Abstract
Serum ferritin reflects total body iron stores, thus a low serum ferritin is used as a parameter of iron deficiency. In healthy adults in Japan, urine ferritin levels were about 5% of serum ferritin levels, with a correlation coefficient of 0.79. It is not known whether a low urine ferritin could serve as a non-invasive screen for iron deficiency. If so, this might be useful for neonates and young children, avoiding phlebotomy to screen for iron deficiency. However, for urinary ferritin screening to be feasible, ferritin must be measurable in the urine and correlate with serum ferritin. Testing should also clarify whether the iron content of ferritin in serum and urine are similar. In this pilot feasibility study we measured ferritin in paired serum and urine samples of healthy adult males, healthy term neonates, growing preterm neonates, and children who had very high serum ferritin levels from liver disorders or iron overload. We detected ferritin in every urine sample, and found a correlation with paired serum ferritin (Spearman correlation coefficient 0.78 of log10-transformed values). These findings suggest merit in further studying urinary ferritin in select populations, as a potential non-invasive screen to assess iron stores.
Keywords: iron, iron deficiency, ferritin, urine, serum
Introduction
When iron deficiency occurs in a newborn infant, long-term neurobehavioral defects are common [1–4]. Iron deficiency can be present at birth among certain high-risk groups, such as infants of diabetic mothers, small for gestational age infants, and very low birth weight premature neonates [5–8]. Unfortunately, the current means of screening for iron deficiency in neonates requires phlebotomy, and is primarily focused on detecting anemia. However, anemia is a rather late effect of iron deficiency in neonates, and likely does not occur until after deficient brain iron deficiency has caused neurological deficits.
Various blood tests can detect early biochemical iron deficiency in neonates before erythropoietic limitation and anemia have occurred [7–10]. A low level of ferritin in serum correlates with low total body iron stores [11], and on that basis is used as a marker for iron deficiency. However, ferritin, like all markers of iron deficiency, is limited in its usefulness for very small infants by the phlebotomy volumes required [12]. Thus, we conducted the present study as a step toward understanding whether measuring the urinary ferritin level could be a feasible, non-invasive, means of screening neonates and young children for iron deficiency or iron overload.
Materials and Methods
The University of Utah Institutional Review Board approved the study protocol. The adults and older children signed informed consent documents. The Board granted a waiver from individual parental consent for the umbilical cord blood and urine from neonates because these were obtained from otherwise discarded blood samples and diapers, and were deidentified with appropriate privacy protection.
Preliminary methods testing of urine collections involved triplicate techniques, where each urine sample was collected in a plastic urine bag, in cotton balls in the diaper, and in rayon balls in the diaper. This testing was to determine whether the collection method affected the ferritin level measured, as could occur with binding of ferritin to plastic or cotton or rayon.
Ferritin concentrations in serum and in urine were measured by ELISA (MP Biomedicals, LLC, Santa Ana, CA) according the manufacture’s specifications. For selected samples, anti-human ferritin monoclonal antibody (ThermoFisher MIF2501, Waltham, MA) was then used to immunoprecipitate ferritin from the samples to quantify ferritin iron content as previously described [13]. Briefly, 50 μL of sample were mixed with antibody and protein A/G PLUS-Agarose (Santa Cruz Biotechnology, Dallas, TX). Samples were rocked overnight at 4°C, agarose pelleted, and washed extensively with phosphate buffered saline followed by ferritin elution using 0.1M glycine pH 2.5, which was neutralized with 1.0 M Trizma base. 0.5 mL of OPTIMA Grade Nitric Acid (Fisher Scientific) was added to eluates (~30 μL). The mixture was allowed to digest overnight, heated until dry, and resuspended in 2% HNO3 for analysis using Inductively Coupled Plasma Mass Spectrometry (ICP-MS).
