Abstract
Microstructural properties of white matter pathways are associated with concurrent reading abilities in children. In this longitudinal study, we asked whether properties of white matter pathways at the onset of learning to read would be associated with reading abilities at older ages. Children (N=37) with a wide range of reading abilities completed standardized measures of language and phonological awareness and diffusion MRI at age 6 years. Mean tract-fractional anisotropy (FA) was extracted from reading-related pathways. At age 8, the same children were reassessed using a standardized reading measure. Using linear regressions, we examined the contribution of tract-FA at age 6 to reading outcome at age 8, beyond known demographic and pre-literacy predictors of reading. Tract-FA of the left arcuate, left and right superior longitudinal fasciculus (SLF), and left inferior cerebellar peduncle (ICP) made unique contributions to reading outcome after consideration of sex and family history of reading delays. Tract-FA of the left and right SLF and left ICP made unique contributions to reading outcome after the addition of preliteracy skills. Thus, cerebellar and bilateral cortical pathways represented a network associated with subsequent reading abilities. Early white matter properties may be associated with other neuropsychological functions that predict reading or may influence reading development, independent of reading-related abilities. Tract-FA at early stages of learning to read may serve as a biomarker of later reading abilities.
Keywords: diffusion MRI, longitudinal study, reading development, tractography
1. Introduction
Reading skills have become critical to academic and occupational success in the information age. Reading performance varies widely across the population (Gilger et al., 1996; Sally E. Shaywitz et al., 1992), and approximately 5–17% of children struggle with learning to read (McCandliss & Noble, 2003). Children with poor reading skills at the early stages of reading rarely catch up with typically developing peers without intervention (O’Connor et al., 2005).
Microstructural properties of white matter pathways in the brain have been associated with concurrent reading abilities in children with dyslexia (Christodoulou et al., 2017; Arrington et al., 2017) and in children with normal reading abilities (Beaulieu et al., 2005; 2015; Travis et al., 2016a, 2015; Vandermosten et al., 2012). Longitudinal studies of prediction of reading from white matter properties have used a variety of analytic approaches. Initial white matter properties have been shown to predict the classification of which children develop dyslexia, the predominant reading disability (Kraft et al., 2016; Vanderauwera et al., 2017). Initial white matter properties have also predicted change in reading scores within children with dyslexia (Hoeft et al., 2011). Change in white matter properties predicted change in reading scores within non-impaired readers (Myers et al., 2014; Yeatman et al., 2012) and in children with dyslexia (Wang et al., 2017). In this longitudinal study, we extended previous findings through assessing whether fractional anisotropy (FA), a sensitive microstructural white matter property, at the onset of learning to read would be associated with reading abilities 2 years later, and whether that contribution would be independent of known predictors of reading ability. This approach allowed us to consider whether the neurobiology at the onset of learning to read would be important to the process of learning to read.
The “simple view of reading” proposes that reading is comprised of two critical subskills, decoding and comprehension (Gough & Tunmer, 1986; Hoover & Gough, 1990). Decoding – mapping orthographic information to phonological information – has been shown to develop from phonological awareness, language skills, and letter knowledge (Ehri, 2005; Perfetti, 1992; Wagner et al., 1997). Comprehension – extracting meaning from text – has been shown to develop from linguistic knowledge and higher-order cognitive processes (Cain, Oakhill, & Bryant, 2004; Kovachy et al., 2015). Moreover, fluency – the ability to read accurately, quickly, and expressively – is needed to achieve skilled decoding, though it is not defined in the simple view of reading and is often neglected in reading instruction (National Institute of Child Health and Human Development, 2000). In this study, we chose a comprehensive measure that assesses accuracy and speed of decoding (fluency), and comprehension as the primary reading outcome.
A distributed network of cerebral cortical regions shows functional activation during reading (Price, 2012). Structural features of white matter pathways connecting these regions have been associated with individual differences in reading abilities (Beaulieu et al., 2005; Travis et al., 2016a, 2015; Vandermosten et al., 2012). A sensitive, non-invasive, and in-vivo method for characterizing microstructural properties of white matter pathways is diffusion magnetic resonance imaging (dMRI). DMRI generates metrics, such as FA, that cannot be detected on conventional MRI (Barnea-Goraly et al., 2005; Mukherjee et al., 2001). Tractography allows identification and characterization of specific white matter pathways. In this study, we restricted the dMRI tractography analyses to cerebral and cerebellar pathways that have previously been associated with reading abilities in children.
The cerebral pathways that have been consistently linked to reading and reading-related abilities are the superior longitudinal fasciculus (SLF) and arcuate fasciculus (Arcuate) (Myers et al., 2014; Saygin et al., 2013; Thiebaut de Schotten et al., 2014; Travis et al., 2016a; Travis et al., 2016b; Vandermosten et al., 2012; Wang et al., 2017; Yeatman et al., 2012), inferior longitudinal fasciculus (ILF) and uncinate fasciculus (UF) (Lebel et al., 2013; Steinbrink et al., 2008; Travis et al., 2016a; Travis et al., 2016b; Yeatman et al., 2012), and corticospinal tract (CST) (Myers et al., 2014; Niogi & McCandliss, 2006; Travis et al., 2016b). Collectively, the SLF and Arcuate have been considered the dorsal stream of the language network (Hickok & Poeppel, 2004; Scott & Wise, 2004), thought to be involved in mapping auditory speech sounds onto articulation (Brauer et al., 2013). Compared to typical readers, children with reading disabilities showed reduced FA in the left Arcuate (Christodoulou et al., 2017; Vanderauwera et al., 2017). Microstructural properties of the same pathway were associated with measures of decoding in both groups (Christodoulou et al., 2017) and improved the prediction of who will develop dyslexia (Kraft et al., 2016; Vanderauwera et al., 2017). In typically developing children, one study showed that change in volume, but not the initial value, of the left Arcuate from kindergarten to 3rd grade predicted children’s reading outcome (Myers et al., 2014). Another longitudinal study of children aged 7 to 15 years used rate of change in FA over time to predict reading ability at baseline: good readers had initially low FA in the left Arcuate that increased over a 3-year-period, while poor readers had initially higher FA that decreased over time (Yeatman et al., 2012). Both studies found that change, rather than white matter properties at time one, predicted reading outcome.
The ILF and UF have been classified as the ventral stream of the language network (Dick, Bernal, & Tremblay, 2014). The ILF is thought to be involved in mapping auditory speech sounds to meaning (Hickok & Poeppel, 2004) and in processing simple syntactic structure (Friederici et al., 2006; Griffiths et al., 2013). As with the Arcuate, in the ILF, good readers showed initial low FA that increased over time whereas poor readers had initial high FA that decreased over time (Yeatman et al., 2012). The UF has been implicated in auditory working memory (Diehl et al., 2008; McDonald et al., 2008), semantic processing (Agosta et al., 2012; Galantucci et al., 2011), and reading in adults and children (Cummine et al., 2015; Travis et al., 2016b). Arrington and colleagues (2017) found FA differences in the left and right UF between children with poor and typical reading skills. FA of the right UF also correlated with reading comprehension and fluency in both groups (Arrington et al., 2017). However, white matter properties of the UF have not been linked to later reading ability.
Though the CST has not been conventionally considered to be within the language network, diffusion properties of the left and right CST correlated with single word reading in children and adolescents (Travis et al., 2016b). Change in white matter volume in the superior corona radiata improved the prediction of reading outcome in children (Myers et al., 2014). In addition, a region of the left centrum semiovale showed increased FA after an intensive reading remediation (Keller & Just, 2009).
Cerebellar gray matter and white matter structures have been implicated in reading (Desmond & Fiez, 1998; Stoodley & Schmahmann, 2009; Travis et al., 2015). The cerebellar cortex shows activation during reading (Fulbright et al., 1999; Petersen et al., 1988, 1989) and the size of the cerebellum, selected anatomic compartments, and lobular structures have been associated with reading abilities (Eckert et al., 2003, 2005; Fernandez et al., 2013; Laycock et al., 2008). Travis and colleagues (2015) found significant associations between measures of decoding and comprehension and FA of the inferior (ICP), superior (SCP), and middle cerebellar peduncles (MCP) that connect association cortices in the cerebrum to the cerebellum (Naidich & Duvernoy, 2009).
It is possible that an association of white matter microstructure and later reading could be found due to associations of these properties with known predictors of reading. For this reason, we needed to control for known predictors of reading abilities. We first considered demographic variables: sex, family history of reading delays, and socioeconomic status (SES). Reading disorders have been found to be more prevalent in boys than in girls (Rutter et al., 2004), though the ratio is higher in clinic-referred as opposed to community-based samples (Shaywitz et al., 1990). Children who have a first degree relative with reading disability or delays in learning to read are more likely to become poor readers than children without such family history (Snowling, Gallagher, & Frith, 2003). These findings suggest a genetic contribution to reading, though the specific genes or interactions between genes and environment remain unclear (Peterson & Pennington, 2012). Children from low-SES backgrounds have been shown to experience greater delays in language skills, phonological awareness, and learning to read than children from middle or high-SES backgrounds (Aikens & Barbarin, 2008). Further, higher SES has been associated with greater vocabulary in children with reading disabilities (Romeo et al., 2017).
