Abstract
Activated regulatory T (Treg) cells express the surface receptor glycoprotein-A repetitions predominant (GARP), which binds and activates latent transforming growth factor-beta (TGF-β). How GARP modulates Treg function in inflammation and cancer remains unclear. Here we demonstrate that loss of GARP in Treg cells leads to spontaneous inflammation with highly activated CD4+ and CD8+ T cells and development of enteritis. Treg cells lacking GARP were unable to suppress pathogenic T cell responses in multiple models of inflammation including T cell transfer colitis. GARP−/− Treg cells were significantly reduced in the gut and exhibited a reduction in CD103 expression, a colon-specific migratory marker. In the colitis-associated colon cancer model, GARP on Treg cells dampened immune surveillance, and mice with GARP−/− Treg cells exhibited improved antitumor immunity. Thus, GARP empowers the functionality of Treg cells and their tissue-specific accumulation, highlighting the importance of cell surface TGF-β in Treg function and GARP as a potential therapeutic target for colorectal cancer therapy.
INTRODUCTION
In order to maintain immune homeostasis and uphold tolerance to self-antigens, CD4+ regulatory T (Treg) cells spearhead the adjustment of the immune responses generated by other effector T cell populations [1]. Treg cells are a potent suppressive cell subset that is classically characterized by expression of the master regulatory transcription factor forkhead box protein 3 (Foxp3) [2]. Loss-of-function mutations in Foxp3 gene lead to a severe systemic autoimmune disorder characterized by fatal tissue and organ damage [3]. In cancer, increased numbers of infiltrating Treg cells into tumor microenvironments suppress anti-tumor responses and have been clinically associated with poor prognosis [4]. Consequently, identifying possible mechanisms that would allow the modulation of Tregs function is urgently needed.
Transforming growth factor β (TGF-β) is a preeminent cytokine with critical immunomodulatory roles [5]. TGF-β is highly implicated in the induction, development, and maintenance of Treg cells. Mice with global TGF-β deletion develop a severe, generalized autoimmune disorder and die within 3–4 weeks after birth [6]. Mice lacking TGF-β-receptor II on T cells showed similar disease characteristics with aberrant T cell activation and increased Th1 and Th2 cell populations [7], which suggests that TGF-β plays an important role in immune surveillance and suppression of effector T cells. Lack of TGF-β receptor on CD4+ T cells leads to disappearance of thymic Treg cells during postnatal days 3–5 [8]. Moreover, peripheral Tregs are significantly reduced in numbers compared to thymic Tregs in 8–10 day old TGF-β-deficient mice [9]. In fact, TGF-β is required to generate peripheral Treg cells [10] by activating the canonical TGF-β/SMADs signaling; mice with Smad2 and Smad3 deletions on CD4+ T cells also developed severe autoimmune disease with reduced Foxp3 expression in the peripheral CD4+ T cells [11]. Further, Treg cells with deficient TGF-β signaling failed to home to the inflammatory sites in a T cell transfer colitis model [12]. Despite the increased knowledge of how TGF-β influences the suppressive function of Treg cells, underlying mechanisms that regulate the bioavailability and activation of TGF-β on Treg cells remain unclear.
Glycoprotein-A repetitions predominant (GARP) – a transmembrane protein encoded by the gene Leucine rich repeat containing protein 32 (Lrrc32) – is a cell surface docking receptor for latent TGF-β, a tetra-peptide complex formed by the TGF-β dimer and two copies of latency associated peptide (LAP) [13]. The release of the biologically active mature TGF-β from this complex can occur by several factors including heat, acidic conditions, and integrins [14]. Earlier reports have shown that GARP deficient CD4+ T cells mice did not impair the suppressive function of Treg cells in vitro [15] and that silencing of its expression by RNA interference does not significantly affect Foxp3 expression in expanded Treg [16]. In contrast, other studies have reported that soluble GARP can have a beneficial effect in sustaining Treg differentiation in xenogeneic Graft-versus-Host Disease [17]. Moreover, a monoclonal antibody directed against the GARP/latent TGF-β complex blocked Treg cell-mediated TGF-β production in the same mouse model [18]. However, the roles of GARP in Treg development, lineage stability, and function have not been completely elucidated.
To address the above question, we generated Treg-specific GARP knockout mice and found that GARP plays an important role in immune homeostasis and aged mice with GARP−/− Treg cells develop spontaneous intestinal inflammation. Tregs lacking GARP show reduced ability to suppress inflammatory responses and less accumulation in the intestinal tract. Using in vitro and in vivo settings, we found that GARP modulates the expression of CD103, an important molecule that is involved in homing of the T cells to the gut. As a result, deletion of GARP on Treg cells significantly improved the antitumor immunity against colorectal cancer. Overall, our data established that GARP plays an important role in empowering Treg cell function and promoting their accumulation in the colon.
MATERIALS AND METHODS
Animals
Lrrc32f/fFoxp3YFP-Cre+ mice were generated in house. Lrrc32f/f mice were obtained from Riken (Japan) [15]. Foxp3YFP-Cre, and Rag2−/− mice were from Jackson Laboratory. Inducible GARP OE and global GARP deletion (R26-creERT2-GARP) mice were previously described [19, 20]. GARP OE was induced with doxycycline (50 μg/ml; Sigma) in 1% sucrose drinking water. GARP deletion in R26-creERT2-GARP cells was induced in vitro using 250 nM 4-Hydroxytamoxifen (4-HT). All mice were on a pure C57BL6/J background. The animal procedures were conducted on an approved protocol by the Medical University of South Carolina Institutional Animal Care and Use Committee.
Isolation of Immune Cells from Tissues
Thymus, spleen, mLN, pLN, were dissociated into a single cell suspension and RBC lysis buffer (Sigma) was used to remove red blood cells. For colons, tissues were dissected incubated for 30 minutes at 37°C with collagenase D (1 mg/ml; Roche), dispase (0.05 U/ml; Worthington), and DNase I (100 mg/ml; Sigma-Aldrich). Lymphocytes were collected from the interface of a 40%/80% Percoll gradient (GE Healthcare).
Flow Cytometry
For surface staining, after Fixable Viability Dye (Affymetrix) and Fc-receptor blocking cells were stained for surface markers. Antibodies against mouse CCR9 (eBioCW-1.2), CD4 (GK1.5), CD8a (53–6.7), CD11b (M1/70), CD44 (IM7), CD62L (MEL-14), CTLA-4 (UC10–4B9), Foxp3 (FJK-16), GARP (YGIC86), GITR (DTA-1), GL7 (GL7), and KLRG1 (2F1) were from ThermoFisher. Those recognizing, CCR2 (48607), CD25 (PC61), Gr1 (RB6–8C5), ICOS (7E.17G9), IFNγ (XMG1.2) Ki67 (SolA15), and TNF (MP6-XT22) were from BD Bioscience. B220 (RA3–6B2) antibody was purchased from Biolegend. Antibodies against human CD4 (RPA-T4), CD25 (M-A251) and CD103 (Ber-ACT8) were purchased from BD Bioscience. Antibodies against human Foxp3 (236A/E7) and GARP (7B11) were purchased from eBioscience and Biolegend, respectively.
For intracellular staining for transcription factors, Foxp3/Transcription Factor Staining Buffer Set (Affymetrix) was used. To assess the expression of the intracellular cytokines, cells were stimulated for 2 hours with 50 ng/ml PMA (phorbol 12-myristate-13-acetate) and 1 μg/ml ionomycin (Sigma-Aldrich) in the presence of 5 μg/ml brefeldin A (BD Biosciences). The staining was then performed using BD Cytofix/Cytoperm Kit according to the manufacturer’s protocol (BD). Samples were analyzed immediately on BD FACSDiva, and data analysis was performed using FlowJo Software (Tree Star).
