Abstract
Background:
Advances in communication technology have enabled new methods of delivering test results to cancer survivors. We sought to determine patient preferences regarding the use of newer technology in delivering test results during cancer surveillance.
Methods:
A single institutional, cross-sectional analysis of the preferences of adult cancer survivors regarding the means (secure digital communication versus phone call or office visit) to receive surveillance test results was undertaken.
Results:
Among 257 respondents, the average age was 59.1 years (SD 13.5) and 61.8% were female. Common malignancies included melanoma/sarcoma (29.5%), thyroid (25.7%), breast (22.8%), and gastrointestinal (22.0%) cancer. Although patients expressed a relative preference to receive normal surveillance results via MyChart or secure e-mail, the majority preferred abnormal imaging (87.2%) or blood results (85.9%) to be communicated by in-office appointments or phone calls irrespective of age or cancer type. Patients with a college degree or higher were more likely to prefer electronic means of communication of abnormal blood results compared with a telephone call or in-person visit (odds ratio 2.18, 95% confidence interval: 1.01−4.73, P < .05). In contrast, patients >65 years were more likely to express a preference for telephone or in-person communication of normal imaging results (odds ratio: 2.03, 95% CI: 1.16−3.56, P < .05) versus patients ≤65 years. Preference also varied according to malignancy type.
Conclusion:
Although many cancer patients preferred to receive “normal” surveillance results electronically, the majority preferred receiving abnormal results via direct conversation with their provider. Shifting routine communication of normal surveillance results to technology-based applications may improve patient satisfaction and decrease health care system costs.
TOC summary
Although many cancer patients preferred to receive “normal” surveillance results electronically, most preferred receiving “abnormal” results via direct communication. Technology-based applications may improve patient satisfaction.
Introduction
With an aging population, development of biomedical innovations, and improvements in targeted tumor treatment, the number of cancer survivors is projected to increase from 15.1 million in 2016 to 26.1 million by 2040.1,2 Cancer surveillance reflects the ongoing, timely, and systematic collection of information on current cancer status.3 Because of the growing population of cancer survivors, current models of cancer surveillance are unlikely to be sustainable.4 In addition, although well intended, intense surveillance strategies have often not correlated with prolonged survival.5,6In fact, among patients who underwent primary treatment for colorectal cancer, there was no association between surveillance intensity and detection of recurrence.7 Other studies among patients with primary and secondary malignancies of the liver have similarly demonstrated that surveillance intensity did not affect time to second procedure or median survival duration.8,9 Rather, surveillance practices can increase patient anxiety, as well as adversely impact quality of life.10
Health care expenditures associated with cancer surveillance can be considerable because of the costs of obtaining repeat blood work and cross-sectional imaging.11 Patients and society can also be economically disadvantaged because of the costs associated with repeat clinic visits, travel, and of time from work.11,12 As such, innovative ways to facilitate cancer surveillance are needed to bend the cost curve.13–16 Although health information technology has been associated with increased adherence to guideline-based care and decreased medication errors, the use of technology to deliver test results related to cancer surveillance has not been well characterized.17 Understanding the role of technology in the delivery of cancer status may have important implications for patients and health care providers. Specifically, such technology may represent a potential avenue to decrease the need for in-person cancer surveillance. Patient preferences for frequency of surveillance, as well as preferred means to receive information related to cancer surveillance, have not been well studied.18,19As such, the objective of the current study was to determine patient preferences around the desired frequency of cancer surveillance, as well as the preferred means of receiving information about cancer surveillance. Specifically, we sought to identify sociodemographic and clinical factors associated with patient preferences regarding the use of technology to deliver test results about cancer surveillance relative to whether the findings were “normal” or “abnormal.”
Methods
Survey instrument design and administration
The study design consisted of a cross-sectional survey of patients using an instrument of validated survey tools augmented with investigator-derived questions.20 Questions related to the patient’s current follow-up care (e.g., frequency of visits) were adapted from the Assessment of Patients' Experience of Cancer Care (APECC) Study.20The APECC was developed by the National Cancer Institute to assess the quality of care from the perspective of the cancer survivor. Published Cronbach’s α statistics on this scale demonstrated good internal consistency (α = .80−.82).20 The full survey was reviewed by a patient advisory committee of cancer patients at The Ohio State University Comprehensive Cancer Center–Arthur G. James Cancer Hospital and Richard J. Solove Research Institute ([OSUCCC-James] Columbus). The final version of the survey consisted of 35 questions (Supplemental Material).
