Abstract
Purpose:
Growth hormone (GH) replacement decreases insulin sensitivity in healthy individuals. However, the effects of GH on organ-specific insulin sensitivity and glucose effectiveness are not well characterized. The purpose of this study was to evaluate the effects of GH administration for 26 weeks on muscle and hepatic insulin sensitivity and glucose effectiveness in healthy older individuals.
Methods:
This report is from a 26-week randomized, double-blind, placebo-controlled parallel-group trial in healthy, ambulatory, community-dwelling older women and men. We compared surrogate indices of insulin sensitivity [The quantitative insulin-sensitivity check index (QUICKI), muscle insulin sensitivity index (MISI), hepatic insulin resistance index (HIRI)] and glucose effectiveness [oral glucose effectiveness index (oGE)] derived from oral glucose tolerance tests (OGTTs) in subjects before and after 26 weeks of administration of GH (n=17) or placebo (n=15) as an exploratory outcome.
Results:
GH administration for 26 weeks significantly increased fasting insulin concentrations and HIRI but did not significantly change MISI or oGE when compared to the placebo group.
Conclusions:
GH administration for 26 weeks in healthy older subjects impairs insulin sensitivity in the liver but not skeletal muscle and does not alter glucose effectiveness.
Keywords: Growth hormone, Insulin resistance, Glucose effectiveness, Aging
INTRODUCTION
Glucose intolerance, insulin resistance, and diabetes mellitus (DM) are frequent clinical manifestations in patients with acromegaly [1,2], in whom increased hepatic glucose production, peripheral insulin resistance, and impaired pancreatic beta cell function contribute to the pathogenesis of glucose intolerance [3–6]. Surgical removal of growth hormone (GH) secreting tumors reverses glucose intolerance and insulin resistance in most patients with acromegaly [3,7]. Administration of pegvisomant, a specific antagonist for the GH receptor (GHR) improves insulin sensitivity in patients with acromegaly [8]. Genetic ablation of the GH receptor (GHR) in mice and GHR deficiency in humans confers increased insulin sensitivity and protection from diabetes mellitus [9,10]. These findings suggest an important role for GH in modulating glucose homeostasis. It appears that intact GH signaling and even physiological increases in GH levels antagonizes insulin action [11].
Despite many years of research, the tissue-specific GH effects that alters insulin sensitivity are unclear [12,13]. Both liver and the adipose tissue have been suggested to be major sites of GH-induced alterations in insulin action in rodent models [12,13]. In humans, acute GH administration stimulates lipolysis and the resulting increase in free fatty acid (FFA) levels contribute to higher hepatic gluconeogenesis and lower peripheral glucose disposal [14–16]. In contrast to these studies suggesting a causal role for enhanced lipolysis in mediating the effects of GH, pegvisomant improves hepatic insulin sensitivity but with no effects on peripheral insulin sensitivity or lipolysis [17]. Thus, in humans, the effects of GH may be mediated by its actions on the liver and adipose tissue. However, the short duration of these studies is a potential limitation and precludes any definitive conclusions. The effects of long-term GH administration on insulin sensitivity in adults with GH deficiency are variable and appear to be dependent on the dose and duration of GH administration and other concomitant hormonal deficiencies [18,19,4]. In contrast, in normal healthy adults, chronic GH administration reduces insulin sensitivity [20,4]. However, the effects of chronic GH administration on tissue-specific insulin sensitivity in healthy individuals are unknown. In this study, we examined the effects of GH administration on indices of muscle and hepatic insulin sensitivity and glucose effectiveness derived from an oral glucose tolerance test (OGTT) in healthy older adults.
METHODS
Study Design and Subjects
Our objective of this exploratory analysis was to study the effects of chronic GH administration on tissue-specific insulin sensitivity in healthy individuals. We previously reported that GH administration to healthy older individuals for six months reduced insulin sensitivity [20]. A detailed description of the original study design, protocol, participants, and hormone interventions has been published [21]. The original study was designed as a randomized, placebo-controlled, double-masked 2×2 factorial, non-crossover trial. Participants received either GH plus placebo, sex steroid (SS) (transdermal estradiol plus oral medroxyprogesterone acetate in women, intramuscular injections of testosterone enanthate in men) plus placebo, GH plus SS, or placebo only. For the GH group, GH (Nutropin; Genentech, Inc., South San Francisco, CA) was administered in a dose of 20 μg/kg body weight and was self-injected subcutaneously three times per week in the evening. For the placebo group, subjects self-injected saline. In the present report, we evaluated surrogate indices of hepatic and muscle insulin sensitivity and glucose effectiveness (exploratory outcomes) before and after treatment only in individuals with normal glucose tolerance who received either GH plus placebo (n=17) or placebo alone (n=15) for 26 weeks.
All subjects were healthy, ambulatory, US community-dwelling individuals aged 65–88 years. Participants were deemed healthy based on history, physical examination, routine serum chemistries, and urinalysis. Medications that affected the GH/IGF-1 axis or glucose metabolism were not permitted. Written informed consent was obtained from each participant. The Institutional Review Board of the Johns Hopkins Bayview Medical Center approved the study protocol.
Body Composition, Fat Distribution, and Cardiovascular Endurance
Body mass index (BMI) was calculated as weight (kg)/height (m2). Total body fat (TBF) and lean body mass (LBM) were measured by DEXA (Lunar model DPX-L; Lunar Radiation, Madison, Wisconsin). Total abdominal fat (TAF), abdominal subcutaneous fat (ASF) and abdominal visceral fat (AVF) were measured by abdominal Magnetic Resonance Imaging at the level of L4–L5 as previously described [22]. Cardiovascular endurance (VO2max) was measured as previously described [21].
Oral Glucose Tolerance Test and Hormone assays
A 75g standard OGTT after an overnight fast was performed at baseline and after 26 weeks of intervention. Plasma glucose and insulin levels were measured at 0, 30, 60, 90, 120 minutes. Impaired glucose tolerance and diabetes mellitus (DM) were defined as per recent ADA guidelines [23]. Plasma insulin and glucose concentrations were measured at the Johns Hopkins Bayview General Clinical Research Center (GCRC) Core Laboratory. Insulin concentrations were determined by RIA (Linco Research Inc., St. Louis, MO). The assay had a sensitivity of 1.2 pmol/liter with a linear range from 12 to 1200 pmol/liter. Intra-assay and inter-assay coefficients of variation (CVs) were 2.1 and 2.8%, respectively. Glucose concentrations were measured using an automated glucose-oxidase assay (Beckman Diagnostics, Fullerton, CA) and exhibited intra-assay and inter-assay CVs of 2.8 and 2.1%, respectively. IGFBP-1 levels were measured by immunoradiometric assay (IRMA) (Diagnostic Systems Laboratories, Webster, TX). The intra- and inter-assay CVs were 2.5 and 9.4%, respectively [24]. Total serum IGF-1 levels were measured by RIA after acid-ethanol extraction (Endocrine Sciences Laboratories, Calabasas Hills, CA). The intra- and inter-assay coefficients of variation (CVs) were, respectively, 5.9 and 7.3% at 289 μg/L and 4.6 and 6.3% at 591 μg/L [24].
