Abstract
The tryptophan-metabolizing enzyme indoleamine 2,3 dioxygenase 1 (IDO1) is frequently overexpressed in epithelial-derived malignancies, where it plays a recognized role in promoting tumor immune tolerance. We previously demonstrated that the IDO1-kynurenine pathway (KP) also directly supports colorectal cancer (CRC) growth by promoting activation of β-catenin and driving neoplastic growth in mice lacking intact adaptive immunity. In this study, we sought to delineate the specific role of epithelial IDO1 in colon tumorigenesis and define how IDO1 and KP metabolites interact with pivotal neoplastic signaling pathways of the colon epithelium. We generated a novel intestinal epithelial-specific IDO1 knockout mouse and utilized established CRC cell lines containing β-catenin-stabilizing mutations, human CRC samples, and human-derived epithelial organoids (colonoids and tumoroids). Mice with intestinal epithelial-specific knockout of IDO1 developed fewer and smaller tumors than wild type littermates in a model of inflammation-driven colon tumorigenesis. Moreover, their tumors exhibited reduced nuclear β-catenin and neoplastic proliferation but increased apoptosis. Mechanistically, kynurenine pathway metabolites (except kynurenic acid) rapidly activated PI3K-Akt signaling in the neoplastic epithelium to promote nuclear translocation of β-catenin, cellular proliferation, and resistance to apoptosis. Together, these data define a novel cell-autonomous function and mechanism by which IDO1 activity promotes CRC progression. These findings may have implications for the rational design of new clinical trials which exploit a synergy of IDO1 inhibitors with conventional cancer therapies for which Akt activation provides resistance such as radiation.
INTRODUCTION
Several types of cancer pathologically exploit tryptophan metabolism along the kynurenine pathway (KP) to promote growth and escape immune surveillance.(1–3) Indoleamine 2, 3 dioxygenase 1 (IDO1) is the most widely studied of tryptophan metabolizing enzymes that catalyze the initial step of the KP. Tumor overexpression of IDO1 increases local kynurenine concentrations and depletes tryptophan levels. These changes promote an immune-tolerant tumor microenvironment by several mechanisms including suppressing tumor-reactive effector T-cell responses and NK cell responses, promoting T-regulatory cell differentiation as well as the expansion and activation of myeloid-derived suppressor cells.(2,4,5) Additionally, a non-enzymatic, pro-tolerance function is also attributed to IDO1 via its interactions with TGF-β.(6)
Human and animal studies illustrate the importance of IDO1 in cancer. In preclinical models, IDO1 expression promotes greater tumor burden of several cancers including those of the colon, lung, skin, pancreas and breast.(7–14) In humans, increased IDO1 expression is associated with poor clinical prognosis across several solid tumor types.(7,15,16) Based on these findings, IDO1 inhibition is currently under evaluation in clinical trials for numerous cancer types.(17,18) The IDO1 functional ortholog, tryptophan dioxygenase (TDO), and evolutionary paralog, IDO2, also metabolize tryptophan and contribute to neoplastic pathogenesis in some models; however, the potential for therapeutic targeting remain less well developed than for IDO1.
IDO1 overexpression is a common feature of human colorectal cancer (CRC), the second leading cause of cancer death in the United States. Pathology studies show that IDO1 expression localizes to CRC infiltrating myeloid derived cells as well as in the neoplastic colon epithelium. (3,19,20) CRC patients also exhibit reduced serum tryptophan levels and increased kynurenine pathway metabolites, indicating increased IDO1 activity.(21–23) Furthermore, high epithelial IDO1 expression at the tumor invasion front is an independent adverse prognostic factor for overall survival and metachronous CRC metastases, while high density of IDO1 expressing cells in the tumor draining lymph nodes was associated with a reduced 5 year survival rates in colon cancer patients. (16,20,24) These findings, which are reviewed more thoroughly elsewhere,(25,26) highlight the relevance of IDO1 as a therapeutic target in human CRC.
We recently examined the role of IDO1 in a model of colitis-associated cancer (CAC) and in cell lines derived from patients with sporadic CRC. This study demonstrated that IDO1 is highly expressed in the neoplastic colon epithelium and that promoted tumor growth (14). Germline genetic deletion of IDO1 and administration of the first generation IDO1 inhibitor (1-methyl tryptophan) decreased tumorigenesis. Two novel mechanistic observations arose from these studies. First, IDO1 blockade reduced tumorigenesis even in mice lacking mature adaptive immunity. Second, IDO1 activity promoted nuclear translocation of epithelial cell β-catenin, a pivotal transcriptional regulator in CRC. Together these findings provided initial evidence that IDO1 expression is a pathogenic driver of CRC progression by a mechanism involving the neoplastic epithelium and one that is complementary to its ability to promote immune tolerance through T-cell suppression.
In the current study, we sought to delineate the role of epithelial IDO1 in colon tumorigenesis and to define the regulators and mechanism of its immune-independent pro-tumorigenic effect. To address this, we developed an epithelial specific IDO1 knockout mouse and examined human CRC samples, established cell lines and human derived colonoids and tumoroids. The data presented herein reveal that epithelial cell IDO1 is key to colon tumorigenesis and that kynurenine pathway metabolites rapidly activate PI3K-Akt signaling in the neoplastic epithelium to promote cellular proliferation and resistance to apoptosis. These findings are highly relevant to IDO1 inhibitors as they move through clinical trials and to the important unmet therapeutic need in advanced colorectal cancer.
MATERIALS AND METHODS
Mice and in vivo modeling of colon cancer:
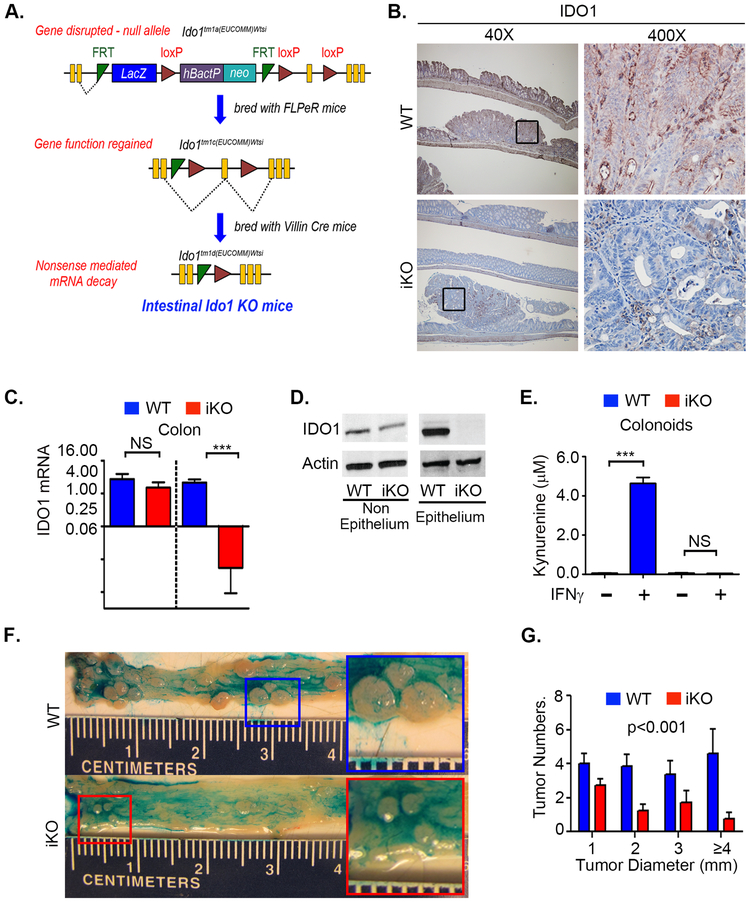
All mice used in this study were on C57BL/6J background. Heterozygous Ido1 “knockout-first” tm1A allele mice; Ido1tm1a(EUCOMM)Wtsi were initially acquired as heterozygotes via Material Transfer Agreement from the Wellcome Trust Sanger Institute [Ido1 (MDCT; EPD0198_1_F02). A schematic of the targeting vector used to create the Ido1 “knockout-first” allele (tm1a) is shown in Figure 1A. As the figure illustrates, subsequent breeding with FLPeR mice and Cre mice with under the control of villin promoter generated mice with intestinal epithelium specific Ido1 knockout (IDO1-iKO). A detailed protocol is given in the supplementary methods. Cre- littermates were used as WT controls.