In Vitro Suppression Assay
Using MACS Treg isolation kit (Miltenyi Biotec), the naïve T (CD4+CD25−) and Treg cells (CD4+CD25+) were purified. A total of 1 × 105 CFSE (ThermoFisher)–labeled WT CD4+CD25− T cells were stimulated with 2 μg/ml anti-CD3 antibody (145–2C11; BD Biosciences) in the presence of 5 × 104 Rag2−/− splenocytes. WT and GARP−/− Treg cells were added in 1:1 to 1:8 Treg/Teff cell ratio. Activated CD4+CD25− T cells only, without Treg cells, were used as a positive control for T cell proliferation. Three days post stimulation, CFSE dilutions of T cells were analyzed and quantified by flow cytometry.
Generation of In Vitro Inducible Treg Cells and Anti-GARP Treatment
CD4+CD25− T cells were isolated from 8- to 10-week-old WT or f/f cre+ mice or from peripheral blood mononuclear cells of healthy individuals using MACS columns (Miltenyi Biotec). 2 × 106 mouse or 4×105 human cells were seeded per well in a 24- or 96-well plate, respectively, and stimulated with 5 μg/ml of plate-bound anti-CD3 (145–2C11; BD Biosciences for mouse, and HITEa; Biolegend for human) and 2 μg/ml anti-CD28 (37.51; BD Biosciences for mouse and CD28.2; Biolegend for human) in RPMI medium supplemented with 10% FBS heat-inactivated, 1000 U/ml of IL-2, anti-IL-4 (5 μg/ml), anti-IFN-γ (5 μg/ml), and TGF-β (10 ng/ml) for 72 hours. Human in vitro-induced Treg cells were treated with a pool of polyclonal mouse anti-human GARP antibody made in our own laboratory (at 10μg/ml each) or their isotype control for up to 10 days. As an additional control, cells were also treated with 10 μg/ml of neutralizing anti-TGFβ antibody. The media was replenished with fresh Ab-containing media every 2 days.
Lupus induction
Female WT and f/f cre+ mice were given a single i.p. injection of Pristane (500 μl/20 grams mouse; Sigma). Mice were sacrificed after 4 months and Peripheral blood, spleen, and lung were collected for further analysis.
T Cell Transfer Colitis
Naïve T cells (CD4+CD45RBhiCD25−) from WT mice were sorted using FACSAria II (BD). A total of 3.5 × 105 CD4+CD45RBhiCD25− T cells per mouse, alone or in combination with 1.5 × 105 WT or GARP−/− Treg cells, were i.p. injected into Rag2−/− mice. After T cell reconstitution, mice were weighed weekly and monitored for signs of disease. At the end of the experiment mice were sacrificed and their organs were examined histologically and by flow cytometry for evidence of colitis and inflammation. Standard H&E-stained sections were examined and scored by an experienced Pathologist (S.S.) in a blinded fashion. Grading was based on acute inflammation as absent (0), minimal (1), mild (2), moderate (3), or severe (4).
Colitis–associated Colon Cancer
A total of 7 to 12 mice per group were given 12.5 mg/kg body weight AOM (azoxymethane) with i.p. injection on day 1. Dextran sodium sulfate (DSS) was added to drinking water was given at 2.5% for 5 days in weeks 2 and 5, and then at 2% for 4 days on week 8. Mice were weighed and monitored weekly for overall health. Mice were euthanized at 12 weeks. Tumors were counted and measured. Tumor infiltrated lymphocytes (TIL) were isolated by collagenase D (Sigma) digestion followed by histopaque-1083 (Sigma) mediated density separation.
Generation of human GARP-Jurkat cells
Human GARP coding sequence was inserted into the BLR retroviral vector via BglII and EcoRI sites (Addgene). The retrovirus was generated in Platinum-A cells (Cell Biolabs) and then transduced into Jurkat cell. Human GARP-Jurkat cells were further selected by blasticidin treatment (Invivogen).
ELISA
For active and total TGF-β: ELISA plates (Corning) were coated O/N at 4°C with anti-mouse/human TGF-β1 capture antibody (BioLegend), followed by blocking with 1% BSA in PBS for 2 hours at RT. Samples were treated with HCl for 10 mins and neutralized with Tris/NaOH to obtain total TGF-β. Both active and total TGF-β were detected using anti-mouse Biotin-TGF-β antibody (BioLegend), Strepdavadin-HRP (BioLegend), and TMB Substrate reagents (BD).
For bacteria specific IgA: A fecal supernatant from μMT mice was used as shown in [20] and the protein concentration was measured using Bradford assay (Bio-Rad). Each well of the ELISA plate was coated with 0.5 μg/ml μMT fecal supernatant. Serum was serially diluted and bacteria specific IgA was detected with anti-mouse IgA-HRP antibody (Southern Biotech).
RNA isolation and quantitative RT-PCR
Total RNA was isolated using TRIzol reagent according to the manufacturer’s instructions (Invitrogen). cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen) and analyzed by quantitative PCR on StepOne Plus machine (Applied BioSystems) using SYBR Green reagent (Bio Rad) and the following primers: human CD103 forward, 5′- TGGGACTGGTGACAATTGTC −3′; human CD103 reverse, 5′- GAGTACAGGTGTGCAGCTCT −3′; human β-actin forward, 5’- CCCTGGACTTCGAGCAA GAG −3′; human β-actin reverse, 5’- CCAGGAAGGAAGGCTGGAAG −3′.
Detection of ANA
NOVA Lite HEp-2-coated slides (INOVA Diagnostics) were incubated with diluted sera (in PBS), followed by FITC-conjugated goat anti-mouse IgG or anti-mouse IgM antibody (ThermoFisher), and examined by fluorescence microscopy. The fluorescence staining intensity was graded as 0 (negative), 1 (slight staining), 2 (moderate staining), and 3 (bright staining).
Histology
Mouse organs were fixed in 4% formalin, embedded in paraffin, sectioned, and stained following standard Hematoxylin and Eosin staining protocol.
Statistics
Data analysis was performed using GraphPad Prism software (GraphPad Software, Inc., California). Results are expressed as mean ± SEM. p value are determined with Student’s t test. Two-way ANOVA with Sidak’s multiple comparison test was used for multiple group comparisons in tumor curves and body weight graphs. p < 0.05 was considered statistically significant; *p < 0.05, **p < 0.01, ***p < 0.005.