Participants were recruited during follow-up visits with their physician at the outpatient clinics at OSUCCC-James. Eligible patients were ≥18 years of age, self-identified as proficient in the English language, had undergone curative-intent resection for a solid tumor, had no evidence of active disease, and were receiving cancer-surveillance care. The study was approved by The Ohio State University Wexner Medical Center Institutional Review Board (protocol # 2017C0190).
Variables and outcomes
Demographic variables were collected including age, race, income, and current relationship status. Cancer demographics were obtained from the patient medical record, including date of cancer diagnosis, operation type, date of cancer operation, and current surveillance schedule. Patient preferences on follow-up care, including frequency, mode of communication (MyChart, secure message, telephone, or in-office visit), and health care provider were assessed with investigator-derived questions. Specifically, preferences on modality of communication (in person, MyChart, phone call, secure message) were ascertained for normal imaging, normal blood, abnormal imaging, and abnormal blood results. Designed to promote continuity of patient-provider communication, MyChart is a secure electronic portal where results and health records are shared, and secure electronic messaging (e-mail) is a feature within MyChart.21 To assess perception of MyChart, secure electronic messages (e-mails), and phone calls during surveillance, questions were formulated using a 5-point Likert scale (1 = not effective at all, 2 = not very effective, 3 = moderately effective, 4 = very effective, 5 = extremely effective). Respondents who did not use any of these tools indicated: “I do not use this tool to share information.” To determine preferences around frequency of surveillance, participants were asked to select their preferred frequency schedule from one of the following: every 3 months, every 6 months, every 9 months, every year, and > 1 year.
Statistical analysis
A cross-sectional descriptive design was used to explore the quantitative survey items. Categorical variables were compared using Pearson’s χ2 test. Unadjusted odds ratios (OR) with 95% confidence intervals (CIs) were also calculated to examine the influence of demographic factors on preference for technology-mediated (MyChart/secure e-mail) versus conventional phone call/in-office visits. All tests of statistical significance were two-sided, with level of statistical significance established at P < .05. Statistical analysis was performed using SPSS (v 24; SPSS, Inc, Chicago, IL).
Results
Among 300 potential participants identified, 271 completed the survey (response rate 90.3%). Based on patient preference, 214 patients (78.9%) completed the survey electronically; whereas 57 patients (21.1%) completed a paper-based survey. After reviewing the data, 14 participants were excluded because of the following: operation not performed at OSUCCC-James (n = 6), surveillance was for a premalignant condition (n = 3), patients did not undergo curative resection (n = 3), and patients failed to complete more than half of the survey (n = 2). The final analytic cohort consisted of 257 participants.
Demographics
The average age of the study participants was 59.1 years (SD = 13.5, range 22–88; Table I). The majority of patients were female (61.5%) and white (94.1%). Less than half of the respondents had a college degree or higher (38.6%), and most individuals had a combined household income of less than $100,000 (<$50,000, 40.1%; $50,000–$99,000, 21%; $100,000–$150,000, 17.9%; >$150,000, 16.7%). The most common diagnosis was melanoma or sarcoma (29.5%). Other cancer diagnoses included thyroid (25.7%), breast (22.8%), and gastrointestinal (22.0%) cancer. Of note, the majority of respondents (82.4%) were not receiving cancer treatment at the time of survey completion. A minority of patients reported currently receiving chemotherapy (7.5%) or radiation therapy (2%), and few patients (8.2%) reported receiving “other” treatments. On average, the survey was completed 4.7 years (SD = 6.42 years, range 0–54) after curative-intent surgery.
Table I.
Demographics of participants (n = 257)
| Age (years)* | 59.11 ± 13.51 | |
| Time after surgery (years)* | 4.70 ± 6.42 | |
| Sex, n (%) | ||
| Male | 99 | (38.5) |
| Female | 158 | (61.5) |
| Race, n (%)† | ||
| White | 241 | (94.1) |
| Non-white | 15 | (5.9) |
| Education, n (%) | ||
| Some high school | 5 | (2.0) |
| High School diploma/General | 57 | (22.7) |
| Education Development test | ||
| Some college/associates/trade school | 92 | (36.7) |
| College degree | 52 | (20.7) |
| Some postgraduate work or degree | 45 | (17.9) |
| Income, n (%) | ||
| <$90,000 | 145 | (59.2) |
| ≥$90,000 | 100 | (40.8) |
| Insurance, n (%)† | ||
| None | 4 | (1.6) |
| Private | 165 | (64.5) |
| Medicare | 85 | (33.2) |
| Both | 2 | (0.8) |
| Malignancy Type, n (%)§ | ||
| Breast | 55 | (22.8) |
| Sarcoma/skin/melanoma | 71 | (29.5) |
| Gastrointestinal | 53 | (22.0) |
| Thyroid | 62 | (25.7) |
Data are presented as mean ± standard deviation.
