants on mental health is not fully characterized in the current era of ARVs where PLWH are also prescribed numerous concomitant non-ARV medications[99].
The studies examining the effectiveness of CBT or antidepressants in PLWH have not examined whether these depression treatments improve cognition. Instead these studies have primarily focused on the efficacy of these treatments, specifically CBT, on ARV adherence which was supported in the case in PLWH[90] but not among PLWH with substance use disorders[91]. Importantly, treating depression in HIV-uninfected individuals improves cognition[100–103].
Gaps in Knowledge and Opportunities
In general, the field of psychiatry has moved away from conceptualizing mental health and disorders as discrete diagnostic categories to being transdiagnostic brain disorders[104, 105], specifically in the Research Domain Criteria (RDoC) framework. This framework is based on the idea that fundamental dysfunctional neurobiological processes or neurobehavioral systems underlie multiple and often comorbid mental health disorders. The advantage of a transdiagnostic approach is that it provides promise in understanding the heterogeneity of mental health disorders, their comorbidities, and functional consequences. Additionally, this approach provides a strong foundation for the development of targeted interventions and treatments to improve mental health. Despite strong scientific evidence supporting the need for a transdiagnostic framework for understanding psychopathology, this approach has not been systematically applied to HIV.
Conclusion
In summary, depression is a highly prevalent disorder that is negatively associated with cognitive function in PLWH. Both a history of depression and current depression are associated with CI among PLWH. The pattern of associations between depression and domain-specific cognitive performance is similar to those reported in HIV− individuals. There remains gaps in knowledge about whether treating depression would improve CI in PLWH and if so what mechanisms (e.g., direct neurobiological, indirect improvement through improved ARV adherence) might explain such improvements. Use of a transdiagnostic approach, which has well-characterized neurobiological substrates for different aspects of depression such as loss, negative valence, and anhedonia could inform this area of research. Thus, this area of research remains a priority and an area that may greatly benefit from utilizing a transdiagnostic approach.
Acknowledgements
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH113512 (Rubin).
Footnotes
Conflict of Interest
The authors declare no conflicts of interest
Compliance with Ethics Guidelines
Human and Animal rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3(11):e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 2001; 58(8):721–728. [DOI] [PubMed] [Google Scholar]
- 3.Orlando M, Burnam MA, Beckman R, Morton SC, London AS, Bing EG, et al. Re-estimating the prevalence of psychiatric disorders in a nationally representative sample of persons receiving care for HIV: results from the HIV Cost and Services Utilization Study. Int J Methods Psychiatr Res 2002; 11(2):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook JA, Burke-Miller JK, Steigman PJ, Schwartz RM, Hessol NA, Milam J, et al. Prevalence, Comorbidity, and Correlates of Psychiatric and Substance Use Disorders and Associations with HIV Risk Behaviors in a Multisite Cohort of Women Living with HIV. AIDS and behavior 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Do AN, Rosenberg ES, Sullivan PS, Beer L, Strine TW, Schulden JD, et al. Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PloS one 2014; 9(3):e92842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherr L, Cluver L. World Health Day focus on HIV and depression - a comorbidity with specific challenges. Journal of the International AIDS Society 2017; 20(1):21956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: a review. Curr Psychiatry Rep 2015; 17(1):530. [DOI] [PubMed] [Google Scholar]
- 8.Grov C, Golub SA, Parsons JT, Brennan M, Karpiak SE. Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS care 2010; 22(5):630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mascolini M Depression Risk Factors With HIV -- Plus Screening and Diagnosis Keys In: The Center for AIDS Information & Advocacy; 2016. [Google Scholar]