Figure 1: Epithelial IDO1 promotes tumor growth and neoplastic proliferation in the colon.
Mice with intestinal epithelial specific Ido1 deletion (IDO1-iKO) and WT (Cre-) littermate controls were examined for gene expression and propensity for colon tumorigenesis when exposed to the AOM/DSS protocol. Mice received 10mg/kg Azoxymethane (AOM) followed by two, week-long cycles of 2.25% DSS in drinking water separated by sterile water for 2 weeks. A) Schematic for generating intestinal knockout (iKO) mice. B) Immunohistochemistry for IDO1 on AOM/DSS induced tumors in iKO and WT mice. C) and D) mRNA and IDO1 protein expression from isolated tracts of non-epithelial and epithelial cellular compartments of mice 48 hours after IDO1 induction with 20 µg IP of CpG DNA E) Measurement of kynurenine production in the supernatants of colon organoid culture from WT and iKO mice. F) Representative colon morphology with tumors highlighted by topically applied Alcian blue. Magnified image of tumors shown in inserts to highlight the difference in the sizes of tumors G) Tumor quantification showing reduced tumor size and number in IDO1-iKO mice. Statistical comparison by two-way ANOVA (D) or Student’s T-test (F). N=5-6 mice/group, 2-4 tumors counted/mouse.
Age (6–12 weeks) matched littermates were used in all experiments with approximately equal representation of male and female mice. Animals were housed in specific pathogen free barrier facility and all protocols were performed under the regulations of and approval of Washington University’s Institutional Animal Care and Use Committee. Induction of colon carcinogenesis by azoxymethane (AOM) followed by cycles of dextran sodium sulfate (DSS), preparation of tissue section for histology and assessment of disease activity index (DAI) were done as per earlier published protocols (14,27,28). Detailed mouse phenotyping data are shown in Figure 1A. Additional phenotyping data and detailed procedures data are provided in supplemental methods and Figure S1A-D.
Organoid Culture:
The collection and use of human tissue for establishing primary epithelial cell culture or organoid culture was approved by the Washington University human research protection office (IRB) and collected by the Washington University Digestive Diseases Research Core Center (DDRCC) Biobank Core. Informed consent was obtained from all patients. Organoid cultures were established from human and mouse tissues and maintained in matrigel (Cat. No. 354234; Corning Life Sciences, Oneonta, NY) as described previously by our group and others (29–31). Human Tumoroids were derived from three CRC specimens from patients with known APC mutations and Familial Adenomatous Polyposis syndrome. Mouse normal colonoids were established from WT and iKO mice, while tumoroids were established from colon tumors of WT mice. Differentiation was induced in normal organoids by maintaining them in 1:10 diluted L-WRN conditioned media with Advanced DMEM/F12 supplemented with 10 µM Y-27632 (ROCK inhibitor; Tocris Bioscience, R&D Systems, Minneapolis, Minnesota) before using them for experiments(29). Tumor organoids were maintained in Advanced DMEM/F12 supplemented with 10 µM Y-27632 (Tocris Bioscience) and 10 µM SB 431542 (TGFBR1 inhibitor; Tocris Bioscience, R&D Systems). For experiments comparing human and mouse normal organoids with tumor organoids, tumor organoids were maintained in 1:10 diluted L-WRN conditioned media to match the growth conditions of normal organoids.
Reagents and assays:
Human colorectal cancer (CRC) cell lines HT-29 (ATCC HTB-38; MSS; KRAS WT), DLD1 (ATCC CCL-221; MSI; KRAS mutant) and HCT116 (ATCC CCL-247; MSI; KRAS and B-Catenin mutant) were purchased from American Type Culture Collection (ATCC, Manassas, VA) at study initiation. Cells were grown in Dulbecco’s Modified Eagle’s medium (Gibco, Thermo Fisher, Waltham, MA) with high glucose supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin (Corning Life Sciences, Tewksbury, MA, USA). Data presented use cell lines from passage 3 and 30 at ~70–80% confluence. Cell lines were checked for mycoplasma contamination in September 2016 at the Tissue Culture Support Center in Washington University School of Medicine. Kynurenine (Kyn), 3-hydroxyanthranillic acid (3HAA), quinolinic acid (QA), picolinic acid (PA), kynurenic acid (KA), xanthurenic acid (XA) and anthranilic acid (AA) were purchased from Millipore Sigma (St. Louis, MO). 3-hydroxykynurenine (3HK) was acquired from Santa Cruz Biotechnology (Dallas, Texas). Each were solubilized according to supplier recommendation. Recombinant Human DKK-1 (Cat#GF170) was purchased from Millipore (Temecular, CA). Total protein was extracted from CRC cell lines using Cell Lysis Buffer (Cell Signaling Technology, Beverly, MA) as per manufacturer’s recommendation. Total protein was extracted from organoids using RIPA Buffer (Sigma) as per published protocols (29). Cytoplasmic and nuclear proteins were extracted from HT-29 cells using NE-PER (Thermo Fisher Scientific). Total protein was estimated using BCA protein assay kit (Thermo Fisher Scientific) before subjecting them to western blot analyses. All phosphorylation specific and corresponding total antibodies used in this study were purchased from Cell Signaling Technologies (Danvers, MA) unless specified. Supplementary Table 1A list all antibodies used in this study.
Signal transduction assays:
CRC cells at ~75% confluency and serum starved 24hrs then incubated with KP metabolites for defined time points followed by total protein or cytoplasmic and nuclear protein extraction. For experiments involving signaling pathway inhibition, HT-29 cells were incubated with 20 µM PI3K (GDC-0941, Selleckchem, Houston, TX) or Akt (MK2206, Selleckchem) inhibitor 4 h prior to KP metabolite incubation. Furthermore, to determine whether activation of Akt signaling by Kyn is intracellular, a putative Kyn transporter LAT1 was blocked using specific inhibitors BCH and JPH203 overnight prior to KP metabolite incubation. Organoids were first isolated from Matrigel using cell recovery solution (Cat. No. 354253; Corning Life Sciences) after overnight serum depletion, then incubated with KP metabolites in serum free DMEM/F12 followed by total protein extraction.
Proliferation and cell viability assay:
CRC cell lines and organoids were plated in flat bottom 96 well plates. Eight hours after plating, CRC cells were serum starved overnight followed by incubation with 100 μM KP metabolites in DMEM containing 1% FBS. Organoids were plated in matrigel and maintained in L-WRN (20% FBS) media for 12 h after plating. The media was then changed to 1% FBS containing differentiation media and maintained for 12 h followed by the addition of 100 μM KP metabolites in 1% FBS differentiation media. Tumor organoids were maintained in advanced DMEM (20% FBS) for 12 h followed by maintenance in 1% FBS containing differentiation media for 12 h. After 12 h, 100 μM KP metabolites were added in 1% FBS containing differentiation media. Proliferation was measured using CCK-8 assay (Dojindo Laboratories, Rockville, MD) after 72 h of incubation with KP metabolites in both CRC cell lines and organoids.