RESULTS
Treg-specific GARP Deletion Leads to Chronic Intestinal Inflammation
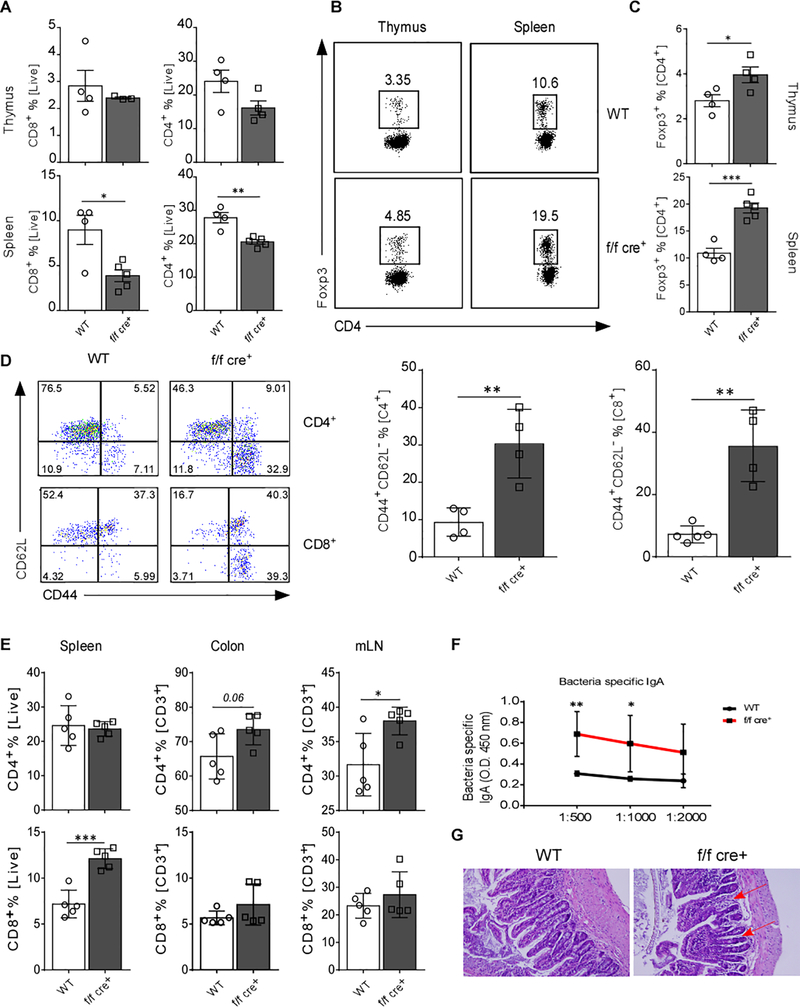
In order to directly determine the role of GARP in Treg-cell-mediated homeostasis, we generated mice with specific deletion of GARP on Treg cells by crossing mice with a conditional allele of Lrrc32 with mice expressing Foxp3YFP-Cre (Lrrc32 flox/flox [f/f] × Foxp3YFP-Cre mice, herein called f/f cre+ mice). TCR activation of CD4+CD25+ Treg cells increased GARP levels (Figure S1A and S1B), in accordance with what has previously reported, that GARP selectively identifies activated Treg cells [16]. Young f/f cre+ mice (4–6 weeks old) developed normally with no apparent changes in the systemic serum levels of active and total TGF-β or any key cytokines including IL-2, IL-6, TNF, and IFNγ (Figure S2A). The percentage of splenic CD4+ and CD8+ T cells was lower in f/f cre+ compared to WT mice. No changes in frequency were observed in the thymic single CD4+ or CD8+ T cell populations (Figure 1A). Notably, the frequencies of CD4+Foxp3+ T cells were elevated in both thymus and spleen of f/f cre+ mice (Figure 1B and 1C). CD4+ and CD8+ T cells displayed a more activated effector-like phenotype with an increase in the CD44+CD62L− population in f/f cre+ mice (Figure 1D). Histologically, slight increases in lymphocyte infiltration were detected in the liver, and the lung (Figure S2B). In addition, the villi in the small intestine were slightly shorter (Figure S2B). Moreover, IgG anti-nuclear antibodies (ANA) in the serum were also increased in the KO mice (Figure S2C).
Figure 1. Deletion of GARP in Foxp3+ T Cells Leads to Systemic Inflammation.
(A) Percentage of CD4+ and CD8+ T cells in thymus and spleen of 6 – 8 weeks old WT and f/f cre+ mice. (B) Representative flow cytometry plots of CD4+Foxp3+ T cells in thymus and spleen. (C) Quantification of percentages of CD4+Foxp3+ T cells in B. (D) Representative flow cytometric plots and quantification of splenic CD4+ and CD8+ T cells based on CD44 and CD62L expression. (E) Percentage of CD4+ and CD8+ T cells in spleen, colon and mLN from 1-year-old WT and f/f cre+ mice (n=5) were determined by flow cytometry and quantified. (F) Gut bacteria-specific IgA was measured in the sera of mice by ELISA (n=5–6). (G) The small intestine of WT and f/f cre+ mice were stained with H&E. Arrows indicate stunted and fused villi. Statistical analyses were performed by unpaired Student’s test, *** p<0.001, ** p<0.01, * p<0.05. Error bars represent mean ± SEM.
To further investigate if these immune alterations predispose the mice to the development of a chronic autoimmune disease, the mice were aged to one year. We observed an increased frequency of CD4+ T cells in both colon (not significant) and mLN, and as well as an increased CD8+ T cell percentage in the spleen (Figure 1E). We also found that aged f/f cre+ mice had significantly higher levels of bacteria-specific IgA in the serum suggesting increased gut permeability due to loss of gut tolerance (Figure 1F). Histologically, we noted aberrant villi structure - thick, shortened, and fused - in small intestine of f/f cre+ mice, which reflects partial villous atrophy that is known to manifest in patients with celiac or other inflammatory bowel diseases [21] (Figure 1G). Thus, lack of GARP on Treg cells resulted in disruption of immune homeostasis and made them incapable of maintaining intestinal homeostasis with aging, suggesting that GARP might play a key role in maintaining Treg-mediated immune regulation.
GARP is Important for Optimal Treg Differentiation and Controlling Lupus-Mediated Inflammation
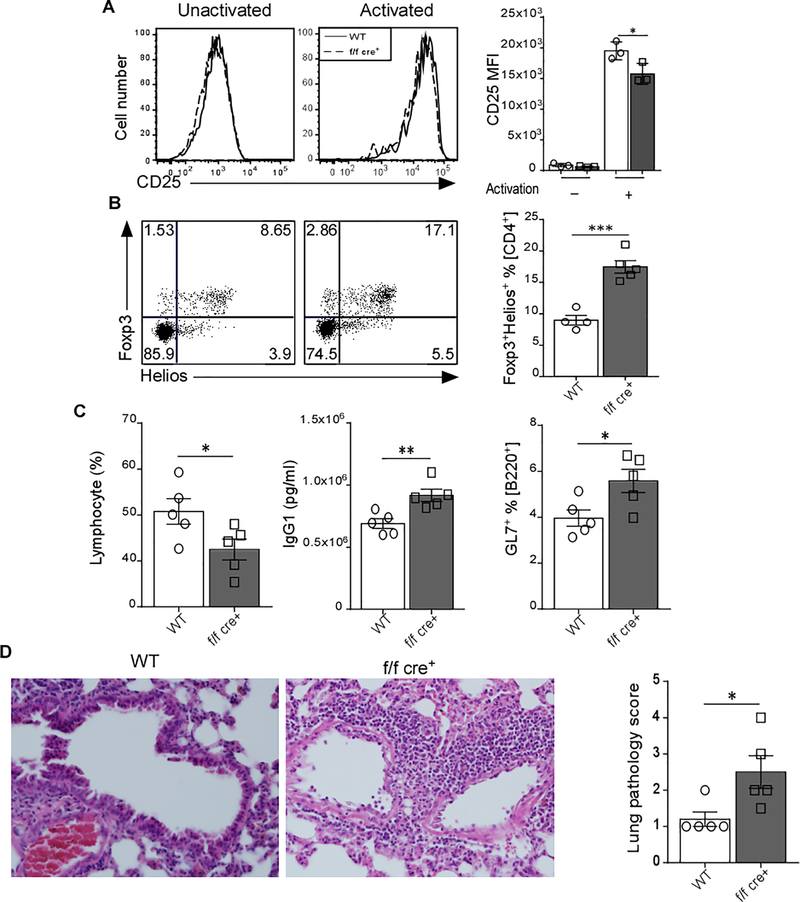
Our data suggest that GARP deletion in Treg cells might affect their suppressive function. Using an in vitro suppression assay, we confirmed Edwards et al. data [15] that the lack of GARP on Treg cells did not alter their suppressive capacity on CD4+ T cells (Figure S3A). However, in vitro Treg suppression assays do not necessarily recapitulate in vivo processes. When we tested the expression of different Treg markers gated on CD4+Foxp3+ cells, we found that CD25 (Figure 2 A), ICOS, and KLRG1 (Figure S3B-C) expression were significantly lower in GARP−/− Treg cells compared to WT cells after 72h TCR-stimulation. In fact, TGF-β signaling has been shown to induce the expression CD25 [22]. KLRG1 defines terminally differentiated Treg cells and is linked to IL-2rα (CD25) [23]. On the other hand, the expression of GITR [24] and CTLA-4 was unchanged (Figure S3D-E). In addition, mice lacking GARP in Treg cells showed increased frequency of Foxp3+Helios+ T cells (Figure 2B); typically defined as thymic Treg cells [25], indicating a possible dysregulation in the generation of peripheral Treg cells which are TGF-β-dependent [10]. Indeed, the frequency of differentiated iTreg cells from primary CD4+CD25− T cells was significantly less in the absence of GARP (Figure S3F). These data suggest that GARP may affect the function of Treg cells.
Figure 2. Lack of GARP Affects the Treg differentiation and Decreases the Ability to Control the Inflammation in Pristane-Induced Lupus Model.