- 10.Slot M, Sodemann M, Gabel C, Holmskov J, Laursen T, Rodkjaer L. Factors associated with risk of depression and relevant predictors of screening for depression in clinical practice: a cross-sectional study among HIV-infected individuals in Denmark. HIV medicine 2015; 16(7):393–402. [DOI] [PubMed] [Google Scholar]
- 11.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA 2001; 285(11):1466–1474. [DOI] [PubMed] [Google Scholar]
- 12.Thames AD, Arentoft A, Rivera-Mindt M, Hinkin CH. Functional disability in medication management and driving among individuals with HIV: a 1-year follow-up study. Journal of clinical and experimental neuropsychology 2013; 35(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thames AD, Kim MS, Becker BW, Foley JM, Hines LJ, Singer EJ, et al. Medication and finance management among HIV-infected adults: the impact of age and cognition. Journal of clinical and experimental neuropsychology 2011; 33(2):200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marquine MJ, Flores I, Kamat R, Johnson N, Umlauf A, Letendre S, et al. A composite of multisystem injury and neurocognitive impairment in HIV infection: association with everyday functioning. Journal of neurovirology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thaler NS, Sayegh P, Arentoft A, Thames AD, Castellon SA, Hinkin CH. Increased neurocognitive intra-individual variability is associated with declines in medication adherence in HIV-infected adults. Neuropsychology 2015; 29(6):919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poquette AJ, Moore DJ, Gouaux B, Morgan EE, Grant I, Woods SP, et al. Prospective memory and antiretroviral medication non-adherence in HIV: an analysis of ongoing task delay length using the memory for intentions screening test. Journal of the International Neuropsychological Society : JINS 2013; 19(2):155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods SP, Weber E, Weisz BM, Twamley EW, Grant I, Group HIVNRP. Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabil Psychol 2011; 56(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott JC, Woods SP, Vigil O, Heaton RK, Schweinsburg BC, Ellis RJ, et al. A neuropsychological investigation of multitasking in HIV infection: implications for everyday functioning. Neuropsychology 2011; 25(4):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zogg JB, Woods SP, Weber E, Iudicello JE, Dawson MS, Grant I, et al. HIV-associated prospective memory impairment in the laboratory predicts failures on a semi-naturalistic measure of health care compliance. Clin Neuropsychol 2010; 24(6):945–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol Rev 2009; 19(2):186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle K, Weber E, Atkinson JH, Grant I, Woods SP, Group HIVNRP. Aging, prospective memory, and health-related quality of life in HIV infection. AIDS and behavior 2012; 16(8):2309–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tozzi V, Balestra P, Murri R, Galgani S, Bellagamba R, Narciso P, et al. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. International journal of STD & AIDS 2004; 15(4):254–259. [DOI] [PubMed] [Google Scholar]
- 23.Tozzi V, Balestra P, Galgani S, Murri R, Bellagamba R, Narciso P, et al. Neurocognitive performance and quality of life in patients with HIV infection. AIDS Res Hum Retroviruses 2003; 19(8):643–652. [DOI] [PubMed] [Google Scholar]
- 24.Osowiecki DM, Cohen RA, Morrow KM, Paul RH, Carpenter CC, Flanigan T, et al. Neurocognitive and psychological contributions to quality of life in HIV-1-infected women. AIDS (London, England) 2000; 14(10):1327–1332. [DOI] [PubMed] [Google Scholar]
- 25.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep 2014; 11(3):291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sin NL, DiMatteo MR. Depression treatment enhances adherence to antiretroviral therapy: a meta-analysis. Ann Behav Med 2014; 47(3):259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. Journal of acquired immune deficiency syndromes (1999) 2011; 58(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS (London, England) 2007; 21(9):1175–1183. [DOI] [PubMed] [Google Scholar]