Hepatic Insulin Resistance Index (HIRI), Muscle Insulin Sensitivity Index (MISI), and Glucose Effectiveness (oGE)
Tissue-specific surrogate indices for insulin sensitivity were derived from plasma insulin and glucose levels from oral glucose tolerance tests [25]. The hepatic insulin resistance index (HIRI) was calculated as {[glucose (mg/dl) AUC0–30] × [insulin (μU/ml) AUC0–30]} where AUC between 0 and 30 minutes was calculated by the trapezoidal method. The muscle insulin sensitivity index (MISI) was derived by dividing the rate of decline in plasma glucose concentration calculated as the slope of the decrease in plasma glucose concentration (dG/dt) from peak to nadir by the mean plasma insulin concentration and measured as 10−2 (mg/dL.min−1/(μU/mL). We identified study participants in whom glucose levels continued to rise from 60 minutes to 120 minutes during the OGTT. Because of the assumption of calculating MISI requires there to be a negative slope from 60 to 120 minutes, 5 subjects from the placebo group and 3 subjects from the GH treated group were excluded from the analyses. Glucose effectiveness is the ability of glucose to facilitate its own disposal at basal insulin concentrations. oGE was calculated from the OGTT as previously described [26,27]. oGE was derived from the equation: oGE = {[PPG_without insulin action and GE] - [PPG with GE but without insulin action] × [2hPG/2hPGE]}/120, where PPG = post-loading plasma glucose, GE = glucose effectiveness, 2hPG = 2-h post-glucose PG, and 2hPGE = expected 2hPG. QUICKI, a measure of insulin sensitivity was calculated as defined previously [28]. QUICKI = 1/[log(I0) + log(G0)], where I0 is fasting insulin (μU/ml) and G0 is fasting glucose (mg/dl). Because QUICKI is the reciprocal of the log-transformed product of fasting glucose and insulin, it is a dimensionless index without units.
Statistical Analysis
The distributions of variables were examined by Q-Q plot, stem-leaf plots, box plots and the D’Agostino and Pearson tests. Variables with normal distributions are expressed as mean ± standard deviation (SD). Variables with a non-normal distribution are expressed as median (IQR). The comparison of baseline characteristics between groups (placebo vs. GH group) was assessed by independent t-test or The Wilcoxon-Mann-Whitney test. Relationships of surrogate indexes of insulin sensitivity / resistance and oGE with body composition, and metabolic profiles were assessed by Spearman correlation. Relationships were assessed in women and men together when slopes of regressions in each sex showed no significant differences. The effect of 26-week treatment (placebo or GH) on MISI and HIRI were assessed by the analysis of covariance (ANCOVA). The dependent variables in the ANCOVAs were the changes (post-pre) in values of the outcome variable being studied. Independent variables included the subject’s age, the initial value of the outcome variable, and treatment group (GH or placebo). A simple contrast method was used for post-hoc, between-group analyses in ANCOVA. A p-value less than 0.05 was considered to be statistically significant in all analyses. Data were analyzed with JMP version 7.0 (SAS Institute, Cary, NC) and GraphPad Prism 7 (GraphPad Software Inc, La Jolla, CA).
RESULTS
Subject characteristics and baseline metabolic profiles
Baseline characteristics and metabolic parameters of the study participants are depicted in Table 1. Baseline characteristics were similar between the placebo and GH groups (Table 2). Our cohort was comprised of lean (31%) and overweight (68%) individuals. In the overall group (GH and placebo combined) there were no differences in age-adjusted QUICKI (p=0.31), MISI (p = 0.36) or HIRI (p = 0.81) between women and men at baseline, whereas oGE was higher in women versus men (adjusted mean ± SEM; 3.52 ± 0.13 vs. 2.46 ± 0.13 mg/dL/min, p<0.0001) (Table 1). IGF-1 binding protein 1 (IGFBP-1), a marker of hepatic insulin sensitivity [29], was higher in women compared with men (73 ± 7 vs. 36 ± 6 ng/mL, p<0.001) (Table 1).
Table 1.
Baseline clinical and metabolic characteristics: Women vs. Men
| Men (n=17) | P Value | |||
|---|---|---|---|---|
| Age, yr | 71 ± 5 | 71 ± 5 | 71 ± 5 | 0.97 |
| Systolic blood pressure, mmHg | 130 ± 15 | 131 ± 18 | 129 ± 13 | 0.72 |
| Diastolic blood pressure, mmHg | 79 ± 8 | 78 ± 9 | 79 ± 6 | 0.79 |
| BMI, kg/m2 | 25.8 ± 2.6 | 24.7 ± 2.8 | 26.7 ± 2.2 | 0.04 |
| Lean body mass (LBM, kg) | 45.7 ± 11.0 | 35.3 ± 3.1 | 55.0 ± 5.6 | <0.0001 |
| Total body fat, % | 34 (29 – 42) | 42 (38–46) | 29 (26 – 33) | <0.0001 |
| Abdominal visceral fat, % of total | 31(21 – 40) | 22 (18 – 28) | 39 (33 – 48) | <0.0001 |
| Abdominal subcutaneous fat, % of total | 69 (60 – 79) | 78 (72 – 82) | 61 (52 – 68) | <0.0001 |
| Maximal oxygen capacity, VO2max (ml/kg/min) | 26 ± 5 | 23 ± 4 | 28 ± 5 | <0.001 |
| Total cholesterol, mg/dL | 188 ± 30 | 203 ± 30 | 174 ± 23 | 0.002 |
| HDL cholesterol, mg/dL | 49 ± 13 | 57 ± 12 | 42 ± 10 | <0.001 |
| LDL cholesterol, mg/dL | 119 ± 30 | 129 ± 34 | 109 ± 22 | 0.04 |
| Triglycerides, mg/dL | 106 ± 38 | 98 ± 34 | 112 ± 41 | 0.29 |
| Fasting glucose, mg/dL | 94 ± 7.6 | 93 ± 7 | 95 ± 8 | 0.44 |
| Fasting insulin, μU/mL | 9.1 (7.4 – 10.6) | 9.0 (7.0 – 11.3) | 9.1 (8.1 – 10.6) | 0.36 |
| 2-hour plasma glucose, OGTT, mg/dL | 114 ± 18 | 110 ± 15 | 118 ± 20 | 0.18 |
| QUICKI | 0.342 ± 0.014 | 0.345 ± 0.015 | 0.340 ± 0.014 | 0.31 |
| Hepatic insulin resistance index (HIRI) | 2.81 (2.21 – 3.66) | 2.68 (2.26 – 2.85) | 3.19 (1.82 – 4.28) | 0.81 |
| Muscle insulin sensitivity index (MISI) | 0.011 (0.006 – 0.019) | 0.009 (0.007 – 0.019) | 0.014 (0.007 – 0.019) | 0.36 |
| Oral glucose effectiveness index, oGE, mg/dL/min | 2.95 (2.51 – 3.43) | 3.44 (3.31 – 3.75) | 2.54 (2.37 – 2.88) | <0.0001 |
| Insulin-like growth factor 1 (IGF-1), μg/mL | 114 (81 – 162) | 84 (59 – 114) | 155 (107 – 207) | <0.001 |
| IGF binding protein-1, ng/mL | 57 (26 – 73) | 69 (58 – 91) | 26 (17 – 61) | <0.001 |
Data are presented as unadjusted arithmetic mean ± SD or as median (IQR); n, no. of subjects; IGF-1, insulin like growth factor-1; and IGFBP-1, IGF binding protein-1. P values indicate significance for comparisons between groups at baseline.