(A) Splenic CD4+CD25+ T cells from WT and f/f cre+ were TCR-activated for 72 hr. The expression level of CD25 was determined by flow cytometry. Representative MFI plots and data quantifications are shown (n=3). (B) Flow cytometry analysis of Foxp3 versus Helios expression in splenic CD4+ T cells. Representative dot plots and bar graphs are presented. (C) Female WT (n=5) and f/f cre+ mice (n=5) were given a single i.p. injection of Pristane. Mice were sacrificed 4 months later. Lymphocyte count was determined by complete blood count (CBC), IgG1 serum level was detected by ELISA, and GL7+ % [B220] was measured by flow cytometry. (D) Lungs of WT and f/f cre+ mice were fixed in 4% paraformaldehyde, sectioned, and stained with H&E. Each point represents an individual mouse. Data represent 3–4 independent experiments. Statistical analyses were performed by unpaired Student’s test, *** p<0.001, ** p<0.01, * p<0.05. Error bars represent mean ± SEM.
To this end and based on data showing increased systemic ANA level in aged f/f cre+ mice (Figure S2C), we hypothesized that mice with GARP−/− Treg cells might be less suppressive under chronic inflammatory conditions such as systemic lupus erythematosus. Therefore, we employed a pristane-induced lupus model, which is a result of induction and proliferation of auto‐reactive lymphocytes that produce pro‐inflammatory cytokines, pathogenic autoantibodies and immuno‐complexes [20, 26]. We observed that deletion of GARP in Treg cells leads to decreased ability to control the disease. Indeed, several hallmarks of autoimmunity, such as lymphocytopenia, systemic IgG, and elevated GL7+ germinal center B cells levels were more prominent in f/f cre+ than WT mice (Figure 2C). In addition, immune cell infiltration into the lungs was more severe in f/f cre+ mice (Figure 2D). These data imply that GARP is crucial for Treg cells to exert their regulatory function during inflammation.
GARP Deletion on Treg Cells Abrogates Their Ability to Suppress T cell Responses during Inflammation
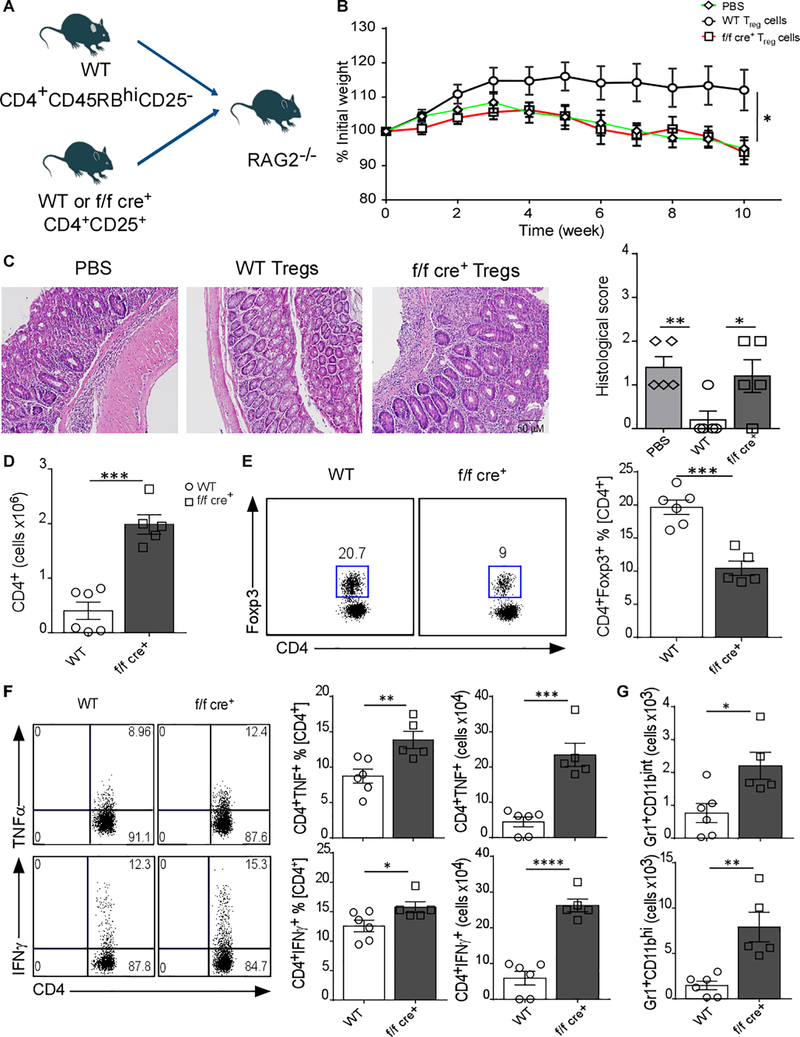
Given that the intestinal compartment was clearly affected in mice lacking GARP on Treg cells, we hypothesized that Treg-GARP might play an important role in managing immunopathology during ongoing intestinal inflammation. To address this possibility, we tested their ability to suppress inflammatory responses in the intestine using a T cell transfer model of colitis. CD4+CD45RBhiCD25− T cells from WT mice were injected i.p. together with either WT or GARP−/− Treg cells into RAG2−/− mice. Functional Treg cells have been shown to reverse the disease in this mouse model [27]. As anticipated, the control group of RAG2−/− mice injected only with CD4+CD45RBhiCD25− T cells without Tregs (Figure 3A) showed severe body weight loss (Figure 3B). Interestingly, GARP−/− Treg cell co-transfer completely failed to control the disease in the recipient mice which showed significant weight loss (Figure 3B). The histological score was also indistinguishable from control mice that did not receive Treg cells (Figure 3C). This was associated with increased CD4+ T cells in the colon (Figure 3D). Notably, the frequency of GARP−/− Treg cells in the colon was significantly lower compared to WT Treg cells (Figure 3E). As expected, this was associated with increased TNFα+, IFNγ+ CD4+ T cells (Figure 3F), tissue inflammatory monocytes/macrophages (CD11b+Gr1int) and neutrophils (CD11b+Gr1hi) (Figure 3G) [28]. These data clearly demonstrate that GARP is important for the suppressive function of Treg cells and may additionally affect their colonic accumulation.
Figure 3. GARP Expression Is Essential for Treg Cell Suppressive Function during Intestinal Inflammation.
(a) Experimental outline; Rag2−/− mice received i.p, 3.5 × 105 CD4+CD45RBhiCD25− WT T cells alone or in combination with 1.5 × 105 WT or GARP−/− Treg cells (CD4+CD25+ T cells). (B) Body weight trend of the RAG2−/− recipient mice upon T cell transfer of WT or GARP−/− Treg cells (five to six mice in each group is displayed; mean ± SEM; *p < 0.05, two-way ANOVA with multiple comparison). (C) Representative H&E staining of colon tissue section and their histology score based on acute inflammation as absent (0), minimal (1), mild (2), moderate (3), or severe (4). (D) Total cell number of colonic CD4+ T cells (E) Representative dot plots and cumulative frequency data of WT and GARP−/− colonic CD4+Foxp3+ T cells. (F) Representative dot plots and bar graphs showing percentage and number of TNFα+ and IFNγ+ CD4+ T cells in the colon. (G) Number of monocytes/macrophages (CD11b+Gr1int) and neutrophils (CD11b+Gr1hi). Each point represents an individual mouse. The results are representative of two independent experiments with five to six mice in each group. Statistical analysis performed by unpaired Student’s test, *** p<0.001, ** p<0.01, * p<0.05. Error bars represent mean ± SEM.