We also wanted to control for cognitive and pre-literacy skills that have been associated with reading proficiency. A recent study found that nonverbal intelligence (IQ) explained nearly 20% of the variance in reading outcome (Wang et al., 2017). Oral language skills, the ability to produce and comprehend spoken language, form the foundation for extracting meaning from text (Snow, 1991). Individual variation in language skills has been shown to be an important predictor of later reading (Nation & Snowling, 2004; Olofsson & Niedersoe, 1999). Phonological awareness – the ability to detect and manipulate sounds within words and syllables – is also a strong predictor of reading outcome (Goswami & Bryant, 1990; Hulme, 2002; Kirby, Parrila, & Pfeiffer, 2003; Schatschneider et al., 2004; Wagner & Torgesen, 1987). These factors may work in tandem to predict reading proficiency. For example, Myers and colleagues (2014) found that the combination of family history of reading difficulty, language, and preliteracy skills accounted for more than 50% of the variance in reading outcome.
In the current study, we examined whether individual variation in FA of specific cerebral and cerebellar white matter pathways at age 6 would be associated with reading abilities at age 8. We sought to determine if these associations would be found after consideration of demographic covariates and after consideration of early cognitive and pre-literacy skills. To our knowledge, no study of typically developing children has yet found that white matter properties of specific pathways at the onset of learning to read correlate with later reading abilities after controlling for known prerequisites. We extend previous research by including cerebellar pathways in these analyses. We used a complex measure of reading that allowed us to interrogate which components of reading would be associated with white matter properties. We analyzed FA and also axial and radial diffusivity in relation to reading outcomes. Many of the previous studies show prediction to dyslexia, a reading disorder. Reading ability and disability fall along a continuum at the level of behavior (Sally E. Shaywitz et al., 1992). In this study, we were interested in whether we could find associations of white matter properties to scores along the full distribution. The clinical significance of such findings would be that properties of white matter pathways could potentially serve as biomarkers of later reading outcomes.
2. Materials and Methods
2.1. Participants
The cross-sectional data of children included in this study were previously described by Travis et al., 2016a. Here, we present the longitudinal data assessing reading abilities in the sample two years later (N = 50). Children were enrolled at age 6 and recruited from the San Francisco Bay Area to assess the neural basis of reading. Exclusionary criteria included significant visual impairment or hearing loss, history of neurological disorders, moderate to severe health conditions, non-English speakers, and children who scored more than two standard deviations below the mean on the WASI-II (Full 4; Wechsler & Hsaio-pin, 2011), equivalent to a score less than 70. Additional children were excluded who did not complete the MRI scan (n = 6) or showed excessive motion, as described below in the methods (n = 1), were lost to follow-up (n = 4), were diagnosed with specific language impairment after age 6 (n = 1), or did not receive formal reading instruction (n = 1). Our final sample included 37 children (15 males, mean age at time 1: 6.15 years ± 0.17 months; mean age at time 2: 8.11 years ± 0.15 months).
Parents completed a comprehensive demographic and health history questionnaire at the first visit (age 6). SES was measured using an updated version of the Hollingshead Four Factor Index of Socioeconomic Status (Hollingshead, 1975), which takes into account both parents’ education and occupation (possible values = 8 to 66). Children were generally from high-SES backgrounds, though the sample included a wide range of SES scores from 30 to 66. Nearly half of the sample was male. Thirty-five out of thirty-seven children were right-handed as measured by the Edinburgh Handedness Inventory (Oldfield, 1971). Further, all children scored above an 85 on a standardized assessment of intelligence (Full 4; Wechsler & Hsaio-pin, 2011). Within the sample, 19 children were exposed to a language other than English either at home or in school, as is highly common in this geographical region. Only two children in the sample were considered English language learners. However, both children scored within the average range on a widely-used and well-validated language assessment. In all cases, children were exposed to English for at least two years, attended an English-speaking school, and completed behavioral testing in English. The majority of children were in kindergarten at time 1. The exact number of children in each subgroup can be found in Online Resource 1.
Parents were asked to report on any first-degree relatives who had difficulty reading and siblings who required special help in reading at school. Children were classified as having a family history of reading delays if a parent was diagnosed or suspected of having a reading disorder or if a sibling was diagnosed with a reading disorder. The research protocol was approved by the Stanford University Institutional Review Board. For all children included in the study, informed consent was obtained from a parent or guardian at the first visit. Children were compensated for each behavioral testing session and for the MRI scan.
2.2. Assessments conducted at age 6 and 8
Children completed a battery of standardized assessments of nonverbal IQ, language, and phonological awareness at age 6 as potential predictor variables for later reading abilities. Nonverbal IQ was assessed with the Performance IQ (PIQ) composite score from the WASI-II (WASI-II; Wechsler and Hsiao-pin, 2011), a nationally-normed measure of intellectual ability. PIQ was derived from the Matrix Reasoning and Block Design subtests. Language skills were assessed using the Clinical Evaluation of Language Fundamentals – Fourth Edition (CELF-4; Wigg et al., 2003). The Core Language Index is a measure of general language ability, comprised of scores from four subtests: Concepts and Following Directions, Word Structure, Recalling Sentences, and Formulating Sentences. Phonological awareness was assessed using the Comprehensive Test of Phonological Processing (CTOPP; Wagner et al., 1999), a nationally-normed and well-established measure of reading-related phonological processing skills. The Phonological Awareness composite score is comprised of the Elision, Blending Words, and Sound Matching subtests. Age-based standard scores were derived for all assessments.
The primary outcome variable was standard score on the Oral Reading Index (ORI) from the Gray Oral Reading Tests - Fifth Edition (GORT-5; Wiederholt and Bryant, 2012) at age 8. This standardized measure of reading ability required children to read passages of increasing difficulty out loud and answer a series of questions, approximating reading in the real world. The ORI is comprised of scaled scores for fluency, based on both accuracy and speed of decoding, and comprehension. The subcomponents, fluency and comprehension, were considered as secondary outcomes in explorative analyses. Table 1 presents characteristics of the final sample including mean standardized test scores, ages of the participants at both time points, and SES.
Table 1.
Characteristics of the sample (n = 37).
| Continuous Variables | M | SD |
|---|---|---|
| Non-Verbal IQ1 | 112.30 | 16.02 |
| Core Language2 | 113.19 | 12.81 |
| Phonological Awareness3 | 113.46 | 11.78 |
| Oral Reading Index4 | 102.22 | 13.16 |
| Fluency4 | 10.89 | 3.23 |
| Comprehension4 | 10.14 | 2.25 |
| Age (years) at Time 1 | 6.15 | 0.17 |
| Age (years) at Time 2 | 8.11 | 0.15 |
| Socioeconomic Status5 | 58.19 | 9.96 |
WASI-II Wechsler Abbreviated Scale of Intelligence, 2nd Edition
CELF 4 Clinical Evaluation of Language Fundamentals, 4th Edition
CTOPP Comprehensive Test of Phonological Processing
GORT 5 Gray Oral Reading Tests, 5th Edition
Measured with the Hollingshead Index
2.3. MRI acquisition
Children were scanned at age 6 at the Center for Cognitive and Neurobiological Imaging at Stanford University (https://cni.stanford.edu), using a 3T MRI scanner (Discovery MR750 scanner, General Electric Healthcare, Milwaukee, WI, USA) with a 32-channel head coil. High resolution T1-weighted anatomical images were acquired using a 3D fast-spoiled gradient (FSPGR) sequence (TR = 7.24 ms; TE = 2.78 ms; FOV = 230 × 230 mm; acquisition matrix = 256 × 256; 0.9 mm isotropic voxels; number of slices = 186 ; orientation = sagihal). Diffusion data were acquired with a dual-spin echo, echo-planar imaging sequence with full brain coverage (TR = 8300 ms; TE = 83.1 ms; FOV = 220 × 220 mm; acquisition matrix = 256 × 256; voxel size: 0.8594 × 0.8594 × 2 mm; number of slices = 70; orientation = axial) using a b-value of 1000 s/mm2, sampling along 30 isotropically distributed diffusion directions. Three additional volumes were acquired at b=0 at the beginning of each scan.
2.4. MR1 data preprocessing.
DMRI pre-processing, analysis of motion, and individual native-space tractography have been previously described in Travis et al. 2016a. All critical steps and modifications to the analysis pipeline in this study are summarized below.
DMRI data were pre-processed using the open-source software mrDiffusion (http://github.com/vistalab/vistasoft/mrDiffusion) implemented in MATLAB R2014a (Mathworks, Natick, MA, USA). To correct for participant’s motion, we used a rigid body transformation (Rohde et al., 2004) to register each diffusion weighted image to the mean of the three non-diffusion (b0) images. The mean b0 image was registered to the participant’s T1-weighted image, which had been aligned to the canonical ac-pc orientation. The combined transform that resulted from motion correction and alignment to the T1 anatomy was applied to the raw data once, and the transformed images were resampled to 2 mm isotropic voxels. Maps of FA, radial diffusivity (RD), and axial diffusivity (AD) were generated using robust tensor fitting and outlier rejection based on the RESTORE procedure (Chang, Jones, & Pierpaoli, 2005) as opposed to a least-squares algorithm used in Travis et al. (2016). In addition, dMRI data were assessed for relative head motion detected during image pre-processing. We quantified the degree of relative head motion in each participant by calculating the magnitude of motion correction (in voxels) in the x-y-z plane of each volume relative to the prior volume. For each dMRI scan (voxel size = 2mm), we counted the number of volumes with translational motion of 1 voxel or more, relative to the prior volume. We then calculated the mean number of volumes with ≥1 voxel of relative motion across the entire sample (M = 1.18 volumes, SD = 2.10 volumes). As described by Dodson and colleagues (2018), participants who deviated from this mean by more than three standard deviations were excluded from analyses. This method identified participants whose movements affected more than 30% of the total number of diffusion volumes. This procedure led to the exclusion of one participant.