Table 2.
Baseline clinical and metabolic characteristics: Placebo vs. GH treatment arms
| GH (n=17) | P Value | ||
|---|---|---|---|
| Age, yr | 73 ± 5 | 70 ± 4 | 0.89 |
| Number of Women/Men | 8/7 | 7/10 | |
| Systolic blood pressure, mmHg | 131 ± 17 | 129 ± 14 | 0.63 |
| Diastolic blood pressure, mmHg | 79 ± 9 | 78 ± 6 | 0.66 |
| BMI, kg/m2 | 25.7 ± 2.1 | 25.8 ± 3.1 | 0.84 |
| Lean body mass (LBM, kg) | 44.9 ± 11.1 | 46.5 ± 11.1 | 0.57 |
| Total body fat, % | 37 (31 – 45) | 32 (26 – 42) | 0.17 |
| Abdominal visceral fat, % of total | 31(21 – 40) | 32 (20 – 41) | 0.89 |
| Abdominal subcutaneous fat, % of total | 69 (59 – 79) | 68 (59 – 79) | 0.97 |
| Maximal oxygen capacity, VO2max (ml/kg/min) | 24 ± 5 | 28 ± 5 | 0.03 |
| Total cholesterol, mg/dL | 188 ± 29 | 187 ± 31 | 0.88 |
| HDL cholesterol, mg/dL | 49 ± 15 | 48 ± 12 | 0.84 |
| LDL cholesterol, mg/dL | 118 ± 32 | 119 ± 26 | 0.95 |
| Triglycerides, mg/dL | 114 ± 46 | 98 ± 29 | 0.24 |
| Fasting glucose, mg/dL | 95 ± 8 | 93 ± 8 | 0.38 |
| Fasting insulin, μU/mL | 9.2 (7.7 – 11.4) | 8.9 (7.1 – 10.6) | 0.33 |
| 2-hour plasma glucose, OGTT, mg/dL | 117 ± 19 | 111 ± 17 | 0.33 |
| QUICKI | 0.339 ± 0.014 | 0.346 ± 0.015 | 0.16 |
| Hepatic insulin resistance index (HIRI) | 3.03 (2.21 – 4.08) | 2.52 (2.13 – 3.11) | 0.17 |
| Muscle insulin sensitivity index (MISI) | 0.009 (0.003 – 0.015) | 0.014 (0.009 – 0.024) | 0.05 |
| Oral glucose effectiveness index, oGE, mg/dL/min | 2.88 (2.51 – 3.44) | 2.97 (2.55 – 3.54) | 0.65 |
| Insulin-like growth factor 1 (IGF-1), μg/mL | 108 (80 – 155) | 140 (82 – 173) | 0.47 |
| IGF binding protein-1, ng/mL | 45 (20 – 89) | 65 (31 – 73) | 0.55 |
Data are presented as unadjusted arithmetic mean ± SD or as median (IQR); n, no. of subjects; IGF-1, insulin like growth factor-1; and IGFBP-1, IGF binding protein-1. P values indicate significance for comparisons between intervention groups at baseline.
Women had higher age-adjusted TBF (42.1 ±0.9 vs. 29.3±0.9 %, p<0.0001) and ASF (77.9 ± 2.2 vs. 60.1± 2.0 %, p<0.0001), but lower AVF (22.0±2.2 vs. 39.8±2.0 %, p<0.0001), LBM (35.2 ± 1.0 vs. 55.0 ± 0.9 kg, p<0.0001), and maximal oxygen capacity, VO2max (23.1 ± 0.9 vs. 28.1 ± 0.8 ml/kg/min, p<0.001) compared with men (Table 1). At baseline in the combined cohort (GH and placebo), oGE was directly related to TBF, ASF, and inversely related to BMI, AVF, HIRI, and plasma concentrations of IGF-1, IGFBP-1, fasting glucose, and fasting insulin (Table 3). HIRI was positively associated with AVF, fasting glucose and insulin levels, and negatively associated with ASF, and oGE. MISI was directly related to VO2max and inversely related to fasting insulin concentrations (Table 3). Circulating IGFBP-1 levels were directly related to oGE, and negatively correlated to HIRI, albeit non-significantly (p=0.07) (Table 3). There was no sexual dimorphism in these relationships.
Table 3.
Relationships between tissue-specific insulin resistance indices, oral glucose effectiveness, and clinical parameters in the entire cohort at baseline.
| HIRI | MISI | oGE | ||||
|---|---|---|---|---|---|---|
| r-value | p | r-value | p | r-value | p | |
| Age, yr | −0.08 | 0.64 | −0.32 | 0.07 | 0.27 | 0.13 |
| Body Mass Index, kg/m2 | 0.09 | 0.58 | −0.12 | 0.52 | −0.64 | <0.0001 |
| Total Body Fat (TBF), % | 0.03 | 0.87 | −0.26 | 0.14 | 0.51 | <0.001 |
| Abdominal visceral fat (AVF), % of total | 0.38 | 0.03 | 0.23 | 0.62 | −0.47 | 0.005 |
| Abdominal subcutaneous fat (ASF), % of total | −0.38 | 0.03 | −0.23 | 0.62 | 0.47 | 0.005 |
| Cardiovascular endurance (VO2max), ml/kg/min | 0.08 | 0.65 | 0.42 | 0.01 | −0.31 | 0.08 |
| Total Cholesterol, mg/dL | −0.09 | 0.59 | 0.17 | 0.35 | 0.23 | 0.19 |
| Triglycerides, mg/dL | 0.21 | 0.25 | 0.08 | 0.63 | −0.23 | 0.21 |
| LDL cholesterol, mg/dL | −0.05 | 0.78 | 0.22 | 0.23 | 0.14 | 0.43 |
| HDL Cholesterol, mg/dL | −0.11 | 0.49 | −0.27 | 0.15 | 0.41 | 0.01 |
| Fasting Glucose, mg/dL | 0.38 | 0.04 | 0.16 | 0.40 | −0.40 | 0.02 |
| Fasting Insulin, μU/mL | 0.50 | <0.001 | −0.42 | 0.01 | −0.41 | 0.02 |
| 2-Hr Plasma Glucose, mg/dL | 0.07 | 0.68 | −0.13 | 0.48 | 0.05 | 0.75 |
| Insulin-like growth factor 1 (IGF-1), μg/mL | 0.04 | 0.78 | 0.16 | 0.40 | −0.54 | <0.0001 |
| IGF binding protein-1, ng/mL | −0.32 | 0.07 | 0.14 | 0.43 | 0.55 | <0.001 |
| HIRI | - | −0.05 | 0.76 | −0.30 | 0.03 | |
| MISI | −0.05 | 0.76 | - | −0.11 | 0.54 | |
| oGE | −0.38 | 0.03 | −0.11 | 0.53 | - | |
Correlation analyses of the relationships between tissue specific insulin resistance indexes, oral glucose effectiveness index, and clinical parameters. HIRI, hepatic insulin resistance index; MISI, muscle insulin sensitivity index; and oGE, oral glucose effectiveness index.