- 29.Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med 2014; 44(10):2029–2040. [DOI] [PubMed] [Google Scholar]
- 30.Rubin LH, Sundermann EE, Cook JA, Martin EM, Golub ET, Weber KM, et al. Investigation of menopausal stage and symptoms on cognition in human immunodeficiency virus-infected women. Menopause (New York, NY) 2014; 21(9):997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin LH, Cook JA, Springer G, Weber KM, Cohen MH, Martin EM, et al. Perceived and post-traumatic stress are associated with decreased learning, memory, and fluency in HIV-infected women. AIDS (London, England) 2017; 31(17):2393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fellows RP, Byrd DA, Morgello S. Effects of information processing speed on learning, memory, and executive functioning in people living with HIV/AIDS. Journal of clinical and experimental neuropsychology 2014; 36(8):806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu SM, Chow DC, Valcour V, Masaki K, Nakamoto B, Kallianpur KJ, et al. The Impact of Depressive Symptoms on Neuropsychological Performance Tests in HIV-Infected Individuals: A Study of the Hawaii Aging with HIV Cohort. World journal of AIDS 2011; 1(4):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braganca M, Palha A. Depression and neurocognitive performance in Portuguese patients infected with HIV. AIDS and behavior 2011; 15(8):1879–1887. [DOI] [PubMed] [Google Scholar]
- 35.Baxter LR Jr., Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46(3):243–250. [DOI] [PubMed] [Google Scholar]
- 36.Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, et al. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res 2004; 50(1):1–11. [DOI] [PubMed] [Google Scholar]
- 37.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res 2009; 201(2):239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry 2007; 61(2):198–209. [DOI] [PubMed] [Google Scholar]
- 39.Thomas EJ, Elliott R. Brain imaging correlates of cognitive impairment in depression. Front Hum Neurosci 2009; 3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci Biobehav Rev 2003; 27(3):233–246. [DOI] [PubMed] [Google Scholar]
- 41.Gold PW, Drevets WC, Charney DS. New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biol Psychiatry 2002; 52(5):381–385. [DOI] [PubMed] [Google Scholar]
- 42.Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R. HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS (London, England) 2014; 28(6):803–811. [DOI] [PubMed] [Google Scholar]
- 43.Del Guerra FB, Fonseca JL, Figueiredo VM, Ziff EB, Konkiewitz EC. Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. Journal of neurovirology 2013; 19(4):314–327. [DOI] [PubMed] [Google Scholar]
- 44.Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun 2015; 45:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65(9):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright E, Brew B, Arayawichanont A, Robertson K, Samintharapanya K, Kongsaengdao S, et al. Neurologic disorders are prevalent in HIV-positive outpatients in the Asia-Pacific region. Neurology 2008; 71(1):50–56. [DOI] [PubMed] [Google Scholar]
- 47.Becker JT, Kingsley L, Mullen J, Cohen B, Martin E, Miller EN, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology 2009; 73(16):1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haddow LJ, Laverick R, Daskalopoulou M, McDonnell J, Lampe FC, Gilson R, et al. Multicenter European Prevalence Study of Neurocognitive Impairment and Associated Factors in HIV Positive Patients. AIDS and behavior 2018; 22(5):1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gascon MRP, Vidal JE, Mazzaro YM, Smid J, Marcusso RMN, Capitao CG, et al. Neuropsychological Assessment of 412 HIV-Infected Individuals in Sao Paulo, Brazil. AIDS patient care and STDs 2018; 32(1):1–8. [DOI] [PubMed] [Google Scholar]
- 50.Bonnet F, Amieva H, Marquant F, Bernard C, Bruyand M, Dauchy FA, et al. Cognitive disorders in HIV-infected patients: are they HIV-related? AIDS (London, England) 2013; 27(3):391–400. [DOI] [PubMed] [Google Scholar]
- 51.Starace F, Bartoli L, Aloisi MS, Antinori A, Narciso P, Ippolito G, et al. Cognitive and affective disorders associated to HIV infection in the HAART era: findings from the NeuroICONA study. Cognitive impairment and depression in HIV/AIDS. The NeuroICONA study. Acta psychiatrica Scandinavica 2002; 106(1):20–26. [DOI] [PubMed] [Google Scholar]