Data available for 256 patients.
Data available for 241 patients.
Patient preferences: Surveillance provider and frequency
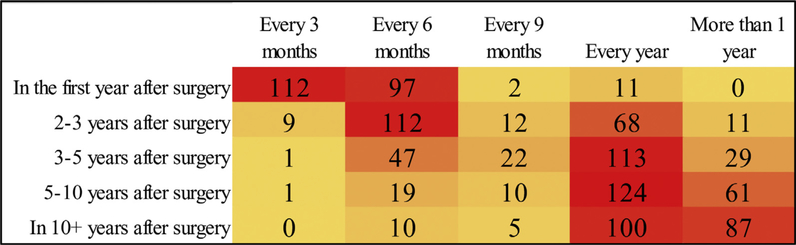
Among the 218 (84.8%) participants who designated a medical provider as the “best” to provide follow-up care for cancer surveillance, the vast majority (n = 197, 90.4%) identified the surgical oncologists as their “best” preference, whereas a minority preferred a primary care provider (n = 9, 4.1%) or an advance practice provider (n = 12, 5.5%). More than half of patients (50.4%) preferred follow-up every 3 months within the first year of surveillance. Of note, as time from curative resection increased, preference on surveillance interval increased concurrently (Fig. 1). Importantly, nearly half of patients (49.5%) still wanted to see a medical provider on an annual basis even 10 or more years after cancer surgery.
Fig. 1.
Heatmap of patient preference for frequency of surveillance visits by elapsed time postsurgery.
Patient preferences: Mode of communication with provider during cancer surveillance
Among 176 respondents (68.5%) who utilized MyChart, the majority of respondents (n = 135, 76.7%) reported MyChart to be a “very effective” to “extremely effective” mode of communication, and 14 (8%) characterized it as” not very effective” to “not effective at all.” Similar trends were noted among the 169 (65.8%) participants who used secure electronic messaging (“very effective” to “extremely effective”: 72.8% versus “not very effective” to “not effective at all”: 9.5%). An overwhelming majority of individuals (89.2%) identified the use of a telephone as an effective means to communicate, whereas a small subset (2.7%) reported that phone calls were “not very effective” or “ineffective.”
Factors associated with preference for surveillance information: Telephone or in person versus MyChart or secure e-mail
When analyzing patient preferences for receiving information on imaging results, the proportion of patients who preferred MyChart or secure e-mail for delivery of normal results was higher compared with abnormal results (normal: 43.9% [n = 102/232] versus abnormal: 12.8% [n = 29/226], P < .001). Similarly, the proportion of patients who preferred MyChart or secure e-mail for delivery of normal blood results was higher versus abnormal results (normal: 50.0% [n = 97/194] versus abnormal: 14.1% [n = 32/226], P < .001).
The influence of demographic factors on preference for technology-mediated (MyChart or secure e-mail) versus conventional phone call or in-office visit was examined for the communication of normal results (Table II). Overall, individuals with a lower education and patients who were accompanied by a family member or friend expressed a preference for telephone or in-person communication of normal surveillance results (all P < .05; Table II). Of note, patients >65 years were more likely to express a preference for telephone/in-person communication of normal imaging results compared with patients ≤65 years (OR: 2.03, 95% CI: 1.16–3.56, P < .05). In contrast, there was no difference in the patient communication preference relative to income level (P > .05; Table II).
Table II.