Effects of GH versus placebo administration on insulin sensitivity and glucose effectiveness
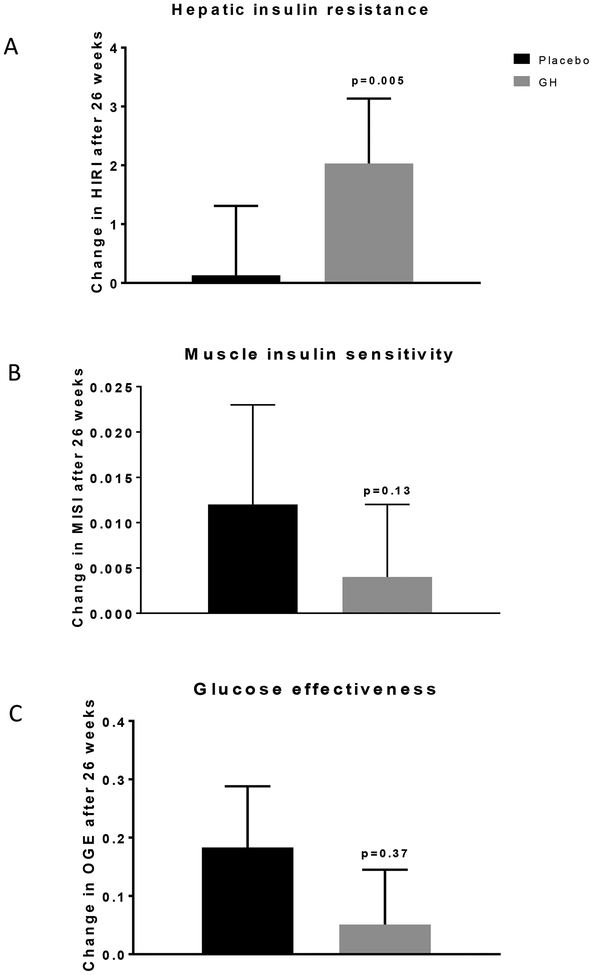
When compared with placebo, GH administration increased IGF-1 levels [110 μg/L (95% CI: 59–160), p<0.001], LBM [1.81 kg (95% CI: 1.01 – 2.60), p<0.001], and VO2max [1.92 ml/kg/min (95% CI: 0.55 – 3.28), p=0.008], and decreased TBF [-3.1 % (95% CI: −5.0 – −1.2), p<0.001] (Table 4) but did not significantly affect AVF or ASF. GH decreased total and LDL cholesterol levels but increased plasma triglyceride concentrations (Table 4). The effects of GH and placebo administration on fasting plasma glucose and insulin levels, 2hr glucose levels during an OGTT, insulin sensitivity/resistance indices, and glucose effectiveness are summarized in Table 5. GH treatment elicited significant increases in fasting insulin levels and HIRI (p<0.001), reduced QUICKI, but did not significantly change MISI or OGE (Figure 1 and Table 5). Levels of IGFBP-1, a marker for hepatic insulin sensitivity were also lower after GH versus placebo [-11.1 (95% CI: −19.3 – −2.8) ng/mL, p=0.01] (Table 5).
Table 4.
Effects of placebo and GH administration for 26 weeks on body composition and IGF-1 and plasma cholesterol concentrations
| P Value | |||
|---|---|---|---|
| BMI, kg/m2 | |||
| Baseline | 25.7 ± 2.1 | 25.8 ± 3.1 | |
| 26 Weeks | 25.8 ± 2.1 | 25.7 ± 3.0 | |
| Change(Δ) | 0.1 ± 0.6 | −0.1 ± 1.3 | |
| Between-group Difference in Δ | −0.2 (−0.9 – 0.6) | 0.62 | |
| Total body fat, % | |||
| Baseline | 37 (31 – 45) | 32 (26 – 42) | |
| 26 Weeks | 37 (31 – 45) | 29 (24 – 36) | |
| Change (Δ) | −0.7 (−2 – 0.2) | −3.0 (−5 – −2) | |
| Between-group Difference in Δ | −3.1 (−5.0 – −1.2) | 0.002 | |
| Lean body mass (LBM, kg) | |||
| Baseline | 44.9 ± 11.1 | 46.5 ± 11.1 | |
| 26 Weeks | 43.1 ± 10.3 | 49.4 ± 11.3 | |
| Change (Δ) | 0.6 ± 0.9 | 2.3 ± 1.1 | |
| Between-group Difference in Δ | 1.8 (1.0 – 2.6) | <0.0001 | |
| Insulin-like growth factor 1 (IGF-1), μg/mL | |||
| Baseline | 108 (80 – 155) | 140 (82 – 173) | |
| 26 Weeks | 87 (75 – 136) | 218 (139 – 325) | |
| Change (Δ) | −16 (−27 – 6) | 59 (31 – 203) | |
| Between-group Difference in Δ | 109 (59 – 160) | 0.0001 | |
| Total cholesterol, mg/dL | |||
| Baseline | 188 ± 29 | 187 ± 31 | |
| 26 Weeks | 196 ± 33 | 180 ± 27 | |
| Change (Δ) | 8 ± 25 | −6 ± 16 | |
| Between-group Difference in Δ | −16 (−31 – 1) | 0.04 | |
| LDL cholesterol, mg/dL | |||
| Baseline | 118 ± 32 | 119 ± 26 | |
| 26 Weeks | 126 ± 34 | 104 ± 21 | |
| Change (Δ) | 8 ± 26 | −14 ± 16 | |
| Between-group Difference in Δ | −23 (−38 – −9) | 0.002 | |
| HDL cholesterol, mg/dL | |||
| Baseline | 49 ± 15 | 48 ± 12 | |
| 26 Weeks | 48 ± 13 | 48 ± 12 | |
| Change (Δ) | −2 ± 8 | 0 ± 8 | |
| Between-group Difference in Δ | 1 (−4 – 7) | 0.62 | |
| Triglycerides, mg/dL | |||
| Baseline | 114 ± 46 | 98 ± 29 | |
| 26 Weeks | 112 ± 34 | 137 ± 71 | |
| Change (Δ) | −2 ± 33 | 40 ± 59 | |
| Between-group Difference in Δ | 37 (-0.2 – 75) | 0.05 | |
Baseline and week 26 data are unadjusted values expressed as arithmetic mean (SD) or median (IQR); Δ, difference in post- and pre-treatment values. Differences in mean Δ between the treatment groups are adjusted for age, baseline value, and treatment group and is expressed as adjusted means (95% CI); n, number of subjects; P values indicate significance for comparisons between the GH and placebo treated groups.
Table 5.