An Agilent 7900 ICP-MS was operated in helium (He) collision cell gas mode for all measurements. Helium cell gas mode is used to remove polyatomic interferences on analytes of interest by Kinetic Energy Discrimination. Fe is measured at its most abundant isotope of 56Fe and He cell gas effectively removes both 40Ar16O and 40Ca16O interferences. Run parameters were as follows: RF power 1600 W, sample depth 8.0 mm, carrier gas 1.05 L/min, sample flow rate: 0.2 mL/min, cell gas flow rate 5 mL/min. Calibration standards and samples were prepared in an acid matrix of 2% OPTIMA grade nitric acid. Calibration standards for Fe were prepared using Agilent Multi-element Calibration Standard 2A to obtain an eight-point calibration curve. Agilent Environmental Calibration Standard was used as an independent measure. Agilent germanium (or scandium) standard(s) were added online to standards, blanks and samples and were used as internal standards to correct for potential sample matrix and/or nebulization effects. The limits of detection for iron by ICP-MS was at an iron concentration of 1.3 ppb (1.3 ng iron/mL solution).
Non-parametric statistical methods were used due to the lack of normality of the data. Spearman’s rank correlation coefficient was used as a measure of correlation between paired ferritin (serum vs. urine) values. The Kruskal-Wallis test was used to compare group medians in the case of greater than two groups, and the Wilcoxon rank-sum test was used to compare group medians in the case of two groups. The Wilcoxon signed-rank test was used to compare a group median to a single value.
Results
Paired blood and urine samples were obtained from five healthy adults, five term neonates at birth (blood) and in the newborn nursery immediately thereafter (urine), eight stable growing preterm neonates, five neonates with congenital liver disorders and high serum ferritin levels, and one child with Beta thalassemia and iron overload. The five neonates with liver disorders included two with hemophagocytic lymphohistiocytosis (HLH), one with enterovirus-associated liver failure and hemochromatosis, one with gestational alloimmune liver disease (GALD) and one with multiple medical and surgical problems who received 22 red blood cell transfusions over the first two months and developed iron overload.
Prior to beginning this study, we measured ferritin levels in otherwise discarded urine of three neonates. Urinary ferritin levels were similar whether collected from a plastic urine bag, or cotton balls or rayon balls in the diaper [sample #1, 231.9 ng/mL (U-bag), 228.4 ng/mL (cotton balls), 232.0 ng/mL (rayon balls); sample #2, 446.7, 451.8, 447.5 ng/mL; sample #3, 19.0, 19.1, 19.2 ng/mL]. Thus, the subsequent study urine collections used any of these three methods.
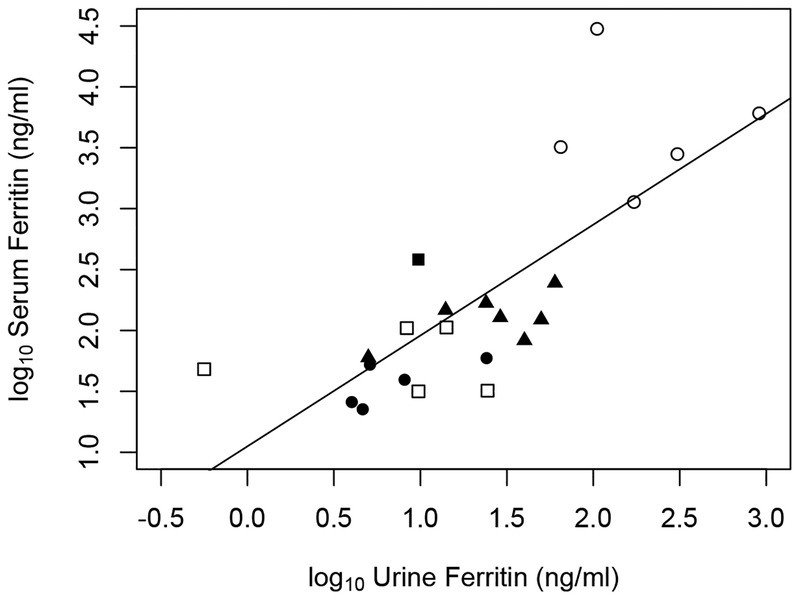
We detected ferritin in all 24 adult and neonatal serum and urine samples. As per the study by Ishikawa et al, the bivariate data were log10-log10 transformed to account for the wide range in values driven primarily in our population by the five neonates with liver disorders (Figure 1). We calculated a Spearman correlation coefficient of rs=0.78 (P-value <0.001).