Apoptosis assessments:
Expression of cleaved PARP was used as a measure of apoptosis by western blot and immunofluorescence. HT-29 cells were grown to ~75% confluency before serum starving overnight. To induce apoptosis, cells with treated with 100ng/ml TNFα (Peprotech, Rocky Hill, NJ) or 2 µM Staurosporine (Cell Signaling Technology, Danvers, MA) in DMEM containing 1% FBS for 12 h with or without combinations of Akt inhibitor (MK2206) and 100 μM Kyn. Cells were pre-treated with 5ng/ml cycloheximide for 1 h before incubating with TNFα or Staurosporine (STP). Cleaved PARP was determined using rabbit anti-cleaved PARP mAb (Cell Signaling Technology) by western blot.
For immunofluorescence, cells were seeded on coverslips in 6-well plates and grown to ~75% confluency. Media was aspirated and coverslips were washed in cold PBS then fixed in fresh 4% paraformaldehyde in PBS for 20 minutes at room temperature. Cells were permeabilized (0.1% Triton X-100 in PBS for 10min), washed in cold PBS, and then blocked with 1% BSA containing 1% normal goat serum for 60 minutes at RT. Cells on coverslips were then probed with anti-cleaved PARP mAb (Cell Signaling Technology) at 1:400 dilution in blocking buffer at 4°C overnight. After washing in cold PBS, goat anti-rabbit AlexaFluor 488 (Thermo Fisher) at 1:600 dilution was added in blocking buffer for 60 minutes at RT and mounted with Southern Biotech DAPI Fluoromount-G.
For analyzing cell viability after apoptosis induction, the CCK-8 assay was used 24 h after TNFα or staurosporine (STP) incubation with or without 100 μM kynurenine co-treatment. TUNEL assay was performed to determine apoptosis in mouse tumors using in situ cell death detection kit (Roche Applied Science, Mannheim, Germany).Immunofluorescence images of cleaved PARP and TUNEL assay were captured with a Zeiss Axioskop 2 MOT microscope equipped with an Apotome module. Immunofluorescence was quantified in ImageJ as follows: black and white images of the blue channel were converted to binary and nuclei were defined using nuclei watershed separation setting, followed by particle analysis with size exclusion of 0.005 in2-infinity. Green channel image thresholds were adjusted until individual nuclei were visible, and particle analysis was performed as above. Data expressed as percent of particles in green channel (positive nuclei) divided by number of particles in blue channel (total nuclei) x 100.
Immunohistochemistry:
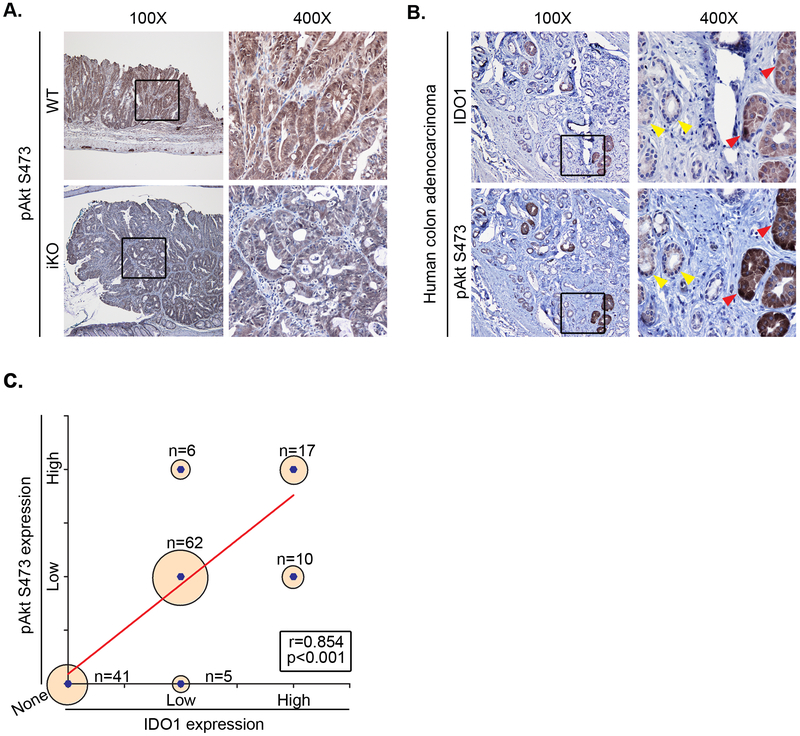
Murine colon tissues were fixed in formalin and stabilized in 2% agar before embedding in paraffin as previously described (27). All antibodies are described in Supplementary Table 1. Ido1 immunostaining with rat anti-mouse IDO1 antibody (Biolegend) was used at 1:50 dilution after antigen retrieval by steaming in sodium citrate buffer (pH 6.0) for 30 min. Trilogy one step antigen retrieval (Millipore Sigma) was performed as per manufacturer’s instructions for pAkt SER473, cyclin D1, β-catenin and survivin immunostaining (Cell signaling technology at 1:50 dilution) on mouse tissue. Tissue sections from post-irradiated, surgically resected human colon adenocarcinoma were obtained from Siteman Cancer Center histopathology core. Trilogy one step heat mediated antigen retrieval was performed for immunostaining IDO1 and pAkt SER473. The relative intensity of immunostaining for human IDO1 and pAkt SER473 was graded based on a point system (No staining=0, low expression=1, high expression=2). CRC pathology samples from 5 patients were analyzed by selecting neoplastic crypts (>10/specimen, 141 total) followed by sequential evaluation of IDO1 staining and pAKT SER473. The grading was confirmed by two authors (SS, data generation; MAC confirmation). Using this data, a correlation curve between IDO1 and pAkt SER473 expression was calculated.
Statistical Analysis:
Animal experiments included 5 to 10 mice in each group and were repeated twice. Cell proliferation assays involved data accumulation from 5 to 8 wells/ group and experiments were repeated at least twice. Wound healing assays included data from 3 wells in each group and the experiments were performed twice. All data were represented as average ± standard error of the mean (SEM). P values were calculated using two-way ANOVA or Student’s t test and a value less than 0.05 was considered as significantly different between groups. The Spearman correlation coefficient was used to analyze the correlation between IDO1 and Akt SER473 based on their immunohistochemical staining scores. Figures and statistical analysis were done using GraphPad Prism version 5.00 for Windows (GraphPad Software, La Jolla, California USA, www.graphpad.com).
RESULTS
IDO1 expression by the neoplastic intestinal epithelium promotes colon tumorigenesis.
We previously showed that germline deletion of Ido1 reduces tumor burden in a mouse model of CRC and that inhibition of IDO1 activity reduces CRC cell proliferation in vitro. These findings suggest that the neoplastic epithelium may be a key cellular source of IDO1 activity for driving CRC growth. To directly examine this issue, we generated a mouse with genetic knockout of Ido1 specifically in the intestinal epithelium (IDO1-iKO) using cre-lox recombination technology (Figure 1A). Mice with a floxed Ido1 gene were crossed to mice expressing cre behind the intestinal epithelial specific villin promoter. The resulting mouse line demonstrated effective knockout of Ido1 protein expression in the colonic epithelium, but not non-epithelial lamina propria cells (Figure 1B,C,D). Colonoids established from normal colons of WT, but not iKO mice showed change in kynurenine production after IFNγ treatment (Figure 1E) illustrating the functional knockout of Ido1 and the relative importance of IDO1 (vs IDO2 or TDO) to the kynurenine pathway in the colon epithelium. Additional details on mouse modeling and Ido1 expression data are presented in Figure S1A-D.