Unadjusted odds ratios (OR) of the communication preferences for normal results stratified by participant demographic factors
| Demographic factors | Imaging | Blood | ||||
|---|---|---|---|---|---|---|
| Phone/in office | Mychart/e-mail | OR (95% CI) | Phone/in office | Mychart/e-mail | OR (95% CI) | |
| Age (years) | 0.49 (0.28–0.86) | 0.65 (0.37–1.13) | ||||
| ≤65 | 75 | 75 | 58 | 91 | ||
| >65 | 55 | 27 | 39 | 40 | ||
| Sex | 1.45 (0.84–2.47) | 1.26 (0.74–2.16) | ||||
| Male | 56 | 35 | 41 | 48 | ||
| Female | 74 | 67 | 56 | 83 | ||
| Education | 1.81 (1.06–3.10) | 2.40 (1.36–4.19) | ||||
| <College degree | 83 | 51 | 67 | 64 | ||
| ≥College degree | 44 | 49 | 28 | 64 | ||
| Income | ||||||
| <$90,000 | 74 | 50 | 1.26 (0.73–2.15) | 57 | 65 | 1.40 (0.81–2.40) |
| ≥$90,000 | 53 | 45 | 37 | 59 | ||
| Accompanied at appointment | 1.81 (1.06–3.09) | 1.88 (1.08–3.27) | ||||
| Yes | 86 | 53 | 66 | 70 | ||
| No | 43 | 48 | 30 | 60 | ||
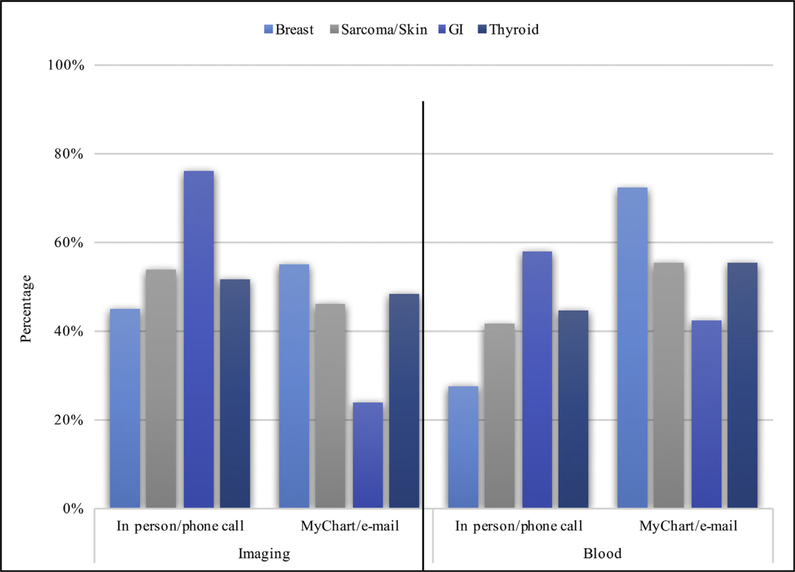
Of interest, there was variation in how patients preferred to receive information about normal surveillance results based on the various malignant diagnoses. In particular, a telephone call or in-person visit was preferred over MyChart or secure e-mail for the communication of normal surveillance results among patients with melanoma or sarcoma (53.8% versus 46.2%, respectively), gastrointestinal (76.1% versus 23.9%, respectively), and thyroid (51.8% versus 48.2%, respectively) cancers (all P < .05). In contrast, patients with breast cancer more often preferred MyChart or secure e-mail (54.9%) versus phone call or in-office visits (44.2%; P < .05; Fig. 2).
Fig. 2.
Distribution of communication preferences for normal blood and imaging results by malignancy type. Significant results for χ2 analysis for patient preference of communication mode by malignancy type for normal imaging (χ2(3) = 10.52, P = .015) and normal blood results (χ2(3) = 9.11, P = .028).
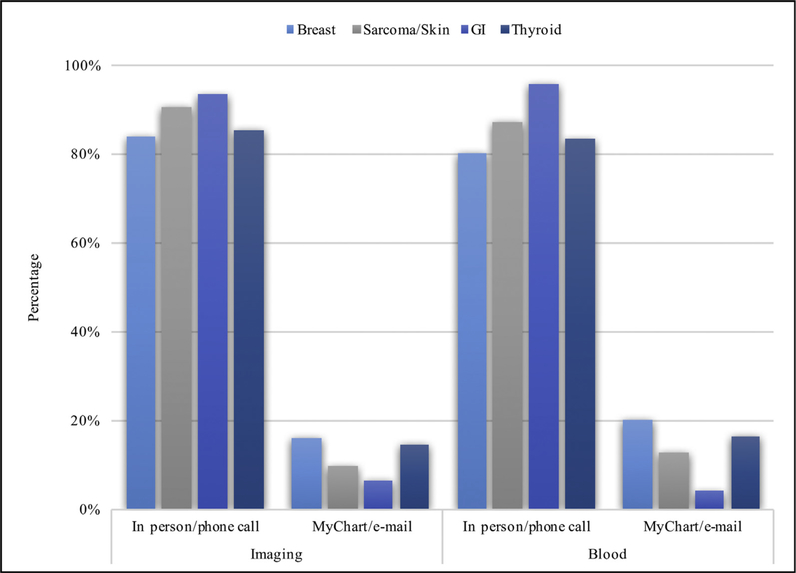
Perhaps not surprising, there was less variation in how patients wanted information communicated about abnormal surveillance findings. Specifically, the overwhelming majority of patients preferred abnormal blood (85.9%, n = 194/226) and imaging results (87.2%, n = 197/226) communicated via a telephone call or in-office visit rather than through the use of technology (P < .001). Of note, factors such as age, sex, and income level were not associated with patient preference of telephone call or in-person versus MyChart or secure e-mail for the communication of abnormal surveillance findings (all P < .05; Table III). Patients with a college degree or higher education level were, however, more likely to prefer electronic means of communication of abnormal blood results compared with a telephone call or in-person visit (OR 2.18, 95% CI: 1.01–4.73, P < .05). The type of malignant diagnosis was not associated with preference for telephone call or in-office visit versus MyChart or secure e-mail as the overwhelming majority preferred nonelectronic-based means of communication of abnormal findings regardless of cancer type (Fig. 3).