GARP−/− Treg Cells Fail to Accumulate in the Colon
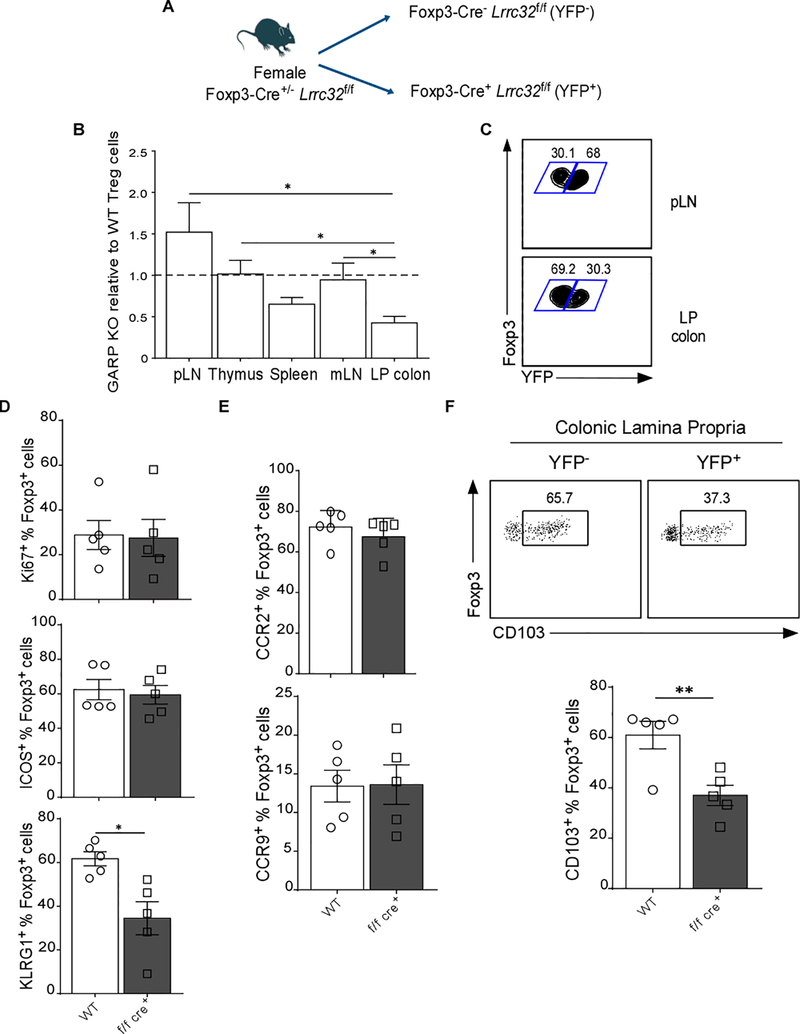
Our data so far led us to hypothesize that GARP expression not only shapes the function of Treg cells, but also impacts their ability to accumulate in the colon. To address this possibility, we examined female GARP-heterozygous mice (f/f cre+/−). Given that Foxp3 is encoded on the X chromosome, one of which undergoes inactivation in females, only 50% of their Treg cells express GARP (Figure 4A). The percentages of WT (YFP−) and GARP−/− (YFP+) of CD4+Foxp3+ Treg cells in thymus, spleen, pLN, mLN, and colon were determined. Consistent with our earlier finding, the ratio of YFP+ to YFP− Treg cells was lower in the colon whereas it was higher in pLN (Figure 4B and 4C) indicating that GARP−/− Treg cells are lacking the ability to migrate to the colon. GARP−/− and WT Treg cells had similar proliferation rate based on Ki67+ staining, as well as survival as indicated by the expression of ICOS (Figure 4D) [29]. KLRG1 expression was lower on colonic GARP−/− Treg cells (Figure 4D). To assess the expression of markers implicated in Treg cell trafficking to the colon, we examined the expression level of chemokine receptors (CCR) such as CCR2 [30], and CCR9 [31] which have been associated with colonic homing. In addition, we determined CD103 expression; CD103 is a TGF-β regulated molecule necessary for cell migration to the colon [12]. No changes in CCR2 or CCR9 expression in GARP−/− Treg cells were observed (Figure 4E); however, CD103 was significantly reduced in the KO mice (Figure 4F). These data show that the ability of GARP−/− Treg cells to accumulate in the colon is altered, possibly due to reduced CD103-mediated homing.
Figure 4. GARP−/− Treg Cells are not Able to Persist within the Colon.
(A) Experimental outline; the Treg cells in Lrrc32f/fFoxp3YFP-Cre+/− female mice constitute of 50% WT Foxp3 and 50% Foxp3-YFP fusion protein due to random X-inactivation. (B) Bar graph shows frequency of GARP−/− relative to WT Treg cells. Value 1 indicates the 1:1 frequency ratio of GARP−/− and WT Treg cells. Student’s unpaired t test are used (paired two-tailed Student’s t test). (C) Representative flow cytometry plots show Foxp3 versus YFP staining gated on CD4+Foxp3+ Treg cells. (D) Bar graphs show frequency of WT or GARP−/− Treg cells expressing, Ki67, ICOS, and KLRG1. (E) Frequencies of WT or GARP−/− Treg cells expressing, Ki67, ICOS, and KLRG1. (F) Representative flow cytometry plots show the percentage of CD103+ cells in WT and GARP−/− Treg cells in the colon, and quantification of Foxp3+CD103+ from Lrrc32f/fFoxp3YFP-Cre+/− mice. Statistical analysis performed by unpaired Student’s test indicated; *** p<0.001, ** p<0.01, * p<0.05
GARP modulates CD103 expression
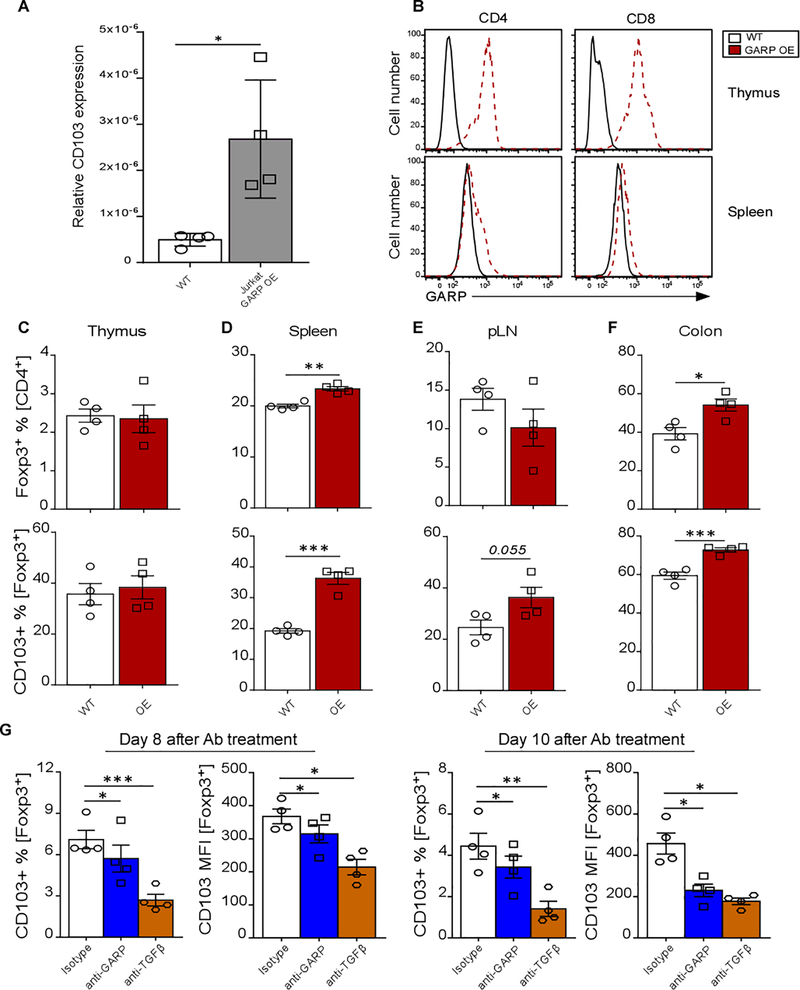
To further assess whether GARP expression could modulate CD103 expression, mRNA level of CD103 was determined in Jurkat human T cells with GARP overexpression (OE). We found that CD103 mRNA levels were higher in GARP OE compared to WT Jurkat cells (Figure 5A). Next, we tested if in vivo GARP OE can also modify CD103 expression. To this end, we employed a doxycycline-inducible GARP OE mouse model where a Tet-on element is knocked into the GARP promoter [19, 20]. After 6 weeks of doxycycline treatment, the mice were analyzed and GARP expression was confirmed to be up-regulated in the thymic and splenic CD4+ and CD8+ T cells (Figure 5B). No differences were observed in the proportion of thymic Treg cells or their CD103 expression (Figure 5C). Notably, the frequencies of CD4+Foxp3+ and CD103+Foxp3+ T cells were more abundant in the periphery of the GARP OE mice, especially in the spleen and the colon (Figure 5D-F). To confirm the modulatory role of GARP on CD103, we used another mouse model for inducible global GARP deletion (ROSA26-creERT2-GARP). Splenocytes were isolated from ERT2-cre+/− and GARP deletion was induced by 4-HT treatment in vitro (Figure S4A). The percentage of CD103+ cells was reduced in ERT2-cre+/− Tregs after 6 days of 4-HT treatment, following GARP deletion. (Figure S4B). As expected, no modulation of either GARP or CD103 was observed in control cells after 4-HT treatment.