Effects of placebo and GH administration for 26 weeks on glucose metabolism and insulin sensitivity
| P Value | |||
|---|---|---|---|
| Fasting glucose, mg/dL | |||
| Baseline | 95 ± 8 | 93 ± 8 | |
| 26 Weeks | 99 ± 21 | 100 ± 9 | |
| Change(Δ) | 3 ± 20 | 7 ± 9 | |
| Between-group Difference in Δ | 3.7 (−8.0 – 15.4) | 0.52 | |
| Fasting insulin, μU/mL | |||
| Baseline | 9.2 (7.7 – 11.4) | 8.9 (7.1 – 10.6) | |
| 26 Weeks | 11.3 (6.6 – 14.6) | 13.3 (10.1 – 15.8) | |
| Change(Δ) | −0.3 (−1.5 – 5.1) | 4.6 (1.9 – 7.9) | |
| Between-group Difference in Δ | 6.7 (−0.5 – 13.7) | 0.01 | |
| 2-hour Plasma glucose, OGTT, mg/dL | |||
| Baseline | 117 ± 19 | 111 ± 17 | |
| 26 Weeks | 139 ± 29 | 138 ± 30 | |
| Change(Δ) | 22 ± 27 | 27 ± 26 | |
| Between-group Difference in Δ | 7 (−13 – 26) | 0.50 | |
| IGF binding protein-1, ng/mL | |||
| Baseline | 45 (20 – 89) | 65 (31 – 73) | |
| 26 Weeks | 42 (24–62) | 34 (23–54) | |
| Change(Δ) | −3 (−27 – 12) | −22 (−34 – −12) | |
| Between-group Difference in Δ | −11 (−19 – −3) | 0.01 | |
| QUICKI | |||
| Baseline | 0.339 ± 0.014 | 0.346 ± 0.015 | |
| 26 Weeks | 0.335 ± 0.023 | 0.318 ± 0.021 | |
| Change(Δ) | −0.003 ± 0.017 | −0.028 ± 0.022 | |
| Between-group Difference in Δ | −0.025 (−0.040 – −0.009) | 0.003 | |
| Hepatic insulin resistance index (HIRI) | |||
| Baseline | 3.03 (2.21 – 4.08) | 2.52 (2.13 – 3.11) | |
| 26 Weeks | 2.57 (1.72 – 4.66) | 4.43 (2.79 – 7.19) | |
| Change(Δ) | 0.03 (-1.28 – 0.98) | 1.80 (-0.10 – 34.46) | |
| Between-group Difference in Δ | 1.89 (0.27 – 3.51) | 0.005 | |
| Muscle insulin s ensitivity index (MISI) | |||
| Baseline | 0.009 (0.003 – 0.015) | 0.014 (0.009 – 0.024) | |
| 26 Weeks | 0.013 (0.007 – 0.035) | 0.011 (0.005 – 0.020) | |
| Change(Δ) | 0.008 (−0.001 – 0.019) | −0.002 (−0.010 – 0.007) | |
| Between-group Difference in Δ | −0.007 (−0.037 – 0.022) | 0.13 | |
| Oral Glucose effectiveness (oGE) | |||
| Baseline | 2.88 (2.51 – 3.44) | 2.97 (2.55 – 3.54) | |
| 26 Weeks | 3.20 (2.69 – 3.52) | 3.06 (2.66 – 3.41) | |
| Change(Δ) | 0.17 (-0.15 – 0.43) | 0.07 (-0.26 – 0.33) | |
| Between-group Difference in Δ | −0.13 (−0.42 – 0.16) | 0.37 | |
Baseline and week 26 data are unadjusted values expressed as arithmetic mean (SD) or median (IQR); Δ, difference in post- and pre-treatment values. Differences in mean Δ between the treatment groups are adjusted for age, baseline value, and treatment group and is expressed as adjusted means (95% CI); n, number of subjects; P values indicate significance for comparisons between the GH and placebo treated groups.
Figure 1.
Effects of GH (n=17) or placebo (n=15) administration on (A) hepatic insulin resistance (HIRI), (B) muscle insulin sensitivity index (MISI), and (C) glucose effectiveness (oGE). GH or placebo was administered to healthy older men and women for 26 weeks. Oral glucose tolerance tests (OGTT) were performed at baseline and after 26 weeks. Changes (post-treatment-baseline) in HIRI, MISI and oGE were calculated from the OGTTs. Differences in mean change between the treatment groups are adjusted for age, baseline value, and treatment group and is expressed as adjusted means ± SEM.
DISCUSSION
Short-term GH administration in healthy older adults increases circulating IGF-1 levels and lean body mass and correspondingly decreases total body fat and abdominal visceral fat mass. These salutary changes would be expected to favorably impact insulin sensitivity, yet GH administration worsens glucose tolerance and insulin sensitivity. In the present study, we found that GH administration decreased hepatic insulin sensitivity, but exerted no significant effect on muscle insulin sensitivity or glucose effectiveness. In addition, we observed novel relationships among glucose effectiveness, hepatic insulin sensitivity and the IGF-1/IGFBP-1 axis.
Clinical Characteristics of Participants
In this study of healthy, non-obese, ambulatory, community dwelling individuals, observed sex differences in BMI and TBF were consistent with previous studies [30–33]; women exhibited a lower BMI and AVF, but higher TBF, compared with men. Glucose per se regulates blood glucose levels by decreasing hepatic glucose production and augmenting peripheral glucose disposal, thereby lowering plasma glucose levels at basal insulin concentrations, typically referred to as glucose effectiveness. Postprandial glucose effectiveness, as determined by the oral glucose minimal model, was higher in women than in men, independent of age [33]. Similarly in a Danish population-based study of 380 young healthy Caucasians, wherein glucose effectiveness (measured by frequently sampled IV glucose tolerance test, FSIVGTT) was 15% higher in women versus men [34]. These studies support our finding of lower glucose effectiveness in men when compared with women. We observed that oGE was inversely related to BMI, AVF, IGF-1, HIRI, and fasting insulin and glucose levels. The relationship with body composition is expected, as adiposity is directly related to glucose intolerance and diabetes. Yet, no previous studies have reported an association between glucose effectiveness and serum concentrations of IGF-1. IGF-1 is an anabolic hormone that is synthesized by hepatocytes in response to GH. Like insulin, IGF-1 promotes peripheral glucose uptake and oxidation and suppresses hepatic glucose production [35,36]. Low plasma IGF-1 concentrations predict impaired insulin-mediated glucose uptake in older individuals [37]. Thus, the increase in glucose effectiveness at lower IGF-1 levels could be a compensatory response to diminished peripheral glucose uptake [38]. Our finding of a negative relationship of OGE with HIRI is consistent with the observation that impaired glucose effectiveness is associated with enhanced hepatic glucose production [39]. The expansion of AVF is hypothesized to contribute to increases in portal free fatty acid FFA and glycerol concentrations, inducing hepatic fatty acid esterification, hepatic steatosis, and hepatic insulin resistance [40,41]. HIRI was directly related to AVF but inversely related to ASF, suggesting a protective effect of ASF [42]. Finally, circulating IGFBP1, has been proposed as a liver-specific surrogate marker of hepatic insulin sensitivity [29]. Consistent with this observation, in the current study, serum IGFBP-1 levels were inversely related to hepatic insulin resistance and directly related to glucose effectiveness.