2.5. White matter tract identification
The open source software, Automated Fiber Quantification (AFQ), was used to track and segment cerebral and cerebellar white matter pathways in each subject’s native space (Yeatman et al., 2012). In brief, AFQ consists of three main processing steps: i) whole-brain tractography, ii) automatic tract segmentation using template ROIs warped to native space, and iii) automatic tract refinement and cleaning (Yeatman et al., 2012). We used the same parameters for deterministic streamline tracking and segmentation of the cerebral tracts as described in Travis et al. (2016a). For segmentation of the cerebellar pathways, ROIs were defined on the JHU MNI template following the procedure described by Travis et al. (2015). We identified the left and right ICP by drawing one ROI on the ICP on an axial plane at the level of the medulla (inferior to the dentate nucleus), and the second ROI on the ipsilateral ICP on an axial plane at the level of the ponto-mesencephalic junction. We captured the left and right SCP by drawing one ROI on the dentate nucleus on an axial plane at the level of the medial pons, and another ROI on the ipsilateral SCP on an axial plane at the level of the ponto-mesencephalic junction. We identified the MCP by drawing two ROIs within the cerebellum on an axial plane at the level of the medial pons: one placed on the central portion of the right MCP and the other placed on the central portion of the left MCP. A non-linear transformation (Friston & Ashburner, 2004) was applied to warp these ROIs from the MNI template space into each individual’s native space. In the way-point ROI procedure, each fiber from the whole-brain tractogram becomes a candidate for a specific fiber group if it passes through two ROIs that define the trajectory of the fiber group (Fig 1). Fiber tract refinement was done by comparing each candidate fiber to an established fiber tract probability map (Hua et al., 2008) and removing candidate streamlines that pass through the regions of white matter having a low probability for belonging to the tract. The core of the tract was calculated by defining 30 sample points along the tract and computing the robust mean position of the corresponding sample points. The robust mean was computed by estimating the three-dimensional Gaussian covariance of the sample points and removing i) fibers that were either more than 5 standard deviations away from the mean position of the tract, or that differed more than 4 standard deviations in length from the mean length of the tract for long cerebral pathways or ii) fibers that were either more than 4 standard deviations away from the mean position of the tract, or that differed more than 1 standard deviations in length from the mean length of the tract for relatively short cerebellar pathways. Fiber renderings for each tract and each child were visually inspected prior to any statistical analyses to ensure that each tract conformed to anatomical norms. Using AFQ, we were able to identify our tracts of interest, namely the left Arcuate as well as left and right SLF, ILF, UF, CST, ICP, SCP and MCP, in all children – with the exception of the left Arcuate and left ICP, which could not be segmented in one child. Mean tract-diffusion metrics were calculated by averaging FA, RD, or AD values between the ROIs used for tract segmentation.
Fig. 1.
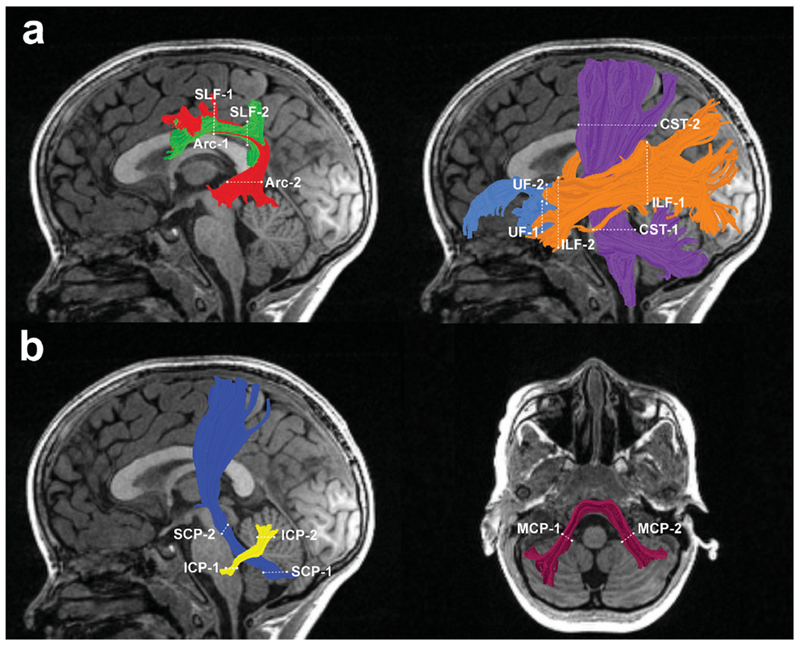
Tractography of cerebral (a) and cerebellar (b) white matter pathways. Left hemisphere tract renderings are displayed on a mid-sagittal T1 image from a representative child in our group. Right hemisphere tract renderings not shown. Dashed lines represent the location of the regions of interest (ROIs) used to segment each pathway from the whole-brain tractogram. (a) Illustrates the arcuate fasciculus (Arc) red, superior longitudinal fasciculus (SLF) green, inferior longitudinal fasciculus (ILF) orange, uncinate fasciculus (UF) light blue, and the corticospinal tract (CST) purple. (b) Illustrates the inferior cerebellar peduncle (ICP) yellow, superior cerebellar peduncle (SCP) dark blue, and middle cerebellar peduncle (MCP) maroon
2.6. Statistical Analyses
Statistical analyses were conducted using IBM SPSS software (version 23.0, IBM Corporation, 2014). Statistical significance was set at p < 0.05. The Shapiro-Wilk test was used to assess whether our behavioral and neurobiological data was normally distributed. Descriptive statistics were used to evaluate the sample. To determine whether demographic variables were important contributors to reading outcome, we compared the mean scores on the ORI at age 8 for the following sub-groups using two-tailed independent samples t-tests: male vs. female, presence vs. absence of family history of reading delays, monolingual vs. bilingual, and kindergarten vs. first grade. We then ran correlations to assess the degree of association between the ORI and SES, nonverbal IQ, language, and phonological awareness at age 6. In the regression models described below, we only included those factors that showed significant sub-group differences or correlations with reading outcome.
To reduce the number of white matter pathways considered in the regression analyses, we similarly conducted correlations assessing the relations between the ORI at age 8 and tract-FA of five cerebral (Arcuate, SLF, ILF, UF, CST) and three cerebellar (ICP, SCP, MCP) pathways at age 6. We applied Bonferroni corrections to account for multiple comparisons between all pathways initially assessed and the GORT ORI. Pathways that survived multiple comparisons were included in subsequent analyses. We refer to the tracts included in regression analyses as “selected pathways.”
To assess the contribution of white matter properties at age 6 to reading abilities at age 8, we performed a series of hierarchical linear regression models. The demographic variables that were significantly associated with reading outcome were included as covariates in all models. We then independently evaluated tract-FA of each of the selected pathways as a potential predictor of reading outcome. Additional regression models included the significant cognitive and pre-literacy variables to determine whether tract-FA contributed to reading outcome after controlling for these prerequisite skills. We ran exploratory analyses using comprehension and fluency as separate outcome variables to assess whether similar patterns persisted when analyzing the subcomponents of the ORI. Finally, we assessed the scaled scores that comprise fluency – accuracy and rate – to delineate whether pathways at age 6 contributed to decoding or reading speed at age 8. We assessed multicollinearity with the variance inflation factor (VIF) for each regression model. If VIF values were greater than ten or the average of VIF values across predictor variables was substantially greater than one, we would conclude that there was multicollinearity within our data (Field, 2013).
3. Results
3.1. Contributions of demographic factors to reading outcome at age 8
All behavioral and neurobiological data were normally distributed; thus parametric tests were used. The mean score on the ORI was within the normal range (Table 1). Subgroup analyses revealed that girls had higher scores on the ORI than boys; children with a family history of reading delays scored lower on the ORI compared to children without a family history of reading delays (Online Resource 1). However, reading scores on the ORI did not differ for children who were enrolled while in kindergarten vs. first grade or for children who were monolingual vs. bilingual (Online Resource 1). SES was not correlated with reading outcome in this sample (p = .111). Therefore, sex and family history of reading delays were entered as covariates in subsequent regression models.
3.2. Simple associations between cognitive and white matter measures at age 6 and reading outcome at age 8
Pearson correlations revealed that the ORI score at age 8 was significantly associated with core language scores and phonological awareness (Online Resource 2) but not with PIQ scores at age 6 (p = .221). Both correlations remained significant after correcting for multiple comparisons using Bonferroni. Therefore, core language and phonological awareness scores were entered as predictor variables within the regression models.