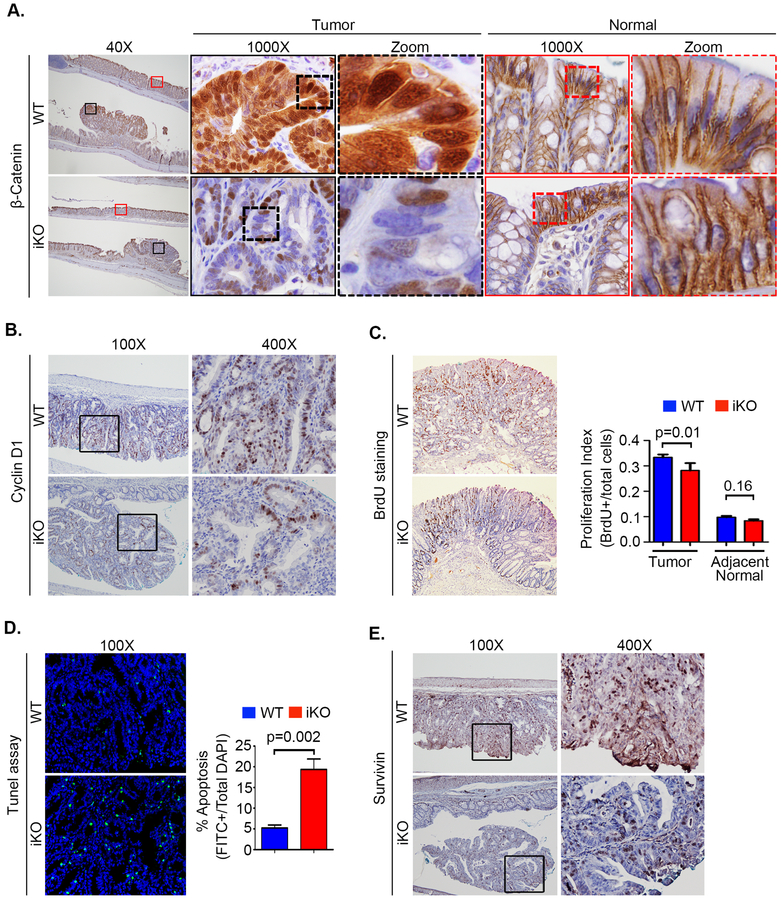
Colon tumorigenesis was induced in IDO1-iKO and littermate WT control mice using the AOM-DSS protocol. No difference in weight loss was observed between the two groups and disease activity index differed only briefly during the first DSS cycle (Figure S1B,C). However, IDO1-iKO mice were found to develop fewer and smaller tumors compared to WT mice (Figure 1F,G). Nuclear β-catenin were visibly reduced in the tumors of IDO1-iKO vs WT mice, as was expression of the target gene Cyclin D1 and the tumor cell proliferation index by 16% (Figure 2A,B,C). These effects were not significant in the adjacent normal colon epithelium, although the colonic crypt epithelial proliferation index trended lower in the IDO1-iKO mice. Tumors from IDO1-iKO mice also demonstrated increased apoptosis (TUNEL staining) and reduced expression of Survivin (a key protein that regulates mitosis and apoptosis) (Figure 2D,E). Together, these data illustrate the importance of epithelial-based IDO1 expression as a driver of neoplastic proliferation, apoptotic resistance and colon tumorigenesis.
Figure 2: Epithelial IDO1 promotes nuclear B-Catenin activation, increases epithelial proliferation and protects from apoptosis.
A) Decreased nuclear β-catenin staining in IDO1-iKO tumors compared to WT. Magnified images of β-Catenin staining by immunohistochemistry in tumors and adjacent normal areas in WT and iKO mice. B) Representative immunohistochemistry images of β-Catenin target gene Cyclin D1. C) Epithelial proliferation by BrdU staining with its quantitation represented as epithelial proliferation index in tumors and adjacent normal epithelium. D) Apoptosis by Tunel assay including representative IF images quantitation. E) Representative images of Survivin immunohistochemistry.
KP metabolites activate β-Catenin through Akt signaling in CRC cells
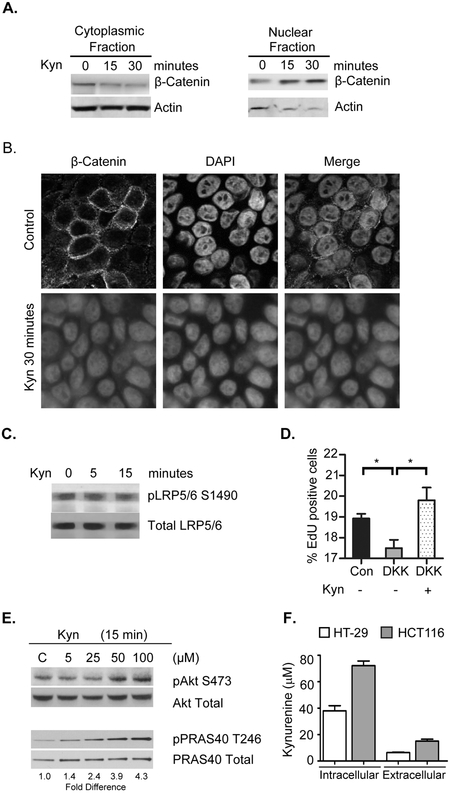
We next sought to examine the signaling mechanism by which IDO1 activity promotes neoplastic epithelial proliferation, nuclear translocation of β-catenin and resistance to apoptosis. In these experiments we examined the impact of kynurenine (Kyn) on CRC cells using in vitro methods. We found that both Kyn rapidly promoted nuclear translocation of β-catenin from the cytoplasm in a time dependent manner (Figures 3A,B).
Figure 3: Kynurenine rapidly induces β-catenin nuclear translocation and activates Akt in CRC cells.
Experiments were completed with HT29 cells treated with kynurenine at 100 µM or at concentrations shown. The impact of kynurenine on β-catenin cellular location was assessed by A) Western blot and B) Immunofluorescence. Kyn did not activate Wnt pathway as evidenced by C) No observed change in the frizzled co-receptor activation as measured by pLRP5/6 S1490 levels and D) Kyn increasing EdU incorporation as a marker of proliferation in the presence of Wnt signaling inhibitor DKK1. *p<0.05. E) Kynurenine did induce dose dependent activation of Akt (pS473) and Akt phosphorylation target PRAS40 at physiological concentrations of kynurenine as measured by F) Intracellular and extracellular levels in CRC cell lines.
Unchecked nuclear β-catenin mediated transcriptional activity is a common feature of CRC and usually attributable to genetically driven aberrant signaling along the canonical Wnt pathway (32). Therefore, we examined whether KP metabolites act as endogenous Wnt ligands. Two approaches were taken, but neither confirmed this mechanism. First, exogenous Kyn did not promote activation of LRP5/6 (lipoprotein receptor-related protein), a co-receptor that is phosphorylated in the presence of Wnt ligands. Secondly, exogenous Kyn promoted enhanced CRC cell proliferation even in the presence of the Wnt antagonist Dickkopf (Figure 3C,D).
We next evaluated whether KP metabolites activate Protein kinase B (Akt), a key signaling intermediate known to inhibit apoptosis while promoting β-catenin nuclear translocation and transcriptional activity. This mechanism involves phosphorylation of Akt at Ser473 and Thr308 residues leading to increased phosphorylation of β-catenin at Ser552 and has been linked to inflammation in CRC (33,34). Indeed, we found that exogenous Kyn application to serum-starved cells promoted rapid and dose dependent Akt activation evidenced by increases in pAKT S472 and phosphorylated PRAS40 pT246, a direct target Akt activity (Figure 3E).
To address the physiologic relevance of these concentrations, we assessed the intracellular kynurenine levels in two CRC cell lines used in this study and found them to reach upwards of 75 µM (Figure 3F). Supporting these findings, prior studies have demonstrated intracellular concentrations of kynurenine to exceed 2 mM in malignant myeloid derived cells (such as those that infiltrate the tumor) and to reach at least 100 µM in WiDr CRC cells.(35) Based on these data, we used 100 µM of kynurenines henceforth to examine the cell signaling pathway.