GH administration affects hepatic but not skeletal muscle insulin sensitivity or glucose effectiveness
Our results demonstrate that short-term GH administration affects insulin resistance in the liver but not skeletal muscle, in healthy adults. Acutely, GH administration in healthy individuals elicits higher hepatic glucose production and lower peripheral glucose disposal [14–16]. Simultaneous administration of acipimox, which lowers FFA levels by inhibition of the hormone-sensitive lipase with GH abolished the negative effects of GH on hepatic insulin sensitivity [14–16,43,44]. Based on these and other studies in GH-deficient adults [45,46,44], the lipolytic effects of GH have been postulated to mediate GH induced insulin resistance. The magnitude and the effects of lipolytic actions of GH after continued GH administration in healthy individuals are unknown. However, in patients with acromegaly, acute administration of pegvisomant, a human GH receptor antagonist, reduced FFA levels and hepatic glucose production but did not alter peripheral glucose disposal [8]. Consistent with the latter finding, GH blockade during fasting also reduced endogenous glucose production and FFA levels in obese individuals [47]. These studies suggest that GH-induced insulin resistance may be primarily confined to the liver. How long-term GH administration induces hepatic but not skeletal muscle insulin resistance is unclear. In one recent study, adipocyte-specific deletion of Janus Kinase 2 (Jak2), an important transducer and activator of the STAT pathway, prevented GH-mediated hepatic insulin resistance in mice [12]. In an elegant set of experiments, Corbit et al. demonstrated that GH-mediated inhibition of insulin-induced suppression of lipolysis (via JAK2) was the proximate cause of hepatic insulin resistance. These recent findings in mice and aforementioned studies in humans suggest that the diabetogenic action of GH is primarily mediated by enhanced lipolysis which leads to increases in hepatic acetyl CoA and accentuated hepatic gluconeogenesis [48]. Moreover, in our study, another marker of hepatic insulin sensitivity, IGFBP-1, was also lower after GH treatment. Insulin inhibits hepatic IGFBP-1 synthesis and thus compensatory hyperinsulinemia following GH administration may explicate the lower circulating IGFBP1 levels [49].
In this study, GH administration did not negatively affect peripheral insulin sensitivity. Several possible mechanisms may explain the differential tissue-specific effects of GH. Favorable body composition changes caused by long-term GH administration, such as increased LBM and decreased fat mass, might exert beneficial effects on peripheral insulin sensitivity [21]. In our study, GH increased maximum exercise capacity when compared with placebo. Improved or high levels of exercise tolerance may promote glucose disposal in skeletal muscle by increasing expression and translocation of the glucose transporter isoform 4 (GLUT-4) [50,51]. Finally, GH-administration may improve peripheral insulin sensitivity by increasing circulating IGF-1 levels, which directly acts on skeletal muscle and facilitates peripheral glucose uptake. The effects of GH on glucose effectiveness in healthy individuals has not been previously examined. However, similar to our findings, six months of GH administration in patients with GH deficiency did not alter glucose effectiveness [52].
Our study had several limitations. First, we used surrogate markers of insulin sensitivity/resistance and glucose effectiveness derived from an OGTT [25–27]. The gold-standard method to evaluate hepatic and peripheral (muscle) insulin sensitivity is a hyperinsulinemic euglycemic clamp with isotope- or radio-labeled glucose tracers [28]. Similarly, somatostatin pancreatic-glucose clamp and minimal model analysis (MM) of frequently sampled intravenous glucose tolerance test (FSIVGTT) are standard procedures used to assess glucose effectiveness. However, these methods are labor-intensive, technically demanding, and not feasible for large studies. The surrogate indexes used in the current study have been widely used in other studies and correspond closely to indexes derived from the reference glucose clamp procedure (HIRI: r = 0.58–0.64; MISI: r = 0.49–0.78; and oGE: r=0.32–0.35) [25–27,53,54]. Second, we consider the changes we observed in tissue-specific insulin sensitivity indexes following GH administration to be exploratory. Thus, the results of our study need to be confirmed in studies where these outcomes are pre-specified as primary outcomes and measured using reference techniques (e.g., glucose clamp). Third, the duration of our study was relatively short (6 months) and whether similar effects occur after longer-term GH administration remains to be determined. Fourth, we did not measure FFA levels and thus cannot address the role of FFA in mediating reduced hepatic insulin sensitivity. Finally, no objective measures of diet intake and physical activity were taken during the trial and participants were simply advised to follow their normal diet and exercise regimen.
In conclusion, GH administration to healthy older women and men for six months decreased hepatic insulin sensitivity but did not significantly affect muscle insulin sensitivity or glucose effectiveness. These observations are consistent with recent findings in rodents and inform our understanding of alterations in glucose metabolism in states of GH excess such as acromegaly, GH deficiency (congenital or acquired), or GH insensitivity/resistance as observed in Laron Syndrome.
Acknowledgments
Funding: This work was supported in part by the Intramural Research Programs of the National Institute on Aging (NIA), Baltimore, Maryland and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Washington, DC, National Institutes of Health Research Grants RO-1 AG11005 (to MRB), and the Research Service, Veterans Affairs Medical Center, Washington DC.
Footnotes
Conflict of Interest: There are no conflicts of interest
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of The Institutional Review Board of the Johns Hopkins Bayview Medical Center and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: was obtained from all individual participants included in the study.