The ORI score at age 8 was also correlated with mean tract-FA at age 6 of the left Arcuate, left and right SLF, and left ICP (Online Resource 2). There were no associations between ORI scores at age 8 and the bilateral ILF, UF, CST, or the bilateral SCP, right ICP, and MCP at age 6. With the exception of the left Arcuate, all correlations remained significant after correcting for multiple comparisons. The Bonferroni adjustment is conservative and the left Arcuate has been repeatedly implicated in reading. Therefore, we decided to include this pathway in subsequent analyses. Hence, tract-FA of the selected pathways, left Arcuate, left and right SLF, and left ICP, were entered as neurobiological predictor variables in the regression models. There were moderate correlations across both behavioral and neurobiological predictor variables (Online Resource 3). However, for all regression models the VIF values were less than 2 and VIF values averaged across predictor variables were not substantially greater than one. Hence, there was no cause for concern regarding multicollinearity (Field, 2013).
3.3. Behavioral and neurobiological factors associated with subsequent reading outcome
Table 2 summarizes the individual and combined contributions of demographic covariates and FA of the selected pathways to reading outcome. Models 1A-1F revealed that family history of reading delays made a contribution to reading outcome while sex did not (Table 2). Models 1B-1E showed that tract-FA of the left Arcuate, left and right SLF, and left ICP at age 6 contributed 14-23% unique variance to reading outcome at age 8. Model 1F demonstrated that when all of the pathways were considered simultaneously, only tract-FA of the left SLF contributed unique variance to the model.
Table 2.
Prediction of mean tract-FA of selected pathways at age 6 to the Oral Reading Index (ORI) at age 8, beyond covariates of sex and family history of reading delays (Unstandardized Coefficients, SE).
| Model 1A | Model IB | Model 1C | Model ID | Model IE | Model IF | |
|---|---|---|---|---|---|---|
| Sex | 5.56 (4.33) | 5.83 (4.04) | 3.59 (3.95) | 6.61 (3.71) | 4.11 (3.94) | 3.72 (3.65) |
| + FH | −10.75 (5.16)* | −10.58 (4.82)* | −9.45 (4.66) | −9.35 (4.43)* | −9.24 (4.69) | −8.06 (4.20) |
| Left Arcuate | - | 180.57 (68.20)* | - | - | - | 7.77 (73.99) |
| Left SLF | - | - | 142.57 (47.59)** | - | - | 103.18 (49.40)* |
| Right SLF | - | - | - | 132.19 (36.07)*** | - | 69.24 (40.43) |
| Left ICP | - | - | - | 148.59 (48.02)** | 99.61 (49.96) | |
| Δ R2 | - | 14.1%* | 16.8%** | 22.8%*** | 18.1%** | 36.8%*** |
| Total R2 | 21.3%* | 35.5%** | 38.2%*** | 44.1%*** | 39.4%*** | 58.2%*** |
| Adjusted R2 | 16.7%* | 29.4%** | 32.5%*** | 39.0%*** | 33.8%*** | 49.2%*** |
+FH = positive family history of reading delays
Arcuate = arcuate fasciculus
SLF = superior longitudinal fasciculus
ICP = inferior cerebellar peduncle
p < .05,
p < .01,
p < .001
Table 3 adds pre-literacy skills to the regression models. Model 2A revealed that the combined contribution of sex, family history of reading delays, core language, and phonological awareness accounted for approximately 46% of the variance in reading outcome at age 8. Model 2B documented that the unique contribution of the left Arcuate reduced to a trend after consideration of pre-literacy skills. Models 2C-2E, however, showed that FA of the left and right SLF and left ICP at age 6 were associated with oral reading scores at age 8, beyond the contribution of the demographic covariates and pre-literacy skills at age 6 (Fig 2). Model 2F included all covariates, pre-literacy skills, and selected pathways to assess the shared versus unique variance amongst these variables. The results showed that core language and the right SLF were both unique contributors to reading outcome. Figure 3 illustrates that the shared variance among all variables accounted for approximately 37% of the total model (Model 2F). Pre-literacy skills and mean FA of the selected pathways accounted for 21% and 37% unique variance in reading abilities at age 8, respectively.
Table 3.
Prediction of mean tract-FA of selected pathways at age 6 to Oral Reading Index (ORI) at age 8, beyond covariates of sex and family history of reading delays and language and phonological awareness at age 6 (Unstandardized Coefficients, SE).
| Model 2A | Model 2B | Model 2C | Model 2D | Model 2E | Model 2F | |
|---|---|---|---|---|---|---|
| Sex | 0.55 (3.95) | 1.22 (3.85) | −0.30 (3.72) | 1.31 (3.08) | 0.15 (3.67) | 0.26 (3.19) |
| + FH | −9.39 (4.44)* | −9.24 (4.30)* | −8.44 (4.18) | −7.65 (3.48)* | −8.41 (4.13) | −6.97 (3.54) |
| Language | 0.33 (0.17) | 0.35 (0.17)* | 0.34 (0.16)* | 0.43 (0.14)** | 0.28 (0.16) | 0.38 (0.14)* |
| Phono Aware | 0.33 (0.18) | 0.21 (0.18) | 0.21 (0.17) | 0.21 (0.14) | 0.28 (0.16) | 0.15 (0.15) |
| Left Arcuate | - | 129.37 (64.58) | - | - | - | –14.72 (63.17) |
| Left SLF | - | - | 104.90 (44.89)* | - | - | 68.55 (42.64) |
| Right SLF | - | - | - | 132.92 (28.54)*** | - | 98.54 (34.77)** |
| Left ICP | - | - | - | - | 115.43 (42.98)* | 62.20 (42.99) |
| Δ R2 | - | 6.3% | 8.0%* | 22.1%*** | 10.4%* | 26.5%*** |
| Total R2 | 46.4%*** | 52.7%*** | 54.4%*** | 68.5%*** | 56.8%*** | 72.9%*** |
| Adjusted R2 | 39.7%*** | 44.8%*** | 47.1%*** | 63.4%*** | 49.6%*** | 64.5%*** |
+FH = positive family history of reading delays
Language = CELF 4 Core Language Index
Phono Aware = CTOPP Phonological Awareness Composite Score
Arcuate = arcuate fasciculus
SLF = anterior superior longitudinal fasciculus
ICP = inferior cerebellar peduncle
p < .05
p < .01
p < .001
Fig. 2.
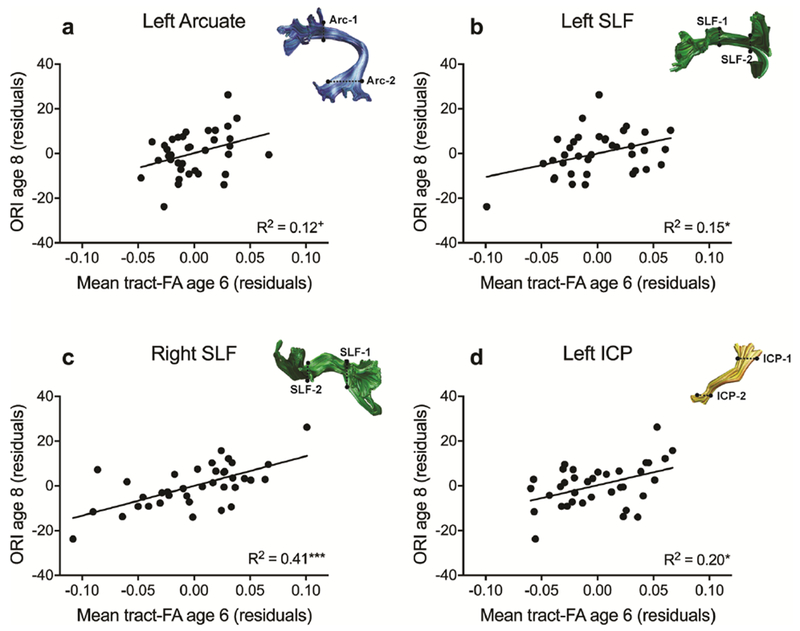
Scatter plots of unstandardized residuals of mean tract-FA of selected pathways at age 6 and ORI at age 8 after consideration of sex, family history of reading delays, core language, and phonological awareness. (a) The left Arcuate approaches statistical significance as a predictor of later reading after controlling for sex and family history of reading delays, language, and phonological awareness. (b-d) The left and right SLF and left ICP are significant predictors of later reading after controlling for sex and family history of reading delays, language, and phonological awareness. *p < .05, **p < .01, ***p < .001
Fig. 3.

An illustration of the unique and shared variance between covariates (sex, family history of reading delays), pre-literacy skills (core language, phonological awareness), and selected pathways (left Arcuate, left and right SLF, left ICP) at age 6 to the ORI at age 8
To determine if the pattern of associations in the selected pathways would be similar for comprehension and fluency, we recalculated Models 2A-2F including tract-FA and pre-literacy measures at age 6 with comprehension (Online Resource 4) or fluency scores at age 8 (Online Resource 5) as the outcome. Similar to Model 2B, tract-FA in the left Arcuate did not significantly contribute unique variance to either subcomponent of the ORI after consideration of pre-literacy skills (Models 3B and 4B). Only tract-FA of the right SLF and left ICP uniquely predicted comprehension (Models 3D-E) while the left and right SLF and left ICP predicted fluency (Models 4C-E). We then assessed accuracy and rate, the subcomponents of fluency, to determine if the associations would be specific to decoding or speed. We observed similar patterns of association seen between tract-FA and fluency (Online Resource 6 and 7).