Intracellular kynurenine destabilizes GSK-3β and activates Akt via PI3K
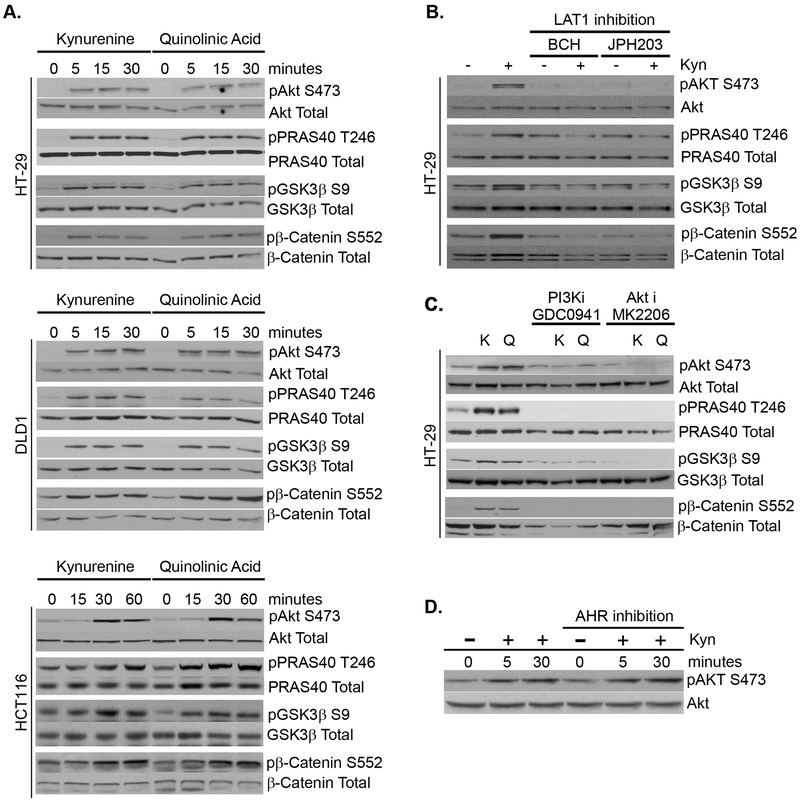
We next sought to understand the pathways upstream and downstream to kynurenine mediated Akt activation as relevant to CRC and to β-catenin activation. Upon activation, Akt signaling is known to interact with β-catenin indirectly by inactivating GSK-3β (36), an enzyme that when in its active state, destabilizes β-catenin towards degredation. Indeed, we identified that initial (kynurenine) and terminal (quinolinic acid) KP metabolites rapidly inactivate GSK-3β by inducing its phosphorylation at Ser9. This mechanism also applied to other CRC cell lines despite known β-catenin stabilizing genetic mutations and regardless of status for microsatellite instability or KRAS mutation (Figure 4A). Together, these data suggest a positive synergy between IDO1 activity in the neoplastic epithelium and the β-catenin stabilizing genetic mutations that drive colon cancer development and progression.
Figure 4: Kyurenine metabolites rapidly activate β-catenin via intracellular activation of PI3K/Akt pathway.
A) HT29, DLD1 and HCT116 colon cancer cells were treated with Kyn or QA for specified time points followed by whole cell protein extraction. Western blots were performed to analyze Akt-Ser473 (activated), pPRAS40-T246 (activated), GSK3β-Ser9 (inactivated) and βCatenin Ser552 (activated) phosphorylation levels. B) Inhibition of the LAT1 amino acid transporter by either BCH or JPH203 blocks kynurenine mediated Akt pathway activation. C) Inhibition of Akt (MK2206) and PI3K (GDC0941) blocks kynurenine mediated phosphorylation of Akt at S473 and prevents downstream targets GSK3β from inactivation (pSer9) and β-catenin from activation (pSer552) in HT-29 cells. D) Inhibition of Aryl hydrocarbon receptor with CH-223191 does not prevent Akt activation in HT-29 CRC cells.
We also aimed to define signaling upstream from Akt activation. First, we identified that intracellular Kyn was required to activate Akt as inhibition of the L-amino acid transporter 1 (LAT1) prevented this effect (Figure 4B). LAT1 is an active transporter of kynurenine and known to be highly upregulated in CRC (37,38). Akt resides at the intersection of numerous signaling pathways including PI3K, PTEN and positive feedback from mTor. We did find that kynurenine promoted mammalian target of rapamycin phosphorylation (pmTor Ser2448) in CRC cells, a form that binds to both raptor and rictor and is shown to be overexpressed in human CRC.(39,40) However, this occurred only after 15 minutes, suggesting this was secondary (rather than proximate) to Akt activation (Figure S2A). While PTEN (phosphatase and tensin homolog) is a negative regulator of Akt activation, phosphatidylinositol-3 kinase (PI3K) directly activates Akt. We confirmed the involvement of PI3K with inhibitor studies and found that Kyn/QA did not induce β-catenin, GSK-3β or PRAS40 phosphorylation in the presence of Akt or PI3K inhibition (Figure 4C). Finally, although activation of the aryl hydrocarbon receptor (AHR) activation is implicated in mediating some of the biologic effects of the IDO1-KP metabolites, we found that rapid Akt activation occurred despite AHR antagonism (Figure 4D). Altogether, these results indicate that kynurenines promote β-catenin activity via a PI3K/AKT/GSK-3β signaling event, rather than AHR mediated transcriptional activity.
IDO1 and activated Akt co-express in murine and human CRC cells
Having shown that KP metabolites rapidly activate Akt signaling in CRC cell lines, we then sought to determine if this mechanism extended in tumor epithelium in vivo. Indeed, we found expression of pAkt Ser473 to be reduced in the neoplastic colon epithelium of iKO vs. WT mice (Figure 5A). We next evaluated a series of five human CRC samples and found that crypt staining for IDO1 mirrored and significantly correlated with that of pAkt Ser473 staining (Figure 5B,C).
Figure 5: IDO1 and activated Akt co-localize in neoplastic epithelium.
A) Tumors and normal epithelium of IDO1-iKO mice demonstrate diminished pAkt S473 immunostaining verses WT. Representative figure from immunostaining in 5 mice/group. B) Representative images of pAkt S473 co-staining with IDO1 immunostaining in human colon tumors. Arrows indicate dual negative crypts (yellow) and dual positive crypts (red). figure from immunostaining for 5 tumor tissues. C) Positive correlation between pAkt and IDO1 immunostaining based co-localization in human CRC. 5 CRCs with a total of 141 crypts compared.
Kynurenine pathway metabolites except for kynurenic acid activate Akt/β-catenin and promote CRC proliferation
IDO1 catalyzes the initial and rate limiting step in tryptophan metabolism to kynurenine initiating a cascade that generates several bioactive downstream kynurenine pathway (KP) metabolites (Figure S3A). We next evaluated the capacity of each of these metabolites to activate the Akt/GSK-3β/β-catenin pathway and to increase CRC cell proliferation. The majority of KP metabolites activated the Akt/GSK-3β/β-catenin pathway and promoted CRC proliferation. Only kynurenic acid (KA) did not activate Akt and pPRAS40 T246 and conversely suppressed CRC proliferation (Figure S3B-D). These findings were confirmed in DLD1 CRC cells as well (Figure S3E,F). Consistent with our findings of KP metabolites potentiating malignant CRC activity, we also confirmed that Kyn and QA promote wound closure (Figure S4 A,B).