Table III.
Unadjusted odds ratios (OR) of the communication preferences for abnormal results stratified by participant demographic factors
| Demographic factors | Imaging | Blood | ||||
|---|---|---|---|---|---|---|
| Phone/in office | Mychart/e-mail | OR (95% CI) | Phone/in office | Mychart/e-mail | OR (95% CI) | |
| Age (years) | 0.46 (0.18–1.19) | 0.49 (0.20–1.20) | ||||
| ≤65 | 126 | 23 | 124 | 25 | ||
| >65 | 71 | 6 | 70 | 7 | ||
| Sex | 1.83 (0.77–4.34) | 1.79 (0.78–4.08) | ||||
| Male | 81 | 8 | 80 | 9 | ||
| Female | 116 | 21 | 114 | 23 | ||
| Education | 1.48 (0.67–3.29) | 2.18 (1.01–4.73) | ||||
| <College degree | 116 | 14 | 117 | 13 | ||
| ≥College degree Income | 78 | 14 | 74 | 18 | ||
| Income | 1.41 (0.62–3.15) | 1.58 (0.71–3.35) | ||||
| <$90,000 | 107 | 13 | 107 | 14 | ||
| ≥$90,000 | 82 | 40 | 79 | 16 | ||
| Accompanied at appointment | 1.24 (0.56–2.73) | 1.59 (0.75–3.38) | ||||
| Yes | 118 | 16 | 118 | 16 | ||
| No | 77 | 13 | 74 | 16 | ||
Fig. 3.
Distribution of communication preferences for abnormal blood and imaging results by malignancy type. Insignificant results for χ2 analysis for patient preference of communication mode by malignancy type for abnormal imaging (χ2(3) = 2.39, P = .496) and abnormal blood results (χ2(3) = 5.51, P = .138).
Discussion
Cancer surveillance, in addition to the sharing of information regarding current cancer status, can be a time-consuming, burdensome, and costly process for both patients and the health care system.3,22–24 Owing to an aging population, the number of cancer survivors is projected to increase and, thus, an even higher number of patients will require ongoing cancer surveillance.1,2The incorporation of technology as a means to communicate cancer surveillance results may alleviate the need for travel, reduce hospital-based costs, and decrease lost work time for patients.25–27 Data on patient preferences about in-person versus technology-based means of communicating regarding cancer surveillance results have not been investigated. The current study was important because we specifically examined patient preferences on surveillance using a cross-sectional survey. Of note, patients preferred more frequent surveillance intervals during the first 3 years after curative-intent resection, whereas most patients preferred yearly in-office visits after year 3. Somewhat surprisingly, many patients still wanted to be seen yearly with in-office visits even beyond 10 years after surgery. Perhaps of even more interest was patient preferences around receiving information on surveillance results. When analyzing patient preferences for receiving information on normal imaging or blood results, more patients preferred MyChart or secure e-mail; however this preference was more prevalent among certain patient subsets (eg, younger, more educated, breast cancer diagnosis). In contrast, when surveillance results were abnormal, the overwhelming majority of patients expressed a preference for a telephone call or in-person visit. These data suggest a relatively modest acceptance of technology-based means to share surveillance information with patients. The opportunity to use technology-based means to share surveillance information appeared largely to be among younger, more educated patients when sharing only normal test results.