Since we included 8 children with a family history of reading delays, regression models might have been unduly influenced by these few children. We re-assessed the associations between tract-FA of reading-related pathways at age 6 and ORI at age 8 and found that the same set of pathways were significantly correlated with reading outcome. We then reran the regression models excluding children with a family history of reading delays; the pattern of associations from selected pathways at age 6 to later reading outcome persisted (Online Resource 6). There were also two children in the sample that were left-handed. Again, if excluded, all results remained unchanged.
While FA is a sensitive measures of white matter organization and integrity, it remains unclear whether the association of initial FA to subsequent reading is driven by greater diffusivity along to the principal direction of the estimated fiber orientation (AD) or reduced diffusivity averaged across the two directions orthogonal to the principal diffusion direction (RD). Therefore, we assessed the relations between initial AD/RD and the ORI at age 8. RD, but not AD, of the four selected pathways (left Arcuate, left and right SLF, left ICP) at age 6 correlated with reading outcome at age 8 (Online Resource 9). We then reran the regression analyses and showed that RD of the selected pathways contributed between 2.7%-12.9% unique variance to the ORI at age 8 (Online Resource 10).
4. Discussion
In this study, we assessed the degree of association between microstructural properties of white matter pathways at age 6 to reading ability at age 8. After controlling for sex and family history of reading delays, we found that FA of the left Arcuate, left and right SLF, and left ICP were significantly associated with the ORI two years later. When pre-literacy skills were introduced the model, the left Arcuate was no longer associated with subsequent reading. However, tract-FA of the left and right SLF and left ICP remained associated with reading outcome, even after consideration of covariates and pre-literacy skills at age 6. When all variables were included, core language and the right SLF made unique contributions to reading abilities.
4.1. White matter pathways associated with later reading
The left Arcuate and left SLF are considered part of the dorsal language network. Both tracts have been consistently implicated in reading and reading development (Myers et al., 2014; Saygin et al., 2013; Thiebaut de Schotten et al., 2014; Travis et al., 2016a; Travis et al., 2016b; Vandermosten et al., 2012; Wang et al., 2017; Yeatman et al., 2012). While tract-FA of the left Arcuate made significate contributions to reading outcome after controlling for demographic covariates, it reduced to a trend (p = .054) when pre-literacy skills were added to the model. This finding is likely due to the high correlation between FA of the left Arcuate with phonological awareness skills at age 6. Many studies have documented the association between phonological awareness and the left Arcuate (Dodson et al., 2018; Saygin et al., 2013; Travis, et al., 2016a; Vandermosten et al., 2012). However, FA of the left SLF remained a unique contributor to later reading abilities. This finding suggests that the left SLF contributes to cognitive processes essential to later reading, beyond its role in phonological awareness. This significant contribution of the left SLF encourages future studies to explore other factors integral to reading development that may be associated with the left SLF. Urger and colleagues (2015), for example, found positive associations between the left SLF and attention and inhibitory control. It remains possible that attention or other related behaviors may also influence the early stages of leaning to read. Further, we extended Wang and colleagues (2017) findings that developmental rates of the left SLF predicted reading fluency and comprehension by demonstrating that initial FA values made significant contributions to both subskills two years later.
Strikingly, we found that tract-FA of the right SLF was associated with reading attainment in children with a range of reading abilities. In selected subsamples, a few studies have found associations between FA of the right SLF and reading. In 14-year-old children with dyslexia, increased activation in the right inferior frontal gyrus combined with FA of the right SLF predicted reading outcome with 72% accuracy. However, the association was not found in the typically developing children (Hoeft et al., 2011). In addition, among children with a familial risk for dyslexia, those children who subsequently developed strong reading abilities had accelerated development in the right SLF compared to those who developed weak reading abilities (Wang et al., 2017). In our sample, we established that the independent contribution of the right SLF was not driven by children with family history of reading delays; when they were excluded all findings remained unchanged. Our results extend previous findings and demonstrate that the right SLF is also important for subsequent reading abilities in typically developing children. Interestingly, the right SLF was not associated with either language or phonological awareness in this sample. We think that the right SLF is likely associated with other behavioral factors involved in learning to read that our regression models did not include. Previous research, for example, found that the right SLF was involved in executive function skills (Urger et al., 2015). Executive functioning skills are needed to plan, organize, engage working memory, maintain and shift attention; these skills have been associated with reading ability (Blair & Razza, 2007; Frye et al., 2009). Other studies researching children with a family risk for dyslexia have hypothesized that the right SLF may be a compensatory mechanism for skilled reading (Hoeft et al., 2011). The right SLF has also been suggested to be a protective factor in children at risk for dyslexia (Wang et al., 2017). In our group of typically developing children, the right SLF was associated with both reading fluency and comprehension. Together, these findings suggest that at the early stages of reading, a bilateral white matter network may be involved in reading development. What we cannot determine on the basis of association data is whether the left and right SLF transmit similar or distinct information within the process of reading. Future research could address this question by assessing the degree of association between a wide range of reading and relating abilities, such as executive function skills, and FA of the left and right SLF to determine if the patterns of association are similar or distinctive in these tracts.
Another novel finding is the association of FA of the left ICP at age 6 with reading abilities at age 8. Our results suggest that cerebellar white matter pathways contribute to cognitive processes that are integral to the development of reading. The cerebellum is a widely-connected region; its white matter pathways provide links to all major divisions of the central nervous system (Buckner et al., 2011; Naidich & Duvernoy, 2009; Stoodley & Schmahmann, 2009). These white matter pathways – in particular those projecting to areas of the frontal, temporal, and parietal lobes engaged in reading – may contribute to observed cerebellar activity during reading (Fulbright et al., 1999; Senaha et al., 2005). Travis and colleagues (2015) were the first to relate FA of the cerebellar pathways with reading abilities. In their study, FA extracted from the left ICP, left and right SCP, and MCP correlated significantly with concurrent reading abilities in older children and adolescents. Contrary to our findings, the associations were negative in the left ICP. Studies performed in children and adults have found both negative (Dougherty et al., 2007; Odegard et al., 2009; Yeatman et al., 2011) and positive associations with reading abilities within several cerebral white matter pathways (Deutsch et al., 2005; Lebel et al., 2013; Yeatman et al., 2012). The direction of correlations of reading with FA may vary as a function of assessing future state rather than concurrent state and may also vary across different age groups. FA is also influenced by a number of different tissue factors (Jones & Cercignani, 2010), which may explain these differences in the direction of the correlation. For example, increased coherence of axons within a voxel could enhance information transmission and increase FA, resulting in a positive association of FA with behavior. By contrast, increased axonal diameter could enhance information processing and decrease FA, resulting in a negative association (Dougherty et al., 2007). By integrating dMRI with imaging methods that provide direct estimates of myelin content and axonal diameter (e.g. Assaf et al., 2008; Mezer et al., 2013), future research could refine our understanding of the relation between white matter tissue properties of the cerebellar peduncles and reading. To our knowledge, this is the first study to document that cerebellar FA of the left ICP at the onset of learning to read are associated with subsequent reading abilities in a longitudinal study. The ICP contains mostly afferent pathways, feeding signals from the spine and the olivary nucleus into the cerebellum; the ICP also contains efferent pathways from the cerebellum towards the vestibular nuclei along the border of the pons and medulla (Naidich & Duvernoy, 2009). We did not find a significant association of FA of the SCP and later reading. The SCP contains primarily efferent pathways that emerge from the deep cerebellar nuclei, travel through the ipsilateral SCP, decussate at the level of the inferior colliculus, and pass through the thalamus projecting to the contralateral cerebral cortex. Hence, the SCPs – rather than ICPs – are thought to convey signals between the cerebellum and inferior prefrontal regions implicated in reading (Jansen et al., 2005; Stoodley, 2012). Learning to read, however, may also depend on other implicit learning processes that enable the execution of new perceptual and motor skills, including articulation and oculomotor control (Stein, Richardson, & Fowler, 2000; Velay et al., 2002). Those implicit learning and feedback processes, which lead to fine-tuned timing and automatization of mechanisms reading is based on, could partly be mediated by the cerebellum, in particular the left ICP.
We showed that the associations of initial FA to later reading were driven by negative associations between RD at age 6 and the ORI at age 8. An increase in FA along with a decrease in RD has been related to a gain in myelination or compactness of fiber bundles (Giorgio et al., 2010; Snook et al., 2005). The combination of positive FA-reading associations and negative RD-reading associations therefore suggest increased myelination or fiber compactness, as opposed to improved fiber coherence or changes in axonal diameter, as the underlying neural substrate.
In this study, we did not observe longitudinal associations between the reading outcome and the initial FA values of the left and right ILF, UF, CST, SCP, and MCP. The lack of associations may be explained by the use of a complex reading measure that tapped into fluency and comprehension as well as decoding rather than measuring isolated subskills of reading. Additionally, we were looking at associations between FA at age 6 and reading at age 8 rather than strictly concurrent associations. Patterns of association may vary across ages.