Figure 1.
log10-transformed ferritin concentrations in paired serum/urine collections from five groups, each represented by a different symbol: healthy adult males (closed circle; n=5), term neonates (open square; n=5), stable, growing, preterm neonates in a NICU (closed triangle; n=8), neonates with very high serum ferritin levels (open circle; n=5) and one child with iron-overload and beta-thalassemia (closed square). The straight line is an “identify” line showing the relationship between serum and urine ferritin. The Spearman correlation coefficient of the log10 values is 0.78.
The median serum ferritin value was lowest in the adults, slightly higher in the term neonates, higher still in the preterm neonates, and highest among neonates with liver disorders (Table 1). Within each group, the median serum ferritin was higher than the median urine ferritin (Table 1). In the entire group of 24 paired samples, the urine ferritin averaged 13% of the serum ferritin. The urine ferritin was lowest (3–5% of the serum ferritin level) among the five neonates and one child with very high serum ferritin levels.
Table 1.
Comparison of ferritin in paired serum vs. urine samples of five study groups (median and interquartile range). The P-value represents a Wilcoxon rank-sum test comparing the median of the healthy adult male group to each of the subsequent groups (except group five) in a pairwise fashion. In the case of group 5, the healthy adult male group was compared to the single value in group 5 with the Wilcoxon signed-rank test.
| Study Group | N | Serum Ferritin (ng/mL) | Urine Ferritin (ng/mL) | Urine Ferritin as a percent of Serum Ferritin | P-value, urine ferritin as a percent of serum Ferritin. Various study groups vs. healthy adults |
|---|---|---|---|---|---|
| #1 Healthy adult males | 5 | 39.4(26.6) | 5.1(3.5 | 13% | |
| #2 Healthy term neonates | 5 | 48.2(72.5) | 9.7(5.8) | 20% | 0.69 |
| #3 Preterm neonates | 8 | 128.0(54.5) | 5.1(35) | 4% | >0.90 |
| #4 Neonates with liver disorders | 5 | 3206(3248.6) | 172.1(201.5) | 5% | 0.06 |
| #5 Child with iron overload | 1 | 382.7 | 9.74 | 3% | 0.03 |
We quantified the iron content of ferritin only in the samples where the serum ferritin concentration exceeded 300 ng/mL. In samples with lower ferritin levels, the iron content was below our assay limits of detectability. The samples with ferritins >300 ng/mL were from the serum of three neonates with liver disease and the child with transfusion-related iron overload. The iron content of serum ferritin had a mean of 7.3 ng iron/100ng ferritin (standard deviation 3.3 ng iron/100 ng ferritin). This is similar to that reported in healthy adults (6–10 ng iron/100 ng ferritin [25,26]). The iron content of urinary ferritin was below detectable limit in all samples.
Discussion
Iron deficiency is the most common micronutrient deficiency in the world [14]. Animal and human studies show that iron deficiency in the fetal and neonatal period causes significant neurodevelopmental and behavioral problems [1–4]. Moreover, these problems persist as permanent defects even after the iron deficiency has been treated [15].
Fetal iron deficiency anemia is rare, owing to the usual transplacental passage of adequate amounts of iron to the fetus even if the mother is iron deficient [16]. However, protection of the fetus from iron deficiency is not absolute. In fact, when mothers are significantly iron deficient, their neonates can be born with a low serum ferritin [17]. In addition, certain obstetrical and fetal conditions seem to retard fetal iron accretion. Specifically, we reported biochemical iron deficiency at birth in 15–20% of infants of diabetic mothers, small for gestational age neonates, and very low birth weight premature neonates [12].