Telemedicine has been increasingly utilized in primary care to deliver results and manage chronic diseases.28–33 For example, Inglis et al34 reviewed 25 randomized clinical trials comparing telephone support or telemonitoring versus usual clinical care of congestive heart failure patients. In their study, the authors noted that both structured telephone support (RR 0.77, 95% CI 0.68–0.87, P < .0001) and telemonitoring (RR 0.79, 95% CI 0.67–0.94, P = .008) reduced congestive heart failure-related hospitalizations and were acceptable to patients.34 In a separate study, Rasmussen et al33 reported on the impact of internet-based monitoring of asthma and noted that this management tool led to better improvement in the internet group compared with the standard of care group regarding asthma symptoms and quality of life.33 Currently, most institutions employ some type of online systems or secure mobile portals that allow patients to access their own health records.35–40 Utilizing these secure portals for cancer surveillance may reduce patient travel costs, unburden busy clinics of routine visits, improve physician workflow, and improve patient satisfaction.25,41–43 To this point, Mair and Whitten44 reported a systematic review of studies on patient satisfaction with telemedicine.44 The authors noted that there were significant methodologic deficiencies among the 32 studies (eg, low sample size, context, study design). Although teleconsultation was acceptable in some circumstances, the authors recommended that further studies were needed to explore the perspective of patients in the context of various health care settings.
To this end, the current study specifically sought to characterize patient perceptions around telemedicine, with a focus on the dissemination of cancer surveillance results. Few earlier studies had sought to evaluate the use of technology for cancer surveillance.45 In one study, Beaver et al45 evaluated traditional hospital visits versus telephone follow-ups among breast cancer patients after completion of primary treatment (surgery, radiotherapy, chemotherapy) and reported that the latter was well received by participants with no evidence of physical or psychologic disadvantage. In a separate study that investigated the use of technological support of patients receiving chemotherapy, Kearney et al46 noted that a computer-based symptom management tool was feasible and acceptable to both patients and health professionals in complementing the care of patients receiving chemotherapy. In a systematic review that examined technology to deliver cancer follow-up, Dickinson et al47 noted that interventions involving technology did not compromise patient safety, but data on patient perception on the acceptability of technology-based delivery of cancer surveillance information was lacking. The current study builds on this previous work because it demonstrated that roughly 50% of surveyed patients reported that telemedicine based (eg, MyChart or secure e-mail) communication was an acceptable means to share cancer surveillance data when the results were normal. Of note, only roughly 1 in 11 patients reported that technologic means (eg, MyChart or secure e-mail) were acceptable when reporting abnormal blood or imaging cancer surveillance results.
Of interest, specific cohorts of patients expressed various preferences around technology versus in-person means to learn about cancer surveillance results. For example, patients >65 years of age were two times more likely to want a telephone call or in-person visit to review normal surveillance results than younger patients. Cimperman et al48 had noted that older patients may have different perceptions of home telehealth services. In particular, older patients may experience computer anxiety that often requires different visual equipment, training, and preemptive reassurances that the mechanisms being used to share health information are trustworthy. Perhaps not surprising, patients with a higher level of education were more amenable to technology-based means to share normal surveillance results. In a model of patient acceptance of health technology for chronic disease management, Dou et al49 noted that education level was an important factor in the acceptance and use of health technology. Unlike other studies, we additionally examined the impact of specific malignant diagnoses on patient preference around communication of surveillance results. Compared with patients with other cancer diagnoses, breast cancer patients were more likely to prefer receiving normal surveillance results by MyChart or secure e-mail. In another report, de Bock et al50 had noted that not all breast patients wanted all types of information or follow-up during routine surveillance after a diagnosis of breast cancer.50 It was important to note, however, that the overwhelming majority (blood: 87.2%, imaging: 85.9%) of patients―regardless of malignant diagnosis―wanted abnormal surveillance blood or imaging results communicated via a telephone call or an in-person visit rather than purely through a technology-based means of communication.
The current study had several limitations that need to be considered when interpreting the results. Similar to other cross-sectional survey studies, the data were subject to selection and recall bias. To limit recall bias, data on cancer diagnoses, treatment, and management were extracted from electronic health records. The majority of patients were recruited from surgical oncology offices, which may have self-selected patients who preferred to have cancer surveillance by a surgeon. In addition, data on how many patients had both surgery and surveillance at OSUCCC-James versus surveillance closer to home were not available. Most patients generally did have surgery and surveillance at our institution. Although some patients may not have chosen an electronic means to receive surveillance information because of a lack of access to the internet/a computer, the overwhelming number of patients were likely to have some means to access the Web (eg, home/community center, smart phone, etc). Finally, given that the survey was administered at the OSUCCC-James, which is a dedicated cancer hospital, patients treated at a matrix cancer hospital and cancer patients cared for in a community setting may have different perceptions regarding surveillance.
In conclusion, many cancer patients still prefer to receive surveillance information in person via a telephone call or an in-person clinic appointment. In particular, the overwhelming majority of patients―regardless of cancer diagnosis―prefer in person communication of abnormal surveillance test results. Acceptance of a technology-based means to communicate normal surveillance results was more common, especially among certain patients (eg, younger, higher level of education). Shifting routine communication of normal cancer surveillance results to technology-based applications may be acceptable to some patients, mitigating patient travel and alleviating clinic congestion.