4.2. Limitations
Despite our intriguing findings, it is important to acknowledge limitations of the current study. The sample size was modest and included children from predominately higher-SES backgrounds. However, we found that language and phonological awareness skills were strongly associated with subsequent reading outcome, similar to many other studies (Hulme, 2002; Nation & Snowling, 2004; Wagner & Torgesen, 1987) and demonstrating that this sample represents a cohort of typically developing children. Future research should investigate whether the pattern of associations persist in a socio-economically diverse sample. Although we included children with a family history of reading delays, we did not have a large enough subsample to adequately address these children in separate analyses. Yet, the pattern of findings remained unchanged after excluding children with family history of reading delays. Due to our sample size, we were also limited in the number of predictor variables that we could include in our regression model(s); it is possible that other genetic, environmental and behavioral factors, which were not included in our study, could also be associated with later reading abilities. We further recognize the risk of overfitting in the regression models, especially models that included all selected pathways (Model 1F and 2F). The intention of these final models was not to assess the strength of the association of all measures in tandem, but rather to examine the shared versus unique variance of the covariates, behavioral measures, and white matter pathways. Future studies using larger independent samples are required to validate our findings.
In recent years, dMRI has become a very popular method to study the microstructural properties of white matter pathways. The DTI model, however, is an oversimplification of white matter anatomy and diffusivity measures are not only affected by biological changes such as myelin thickness, axon size, and axon density (Beaulieu, 2002), but also by methodological confounds such as the presence of crossing fibers (Wheeler-Kingshott & Cercignani, 2009) and partial volume effects (Vos et al., 2011). As a result, the interpretation of associations between FA and behavior is difficult and rests on knowledge of what is known to drive diffusion anisotropy. Finally, we assessed the FA measures averaged across the 30 nodes along each white matter pathway. Because diffusion measures vary along the trajectory of these pathways, averaging can obscure important information (Yeatman et al., 2012). Hence, mean values may not be sensitive enough to reveal more focal associations present in other cerebral and/or cerebellar pathways.
4.3. Conclusions and Future Directions
To our knowledge, this study is the first demonstration that white matter properties of cerebral and cerebellar pathways at the onset of learning to read are associated with later reading in a sample of typically developing children with a range of reading abilities. FA of the left and right SLF and left ICP at age 6 contributed to reading abilities at age 8 even after consideration of demographic covariates and individual variation in language and phonological awareness. While other studies have found that developmental change in measures of cerebral white matter predict outcome, we have shown that FA values from a single point in time, early in the process of learning to read, contribute to the variance in reading abilities two years later. One explanation for these findings is that additional behavioral factors associated with the left and right SLF and left ICP are involved in development or reading, such as executive function skills and oculomotor control. Another possibility is that individual variation in these white matter pathways are directly related to efficiencies in learning to read, independent of other specific neuropsychological processes. This latter possibility may be difficult to prove experimentally. One avenue for differentiating between these possibilities would be to conduct a similar study with clinical populations who experience white matter injury. We could then determine if white matter pathways show similar patterns of associations to reading outcomes. Research should also focus on the association between executive functioning and FA of right SLF to examine its potential role in reading development. Moreover, future research should assess which neurobiological factors affect the subcomponents of FA that enhance reading abilities. Quantitative MRI protocols that provide myelin-sensitive T1 values (Mezer et al., 2013) and advanced dMRI analysis methods such as spherical deconvolution and probabilistic tractography have dramatically improved the potential of neuroimaging to characterize white matter fiber bundles. Combining diffusion measures with estimates of T1 will further elucidate microstructural properties of cerebral and cerebellar pathways and their functional relevance to learning to read.
Supplementary Material
Acknowledgments
We would like the thank the children and families who participated in this longitudinal project. We would also like to thank Ms. Vanessa N. Kovachy for initial recruitment and data collection.
Funding
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (RO1 HD069162) and the Stanford Transdisciplinary Initiatives Program, Child Health Research Institute.
Footnotes
Compliance with Ethical Standards
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
“The authors declare that they have no conflict of interest.”
References
- Agosta F, Scola E, Canu E, Marcone A, Magnani G, Sarro L, … Filippi M (2012). White Matter Damage in Frontotemporal Lobar Degeneration Spectrum. Cerebral Cortex, 22(12), 2705–2714. 10.1093/cercor/bhr288 [DOI] [PubMed] [Google Scholar]
- Aikens NL, & Barbarin O (2008). Socioeconomic differences in reading trajectories: The contribution of family, neighborhood, and school contexts. Journal of Educational Psychology, 100(2), 235–251. https://doi.Org/10.1037/0022-0663.100.2.235 [Google Scholar]
- Assaf Y, Blumenfeld-Katzir T, Yovel Y, & Basser PJ (2008). Axcaliber: A method for measuring axon diameter distribution from diffusion MRI. Magnetic Resonance in Medicine, 59(6), 1347–1354. 10.1002/mrm.21577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, … Reiss AL (2005). White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral Cortex (New York, N.Y. : 1991), 15(12), 1848–54. 10.1093/cercor/bhi062 [DOI] [PubMed] [Google Scholar]
- Beaulieu C (2002). The basis of anisotropic water diffusion in the nervous system - a technical review. - PubMed - NCBI. NMR in Biomedicine, 15(7–8), 435–55. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, & Phillips L (2005). Imaging brain connectivity in children with diverse reading ability. Neuroimage, 25(4), 1266–1271. https://doi.Org/10.1016/J.NEUROIMAGE.2004.12.053 [DOI] [PubMed] [Google Scholar]
- Blair C, & Razza RP (2007). Relating Effortful Control, Executive Function, and False Belief Understanding to Emerging Math and Literacy Ability in Kindergarten. Child Development, 78(2), 647–663. 10.1111/j.1467-8624.2007.01019.X [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Perani D, & Friederici AD (2013). Dorsal and ventral pathways in language development. Brain and Language, 1–7. https://doi.Org/10.1016/j.bandl.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, & Yeo BTT (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(5), 2322–2345. 10.1152/jn.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain K, Oakhill J, & Bryant P (2004). Children’s Reading Comprehension Ability: Concurrent Prediction by Working Memory, Verbal Ability, and Component Skills. Journal of Educational Psychology. [Google Scholar]
- Cain Kate: Department of Psychology, University of Essex, Wivenhoe Park, Colchester, United Kingdom, C04 3SQ, kcain@essex.ac.uk: American Psychological Association. 10.1037/0022-0663.96.1.31 [DOI] [Google Scholar]
- Chang L-C, Jones DK, & Pierpaoli C (2005). RESTORE: Robust estimation of tensors by outlier rejection. Magnetic Resonance in Medicine, 53(5), 1088–1095. 10.1002/mrm.20426 [DOI] [PubMed] [Google Scholar]
- Christodoulou JA, Murtagh J, Cyr A, Perrachione TK, Chang P, Halverson K, … Gabrieli JDE (2017). Relation of white-matter microstructure to reading ability and disability in beginning readers. Neuropsychology, 31(5), 508–515. 10.1037/neu0000243 [DOI] [PubMed] [Google Scholar]
- Cummine J, Dai W, Borowsky R, Gould L, Rollans C, & Boliek C (2015). Investigating the ventral-lexical, dorsal-sublexical model of basic reading processes using diffusion tensor imaging. Brain Structure and Function, 220(1), 445–455. https://doi.Org/10.1007/s00429-013-0666-8 [DOI] [PubMed] [Google Scholar]
- Desmond JE, & Fiez JA (1998). Neuroimaging studies of the cerebellum: language, learning and memory. Trends in Cognitive Sciences, 2(9), 355–362. 10.1016/S1364-6613(98)01211-X [DOI] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JDE, & Wandell B (2005). Children’s Reading Performance is Correlated with White Matter Structure Measured by Diffusion Tensor Imaging. Cortex, 41(3), 354–363. 10.1016/S0010-9452(08)70272-7 [DOI] [PubMed] [Google Scholar]
- Dick AS, Bernal B, & Tremblay P (2014). The Language Connectome. The Neuroscientist, 20(5), 453–467. 10.1177/1073858413513502 [DOI] [PubMed] [Google Scholar]
- Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, & Lüders HO (2008). Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia, 49(8), 1409–1418. https://doi.Org/10.1111/j.1528-1167.2008.01596.x [DOI] [PubMed] [Google Scholar]