Kynurenine protects CRC cells from apoptosis
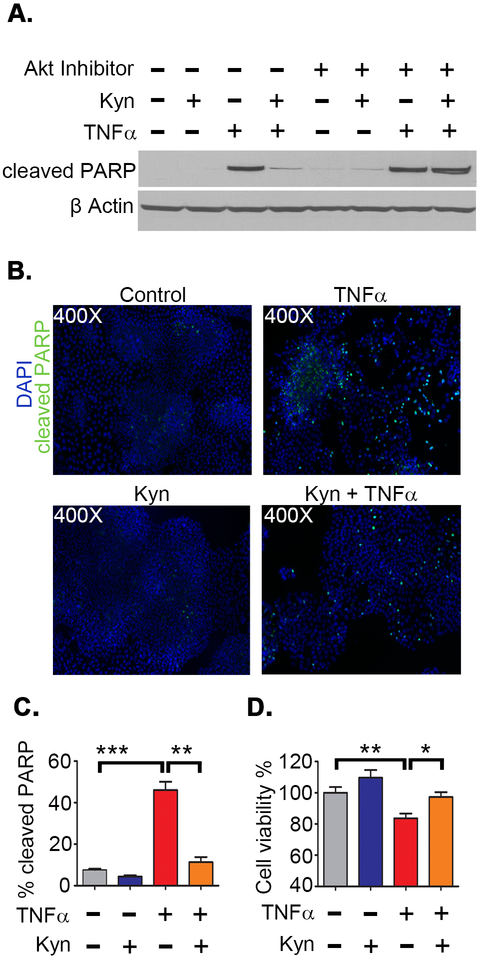
PI3K/Akt pathway activation is a recognized mechanism by which CRC cells resist stress-induced apoptosis (41–43). We thus sought to determine if KP metabolites offer protection against apoptosis in CRC cells. Indeed, Kyn co-treatment reduced TNFα induced apoptosis as measured by cleaved PARP and enhanced CRC cell viability (Figure 6A-D). Importantly, we found that kynurenine did not prevent TNFα induced apoptosis in the presence of an Akt inhibitor. We also found that Kyn reduced staurosporine induced apoptosis (Figure S5A-D). These results complement the in vivo data presented in Figure 2 and collectively indicate that KP metabolites promote tumor progression by promoting Akt induced resistance to apoptosis and increasing neoplastic survival during inflammatory and cellular stress.
Figure 6: Kynurenine protects CRC cells from apoptosis and promotes viability during stress.
Apoptosis was induced in HT29 cells by TNFα (100 ng/ml) in the presence and absence of kynurenine (100 µM) and the Akt inhibitor MK-2206. Apoptosis was measured by cleaved PARP expression 12 hours after listed treatment A) Western blot. B,C) Immunofluorescence staining images and quantitation of cleaved PARP as a percentage of DAPI positive cells. D) Cell viability 24 hours after treatement measured by WST-8 assay. *P<0.05, **P<0.01, ***P<0.001
Kynurenine mediated Akt activation differentiates tumor cells from normal cells
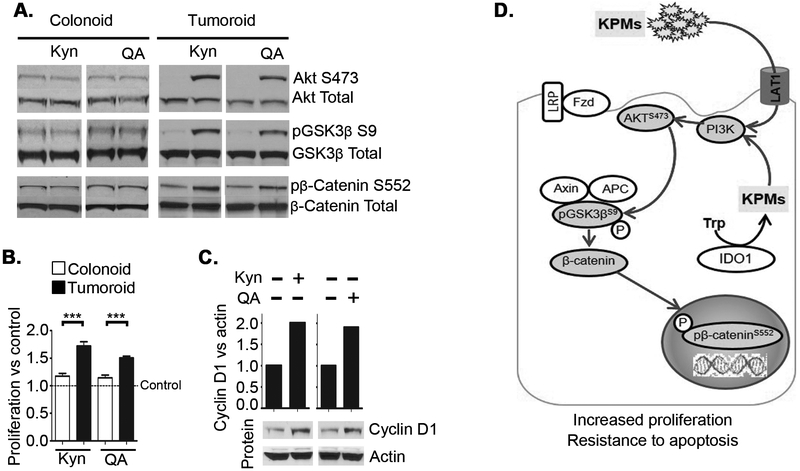
In IDO1-iKO mice, the epithelial cell proliferation index was significantly reduced in the tumor, but the difference was less pronounced in adjacent normal crypts (Figure 2C). To further explore potential differences in how KP metabolites impact normal verses neoplastic epithelial cells and to extend the findings further to human disease, we used and human derived colon tumoroid and normal colonoid cultures. Similar to our observations in monolayer CRC cell cultures, Kyn and QA induced rapid Akt/GSK-3β/β-catenin signaling in human CRC derived tumoroids (Figure 7A). However, in colonoids derived from normal colon epithelial crypts, Kyn and QA provoked no rapid response in phosphorylation. Enhanced proliferation and CyclinD1 levels were also observed in CRC derived tumoroids compared to minimal change in proliferation in normal colonoids (Figure 7B,C). These data indicate that neoplastic transformation drives an augmented response of the colon epithelium to IDO1 pathway activity. A summary illustration of the overall findings is shown in Figure 7D.
Figure 7: Kynurenine mediated rapid activation of Akt and β-catenin differentiates tumor cells from normal cells.
Western blot was used to detect total and phosphorylated proteins in A) Normal human colon crypt derived organoids (colonoids) and tumoroids derived from colon cancer tissue derived from patients with FAP. B) Proliferation in human colonoids and tumoroids measured by WST assay after 72 hours of treatment with Kyn or QA. C) Representative western blot of tumoroid cyclin D1 expression 12 hours after Kyn or QA treatment with mean densitometry quantitation. D) Model for KP metabolites induced PI3K/Akt activation and its effect on tumor cells and nuclear β-catenin activation. Data shown are a representative data from 3 experiments. ***P<0.001.
DISCUSSION
The immunometabolic IDO1 pathway has emerged as an important therapeutic immune-oncology target for solid tumors including colon cancer (17). The recognized capacity of this enzyme to suppress anti-tumoral immunity provides the mechanistic basis for current clinical trials of IDO1 inhibitors. In the current study, we define a novel, epithelial cell-centric mechanism and signaling cascade by which IDO1, through kynurenines, interacts directly with the signaling pathways of the neoplastic cells to promote tumor growth. Using cell culture, animal models, and human CRC tissues, we illustrate that epithelial IDO1 activity and several kynurenine pathway (KP) metabolites directly promote colon tumorigenesis by activating the pro-proliferative/anti-apoptotic PI3K/Akt pathway in the neoplastic epithelium. These findings provide mechanistic insight and strengthen justification for clinical trials evaluating IDO1 inhibitors as adjuvant therapeutics in colon cancer. Furthermore, these findings may have implications for the rational design of future clinical trials that would exploit a synergy of IDO1 inhibitors and conventional cancer therapies where Akt activation provides resistance such as radiation.
Linking IDO1 activity and KP metabolites to PI3K/Akt signaling provides a new mechanistic insight to how inflammation fuels neoplastic growth. Activation of Akt signaling or impaired expression of phosphatase and tensin homolog (PTEN) (a negative regulator of Akt) is reported in a majority of human colon cancers, while inhibitors of PI3K/Akt signaling are considered potential therapeutic agents (44). We demonstrated that intracellular KP activation rapidly initiates several Akt regulated pro-tumorigenic effects including β-catenin activation and proliferative cell cycle signaling, prevention of apoptosis from internal and extrinsic sources of cellular stress, and activation of the mTOR signaling pathway. These findings also define the upstream signaling pathway for our previous observations linking IDO1 to β-catenin activation as being mediated through PI3K/Akt signaling which is implicated in colitis and CRC (34,45). The precise mechanism by which KPs rapidly activate PI3K is an area of future study with our preliminary investigations not implicating the common activator Src (Figure S6A). However, the rapidity of these events (initiated within 5 minutes) do point to activation of cell signaling cascades rather than activating translational regulators such as AHR or induction of COX2 which kynurenine has been tied to in inflammation and malignancy (46,47). Regarding COX2, we confirmed the recent observation that kynurenine induced its expression, but required a longer time course ranging from hours, rather than minutes (Figure S6B).