- 52.Pinheiro CA, Souza LD, Motta JV, Kelbert EF, Souza MS, Martins CS, et al. Depression and diagnosis of neurocognitive impairment in HIV-positive patients. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 2016; 49(10):e5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vo QT, Cox C, Li X, Jacobson LP, McKaig R, Sacktor N, et al. Neuropsychological test performance before and after HIV-1 seroconversion: the Multicenter AIDS Cohort Study. Journal of neurovirology 2013; 19(1):24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bloch M, Kamminga J, Jayewardene A, Bailey M, Carberry A, Vincent T, et al. A Screening Strategy for HIV-Associated Neurocognitive Disorders That Accurately Identifies Patients Requiring Neurological Review. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2016; 63(5):687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tymchuk S, Gomez D, Koenig N, Gill MJ, Fujiwara E, Power C. Associations between Depressive Symptomatology and Neurocognitive Impairment in HIV/AIDS. Canadian journal of psychiatry Revue canadienne de psychiatrie 2018; 63(5):329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castellon SA, Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, et al. Components of depression in HIV-1 infection: their differential relationship to neurocognitive performance. Journal of clinical and experimental neuropsychology 2006; 28(3):420–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cysique LA, Deutsch R, Atkinson JH, Young C, Marcotte TD, Dawson L, et al. Incident major depression does not affect neuropsychological functioning in HIV-infected men. Journal of the International Neuropsychological Society : JINS 2007; 13(1):1–11. [DOI] [PubMed] [Google Scholar]
- 58.Kelly CM, van Oosterhout JJ, Ngwalo C, Stewart RC, Benjamin L, Robertson KR, et al. HIV associated neurocognitive disorders (HAND) in Malawian adults and effect on adherence to combination anti-retroviral therapy: a cross sectional study. PloS one 2014; 9(6):e98962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schouten J, Su T, Wit FW, Kootstra NA, Caan MW, Geurtsen GJ, et al. Determinants of reduced cognitive performance in HIV-1-infected middle-aged men on combination antiretroviral therapy. AIDS (London, England) 2016; 30(7):1027–1038. [DOI] [PubMed] [Google Scholar]
- 60.Janssen MA, Meulenbroek O, Steens SC, Goraj B, Bosch M, Koopmans PP, et al. Cognitive functioning, wellbeing and brain correlates in HIV-1 infected patients on long-term combination antiretroviral therapy. AIDS (London, England) 2015; 29(16):2139–2148. [DOI] [PubMed] [Google Scholar]
- 61.Ammassari A, Antinori A, Aloisi MS, Trotta MP, Murri R, Bartoli L, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics 2004; 45(5):394–402. [DOI] [PubMed] [Google Scholar]
- 62.Nakasujja N, Skolasky RL, Musisi S, Allebeck P, Robertson K, Ronald A, et al. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda. BMC psychiatry 2010; 10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gibbie T, Mijch A, Ellen S, Hoy J, Hutchison C, Wright E, et al. Depression and neurocognitive performance in individuals with HIV/AIDS: 2-year follow-up. HIV medicine 2006; 7(2):112–121. [DOI] [PubMed] [Google Scholar]
- 64.Lawler K, Mosepele M, Ratcliffe S, Seloilwe E, Steele K, Nthobatsang R, et al. Neurocognitive impairment among HIV-positive individuals in Botswana: a pilot study. Journal of the International AIDS Society 2010; 13:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gandhi NS, Moxley RT, Creighton J, Roosa HV, Skolasky RL, Selnes OA, et al. Comparison of scales to evaluate the progression of HIV-associated neurocognitive disorder. HIV therapy 2010; 4(3):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS (London, England) 2010; 24(9):1243–1250. [DOI] [PubMed] [Google Scholar]
- 67.Cysique LA, Dermody N, Carr A, Brew BJ, Teesson M. The role of depression chronicity and recurrence on neurocognitive dysfunctions in HIV-infected adults. Journal of neurovirology 2016; 22(1):56–65. [DOI] [PubMed] [Google Scholar]