- Dodson C, Travis K, Borchers L, Marchman V, Ben-Shachar M, & Feldman H (2018). White matter properties associated with prereading skills in 6-year-old children born preterm and at term. Developmental Medicine and Child Neurology. 10.1111/dmcn.13783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, & Wandell BA (2007). Temporal-callosal pathway diffusivity predicts phonological skills in children. Proceedings of the National Academy of Sciences, 104(20), 8556–8561. https://doi.org/www.pnas.org?cgi?doi?10.1073?pnas.0608961104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, & Berninger VW (2003). Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain, 126(2), 482–494. 10.1093/brain/awg026 [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, & Berninger V (2005). Anatomical Signatures of Dyslexia in Children: Unique Information from Manual and Voxel Based Morphometry Brain Measures. Cortex, 41(3), 304–315. 10.1016/S0010-9452(08)70268-5 [DOI] [PubMed] [Google Scholar]
- Ehri LC (2005). Learning to Read Words: Theory, Findings, and Issues. Scientific Studies of Reading, 9(2), 167–188. 10.1207/s1532799xssr0902_4 [DOI] [Google Scholar]
- Feldman HM, Lee ES, Yeatman JD, & Yeom KW (2012). Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia, 50(14), 3348–62. 10.1016/j.neuropsychologia.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez VG, Stuebing K, Juranek J, & Fletcher JM (2013). Volumetric Analysis of Regional Variability in the Cerebellum of Children with Dyslexia. The Cerebellum, 12(6), 906–915. 10.1007/s12311-013-0504-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A (2013). Discovering statistics using IBM SPSS statistics. sage. [Google Scholar]
- Friederici AD, Bahlmann J, Heim S, Schubotz RI, & Anwander A (2006). The brain differentiates human and non-human grammars: functional localization and structural connectivity. Proceedings of the National Academy of Sciences of the United States of America, 103(7), 2458–63. 10.1073/pnas.0509389103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, & Ashburner J (2004). Generative and recognition models for neuroanatomy. NeuroImage, 23(1), 21–24. 10.1016/j.neuroimage.2004.04.021 [DOI] [PubMed] [Google Scholar]
- Frye RE, Landry SH, Swank PR, & Smith KE (2009). Executive Dysfunction in Poor Readers Born Prematurely at High Risk. Developmental Neuropsychology, 34(3), 254–271. 10.1080/87565640902805727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulbright RK, Jenner AR, Mencl WE, Pugh KR, Shaywitz BA, Shaywitz SE, … Gore JC (1999). The cerebellum’s role in reading: a functional MR imaging study. AJNR. American Journal of Neuroradiology, 20(10), 1925–30. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10588120 [PMC free article] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, … Gorno-Tempini ML (2011). White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain, 134(10), 3011–3029. 10.1093/brain/awr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilger JW, Hanebuth E, Smith SD, & Pennington BF (1996). Differential risk for developmental reading disorders in the offspring of compensated versus noncompensated parents. Reading and Writing, 8(5), 407–417. 10.1007/bf00404002 [DOI] [Google Scholar]
- Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, … James AC. (2010). Longitudinal changes in grey and white matter during adolescence. NeuroImage, 49(1), 94–103. 10.1016/j.neuroimage.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Goswami U, & Bryant P (1990). Phonological skills and learning to read. Journal of Child Psychology and Psychiatry, 32(7), 1173–1176. 10.1111/j.1469-7610.1991.tb00359.x [DOI] [Google Scholar]
- Gough PB, & Tunmer WE (1986). Decoding, Reading, and Reading Disability. Remedial and Special Education, 7(1), 6–10. 10.1177/074193258600700104 [DOI] [Google Scholar]
- Griffiths JD, Marslen-Wilson WD, Stamatakis EA, & Tyler LK (2013). Functional Organization of the Neural Language System: Dorsal and Ventral Pathways Are Critical for Syntax. Cerebral Cortex, 23(1), 139–147. 10.1093/cercor/bhr386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, & Poeppel D (2004). Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition, 92(1–2), 67–99. 10.1016/J.COGNITION.2003.10.011 [DOI] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, … Gabrieli JDE (2011). Neural systems predicting long-term outcome in dyslexia. Proceedings of the National Academy of Sciences, 108(1), 361–366. 10.1073/pnas.1008950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WA, & Gough PB (1990). The simple view of reading. Reading and Writing, 2(2), 127–160. 10.1007/BF00401799 [DOI] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, … Mori S (2008). Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. NeuroImage, 39(1), 336–347. 10.1016/J.NEUROIMAGE.2007.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme C (2002). Phonemes, Rimes, and the Mechanisms of Early Reading Development. Journal of Experimental Child Psychology, 82(1), 58–64. 10.1006/JECP.2002.2674 [DOI] [PubMed] [Google Scholar]
- Jansen A, Flöel A, Van Randenborgh J, Konrad C, Rotte M, Förster A-F, … Knecht S (2005). Crossed cerebro-cerebellar language dominance. Human Brain Mapping, 24(3), 165–172. 10.1002/hbm.20077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, & Cercignani M (2010). Twenty-five pitfalls in the analysis of diffusion MRI data. NMR in Biomedicine, 23(7), 803–820. 10.1002/nbm.1543 [DOI] [PubMed] [Google Scholar]
- Keller TA, & Just MA (2009). Altering Cortical Connectivity: Remediation-Induced Changes in the White Matter of Poor Readers. Neuron, 64(5), 624–631. 10.1016/J.NEURON.2009.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby JR, Parrila RK, & Pfeiffer SL (2003). Naming speed and phonological awareness as predictors of reading development. Journal of Educational Psychology [Google Scholar]
- Kirby John R.: Queen’s U, Faculty of Education, Kingston, ON, Canada, K7L 3N6, kirbyj@educ.queensu.ca: American Psychological Association. 10.1037/0022-0663.95.3.453 [DOI] [Google Scholar]
- Kovachy VN, Adams JN, Tamaresis JS, & Feldman HM (2015). Reading abilities in school-aged preterm children: a review and meta-analysis. Developmental Medicine & Child Neurology, 57(5), 410–419. 10.1111/dmcn.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft I, Schreiber J, Cafiero R, Metere R, Schaadt G, Brauer J, … Skeide MA. (2016). Predicting early signs of dyslexia at a preliterate age by combining behavioral assessment with structural MRI. NeuroImage, 143, 378–386. 10.1016/j.neuroimage.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Langer N, Peysakhovich B, Zuk J, Drottar M, Sliva DD, Smith S, … Gaab N (2015). White Matter Alterations in Infants at Risk for Developmental Dyslexia. Cerebral Cortex, 27(2), bhv281 10.1093/cercor/bhv281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock SK, Wilkinson ID, Wallis LI, Darwent G, Wonders SH, Fawcett AJ, … Nicolson RI (2008). Cerebellar Volume and Cerebellar Metabolic Characteristics in Adults with Dyslexia. Annals of the New York Academy of Sciences, 1145(1), 222–236. 10.1196/annals.1416.002 [DOI] [PubMed] [Google Scholar]
- Lebel C, Shaywitz B, Holahan J, Shaywitz S, Marchione K, & Beaulieu C (2013). Diffusion tensor imaging correlates of reading ability in dysfluent and non-impaired readers. Brain and Language, 125(2), 215–222. 10.1016/J.BANDL.2012.10.009 [DOI] [PubMed] [Google Scholar]
- McCandliss BD, & Noble KG (2003). The development of reading impairment: A cognitive neuroscience model. Mental Retardation and Developmental Disabilities Research Reviews, 9(3), 196–204. 10.1002/mrdd.10080 [DOI] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, … Halgren E (2008). Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology, 71(23), 1869–76. 10.1212/01.wnl.0000327824.05348.3b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezer A, Yeatman JD, Stikov N, Kay KN, Cho N-J, Dougherty RF, … Wandell BA (2013). Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nature Medicine, 19(12), 1667–1672. 10.1038/nm.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, & McKinstry RC (2001). Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology, 221(2), 349–358. 10.1148/radiol.2212001702 [DOI] [PubMed] [Google Scholar]
- Myers CA, Vandermosten M, Farris EA, Hancock R, Gimenez P, Black JM, … Hoeft F. (2014). White Matter Morphometric Changes Uniquely Predict Children’s Reading Acquisition. Psychological Science, 25(10), 1870–1883. 10.1177/0956797614544511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidich TP, & Duvemoy HM (2009). Duvernoy’s atlas of the human brain stem and cerebellum : high-field MR1 : surface anatomy, internal structure, vascularization and 3D sectional anatomy. Springer. [Google Scholar]
- Nation K, & Snowling MJ (2004). Beyond phonological skills: broader language skills contribute to the development of reading. Journal of Research in Reading, 27(4), 342–356. 10.1111/j.1467-9817.2004.00238.x [DOI] [Google Scholar]
- Nikki Arrington C, Kulesz PA, Juranek J, Cirino PT, & Fletcher JM (2017). White matter microstructure integrity in relation to reading proficiency☆. Brain and Language, 174(August). 103–111. https://doi.Org/10.1016/j.bandl.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, & McCandliss BD (2006). Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia, 44(11), 2178–2188. 10.1016/J.NEUROPSYCHOLOGIA.2006.01.011 [DOI] [PubMed] [Google Scholar]
- O’Connor RE, Fulmer D, Harty KR, & Bell KM (2005). Layers of Reading Intervention in Kindergarten Through Third Grade. Journal of Learning Disabilities, 38(5), 440–455. [DOI] [PubMed] [Google Scholar]