To our knowledge, this is the first study to demonstrate a specific functional role for epithelial-based IDO1 as an independent promoter of tumorigenesis using tissue specific mouse modeling. Mice with genetic deletion of colon epithelial IDO1 demonstrate fewer tumors, significantly reduced tumor proliferation and showed higher apoptosis than WT mice. Furthermore, KP metabolites directly promoted CRC cell proliferation, resistance to apoptosis and restoration after injury. Given that the levels of intracellular kynurenine within cancer cells can reach high µM to low mM concentrations (35), it should not be surprising that they drive biologic effects within the neoplastic cells. Our own analysis shows on intracellular concentrations of kyn show that the levels can reach >70µM, which when applied exogenously activated Akt (Figure 3). Thus, together with our published work showing IDO1 inhibition directly suppresses proliferation in CRC cells in vitro, these findings support the hypothesis that epithelial IDO1 and kynurenines are both sufficient and necessary to drive colon tumor growth. The pro-tumorigenic, epithelial cell-intrinsic effects of IDO1 activity no doubt augment the pathogenic immunosuppressive effect of IDO1 expressing bone marrow derived monocytic cells and tumor cells.
Differences in IDO1 mediated effects between normal and neoplastic epithelial cells are also highlighted by the current data. In vivo, tumor cell proliferation is higher in WT than IDO1-iKO mice, but not significantly higher in the adjacent normal epithelial crypts. In vitro, KP metabolites robustly activate Akt and potentiate β-catenin activity to promote proliferation in CRC cell lines and colon tumoroids derived from patients with APC mutations. However, only delayed and modest effects were observed in colonoids derived from normal tissue. Prior studies had already identified that expression of IDO1 and other Kyn producing enzymes is often high and constitutively active after neoplastic transformation, compared to the non-transformed state (3,25,26,46). Intriguing studies have implicated COX2 and inflammation-associated AHR signaling as regulators of this phenomenon (47,48) Our now studies now illustrate a functional significance to this observed difference in expression by demonstrating that the pro-growth, epithelial cell-centric effects of IDO1 activity are also more pronounced in neoplastic over normal epithelial cells. Taken together, these data suggest a positive synergy between acquisition of constitutive IDO1 activity and the genetic mutations that drive colon cancer progression, including those that promote stabilized β-catenin. Thus, while the in vivo AOM/DSS model of colitis-associated cancer was used in this study, we predict that the phenotype extend to APC and β-catenin mutation driven sporadic colon cancers as well.
As an immunomodulatory pathway, there is existing rationale and clinical precedence for examining IDO1 inhibition in CRC. High tumor IDO1 expression is observed in a significant subset of CRC patients and high IDO1 expression at the tumor invasion front is an independent adverse prognostic factor for overall survival and metachronous metastases (16,20,25,26). As promising as this sounds, IDO1 inhibition as a monotherapy appears inadequate in colorectal and other cancers. A recently published phase I trial examined a novel hydroxyamidine small molecule IDO1 inhibitor (Epacadostat, Incyte Corp., Wilmington, DE) (11,49) in individuals with advanced solid tumors failing prior therapies. Twenty nine of the 52 patients enrolled (56%) had CRC (50). While the study met safety and biochemical efficacy endpoints, no patients achieved complete or partial response. Current studies with Epacadostat are examining it in combination with other immune-checkpoint inhibitors targeting CTLA-4 and the programmed cell death (PD-1) pathway.
Our findings provide new mechanistic rationale for targeting IDO1 inhibition in CRC and potentially add directionality to future clinical studies. IDO1 inhibitors may synergize with or augment the effects of cytotoxic chemo- or radiation therapy as the Akt pathway is intimately involved in preventing apoptotic or radiation induced cell death in CRC (51), Moreover, the specificity of IDO1 expression in neoplastic verses normal colon cells may provide a target that could enhance therapeutic efficacy without enhancing toxicity. Also relevant to cytotoxic cancer therapies, the IDO1-kynurenine pathway provides an important source for de novo generation of NAD+ (52). NAD+ is an important enzymatic cofactor for enzymes involved in DNA repair and inhibitors of its generation are recognized targets in cancer therapy (53,54). Finally, it is intriguing to think that IDO1 inhibitors might have a therapeutic role in CRC when combined with other well-tolerated small molecule drugs, such as inhibitors of the COX2 pathway with which our study also confirmed this association (47).
Kynurenic acid (KA) unlike other KP metabolites, did not activate Akt and in HT29 cells decreased proliferation. This finding is consistent with published literature which suggested that KA may have chemopreventive effects in CRC after demonstrating that KA reduced PI3K/Akt signaling (55). Notably, these effects were observed using high (millimolar) doses of KA. Still, these findings are intriguing and potentially suggest that pharmacologic shunting of the kynurenine pathway toward KA production and away from the Akt activating KP metabolites, may have a potentiating effect for IDO1 inhibitors or in combination with other therapeutics.
In summary, our results demonstrate a novel mechanistic link between IDO1 activity, kynurenine pathway metabolites and the PI3K/Akt pro-proliferative and anti-apoptotic pathways in colorectal cancer including activated β-catenin signaling. These findings demonstrate the importance of epithelial IDO1 activity in driving colon tumorigenesis and extend from cell lines and mouse models to human CRC. Together, these findings underscore the value of investigating IDO1 inhibition in colorectal cancer, provide insights into mechanism of action and may stimulate consideration for future clinical trials that may exploit a synergy of IDO1 inhibitors and therapies where Akt activation provides resistance.
Supplementary Material
Study Significance: This study identifies a new mechanistic link between IDO1 activity and PI3K/AKT signaling, both of which are important pathways involved in cancer growth and resistance to cancer therapy.
ACKNOWLEDGEMENTS
M. Ciorba has support from a Crohn’s and Colitis Foundation Daniel H Present Senior Research Award, Ref. 370763, NIH grants (DK109384, DK100737 and AI095776), philanthropic support from the Givin’ it all for Guts Foundation https://givinitallforguts.org/, and a Central Society for Clinical Research Early Career Development Award. B. Dieckgraefe was supported by I01 BX003072. Core support from The Washington University Digestive Diseases Research Core Center (P30 DK052574) and Siteman Cancer Center (P30 CA91842). D. Alvarado was supported by DK077653 and The Lawrence C. Pakula MD IBD Innovation Fellowship. We acknowledge technical assistance from Srikanth Santhanam PhD. Finally, we are grateful to Nicholas O. Davidson MD for his unwavering and enthusiastic support of gastroenterology research at Washington University and for his insightful comments on this project.
Footnotes
COI: MAC has received investigator-initiated research support from Incyte, Corp (Wilmington, DE) for work independent of that presented herein. All other authors declare no potential conflicts of interest.