REFERENCES
- 1.Fieffe S, Morange I, Petrossians P, Chanson P, Rohmer V, Cortet C, Borson-Chazot F, Brue T, Delemer B, French Acromegaly R: Diabetes in acromegaly, prevalence, risk factors, and evolution: data from the French Acromegaly Registry. European journal of endocrinology 164(6), 877–884 (2011). doi: 10.1530/EJE-10-1050 [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou O, Bex M, Kamenicky P, Mvoula AB, Chanson P, Maiter D: Prevalence and risk factors of impaired glucose tolerance and diabetes mellitus at diagnosis of acromegaly: a study in 148 patients. Pituitary 17(1), 81–89 (2014). doi: 10.1007/s11102-013-0471-7 [DOI] [PubMed] [Google Scholar]
- 3.Moller N, Schmitz O, Joorgensen JO, Astrup J, Bak JF, Christensen SE, Alberti KG, Weeke J: Basal- and insulin-stimulated substrate metabolism in patients with active acromegaly before and after adenomectomy. J Clin Endocrinol Metab 74(5), 1012–1019 (1992). doi: 10.1210/jcem.74.5.1569148 [DOI] [PubMed] [Google Scholar]
- 4.Moller N, Jorgensen JO: Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30(2), 152–177 (2009). doi: 10.1210/er.2008-0027 [DOI] [PubMed] [Google Scholar]
- 5.Hansen I, Tsalikian E, Beaufrere B, Gerich J, Haymond M, Rizza R: Insulin resistance in acromegaly: defects in both hepatic and extrahepatic insulin action. The American journal of physiology 250(3 Pt 1), E269–273 (1986). doi: 10.1152/ajpendo.1986.250.3.E269 [DOI] [PubMed] [Google Scholar]
- 6.Kasayama S, Otsuki M, Takagi M, Saito H, Sumitani S, Kouhara H, Koga M, Saitoh Y, Ohnishi T, Arita N: Impaired beta-cell function in the presence of reduced insulin sensitivity determines glucose tolerance status in acromegalic patients. Clinical endocrinology 52(5), 549–555 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita Y, Fujii H, Takeshita A, Taguchi M, Miyakawa M, Oyama K, Yamada S, Takeuchi Y: Impaired glucose metabolism in Japanese patients with acromegaly is restored after successful pituitary surgery if pancreatic {beta}-cell function is preserved. European journal of endocrinology 164(4), 467–473 (2011). doi: 10.1530/EJE-10-1096 [DOI] [PubMed] [Google Scholar]
- 8.Higham CE, Rowles S, Russell-Jones D, Umpleby AM, Trainer PJ: Pegvisomant improves insulin sensitivity and reduces overnight free fatty acid concentrations in patients with acromegaly. J Clin Endocrinol Metab 94(7), 2459–2463 (2009). doi: 10.1210/jc.2008-2086 [DOI] [PubMed] [Google Scholar]
- 9.List EO, Sackmann-Sala L, Berryman DE, Funk K, Kelder B, Gosney ES, Okada S, Ding J, Cruz-Topete D, Kopchick JJ: Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr Rev 32(3), 356–386 (2011). doi: 10.1210/er.2010-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD: Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Science translational medicine 3(70), 70ra13 (2011). doi: 10.1126/scitranslmed.3001845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizza RA, Mandarino LJ, Gerich JE: Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes 31(8 Pt 1), 663–669 (1982). [DOI] [PubMed] [Google Scholar]
- 12.Corbit KC, Camporez JP, Tran JL, Wilson CG, Lowe DA, Nordstrom SM, Ganeshan K, Perry RJ, Shulman GI, Jurczak MJ, Weiss EJ: Adipocyte JAK2 mediates growth hormone-induced hepatic insulin resistance. JCI Insight 2(3), e91001 (2017). doi: 10.1172/jci.insight.91001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominici FP, Argentino DP, Munoz MC, Miquet JG, Sotelo AI, Turyn D: Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society 15(5), 324–336 (2005). doi: 10.1016/j.ghir.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 14.Moller N, Butler PC, Antsiferov MA, Alberti KG: Effects of growth hormone on insulin sensitivity and forearm metabolism in normal man. Diabetologia 32(2), 105–110 (1989). [DOI] [PubMed] [Google Scholar]
- 15.Piatti PM, Monti LD, Caumo A, Conti M, Magni F, Galli-Kienle M, Fochesato E, Pizzini A, Baldi L, Valsecchi G, Pontiroli AE: Mediation of the hepatic effects of growth hormone by its lipolytic activity. J Clin Endocrinol Metab 84(5), 1658–1663 (1999). doi: 10.1210/jcem.84.5.5685 [DOI] [PubMed] [Google Scholar]
- 16.Orskov L, Schmitz O, Jorgensen JO, Arnfred J, Abildgaard N, Christiansen JS, Alberti KG, Orskov H: Influence of growth hormone on glucose-induced glucose uptake in normal men as assessed by the hyperglycemic clamp technique. J Clin Endocrinol Metab 68(2), 276–282 (1989). doi: 10.1210/jcem-68-2-276 [DOI] [PubMed] [Google Scholar]
- 17.Thankamony A, Tossavainen PH, Sleigh A, Acerini C, Elleri D, Dalton RN, Jackson NC, Umpleby AM, Williams RM, Dunger DB: Short-term administration of pegvisomant improves hepatic insulin sensitivity and reduces soleus muscle intramyocellular lipid content in young adults with type 1 diabetes. J Clin Endocrinol Metab 99(2), 639–647 (2014). doi: 10.1210/jc.2013-3264 [DOI] [PubMed] [Google Scholar]
- 18.Svensson J, Fowelin J, Landin K, Bengtsson BA, Johansson JO: Effects of seven years of GH-replacement therapy on insulin sensitivity in GH-deficient adults. J Clin Endocrinol Metab 87(5), 2121–2127 (2002). doi: 10.1210/jcem.87.5.8482 [DOI] [PubMed] [Google Scholar]
- 19.Rosenfalck AM, Maghsoudi S, Fisker S, Jorgensen JO, Christiansen JS, Hilsted J, Volund AA, Madsbad S: The effect of 30 months of low-dose replacement therapy with recombinant human growth hormone (rhGH) on insulin and C-peptide kinetics, insulin secretion, insulin sensitivity, glucose effectiveness, and body composition in GH-deficient adults. J Clin Endocrinol Metab 85(11), 4173–4181 (2000). doi: 10.1210/jcem.85.11.6930 [DOI] [PubMed] [Google Scholar]
- 20.Munzer T, Harman SM, Sorkin JD, Blackman MR: Growth hormone and sex steroid effects on serum glucose, insulin, and lipid concentrations in healthy older women and men. J Clin Endocrinol Metab 94(10), 3833–3841 (2009). doi: 10.1210/jc.2009-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O’Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM: Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 288(18), 2282–2292 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Munzer T, Harman SM, Hees P, Shapiro E, Christmas C, Bellantoni MF, Stevens TE, O’Connor KG, Pabst KM, St Clair C, Sorkin JD, Blackman MR: Effects of GH and/or sex steroid administration on abdominal subcutaneous and visceral fat in healthy aged women and men. J Clin Endocrinol Metab 86(8), 3604–3610 (2001). doi: 10.1210/jcem.86.8.7773 [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes A: Standards of Medical Care in Diabetes-2017 Abridged for Primary Care Providers. Clinical diabetes: a publication of the American Diabetes Association 35(1), 5–26 (2017). doi: 10.2337/cd16-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munzer T, Rosen CJ, Harman SM, Pabst KM, St Clair C, Sorkin JD, Blackman MR: Effects of GH and/or sex steroids on circulating IGF-I and IGFBPs in healthy, aged women and men. Am J Physiol Endocrinol Metab 290(5), E1006–1013 (2006). doi: 10.1152/ajpendo.00166.2005 [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA: Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 30(1), 89–94 (2007). doi: 10.2337/dc06-1519 [DOI] [PubMed] [Google Scholar]
- 26.Nagasaka S, Kusaka I, Yamashita K, Funase Y, Yamauchi K, Katakura M, Ishibashi S, Aizawa T: Index of glucose effectiveness derived from oral glucose tolerance test. Acta diabetologica 49 Suppl 1, S195–204 (2012). doi: 10.1007/s00592-012-0417-y [DOI] [PubMed] [Google Scholar]