- Odegard TN, Farris EA, Ring J, McColl R, & Black J (2009). Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia, 47(8–9), 1972–1977. https://doi.Org/10.1016/J.NEUROPSYCHOLOGIA.2009.03.009 [DOI] [PubMed] [Google Scholar]
- Olofsson Å, & Niedersøe J (1999). Early Language Development and Kindergarten Phonological Awareness as Predictors of Reading Problems. Journal of Learning Disabilities, 32(5), 464–472. 10.1177/002221949903200512 [DOI] [PubMed] [Google Scholar]
- Perfetti CA (1992). The representation problem in reading acquisition Reading Acquisition. Hillsdale, NJ, US: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, & Raichle ME (1988). Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature, 331(6157), 585–589. 10.1038/331585a0 [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, & Raichle ME (1989). Positron Emission Tomographic Studies of the Processing of Singe Words. Journal of Cognitive Neuroscience, 1(2), 153–170. 10.1162/jocn.1989.1.2.153 [DOI] [PubMed] [Google Scholar]
- Peterson RL, & Pennington BF (2012). Developmental dyslexia. Www.thelancet.com Lancet, 379, 1997–2007. 10.1016/S0140-6736(12)60198-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage, 62(2), 816–847. https://doi.Org/10.1016/J.NEUROIMAGE.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde GK, Barnett AS, Basser PJ, Marenco S, & Pierpaoli C (2004). Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magnetic Resonance in Medicine, 51(1), 103–114. 10.1002/mrm.10677 [DOI] [PubMed] [Google Scholar]
- Romeo RR, Christodoulou JA, Halverson KK, Murtagh J, Cyr AB, Schimmel C, … Gabrieli JDE (2017). Socioeconomic Status and Reading Disability: Neuroanatomy and Plasticity in Response to Intervention. Cerebral Cortex, 1–16. 10.1093/cercor/bhxl31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Fergusson D, Horwood LJ, Goodman R, Maughan B, … Carroll J (2004). Sex Differences in Developmental Reading Disability. JAMA, 291(16), 2007 10.1001/jama.291.16.2007 [DOI] [PubMed] [Google Scholar]
- Saygin ZM, Norton ES, Osher DE, Beach SD, Cyr AB, Ozemov-Palchik O, … Gabrieli JDE (2013a). Tracking the Roots of Reading Ability: White Matter Volume and Integrity Correlate with Phonological Awareness in Prereading and Early-Reading Kindergarten Children. The Journal of Neuroscience, 33(33), 13251–13258. 10.1523/JNEUROSCI.4383-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin ZM, Norton ES, Osher DE, Beach SD, Cyr AB, Ozemov-Palchik O, … Gabrieli JDE (2013b). Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33(33), 13251–8. 10.1523/JNEUROSCI.4383-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatschneider C, Fletcher JM, Francis DJ, Carlson CD, & Foorman BR (2004). Kindergarten Prediction of Reading Skills: A Longitudinal Comparative Analysis. Journal of Educational Psychology, 96(2), 265–282. https://doi.Org/10.1037/0022-0663.96.2.265 [Google Scholar]
- Scott SK, & Wise RJS (2004). The functional neuroanatomy of prelexical processing in speech perception. Cognition, 92(1–2), 13–45. https://doi.Org/10.1016/J.COGNITION.2002.12.002 [DOI] [PubMed] [Google Scholar]
- Senaha MLH, Martin MGM, Amaro E Jr., Campi C, & Caramelli P (2005). Patterns of cerebral activation during lexical and phonological reading in Portuguese. Brazilian Journal of Medical and Biological Research, 38(12), 1847–1856. 10.1590/S0100-879X2005001200013 [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Escobar MD, Shaywitz BA, Fletcher JM, & Makuch R (1992). Evidence That Dyslexia May Represent the Lower Tail of a Normal Distribution of Reading Ability. New England Journal of Medicine, 326(3), 145–150. 10.1056/NEJM199201163260301 [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fletcher JM, & Escobar MD (1990). Prevalence of reading disability in boys and girls.Results of the Connecticut Longitudinal Study. Journal of American Medical Association, 264(1), 998–1002. https://doi.Org/10.1001/jama.264.8.998 [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, & Beaulieu C (2005). Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage, 26(4), 1164–1173. 10.1016/j.neuroimage.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Snow CE (1991). The Theoretical Basis For Relationships Between Language and Literacy in Development. Journal of Research in Childhood Education, 6(1), 5–10. 10.1080/02568549109594817 [DOI] [Google Scholar]
- Snowling MJ, Gallagher A, & Frith U (2003). Family Risk of Dyslexia Is Continuous: Individual Differences in the Precursors of Reading Skill. Child Development, 74(2), 358–373. 10.1111/1467-8624.7402003 [DOI] [PubMed] [Google Scholar]
- Stein JF, Richardson AJ, & Fowler MS (2000). Monocular occlusion can improve binocular control and reading in dyslexics. Brain, 123(1), 164–170. 10.1093/brain/123.1.164 [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Muller H-P, Juengling FD, Kassubek J, & Riecker A (2008). The contribution of white and gray matter differences to developmental dyslexia: Insights from DTI and VBM at 3.0 T. Neuropsychologia, 46(13), 3170–3178. https://doi.Org/10.1016/J.NEUROPSYCHOLOGIA.2008.07.015 [DOI] [PubMed] [Google Scholar]
- Stoodley CJ (2012). The Cerebellum and Cognition: Evidence from Functional Imaging Studies. The Cerebellum, 11(2), 352–365. 10.1007/sl2311-011-0260-7 [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, & Schmahmann JD (2009). Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage, 44(2), 489–501. https://doi.Org/10.1016/J.NEUROIMAGE.2008.08.039 [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Cohen L, Amemiya E, Braga LW, & Dehaene S (2014). Learning to Read Improves the Structure of the Arcuate Fasciculus. Cerebral Cortex, 24(4), 989–995. 10.1093/cercor/bhs383 [DOI] [PubMed] [Google Scholar]
- Travis KE, Adams JN, Kovachy VN, Ben-Shachar M, & Feldman HM (2016). White matter properties differ in 6-year old Readers and Pre-readers. Brain Structure and Function, 1–19. 10.1007/s00429-016-1302-l [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis KE, Ben-Shachar M, Myall NJ, & Feldman HM (2016). Variations in the neurobiology of reading in children and adolescents born full term and preterm. NeuroImage: Clinical, 11, 555–565. https://doi.Org/10.1016/J.NICL.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis KE, Leitner Y, Feldman HM, & Ben-Shachar M (2015). Cerebellar white matter pathways are associated with reading skills in children and adolescents. Human Brain Mapping, 36(4), 1536–1553. 10.1002/hbm.22721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urger SE, De Beilis MD, Hooper SR, Woolley DP, Chen SD, & Provenzale J (2015). The Superior Longitudinal Fasciculus in Typically Developing Children and Adolescents. Journal of Child Neurology, 30(1), 9–20. 10.1177/0883073813520503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera J, Wouters J, Vandermosten M, & Ghesquiere P (2017). Early dynamics of white matter deficits in children developing dyslexia. Developmental Cognitive Neuroscience, 27(August), 69–77. https://doi.Org/10.1016/j.dcn.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Wouters J, & Ghesquiere P (2012). A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neuroscience & Biobehavioral Reviews, 36(6), 1532–1552. https://doi.Org/10.1016/J.NEUBIOREV.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Velay J-L, Daffaure V, Giraud K, & Habib M (2002). Interhemispheric sensorimotor integration in pointing movements: a study on dyslexic adults. Neuropsychologia, 40(1), 827–834. 10.1016/S0028-3932(01)00177-4 [DOI] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Viergever MA, & Leemans A (2011). Partial volume effect as a hidden covariate in DTI analyses. Neuroimage, 55(4), 1566–76. https://doi.Org/10.1016/j.neuroimage.2011.01.048 [DOI] [PubMed] [Google Scholar]
- Wagner RK, & Torgesen JK (1987). The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin, 101(2), 192–212. https://doi.Org/10.1037/0033-2909.101.2.192 [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA, Hecht SA, Barker TA, Burgess SR, … Garon T (1997). Changing relations between phonological processing abilities and word-level reading as children develop from beginning to skilled readers: A 5-year longitudinal study. Developmental Psychology, 33(3), 468–479. https://doi.Org/10.1037/0012-1649.33.3.468 [DOI] [PubMed] [Google Scholar]
- Wang Y, Mauer MV, Raney T, Peysakhovich B, Becker BLC, Sliva DD, & Gaab N (2017). Development of Tract-Specific White Matter Pathways During Early Reading Development in At-Risk Children and Typical Controls. Cerebral Cortex (New York, N. Y.: 1991), 27(4), 2469–2485. 10.1093/cercor/bhw095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott CAM, & Cercignani M (2009). About “axial” and “radial” diffusivities. Magnetic Resonance in Medicine, 61(5), 1255–60. 10.1002/mrm.21965 [DOI] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Ben-Shachar M, & Wandell BA (2012). Development of white matter and reading skills. Proceedings of the National Academy of Sciences, 109(44), E3045–E3053. 10.1073/pnas.1206792109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, & Feldman HM (2012). Tract profiles of white matter properties: automating fiber-tract quantification. PloS One, 7(11), e49790 10.1371/journal.pone.0049790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Rykhlevskaia E, Sherbondy AJ, Deutsch GK, Wandell BA, & Ben-Shachar M (2011). Anatomical Properties of the Arcuate Fasciculus Predict Phonological and Reading Skills in Children. Journal of Cognitive Neuroscience, 23(11), 3304–3317. 10.1162/jocn_a_00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