Supplementary Material
Acknowledgments
Dr. Onuma is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number 1T32AI106704–01A1 (Advanced Research Training in Immunology for Surgery Trainees). The research reported in this publication and the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.surg.2018.12.021.
Presented at the 2019 Annual Academic Surgical Congress.
References
- 1.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the "Silver Tsunami": prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heymach J, Krilov L, Alberg A, et al. Clinical cancer advances 2018: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2018;36:1020–1044. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer surveillance programs in the United States: American Cancer Society Web site. https://www.cancer.org/cancer/cancerbasics/cancer-surveillance-programs-and-registries-in-the-united-states.html. Accessed November 1, 2018.
- 4.Hewitt M, Greenfield S, Stovall E. From cancer patient to cancer survivor: lost in transition. Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 5.Jeffery M, Hickey BE, Hider PN, See AM. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11:CD002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007;50:1783–1799. [DOI] [PubMed] [Google Scholar]
- 7.Snyder RA, Hu CY, Cuddy A, et al. Association between intensity of posttreatment surveillance testing and detection of recurrence in patients with colorectal cancer. JAMA. 2018;319:2104–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyder O, Dodson RM, Mayo SC, et al. Post-treatment surveillance of patients with colorectal cancer with surgically treated liver metastases. Surgery. 2013;154:256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyder O, Dodson RM, Weiss M, et al. Trends and patterns of utilization in posttreatment surveillance imaging among patients treated for hepatocellular carcinoma. J Gastrointest Surg. 2013;17:1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lampic C, Wennberg A, Schill JE, Brodin O, Glimelius B, Sjoden PO. Anxiety and cancer-related worry of cancer patients at routine follow-up visits. Acta Oncol. 1994;33:119–125. [DOI] [PubMed] [Google Scholar]
- 11.Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20:1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guy GP Jr, Ekwueme DU, Yabroff KR, et al. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013;31: 3749–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinberg BA, Lang J, Grzegorczyk J, et al. Implementation of cancer clinical care pathways: a successful model of collaboration between payers and providers. J Oncol Pract. 2012;8(3 Suppl):e38s–e43s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. [DOI] [PubMed] [Google Scholar]
- 15.Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med 2011;364:2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkin EB, Bach PB. Cancer's next frontier: addressing high and increasing costs. JAMA 2010;303:1086–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med 2006;144:742–752. [DOI] [PubMed] [Google Scholar]
- 18.Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24:626–634. [DOI] [PubMed] [Google Scholar]
- 19.Weeks JC, Cook EF, O'Day SJ, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA. 1998;279: 1709–1714. [DOI] [PubMed] [Google Scholar]
- 20.Kent EE, Mitchell SA, Oakley-Girvan I, Arora NK. The importance of symptom surveillance during follow-up care of leukemia, bladder, and colorectal cancer survivors. Support Care Cancer. 2014;22:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MyChart frequently asked questions Web site. https://mychart.osu.edu/osumc/default.asp?mode=stdfile&option=faq. [Google Scholar]
- 22.Wingo PA, Howe HL, Thun MJ, et al. A national framework for cancer surveillance in the United States. Cancer Causes Control. 2005;16:151–170. [DOI] [PubMed] [Google Scholar]
- 23.Paulson EC, Veenstra CM, Vachani A, Ciunci CA, Epstein AJ. Trends in surveillance for resected colorectal cancer, 2001–2009. Cancer. 2015;121: 3525–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis RA, Neal RD, Williams NH, et al. Follow-up of cancer in primary care versus secondary care: systematic review. Br J Gen Pract. 2009;59:e234–e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thaker DA, Monypenny R, Olver I, Sabesan S. Cost savings from a telemedicine model of care in northern Queensland, Australia. Med J Aust. 2013;199: 414–417. [DOI] [PubMed] [Google Scholar]
- 26.Doolittle GC, Williams AR, Spaulding A, Spaulding RJ, Cook DJ. A cost analysis of a tele-oncology practice in the United States. J Telemed Telecare. 2004;10(Suppl 1):27–29. [DOI] [PubMed] [Google Scholar]
- 27.Jacklin PB, Roberts JA, Wallace P, et al. Virtual outreach: economic evaluation of joint teleconsultations for patients referred by their general practitioner for a specialist opinion. BMJ. 2003;327:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neubeck L, Redfern J, Fernandez R, Briffa T, Bauman A, Freedman SB. Telehealth interventions for the secondary prevention of coronary heart disease: a systematic review. Eur J Cardiovasc Prev Rehabil. 2009;16:281–289. [DOI] [PubMed] [Google Scholar]