REFERENCES
- 1.Huang L, Mellor AL. Metabolic control of tumour progression and antitumour immunity. Curr Opin Oncol 2014;26:92–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer immunology, immunotherapy : CII 2014;63:721–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003;9:1269–74 [DOI] [PubMed] [Google Scholar]
- 4.Zhai L, Spranger S, Binder DC, Gritsina G, Lauing KL, Giles FJ, et al. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clin Cancer Res 2015;21:5427–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, et al. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell reports 2015;13:412–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fallarino F, Grohmann U, Puccetti P. Indoleamine 2,3-dioxygenase: from catalyst to signaling function. Eur J Immunol 2012;42:1932–7 [DOI] [PubMed] [Google Scholar]
- 7.Munn DH. Blocking IDO activity to enhance anti-tumor immunity. Frontiers in bioscience 2012;4:734–45 [DOI] [PubMed] [Google Scholar]
- 8.Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA 3rd, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A 2008;105:17073–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer discovery 2012;2:722–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest 2004;114:280–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koblish HK, Hansbury MJ, Bowman KJ, Yang G, Neilan CL, Haley PJ, et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther 2010;9:489–98 [DOI] [PubMed] [Google Scholar]
- 12.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res 2007;67:792–801 [DOI] [PubMed] [Google Scholar]
- 13.Zheng X, Koropatnick J, Chen D, Velenosi T, Ling H, Zhang X, et al. Silencing IDO in dendritic cells: a novel approach to enhance cancer immunotherapy in a murine breast cancer model. Int J Cancer 2013;132:967–77 [DOI] [PubMed] [Google Scholar]
- 14.Thaker AI, Rao MS, Bishnupuri KS, Kerr TA, Foster L, Marinshaw JM, et al. IDO1 metabolites activate beta-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology 2013;145:416–25 e1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godin-Ethier J, Hanafi L-A, Piccirillo CA, Lapointe R. Indoleamine 2,3-Dioxygenase Expression in Human Cancers: Clinical and Immunologic Perspectives. Clinical Cancer Research 2011;17:6985–91 [DOI] [PubMed] [Google Scholar]
- 16.Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res 2006;12:1144–51 [DOI] [PubMed] [Google Scholar]
- 17.Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 Inhibitors: From Bench to Bedside. Cancer Res 2017;77:6795–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornyák L, Dobos N, Koncz G, Karányi Z, Páll D, Szabó Z, et al. The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Frontiers in immunology 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theate I, van Baren N, Pilotte L, Moulin P, Larrieu P, Renauld JC, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res 2014 [DOI] [PubMed] [Google Scholar]
- 20.Ferdinande L, Decaestecker C, Verset L, Mathieu A, Moles Lopez X, Negulescu AM, et al. Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. British journal of cancer 2012;106:141–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010;115:3520–30 [DOI] [PubMed] [Google Scholar]
- 22.Walczak K, Dabrowski W, Langner E, Zgrajka W, Pilat J, Kocki T, et al. Kynurenic acid synthesis and kynurenine aminotransferases expression in colon derived normal and cancer cells. Scand J Gastroenterol 2011;46:903–12 [DOI] [PubMed] [Google Scholar]
- 23.Engin AB, Karahalil B, Karakaya AE, Engin A. Helicobacter pylori and serum kynurenine-tryptophan ratio in patients with colorectal cancer. World J Gastroenterol 2015;21:3636–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao YF, Peng RQ, Li J, Ding Y, Zhang X, Wu XJ, et al. The paradoxical patterns of expression of indoleamine 2,3-dioxygenase in colon cancer. J Transl Med 2009;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santhanam S, Alvarado DM, Ciorba MA. Therapeutic targeting of inflammation and tryptophan metabolism in colon and gastrointestinal cancer. Transl Res 2016;167:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarado DM, Santhanam S, Ciorba MA. Role of Kynurenine Pathway in Gastrointestinal Diseases In: Mittal S, editor. Targeting the Broadly Pathogenic Kynurenine Pathway. Cham: Springer International Publishing; 2015. p 157–67. [Google Scholar]
- 27.Thaker AI, Shaker A, Rao MS, Ciorba MA. Modeling colitis-associated cancer with azoxymethane (AOM) and dextran sulfate sodium (DSS). J Vis Exp 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciorba MA, Bettonville EE, McDonald KG, Metz R, Prendergast GC, Newberry RD, et al. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol 2010;184:3907–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 2015;64:911–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc 2013;8:2471–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi H, Ajima R, Luo CT, Yamaguchi TP, Stappenbeck TS. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science 2012;338:108–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Najdi R, Holcombe RF, Waterman ML. Wnt signaling and colon carcinogenesis: beyond APC. Journal of carcinogenesis 2011;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 2007;282:11221–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee G, Goretsky T, Managlia E, Dirisina R, Singh AP, Brown JB, et al. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology 2010;139:869–81, 81 e1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knowles RG, Clarkson NA, Pogson CI, Salter M, Duch DS, The MPE Role of Tryptophan and Kynurenine Transport in the Catabolism of Tryptophan Through Indoleamine 2,3-Dioxygenase In: Schwarcz R YSN Brown R.R. (eds), editor. Kynurenine and Serotonin Pathways Advances in Experimental Medicine and Biology. Boston, MA: Springer; 1991. p pp 161–6. [DOI] [PubMed] [Google Scholar]
- 36.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995;378:785–9 [DOI] [PubMed] [Google Scholar]
- 37.Walker AK, Wing E, Banks WA, Dantzer R. 84. Targeting the kynurenine blood-to-brain transport system to treat inflammation-induced fatigue and depression. Brain, Behavior, and Immunity 2014;40:e24–e5 [Google Scholar]
- 38.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, et al. l-type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci 2008;99:2380–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosner M, Siegel N, Valli A, Fuchs C, Hengstschlager M. mTOR phosphorylated at S2448 binds to raptor and rictor. Amino Acids 2010;38:223–8 [DOI] [PubMed] [Google Scholar]
- 40.Johnson SM, Gulhati P, Rampy BA, Han Y, Rychahou PG, Doan HQ, et al. Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. Journal of the American College of Surgeons 2010;210:767–76, 76–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene 2003;22:8983–98 [DOI] [PubMed] [Google Scholar]
- 42.Kaliszczak M, Trousil S, Ali T, Aboagye EO. AKT activation controls cell survival in response to HDAC6 inhibition. Cell death & disease 2016;7:e2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S, Deng Z, Yao C, Huang P, Zhang Y, Cao S, et al. AT7867 Inhibits Human Colorectal Cancer Cells via AKT-Dependent and AKT-Independent Mechanisms. PLoS One 2017;12:e0169585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandurangan AK. Potential targets for prevention of colorectal cancer: a focus on PI3K/Akt/mTOR and Wnt pathways. Asian Pacific journal of cancer prevention : APJCP 2013;14:2201–5 [DOI] [PubMed] [Google Scholar]
- 45.Goretsky T, Bradford EM, Ye Q, Lamping OF, Vanagunas T, Moyer MP, et al. Beta-catenin cleavage enhances transcriptional activation. Scientific reports 2018;8:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011;478:197–203 [DOI] [PubMed] [Google Scholar]
- 47.Hennequart M, Pilotte L, Cane S, Hoffmann D, Stroobant V, De Plaen E, et al. Constitutive IDO1 Expression in Human Tumors Is Driven by Cyclooxygenase-2 and Mediates Intrinsic Immune Resistance. Cancer Immunol Res 2017 [DOI] [PubMed] [Google Scholar]
- 48.Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M, et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget 2014;5:1038–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newton RC, Scherle PA, Bowman K, Liu X, Beatty GL, O’Dwyer PJ, et al. Pharmacodynamic assessment of INCB024360, an inhibitor of indoleamine 2,3-dioxygenase 1 (IDO1), in advanced cancer patients. J Clin Oncol 2012;30 (Abstract 2500)23169502 [Google Scholar]
- 50.Beatty GL, O’Dwyer PJ, Clark J, Shi JG, Bowman KJ, Scherle P, et al. First-in-Human Phase 1 Study of the Oral Inhibitor of Indoleamine 2,3-dioxygenase-1 Epacadostat (INCB024360) in Patients With Advanced Solid Malignancies. Clin Cancer Res 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 2002;94:3127–34 [DOI] [PubMed] [Google Scholar]
- 52.Sahm F, Oezen I, Opitz CA, Radlwimmer B, von Deimling A, Ahrendt T, et al. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res 2013;73:3225–34 [DOI] [PubMed] [Google Scholar]
- 53.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res 2003;63:7436–42 [PubMed] [Google Scholar]
- 54.Espindola-Netto JM, Chini CCS, Tarrago M, Wang E, Dutta S, Pal K, et al. Preclinical efficacy of the novel competitive NAMPT inhibitor STF-118804 in pancreatic cancer. Oncotarget 2017;8:85054–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walczak K, Turski WA, Rajtar G. Kynurenic acid inhibits colon cancer proliferation in vitro: effects on signaling pathways. Amino Acids 2014;46:2393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.