Identifying iron deficient neonates, and instituting effective treatment, is a priority of the American Academy of Pediatrics [18] and the World Health Organization [19]. Unfortunately, most screening programs to detect neonatal iron deficiency focus on identifying anemia [18,19]. However, in neonates, anemia is a rather late consequence of iron deficiency [11]. Once iron deficiency anemia has developed in a neonate, it might be too late to prevent the neurodevelopmental delays caused by biochemical iron deficiency. Therefore, screening tests to identify early biochemical iron deficiency, not anemia, are needed if iron-deficient neurodevelopmental delay is to be prevented [5].
Iron overload and high serum ferritin levels can occur in young children who chronically require erythrocyte transfusions, and in neonates with certain liver disorders such as GALD and HLH [20,21]. The usual means of screening young children for iron deficiency or iron overload necessitate phlebotomy. For serum ferritin quantification, clinical laboratories typically require 1 mL of serum (at least 2 mL blood). Similarly, 1 mL of serum is typically required for soluble transferrin receptor quantification, and an additional 1 mL of blood is typically required for zinc protoporphyrin to heme ratio (http://ltd.aruplab.com/Tests/Pub/0070065; http://ltd.aruplab.com/Tests/Pub/0020605). Thus, ≥5 mL of blood are needed for a battery of all three tests. For the smallest preterm infants this phlebotomy is about 10% of their blood volume.
We postulated that measuring ferritin in urine of infants might serve as a non-invasive screen for abnormal iron stores. We recognized pitfalls in that approach, and explored some of these before planning a definitive study. First, we were aware of no studies reporting whether ferritin can be measured in the urine of healthy or ill, term or preterm neonates, or testing a relationship between serum and urine ferritin in neonates. Ishikawa et al. found, in healthy adults, that urine ferritin was about 5% of serum levels [22], but this has not been repeated, or reported from neonates.
Another problem is that ferritin is an acute phase reactant; thus, levels can increase during inflammation. This principle was illustrated both by Migliari et al. and Tang et al. when they showed increased urine ferritin in patients with bladder cancer [23,24]. Consequently, false negative screening of urine might be an issue. However very low levels of urinary ferritin might identify a group of neonates where serum values should be obtained to assess the iron status before treatment is instituted.
Another potential pitfall in using urinary ferritin as a screening tool is that the ferritin assay typically used in clinical laboratories involves antibodies to the heavy and light chains of the ferritin shell proteins. Ferritin is composed of 24 protein subunits, termed H and L, the molecular masses of which are 21,000 Da and 20,000 Da, respectively [25]. The relative molecular mass of assembled apoferritin is 450,000–480,000 Da, and the maximal number of iron molecules per molecule of ferritin is reported to be around 4500 [26]. Studies from the Netherlands indicate that serum ferritin of healthy adults typically has 19–25% of the maximal iron content, or is 19–25% saturated with iron. Studies from Hamburg agree, and report that 100 ng of serum ferritin in healthy adults has about 8 ng of iron, but that ferritin saturation levels may be as low as 3–4% [27]. In rats, the iron content of ferritin varies with site; such that 100 ng of serum ferritin has about 1.8 ng of iron, whereas 100 ng of ferritin in liver contains about 23 ng of iron [28]. We found that in children with liver diseases who had very high serum ferritin levels, their ferritin was not iron-loaded. In fact, the iron content of their ferritin was on average, only 7.8 ng iron per 100 ng ferritin, similar to the iron content found in serum ferritin of healthy adults.
We recognize other shortcomings in our study. The small number of subjects in each group makes statistical comparisons tenuous. In addition, the ferritin we measured in serum and urine is likely quite heterogeneous and may not always be intact ferritin, but pieces of the ferritin shell. This may be particularly true in urine where a molecule as large as ferritin is not expected to normally be filtered by the glomerulus. Additionally, the ferritin in many of our samples had an iron content that was below the detectability of our ICP-MS assay. Perhaps this could be remedied in future studies by utilizing larger sample volumes
Obviously, much work remains before we know if there is value in measuring urinary ferritin in young children as a screen for iron stores. However, we maintain that our study does provide a few clear answers. For instance, we found ferritin in all 24 urine samples tested. This answered one of our initial questions. A correlation between urinary and serum ferritin levels was observed. We hypothesize that the correlation might be improved by correcting urinary ferritin levels using urinary creatinine or some other factors, and we intend to evaluate this in future studies.