- 29.Clark RA, Inglis SC, McAlister FA, Cleland JG, Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ. 2007;334:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnett TE, Chumbler NR, Vogel WB, Beyth RJ, Qin H, Kobb R. The effectiveness of a care coordination home telehealth program for veterans with diabetes mellitus: a 2-year follow-up. Am J Manag Care. 2006;12:467–474. [PubMed] [Google Scholar]
- 31.Biermann E, Dietrich W, Rihl J, Standl E. Are there time and cost savings by using telemanagement for patients on intensified insulin therapy? A randomised, controlled trial. Comput Methods Programs Biomed. 2002;69: 137–146. [DOI] [PubMed] [Google Scholar]
- 32.Louis AA, Turner T, Gretton M, Baksh A, Cleland JG. A systematic review of telemonitoring for the management of heart failure. Eur J Heart Fail. 2003;5: 583–590. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen LM, Phanareth K, Nolte H, Backer V. Internet-based monitoring of asthma: a long-term, randomized clinical study of 300 asthmatic subjects. J Allergy Clin Immunol. 2005;115:1137–1142. [DOI] [PubMed] [Google Scholar]
- 34.Inglis SC, Clark RA, McAlister FA, et al. Structured telephone support or telemonitoring programmes for patients with chronic heart failure. Cochrane Database Syst Rev 2010:CD007228. [DOI] [PubMed] [Google Scholar]
- 35.Haun JN, Lind JD, Shimada SL, et al. Evaluating user experiences of the secure messaging tool on the Veterans Affairs' patient portal system. J Med Internet Res. 2014;16:e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung C, Padman R, Shevchik G, Paone S. Who are portal users vs. early e-Visit adopters? A preliminary analysis. AMIA Annu Symp Proc 2011;2011: 1070–1079. [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou YY, Garrido T, Chin HL, Wiesenthal AM, Liang LL. Patient access to an electronic health record with secure messaging: Impact on primary care utilization. Am J Manag Care. 2007;13:418–424. [PubMed] [Google Scholar]
- 38.Ross SE, Moore LA, Earnest MA, Wittevrongel L, Lin CT. Providing a web-based online medical record with electronic communication capabilities to patients with congestive heart failure: randomized trial. J Med Internet Res. 2004; 6:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wade-Vuturo AE, Mayberry LS, Osborn CY. Secure messaging and diabetes management: experiences and perspectives of patient portal users. J Am Med Inform Assoc. 2013;20:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin CT, Wittevrongel L, Moore L, Beaty BL, Ross SE. An internet-based patient-provider communication system: randomized controlled trial. J Med Internet Res. 2005;7:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox A, Lucas G, Marcu A, et al. Cancer survivors' experience with telehealth: a systematic review and thematic synthesis. J Med Internet Res. 2017;19:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viers BR, Lightner DJ, Rivera ME, et al. Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. Eur Urol. 2015;68:729–735. [DOI] [PubMed] [Google Scholar]
- 43.Sabesan S Medical models of teleoncology: current status and future directions. Asia Pac J Clin Oncol. 2014;10:200–204. [DOI] [PubMed] [Google Scholar]
- 44.Mair F, Whitten P. Systematic review of studies of patient satisfaction with telemedicine. BMJ. 2000;320:1517–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beaver K, Tysver-Robinson D, Campbell M, et al. Comparing hospital and telephone follow-up after treatment for breast cancer: randomised equivalence trial. BMJ. 2009;338:a3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kearney N, Kidd L, Miller M, et al. Utilising handheld computers to monitor and support patients receiving chemotherapy: results of a UK-based feasibility study. Support Care Cancer. 2006;14:742–752. [DOI] [PubMed] [Google Scholar]
- 47.Dickinson R, Hall S, Sinclair JE, Bond C, Murchie P. Using technology to deliver cancer follow-up: a systematic review. BMC Cancer. 2014;14:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cimperman M, Brenčič MM, Trkman P, Stanonik MeL. Older adults' perceptions of home telehealth services. Telemed J E Health. 2013;19:786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dou K, Yu P, Deng N, et al. Patients' acceptance of smartphone health technology for chronic disease management: a theoretical model and empirical test. JMIR Mhealth Uhealth. 2017;5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Bock GH, Bonnema J, Zwaan RE, van de Velde CJ, Kievit J, Stiggelbout AM. Patient's needs and preferences in routine follow-up after treatment for breast cancer. Br J Cancer. 2004;90:1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.