- 27.Weiss R, Magge SN, Santoro N, Giannini C, Boston R, Holder T, Shaw M, Duran E, Hershkop KJ, Caprio S: Glucose effectiveness in obese children: relation to degree of obesity and dysglycemia. Diabetes Care 38(4), 689–695 (2015). doi: 10.2337/dc14-2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muniyappa R, Lee S, Chen H, Quon MJ: Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294(1), E15–26 (2008). doi: 10.1152/ajpendo.00645.2007 [DOI] [PubMed] [Google Scholar]
- 29.Kotronen A, Lewitt M, Hall K, Brismar K, Yki-Jarvinen H: Insulin-like growth factor binding protein 1 as a novel specific marker of hepatic insulin sensitivity. J Clin Endocrinol Metab 93(12), 4867–4872 (2008). doi: 10.1210/jc.2008-1245 [DOI] [PubMed] [Google Scholar]
- 30.Kuk JL, Saunders TJ, Davidson LE, Ross R: Age-related changes in total and regional fat distribution. Ageing Res Rev 8(4), 339–348 (2009). doi: 10.1016/j.arr.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 31.Geer EB, Shen W: Gender differences in insulin resistance, body composition, and energy balance. Gend Med 6 Suppl 1, 60–75 (2009). doi: 10.1016/j.genm.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U: Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest 72(3), 1150–1162 (1983). doi: 10.1172/JCI111040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, Cobelli C, Rizza RA: Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes 55(7), 2001–2014 (2006). doi: 10.2337/db05-1692 [DOI] [PubMed] [Google Scholar]
- 34.Clausen JO, Borch-Johnsen K, Ibsen H, Bergman RN, Hougaard P, Winther K, Pedersen O: Insulin sensitivity index, acute insulin response, and glucose effectiveness in a population-based sample of 380 young healthy Caucasians. Analysis of the impact of gender, body fat, physical fitness, and life-style factors. J Clin Invest 98(5), 1195–1209 (1996). doi: 10.1172/JCI118903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolaczynski JW, Caro JF: Insulin-like growth factor-1 therapy in diabetes: physiologic basis, clinical benefits, and risks. Ann Intern Med 120(1), 47–55 (1994). [DOI] [PubMed] [Google Scholar]
- 36.Pratipanawatr T, Pratipanawatr W, Rosen C, Berria R, Bajaj M, Cusi K, Mandarino L, Kashyap S, Belfort R, DeFronzo RA: Effect of IGF-I on FFA and glucose metabolism in control and type 2 diabetic subjects. Am J Physiol Endocrinol Metab 282(6), E1360–1368 (2002). doi: 10.1152/ajpendo.00335.2001 [DOI] [PubMed] [Google Scholar]
- 37.Paolisso G, Tagliamonte MR, Rizzo MR, Carella C, Gambardella A, Barbieri M, Varricchio M: Low plasma insulin-like growth factor-1 concentrations predict worsening of insulin-mediated glucose uptake in older people. J Am Geriatr Soc 47(11), 1312–1318 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Henriksen JE, Levin K, Thye-Ronn P, Alford F, Hother-Nielsen O, Holst JJ, BeckNielsen H: Glucose-mediated glucose disposal in insulin-resistant normoglycemic relatives of type 2 diabetic patients. Diabetes 49(7), 1209–1218 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Kehlenbrink S, Koppaka S, Martin M, Relwani R, Cui MH, Hwang JH, Li Y, Basu R, Hawkins M, Kishore P: Elevated NEFA levels impair glucose effectiveness by increasing net hepatic glycogenolysis. Diabetologia 55(11), 3021–3028 (2012). doi: 10.1007/s00125-012-2662-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giorgino F, Laviola L, Eriksson JW: Regional differences of insulin action in adipose tissue: insights from in vivo and in vitro studies. Acta Physiol Scand 183(1), 13–30 (2005). doi: 10.1111/j.1365-201X.2004.01385.x [DOI] [PubMed] [Google Scholar]
- 41.Garg A, Misra A: Hepatic steatosis, insulin resistance, and adipose tissue disorders. J Clin Endocrinol Metab 87(7), 3019–3022 (2002). doi: 10.1210/jcem.87.7.8736 [DOI] [PubMed] [Google Scholar]
- 42.McLaughlin T, Lamendola C, Liu A, Abbasi F: Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 96(11), E1756–1760 (2011). doi: 10.1210/jc.2011-0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jessen N, Djurhuus CB, Jorgensen JO, Jensen LS, Moller N, Lund S, Schmitz O: Evidence against a role for insulin-signaling proteins PI 3-kinase and Akt in insulin resistance in human skeletal muscle induced by short-term GH infusion. Am J Physiol Endocrinol Metab 288(1), E194–199 (2005). doi: 10.1152/ajpendo.00149.2004 [DOI] [PubMed] [Google Scholar]
- 44.Raben MS: Growth hormone. 1. Physiologic aspects. The New England journal of medicine 266, 31–35 (1962). doi: 10.1056/NEJM196201042660109 [DOI] [PubMed] [Google Scholar]
- 45.Nielsen S, Moller N, Christiansen JS, Jorgensen JO: Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes 50(10), 2301–2308 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Segerlantz M, Bramnert M, Manhem P, Laurila E, Groop LC: Inhibition of the rise in FFA by Acipimox partially prevents GH-induced insulin resistance in GH-deficient adults. J Clin Endocrinol Metab 86(12), 5813–5818 (2001). doi: 10.1210/jcem.86.12.8096 [DOI] [PubMed] [Google Scholar]
- 47.Pedersen MH, Svart MV, Lebeck J, Bidlingmaier M, Stodkilde-Jorgensen H, Pedersen SB, Moller N, Jessen N, Jorgensen JOL: Substrate Metabolism and Insulin Sensitivity During Fasting in Obese Human Subjects: Impact of GH Blockade. J Clin Endocrinol Metab 102(4), 1340–1349 (2017). doi: 10.1210/jc.2016-3835 [DOI] [PubMed] [Google Scholar]
- 48.Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, Ruan HB, Yang X, Caprio S, Kaech SM, Sul HS, Birnbaum MJ, Davis RJ, Cline GW, Petersen KF, Shulman GI: Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160(4), 745–758 (2015). doi: 10.1016/j.cell.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wheatcroft SB, Kearney MT: IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends in endocrinology and metabolism: TEM 20(4), 153–162 (2009). doi: 10.1016/j.tem.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 50.Goedecke JH, Micklesfield LK: The effect of exercise on obesity, body fat distribution and risk for type 2 diabetes. Med Sport Sci 60, 82–93 (2014). doi: 10.1159/000357338 [DOI] [PubMed] [Google Scholar]
- 51.Moore MC, Cherrington AD, Wasserman DH: Regulation of hepatic and peripheral glucose disposal. Best Pract Res Clin Endocrinol Metab 17(3), 343–364 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Laursen T, Gravholt CH, Heickendorff L, Drustrup J, Kappelgaard AM, Jorgensen JO, Christiansen JS: Long-term effects of continuous subcutaneous infusion versus daily subcutaneous injections of growth hormone (GH) on the insulin-like growth factor system, insulin sensitivity, body composition, and bone and lipoprotein metabolism in GH-deficient adults. J Clin Endocrinol Metab 86(3), 1222–1228 (2001). doi: 10.1210/jcem.86.3.7323 [DOI] [PubMed] [Google Scholar]
- 53.Vangipurapu J, Stancakova A, Kuulasmaa T, Paananen J, Kuusisto J, Group E-RS, Ferrannini E, Laakso M: A novel surrogate index for hepatic insulin resistance. Diabetologia 54(3), 540–543 (2011). doi: 10.1007/s00125-010-1966-7 [DOI] [PubMed] [Google Scholar]
- 54.Bastard JP, Faraj M, Karelis AD, Lavasseur J, Garrel D, Prud’homme D, Rabasa-Lhoret R: Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test: response to Abdul-Ghani et al. Diabetes Care 30(7), e83; author reply e84 (2007). doi: 10.2337/dc07-0622 [DOI] [PubMed] [Google Scholar]