Figure 5. GARP Modulates CD103 Expression.
(A) Expression of CD103 was determined in WT and GARP OE Jurkat cells by qPCR. (B) The expression levels of GARP in thymic and splenic CD4+ and CD8+ T cells in WT and Tet-inducible GARP-overexpressing (OE) mice were determined by flow cytometry. Representative MFI plots are shown. (C-F) Percentage of Treg cells and CD103+ Treg cells in the thymus, spleen, pLN, and colon was determined by flow cytometry and quantified. (G) Graph bars show the change in the percentage of CD103+ and CD103 MFI in human Tregs treated with GARP antibodies. Human Treg cells were generated in vitro from CD4+CD25− T cells of 4 different healthy individuals in the presence of anti-CD28/CD3, IL-2, TGF-β, anti-IL-4, and anti-IFNγ. After 72h, the media was removed and replenished with fresh T-cell media containing IL-2 and either anti-GARP antibody mix or isotype antibody or anti-TGF-β neutralizing antibody. The cells were harvested after 8 and 10 days of antibody treatment, stained and analyzed by flow cytometry. Statistical analysis was performed by unpaired Student’s test (A-F) or paired t-test (G), *** p<0.001, ** p<0.01, * p<0.05. Error bars represent mean ± SEM.
To validate that in human setting, in vitro-induced human Treg cells were generated from peripheral blood CD4+CD25− cells from four healthy subjects and treated with a pool of polyclonal mouse anti-human GARP antibody made in our own laboratory (at 10μg/ml each) or their isotype control for up to 10 days. As an additional control, cells were also treated with 10μg/ml of neutralizing anti-TGFβ antibody. The media was replenished with fresh Ab-containing media every 2 days. Both the percentage of CD103+ Treg cells and their expression level of CD103 were significantly decreased after 8 and 10 days of either anti-GARP or anti-TGFβ antibody treatment compared to treatment with the isotype antibody for the same period of time (Figure 5G). These data further support the modulatory function of GARP on CD103 expression.
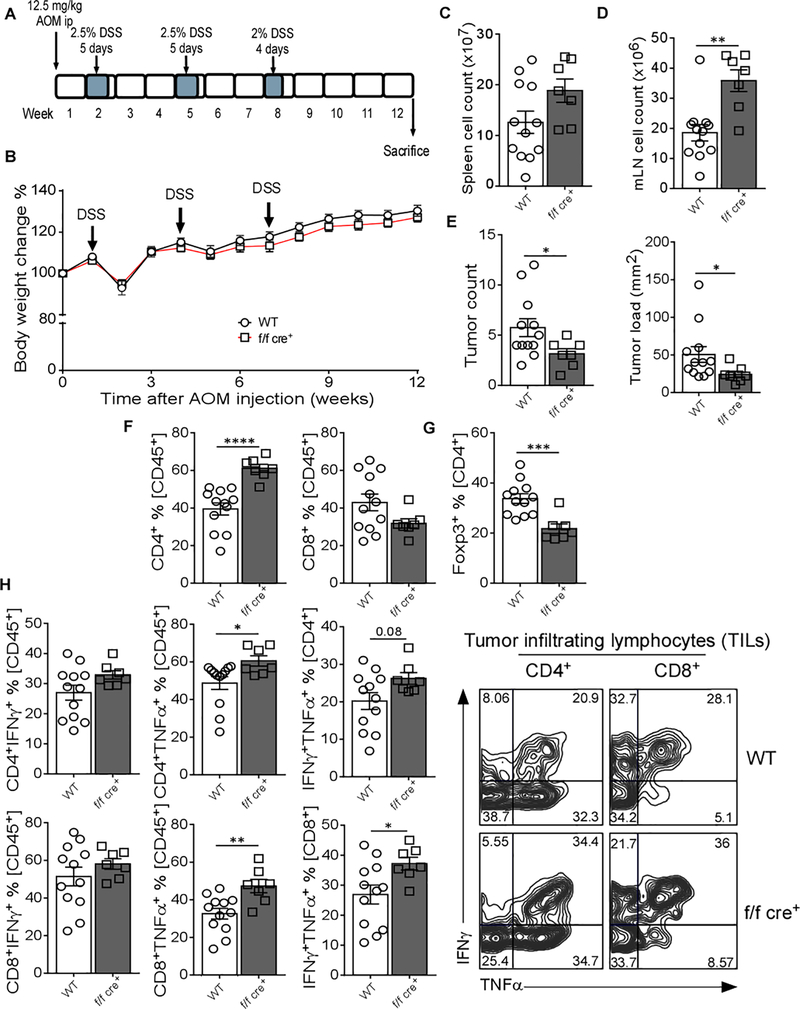
Deletion of GARP in Treg Cells Leads to Reduced Colitis-associated Colon Cancer Development
We next investigate the impact of GARP on the colonic accumulation of Treg cells in the context of colorectal tumor. WT and f/f cre+ littermates were subjected to colitis-associated colon cancer (CAC) based on a combination of the mutagenic agent azoxymethane (AOM) and dextran sodium sulfate (DSS) [32]. CAC was induced by injecting a single dose of AOM, followed by three cycles of DSS administration (Figure 6A). The mice were sacrificed at week 12 post initial AOM injection. No weight difference was observed between WT and f/f cre+ mice (Figure 6B). While, no changes were observed in splenocyte cell counts (Figure 6C), the cell numbers were significantly increased in the mLN of f/f cre+ mice (Figure 6D). Interestingly, mice with GARP deletion in Treg cells developed fewer tumors and the tumor load in these mice was significantly reduced compared with the WT mice (Figure 6E). Notably, this was associated with a significant increase in the percentage of CD4+ T cells, but not CD8+ T cells, within the tumor infiltrating lymphocytes (TIL) population (Figure 6F). Indeed, TIL from f/f cre+ mice had a significant decrease in the percentage of Treg cells compared to control littermates (Figure 6G). Functional analysis of TIL also showed that both CD4+ and CD8+ T cells had higher TNFα expression (Figure 6H). Altogether, these data are in accordance with the aforementioned results that GARP improves the accumulation of Treg cells in the gut.
Figure 6. Mice with GARP Deletion in Treg Cells Have Better Protective Tumor Immunity in a Mouse Colon Cancer Model.
(A) Experimental outline; 8–12 weeks old WT and f/f cre+ mice were i.p. injected with 12.5 mg/kg azoxymethane (AOM), followed by three cycles of dextran sodium sulfate (DSS) administration. Mice were sacrificed at week 12 after AOM injection. The results are representative of two independent experiments. (B) Body weight change during the course of the experiment. (C) Total cells count in spleen. (D) Total cells count in mLN. (E) Total tumor count in each individual mouse and tumor load defined by the total tumor size in each mouse. (F) CD4+ and CD8+ T cell percentage in TILs gated on CD45+ cells. (G) CD4+Foxp3+ T cells percentage in TILs gated on CD45+ cells. (H) Bar graphs and representative flow cytometry plots show IFNγ and TNFα expression in CD4+ and CD8+ T cells in TILs gated on CD45+ cells. Statistical analysis was performed by unpaired Student’s test, *** p<0.001, ** p<0.01, * p<0.05. Error bars represent mean ± SEM.