Conclusions
The findings of our pilot study suggest merit in pursuing the possibility of measuring urinary ferritin as a screening tool to assess iron stores where screening by phlebotomy can be problematic.
Acknowledgments
The authors thank Jennifer Brereton of Brigham Young University (Provo, UT, USA) for helpful discussions about ferritin and insights regarding this study. Metal analysis was performed at the Iron and Heme Core facility at the University of Utah (Salt Lake City, UT, USA), supported in part by a grant from the NIH National Institute of Diabetes and Digestive and Kidney Diseases, Grant number U54DK110858.
Funding: The study was supported in part by grants U54DK110858 and DK030534 (to DMW) from the US Public Health Service, and by funds from the Department of Pediatrics (to TMB), University of Utah Health, Salt Lake City, UT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this study to disclose.
References
- [1].Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG, Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age, The Journal of Pediatrics. 140 (2002) 165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- [2].Fleming RE, Cord serum ferritin levels, fetal iron status, and neurodevelopmental outcomes: Correlations and confounding variables, The Journal of Pediatrics. 140 (2002) 145–148. doi: 10.1067/mpd.2002.121931. [DOI] [PubMed] [Google Scholar]
- [3].Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier R-A, Iron Deficiency Alters Auditory Recognition Memory in Newborn Infants of Diabetic Mothers, Pediatric Research. 55 (2004) 1034–1041. doi: 10.1203/01.pdr.0000127021.38207.62. [DOI] [PubMed] [Google Scholar]
- [4].Grantham-McGregor S, Ani C, A Review of Studies on the Effect of Iron Deficiency on Cognitive Development in Children, The Journal of Nutrition. 131 (2001) 649S–668S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- [5].Cusick S, Georgieff M, Rao R, Approaches for Reducing the Risk of Early-Life Iron Deficiency-Induced Brain Dysfunction in Children, Nutrients. 10 (2018) 227. doi: 10.3390/nu10020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zamora TG, Guiang SF, Widness JA, Georgieff MK, Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs, Pediatric Research. 79 (2016) 922–928. doi: 10.1038/pr.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lundström U, Siimes MA, Dallman PR, At what age does iron supplementation become necessary in low-birth-weight infants?, The Journal of Pediatrics. 91 (1977) 878–883. doi: 10.1016/S0022-3476(77)80881-0. [DOI] [PubMed] [Google Scholar]
- [8].Bishara N, Ohls RK, Current Controversies in the Management of the Anemia of Prematurity, Seminars in Perinatology. 33 (2009) 29–34. doi: 10.1053/j.semperi.2008.10.006. [DOI] [PubMed] [Google Scholar]
- [9].Goodnough LT, Skikne B, Brugnara C, Erythropoietin, iron, and erythropoiesis, 96 (2000) 11. [PubMed] [Google Scholar]
- [10].Kasper DC, Widness JA, Haiden N, Berger A, Hayde M, Pollak A, Herkner KR, Characterization and Differentiation of Iron Status in Anemic Very Low Birth Weight Infants Using a Diagnostic Nomogram, Neonatology. 95 (2009) 164–171. doi: 10.1159/000153101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK, The Assessment of Newborn Iron Stores at Birth: A Review of the Literature and Standards for Ferritin Concentrations, Neonatology. 92 (2007) 73–82. doi: 10.1159/000100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].MacQueen BC, Christensen RD, Ward DM, Bennett ST, O’Brien EA, Sheffield MJ, Baer VL, Snow GL, Weaver Lewis KA, Fleming RE, Kaplan J, The iron status at birth of neonates with risk factors for developing iron deficiency: a pilot study, Journal of Perinatology. 37 (2017) 436–440. doi: 10.1038/jp.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Smith SM, Gillman PL, Quantitating Iron in Serum Ferritin by Use of ICP-MS, NASA Tech Brief. (2003). https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20110023580.pdf (accessed November 21, 2018).