- 68.Grant I, Franklin DR Jr., Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 2014; 82(23):2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blackstone K, Moore DJ, Heaton RK, Franklin DR Jr, Woods SP, Clifford DB, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. Journal of the International Neuropsychological Society : JINS 2012; 18(1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yusuf AJ, Hassan A, Mamman AI, Muktar HM, Suleiman AM, Baiyewu O. Prevalence of HIV-Associated Neurocognitive Disorder (HAND) among Patients Attending a Tertiary Health Facility in Northern Nigeria. Journal of the International Association of Providers of AIDS Care 2017; 16(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Judd A, Le Prevost M, Melvin D, Arenas-Pinto A, Parrott F, Winston A, et al. Cognitive Function in Young Persons With and Without Perinatal HIV in the AALPHI Cohort in England: Role of Non-HIV-Related Factors. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2016; 63(10):1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robinson-Papp J, Byrd D, Mindt MR, Oden NL, Simpson DM, Morgello S, et al. Motor function and human immunodeficiency virus-associated cognitive impairment in a highly active antiretroviral therapy-era cohort. Archives of neurology 2008; 65(8):1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richardson JL, Nowicki M, Danley K, Martin EM, Cohen MH, Gonzalez R, et al. Neuropsychological functioning in a cohort of HIV- and hepatitis C virus-infected women. AIDS (London, England) 2005; 19(15):1659–1667. [DOI] [PubMed] [Google Scholar]
- 74.Akolo C, Royal W, 3rd, Cherner M, Okwuasaba K, Eyzaguirre L, Adebiyi R, et al. Neurocognitive impairment associated with predominantly early stage HIV infection in Abuja, Nigeria. Journal of neurovirology 2014; 20(4):380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenthal LS, Skolasky RL, Moxley RTt, Roosa HV, Selnes OA, Eschman A, et al. A novel computerized functional assessment for human immunodeficiency virus-associated neurocognitive disorder. Journal of neurovirology 2013; 19(5):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. Journal of neurovirology 2011; 17(2):176–183. [DOI] [PubMed] [Google Scholar]
- 77.Heaton RK, Franklin DR Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2015; 60(3):473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bryant VE, Whitehead NE, Burrell LE, 2nd, Dotson VM, Cook RL, Malloy P, et al. Depression and Apathy Among People Living with HIV: Implications for Treatment of HIV Associated Neurocognitive Disorders. AIDS and behavior 2015; 19(8):1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harrison JD, Dochney JA, Blazekovic S, Leone F, Metzger D, Frank I, et al. The nature and consequences of cognitive deficits among tobacco smokers with HIV: a comparison to tobacco smokers without HIV. Journal of neurovirology 2017; 23(4):550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69(18):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59 Suppl 20:22–33;quiz 34–57. [PubMed] [Google Scholar]
- 82.Armstrong NM, Surkan PJ, Treisman GJ, Sacktor NC, Irwin MR, Teplin LA, et al. Association of long-term patterns of depressive symptoms and attention/executive function among older men with and without human immunodeficiency virus. Journal of neurovirology 2017; 23(4):558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Molsberry SA, Lecci F, Kingsley L, Junker B, Reynolds S, Goodkin K, et al. Mixed membership trajectory models of cognitive impairment in the multicenter AIDS cohort study. AIDS (London, England) 2015; 29(6):713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Turner BJ, Laine C, Cosler L, Hauck WW. Relationship of gender, depression, and health care delivery with antiretroviral adherence in HIV-infected drug users. J Gen Intern Med 2003; 18(4):248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aljassem K, Raboud JM, Hart TA, Benoit A, Su D, Margolese SL, et al. Gender Differences in Severity and Correlates of Depression Symptoms in People Living with HIV in Ontario, Canada. Journal of the International Association of Providers of AIDS Care 2016; 15(1):23–35. [DOI] [PubMed] [Google Scholar]
- 86.Semple SJ, Patterson TL, Straits-Troster K, Atkinson JH, McCutchan JA, Grant I. Social and psychological characteristics of HIV-infected women and gay men. HIV Neurobehavioral Research Center (HNRC) Group. Women Health 1996; 24(2):17–41. [DOI] [PubMed] [Google Scholar]