To further confirm the association of GARP/CD103 and colonic Treg cells, MC38 colon cancer cells were implanted subcutaneously into the flanks of WT and f/f cre+ mice. The tumor growth was similar (Figure S5A) with no changes in tumor-infiltrating CD4+, CD8+, or CD4+Foxp3+ T cells (Figure S5B and S5C). The percentage of Foxp3+CD103+ Treg cells was also comparable (Figure S5D), consistent with the previous data that GARP is more important for the accumulation of Treg cells specifically in the gut.
DISCUSSION
The role of TGF-β in the development of Treg cells and suppression of various immune cell subsets including T cells, dendritic cells, B cells, NK cells, and myeloid cells is well-established [33–35]. The presence of GARP specifically on Treg cells underlines its importance for these cells. However, it is not clear how the absence of GARP on Treg cells modulates their function and ability to operate under inflammatory conditions. Our study is the first comprehensive research done on the role of GARP in the generation, maintenance, suppressive function, and migration of Treg cells in various inflammatory and cancer settings. We found that GARP is required to maintain systemic immune homeostasis, particularly in the intestine. GARP is also important for optimal generation of Treg cells from naïve CD4+ T cells. We observed that even though GARP deletion affected the expression level of Foxp3 after activation, GARP−/− Treg cells were able to control the proliferation of CD4+ T cells in vitro. However, they were unable to control chronic inflammatory diseases such as inducible lupus. GARP−/− Treg cells were also unable to suppress T cell-mediated colitis or anti-tumor responses in the colon. This was not only due to the defect in the suppressive function, but also because of their defective accumulation in the colon, which might be CD103-mediated.
GARP was previously shown to be expressed on activated Treg cells [16]. The main function of GARP is to bind latent TGF-β indicating that GARP might play a role in tuning the function of Treg cells. In our work, we used a transgenic mouse model in which GARP was specifically deleted on Foxp3 Treg cells which allowed us to elucidate important and previously unknown functions of this molecule on Treg cells. In particular, Foxp3 expression in GARP−/− Treg cells was not affected under homeostatic conditions. When the cells were activated by TCR-stimulation, we noticed that the expression levels of key markers such as CD25, ICOS, and KLRG1 were reduced which might be associated with the compromised function and stability of Treg cells. Accordingly, in chronic inflammatory disease models such as the pristine-induced lupus model, GARP−/− Treg cells were not able to control the disease. In fact, previous studies have shown that Treg cells from active lupus patients exhibited functional and stability abnormalities [36, 37]. In parallel, the TGF-β-mediated signaling in the cells defined by SMAD-activation was consequently weaker. This signaling pathway is critically involved in the induction of Foxp3 since deletion of both SMAD2 and SMAD3 in CD4+ T cells leads to their failure to up-regulate Foxp3 in response to TGF-β [11]. Another evidence of the abnormal TGF-β signaling as a consequence of GARP deletion is that in vitro generation of Treg cells which is largely TGF-β-dependent differentiation mechanism, was significantly reduced. In other words, by regulating of TGF-β bioavailability, GARP might modulate the function of Treg cells. Although, TGF-β is important for the generation, maintenance, and function of Treg cells, it has been reported that cell specific-deletion of TGF-β did not result in any immune abnormalities and the mice developed normally [38]. Thus, more work is needed to clarify these controversial findings.
Another key observation from our study is that the expression of GARP on Treg cells is a crucial factor to maintain the intestinal homeostasis. We observed that young mice with GARP−/− Treg cells had shorter villi and increased lymphocytes. Upon aging, the mice developed an intestinal inflammation mimicking celiac disease in humans with thick, shortened, and fused villi. We also detected significantly increased bacterial-specific IgA in the serum of aged mice indicating increased intestinal barrier permeability compared to WT mice. This chronic disease development is induced by TGF-β signaling as also was shown in a recent study in mice lacking Tgfbr1 on Treg cells [12]. In addition, aging itself has been reported to decrease the function of Treg cells [39, 40]. Therefore, further studies are needed to determine how the age can affect the expression profile for TGF-β-associated mediators including GARP on Treg cells.
A prior study [41] demonstrated that deletion of GARP on CD4+ T cells leads to a reduction Treg cells in the mLN and Peyer patches during an oral tolerance disease model. It suggests that the generation of Treg cells in these tissues is affected due to GARP deletion. Our study brings to light unknown effects of GARP expression on Treg cells. In fact, not only it is necessary for the generation of new Treg cells but it is also important for the optimal expression of CD103 which is an important factor in cell recruitment to the intestine. Indeed, GARP−/− Treg cells were not able to suppress T cell transfer colitis because of the failure of GARP−/− Treg cells to accumulate in the colon. Moreover, mice with specific GARP deletion in Treg cells exhibit better anti-tumor responses in the AOM/DSS colon tumor model. Our finding is well in line with previous murine and human studies showing that depletion of Treg cells or a low density of Foxp3+ cells in the colon is associated with better colorectal antitumor immunity [42, 43]. Furthermore, the role of TGF-β in the induction of CD103 has been previously reported in a variety of immune cells [44, 45]. CD103 is not just functioning as a homing marker. Recent findings show that the Foxp3 expression level in Treg cells from CD103−/− mice was significantly reduced and these Treg cells were not able to suppress murine contact hypersensitivity reactions during the elicitation phase [46]. Moreover, in vitro-generated alloantigen-primed CD4+CD103+ cells co-expressed CD25. These cells suppressed T cell activation, and contained more Foxp3 mRNA than the CD103− CD25+ cells isolated from the same cultures [47]. The link between GARP and CD103 is therefore remarkable. Our findings have a translational relevance to imply GARP as a potential target for combination therapy against cancer to improve the current immunotherapy outcomes, especially because Treg cells constitute a major challenge in antitumor immunity. To this end, our lab has previously reported that targeting GARP potentiates protective immunity against both melanoma and colon cancer [48]. Additionally, it also limited metastasis in an orthotopic model of human breast cancer [49]. Therefore, we believe that our study uncovers several novel aspects of GARP as a functionality-tuning and trafficking enhancer by empowering TGF-β signaling. It is an interesting feature that can be utilized as a treatment strategy in immunotherapy combinations for autoimmune diseases and cancer especially after the recent findings which give clear structural and mechanistic insights on how GARP binds to TGF-β on human Treg cells [50].
Supplementary Material
SIGNIFICANCE:
Findings uncover functions of membrane-bound TGF-β and GARP that tune the activity of Treg cells, highlighting a potential treatment strategy in autoimmune diseases and cancer.
ACKNOWLEDGMENTS
We thank the FACS core of Hollings Cancer Center, MUSC for their technical assistance.
Financial support: The research was funded by multiple grants from the NIH: R01AI077283 (Z.L.); R01CA213290 (Z.L.); R01CA188419 (Z.L.) and P01CA186866 (Z.L.)
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare no competing interests.