- [14].Bailey RL, West KP Jr., Black RE, The Epidemiology of Global Micronutrient Deficiencies, Annals of Nutrition and Metabolism. 66 (2015) 22–33. doi: 10.1159/000371618. [DOI] [PubMed] [Google Scholar]
- [15].Georgieff MK, The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus, Biochemical Society Transactions. 36 (2008) 1267–1271. doi: 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McArdle HJ, Gambling L, Kennedy C, Iron deficiency during pregnancy: the consequences for placental function and fetal outcome, Proceedings of the Nutrition Society. 73 (2014) 9–15. doi: 10.1017/S0029665113003637. [DOI] [PubMed] [Google Scholar]
- [17].Terefe B, Birhanu A, Nigussie P, Tsegaye A, Effect of Maternal Iron Deficiency Anemia on the Iron Store of Newborns in Ethiopia, Anemia. 2015 (2015) 1–6. doi: 10.1155/2015/808204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baker RD, Greer FR, The Committee on Nutrition, Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0–3 Years of Age), PEDIATRICS. 126 (2010) 1040–1050. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- [19].WHO, Global nutrition targets 2025: anaemia policy brief (WHO/NMH/NHD/14.4), (2014).
- [20].Rath MEA, Smits-Wintjens VEHJ, Oepkes D, Walther FJ, Lopriore E, Iron status in infants with alloimmune haemolytic disease in the first three months of life, Vox Sanguinis. 105 (2013) 328–333. doi: 10.1111/vox.12061. [DOI] [PubMed] [Google Scholar]
- [21].Grabhorn E, Richter A, Burdelski M, Rogiers X, Ganschow R, Neonatal Hemochromatosis: Long-term Experience With Favorable Outcome, PEDIATRICS. 118 (2006) 2060–2065. doi: 10.1542/peds.2006-0908. [DOI] [PubMed] [Google Scholar]
- [22].Ishikawa K, Narita O, Saito H, Kato K, Determination of ferritin in urine and in serum of normal adults with a sensitive enzyme immunoassay, Clinica Chimica Acta. 123 (1982) 73–81. doi: 10.1016/0009-8981(82)90115-2. [DOI] [PubMed] [Google Scholar]
- [23].Migliari R, Mela Q, Ruggiero V, Scarpa RM, Migliari M, Pitzus F, Usai E, Serum and urine ferritin in patients with transitional cell carcinoma of the bladder, Arch Ital Urol Nefrol Androl. 63 (1991) 141–145. [PubMed] [Google Scholar]
- [24].Tang DJ, Fan XY, Huang XB, Chen PH, Liang Y, Lang YM, Clinical significance of urine ferritin determination in urologic malignancies, Chin. Med. J 102 (1989) 356–360. [PubMed] [Google Scholar]
- [25].Halliday JW, Powell LW, Ferritin metabolism and the liver, Semin. Liver Dis 4 (1984) 207–216. doi: 10.1055/s-2008-1041771. [DOI] [PubMed] [Google Scholar]
- [26].ten Kate J, Wolthuis A, Westerjhuis B, van Duersen C, The iron content of serum ferritin: physiological importance and diagnostic value., Eur J Chem Clin Biochem. 35 (1997) 53–56. [DOI] [PubMed] [Google Scholar]
- [27].Nielsen P, Günther U, Dürken M, Fischer R, Düllmann J, Serum ferritin iron in iron overload and liver damage: Correlation to body iron stores and diagnostic relevance, Journal of Laboratory and Clinical Medicine. 135 (2000) 413–418. doi: 10.1067/mlc.2000.106456. [DOI] [PubMed] [Google Scholar]
- [28].Watanabe K, Yamashita Y, Ohgawara H, Sekiguchi M, Satake N, Orino K, Yamamoto S, Iron Content of Rat Serum Ferritin., Journal of Veterinary Medical Science. 63 (2001) 587–589. doi: 10.1292/jvms.63.587. [DOI] [PubMed] [Google Scholar]