- 87.Rabkin J, Rabkin R. [Depression and HIV]. Sidahora 1997:19–22. [PubMed] [Google Scholar]
- 88.Robertson K, Bayon C, Molina JM, McNamara P, Resch C, Munoz-Moreno JA, et al. Screening for neurocognitive impairment, depression, and anxiety in HIV-infected patients in Western Europe and Canada. AIDS care 2014; 26(12):1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sherr L, Clucas C, Harding R, Sibley E, Catalan J. HIV and depression--a systematic review of interventions. Psychol Health Med 2011; 16(5):493–527. [DOI] [PubMed] [Google Scholar]
- 90.Safren SA, O’Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol 2009; 28(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: a randomized controlled trial. J Consult Clin Psychol 2012; 80(3):404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grelotti DJ, Hammer GP, Dilley JW, Karasic DH, Sorensen JL, Bangsberg DR, et al. Does substance use compromise depression treatment in persons with HIV? Findings from a randomized controlled trial. AIDS care 2017; 29(3):273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Himelhoch S, Medoff D, Maxfield J, Dihmes S, Dixon L, Robinson C, et al. Telephone based cognitive behavioral therapy targeting major depression among urban dwelling, low income people living with HIV/AIDS: results of a randomized controlled trial. AIDS and behavior 2013; 17(8):2756–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Himelhoch S, Mohr D, Maxfield J, Clayton S, Weber E, Medoff D, et al. Feasibility of telephone-based cognitive behavioral therapy targeting major depression among urban dwelling African-American people with co-occurring HIV. Psychol Health Med 2011; 16(2):156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Himelhoch S, Medoff DR. Efficacy of antidepressant medication among HIV-positive individuals with depression: a systematic review and meta-analysis. AIDS patient care and STDs 2005; 19(12):813–822. [DOI] [PubMed] [Google Scholar]
- 96.Eshun-Wilson I, Siegfried N, Akena DH, Stein DJ, Obuku EA, Joska JA. Antidepressants for depression in adults with HIV infection. Cochrane Database Syst Rev 2018; 1:CD008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ngo VK, Wagner GJ, Nakasujja N, Dickens A, Aunon F, Musisi S. Effectiveness of antidepressants and predictors of treatment response for depressed HIV patients in Uganda. International journal of STD & AIDS 2015; 26(14):998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mills JC, Harman JS, Cook RL, Marlow NM, Harle CA, Duncan RP, et al. Comparative effectiveness of dual vs. single-action antidepressants on HIV clinical outcomes in HIV-infected people with depression. AIDS (London, England) 2017; 31(18):2515–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Radtke KK, Bacchetti P, Anastos K, Merenstein D, Crystal H, Karim R, et al. Use of Nonantiretroviral Medications That May Impact Neurocognition: Patterns and Predictors in a Large, Long-Term HIV Cohort Study. Journal of acquired immune deficiency syndromes (1999) 2018; 78(2):202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rosenblat JD, Kakar R, McIntyre RS. The Cognitive Effects of Antidepressants in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int J Neuropsychopharmacol 2015; 19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baune BT, Brignone M, Larsen KG. A Network Meta-Analysis Comparing Effects of Various Antidepressant Classes on the Digit Symbol Substitution Test (DSST) as a Measure of Cognitive Dysfunction in Patients with Major Depressive Disorder. Int J Neuropsychopharmacol 2018; 21(2):97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martin DM, McClintock SM, Forster JJ, Lo TY, Loo CK. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: A systematic review and meta-analysis of individual task effects. Depress Anxiety 2017; 34(11):1029–1039. [DOI] [PubMed] [Google Scholar]
- 103.McIntyre RS, Harrison J, Loft H, Jacobson W, Olsen CK. The Effects of Vortioxetine on Cognitive Function in Patients with Major Depressive Disorder: A Meta-Analysis of Three Randomized Controlled Trials. Int J Neuropsychopharmacol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167(7):748–751. [DOI] [PubMed] [Google Scholar]
- 105.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci 2012; 14(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