REFERENCES
- 1.Grant CR, Liberal R, Mieli-Vergani G, Vergani D, Longhi MS. Regulatory T-cells in autoimmune diseases: challenges, controversies and--yet--unanswered questions. Autoimmun Rev. 2015;14(2):105–16. [DOI] [PubMed] [Google Scholar]
- 2.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. [DOI] [PubMed] [Google Scholar]
- 3.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–1. [DOI] [PubMed] [Google Scholar]
- 4.Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22(6):679–84. [DOI] [PubMed] [Google Scholar]
- 5.Chen W,Dijke P Ten. Immunoregulation by members of the TGFbeta superfamily. Nat Rev Immunol. 2016;16(12):723–740. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993;90(2):770–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorelik L,Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12(2):171–81. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9(6):632–40. [DOI] [PubMed] [Google Scholar]
- 9.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201(7):1061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takimoto T, Wakabayashi Y, Sekiya T, Inoue N, Morita R, Ichiyama K, et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J Immunol. 2010;185(2):842–55. [DOI] [PubMed] [Google Scholar]
- 12.Konkel JE, Zhang D, Zanvit P, Chia C, Zangarle-Murray T, Jin W, et al. Transforming Growth Factor-beta Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity. 2017;46(4):660–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106(32):13445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metelli A, Salem M, Wallace CH, Wu BX, Li A, Li X, et al. Immunoregulatory functions and the therapeutic implications of GARP-TGF-beta in inflammation and cancer. J Hematol Oncol. 2018;11(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards JP, Fujii H, Zhou AX, Creemers J, Unutmaz D, Shevach EM. Regulation of the expression of GARP/latent TGF-beta1 complexes on mouse T cells and their role in regulatory T cell and Th17 differentiation. J Immunol. 2013;190(11):5506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106(32):13439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn SA, Stahl HF, Becker C, Correll A, Schneider FJ, Tuettenberg A, et al. Soluble GARP has potent antiinflammatory and immunomodulatory impact on human CD4(+) T cells. Blood. 2013;122(7):1182–91. [DOI] [PubMed] [Google Scholar]
- 18.Cuende J, Lienart S, Dedobbeleer O, van der Woning B, De Boeck G, Stockis J, et al. Monoclonal antibodies against GARP/TGF-beta1 complexes inhibit the immunosuppressive activity of human regulatory T cells in vivo. Sci Transl Med. 2015;7(284):284ra56. [DOI] [PubMed] [Google Scholar]
- 19.Wu BX, Li A, Lei L, Kaneko S, Wallace C, Li X, et al. Glycoprotein A repetitions predominant (GARP) positively regulates transforming growth factor (TGF) beta3 and is essential for mouse palatogenesis. J Biol Chem. 2017;292(44):18091–18097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace CH, Wu BX, Salem M, Ansa-Addo EA, Metelli A, Sun S, et al. B lymphocytes confer immune tolerance via cell surface GARP-TGF-beta complex. JCI Insight. 2018;3(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catassi C, Kryszak D, Bhatti B, Sturgeon C, Helzlsouer K, Clipp SL, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med. 2010;42(7):530–8. [DOI] [PubMed] [Google Scholar]
- 22.Dupuy d’Angeac A, Reme T, Monier S, Gao Q, Duperray C, Jullien P, et al. Contrasting effect of transforming growth factor type beta 1 (TGF-beta 1) on proliferation and interleukin-2 receptor expression in activated and rapidly cycling immature (CD3-CD4-CD8-) thymocytes. J Cell Physiol. 1993;154(1):44–52. [DOI] [PubMed] [Google Scholar]
- 23.Cheng G, Yuan X, Tsai MS, Podack ER, Yu A, Malek TR. IL-2 receptor signaling is essential for the development of Klrg1+ terminally differentiated T regulatory cells. J Immunol. 2012;189(4):1780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronchetti S, Ricci E, Petrillo MG, Cari L, Migliorati G, Nocentini G, et al. Glucocorticoid-induced tumour necrosis factor receptor-related protein: a key marker of functional regulatory T cells. J Immunol Res. 2015;2015:171520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elkord E,Al-Ramadi BK. Helios expression in FoxP3(+) T regulatory cells. Expert Opin Biol Ther. 2012;12(11):1423–5. [DOI] [PubMed] [Google Scholar]
- 26.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–21. [DOI] [PubMed] [Google Scholar]
- 27.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170(8):3939–43. [DOI] [PubMed] [Google Scholar]
- 28.Griseri T, McKenzie BS, Schiering C, Powrie F. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity. 2012;37(6):1116–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redpath SA, van der Werf N, Cervera AM, MacDonald AS, Gray D, Maizels RM, et al. ICOS controls Foxp3(+) regulatory T-cell expansion, maintenance and IL-10 production during helminth infection. Eur J Immunol. 2013;43(3):705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johdi NA, Ait-Tahar K, Sagap I, Jamal R. Molecular Signatures of Human Regulatory T Cells in Colorectal Cancer and Polyps. Front Immunol. 2017;8:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wermers JD, McNamee EN, Wurbel MA, Jedlicka P, Rivera-Nieves J. The chemokine receptor CCR9 is required for the T-cell-mediated regulation of chronic ileitis in mice. Gastroenterology. 2011;140(5):1526–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2(8):1998–2004. [DOI] [PubMed] [Google Scholar]
- 33.Tran DQ. TGF-beta: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol. 2012;4(1):29–37. [DOI] [PubMed] [Google Scholar]
- 34.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105(29):10113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu A, Liu Y, Chen W, Wang J, Xue Y, Huang F, et al. TGF-beta-Induced Regulatory T Cells Directly Suppress B Cell Responses through a Noncytotoxic Mechanism. J Immunol. 2016;196(9):3631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyssuk EY, Torgashina AV, Soloviev SK, Nassonov EL, Bykovskaia SN. Reduced number and function of CD4+CD25highFoxP3+ regulatory T cells in patients with systemic lupus erythematosus. Adv Exp Med Biol. 2007;601:113–9. [PubMed] [Google Scholar]
- 37.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178(4):2579–88. [DOI] [PubMed] [Google Scholar]
- 38.Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity. 2011;34(3):396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol. 2007;81(6):1386–94. [DOI] [PubMed] [Google Scholar]
- 40.Sun L, Hurez VJ, Thibodeaux SR, Kious MJ, Liu A, Lin P, et al. Aged regulatory T cells protect from autoimmune inflammation despite reduced STAT3 activation and decreased constraint of IL-17 producing T cells. Aging Cell. 2012;11(3):509–19. [DOI] [PubMed] [Google Scholar]
- 41.Edwards JP, Hand TW, Morais da Fonseca D, Glass DD, Belkaid Y, Shevach EM. The GARP/Latent TGF-beta1 complex on Treg cells modulates the induction of peripherally derived Treg cells during oral tolerance. Eur J Immunol. 2016;46(6):1480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pastille E, Bardini K, Fleissner D, Adamczyk A, Frede A, Wadwa M, et al. Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res. 2014;74(16):4258–69. [DOI] [PubMed] [Google Scholar]
- 43.Yoon HH, Orrock JM, Foster NR, Sargent DJ, Smyrk TC, Sinicrope FA. Prognostic impact of FoxP3+ regulatory T cells in relation to CD8+ T lymphocyte density in human colon carcinomas. PLoS One. 2012;7(8):e42274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bain CC, Montgomery J, Scott CL, Kel JM, Girard-Madoux MJH, Martens L, et al. TGFbetaR signalling controls CD103(+)CD11b(+) dendritic cell development in the intestine. Nat Commun. 2017;8(1):620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anz D, Mueller W, Golic M, Kunz WG, Rapp M, Koelzer VH, et al. CD103 is a hallmark of tumor-infiltrating regulatory T cells. Int J Cancer. 2011;129(10):2417–26. [DOI] [PubMed] [Google Scholar]
- 46.Braun A, Dewert N, Brunnert F, Schnabel V, Hardenberg JH, Richter B, et al. Integrin alphaE(CD103) Is Involved in Regulatory T-Cell Function in Allergic Contact Hypersensitivity. J Invest Dermatol. 2015;135(12):2982–2991. [DOI] [PubMed] [Google Scholar]
- 47.Allakhverdi Z, Fitzpatrick D, Boisvert A, Baba N, Bouguermouh S, Sarfati M, et al. Expression of CD103 identifies human regulatory T-cell subsets. J Allergy Clin Immunol. 2006;118(6):1342–9. [DOI] [PubMed] [Google Scholar]
- 48.Rachidi S, Metelli A, Riesenberg B, Wu BX, Nelson MH, Wallace C, et al. Platelets subvert T cell immunity against cancer via GARP-TGFbeta axis. Sci Immunol. 2017;2(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Metelli A, Wu BX, Fugle CW, Rachidi S, Sun S, Zhang Y, et al. Surface Expression of TGFbeta Docking Receptor GARP Promotes Oncogenesis and Immune Tolerance in Breast Cancer. Cancer Res. 2016;76(24):7106–7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lienart S, Merceron R, Vanderaa C, Lambert F, Colau D, Stockis J, et al. Structural basis of latent TGF-beta1 presentation and activation by GARP on human regulatory T cells. Science. 2018;362(6417):952–956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.