Abstract
Background:
The effectiveness of 5-fluorouracil compared to imiquimod for preventing keratinocyte carcinoma is unknown.
Objective:
To compare the effectiveness of 5-fluorouracil and imiquimod in preventing keratinocyte carcinoma in a real-world practice setting.
Methods:
We identified 5,700 subjects who filled prescriptions for 5-fluorouracil or imiquimod for actinic keratosis treatment in 2007. An intention-to-treat analysis controlling for potential confounding variables was used to calculate 2- and 5-year cumulative risk differences for subsequent keratinocyte carcinoma overall and in field-treated areas.
Results:
5-fluorouracil was associated with a statistically significant decreased risk of any keratinocyte carcinoma compared to imiquimod (adjusted hazard ratio [aHR] 0.86, 95% CI 0.76–0.97), but there were no significant differences in risk by tumor subtype (squamous cell carcinoma aHR 0.89, 95% CI 0.74–1.07; basal cell carcinoma aHR 0.87, 95% CI 0.74–1.03), or site-specific keratinocyte carcinoma (aHR 0.96, 95% CI 0.81–1.14). There were no significant differences in 2- or 5-year cumulative risk for keratinocyte carcinoma among those treated with 5-fluorouracil versus imiquimod.
Limitations:
Generalizability to other practice settings may be limited.
Conclusions:
Whereas 5-fluorouracil was more effective in reducing keratinocyte carcinoma risk overall, we found no differences in the short- or long-term risk of subsequent site-specific keratinocyte carcinoma in a real-world practice setting.
Keywords: 5-fluorouracil, Actinic keratosis, Basal cell carcinoma, Comparative effectiveness, Imiquimod, Keratinocyte carcinoma, Skin cancer, Squamous cell carcinoma
INTRODUCTION
Actinic keratoses (AKs) are precancerous keratinocyte-derived cutaneous neoplasms caused by chronic sun exposure. AKs are prevalent and costly, arising in nearly 40 million Americans per year1–3 and accounting for >$1 billion in healthcare expenditures annually in the United States (US).3 AKs are one of the most common reasons why patients visit a dermatologist4 and are responsible for over 3.7 million ambulatory care office visits in the US each year.5 A natural history study of AKs has shown that they can progress to both basal cell carcinoma (BCCs) and squamous cell carcinoma (SCC), collectively termed keratinocyte carcinomas (KC).6 Estimates of lifetime progression rates range from as low as 0.1% to as high as 20%.6–9 Data from a prospective trial showed that the rate of malignant transformation of an untreated AK over a 5-year period was 5.58% (95% confidence interval [CI], 4.54–6.86).6 One of the primary goals of AK treatment is to prevent progression to KC, which can not only become locally invasive, destructive, and deforming, but also metastasize.
If a patient has multiple AKs on an anatomic unit, field treatment is often recommended to reduce AK burden and risk of subsequent AKs and KCs in the treated area. Multiple modalities for field treatment of AKs are used clinically, including various topical agents and light-based treatments. Two commonly prescribed topical field-based treatments for multiple AKs include 5-fluorouracil (5-FU) and imiquimod (imq).
There is no agreement on the best AK field treatment,10 and there is insufficient data to guide clinicians as to which topical AK field treatment is most effective in preventing KCs.11 A recent review on AKs stated that “more head-to-head comparisons of alternative treatment strategies for AK are needed to determine the best treatment,”10 while a Cochrane review on AK treatments concluded that “more direct comparisons…are needed to determine the best therapeutic approach.”11 A recent randomized controlled trial suggested that 5-FU could reduce SCC risk at one year.12 However, previous studies have not compared the effectiveness of 5-FU and imq in preventing KCs. The goal of this study was to compare the effectiveness of two widely used topical field-based AK treatments in preventing subsequent KC using a real-world setting to help inform treatment selection.
MATERIALS AND METHODS
We conducted a retrospective longitudinal cohort study of all Kaiser Permanente Northern California (KPNC) health plan members aged ≥18 years who were diagnosed with an AK (International Statistical Classification of Diseases and Related Health Problems, 9th revision [ICD-9] code 702.0) in 2007 and subsequently filled a prescription for 5-FU (n=5,062) or imq (n=638) for AK treatment. Cohort members were followed for subsequent KC (any KC, any BCC, any SCC, and site-specific KC arising in the treated field).
Study Setting
KPNC is a pre-paid, integrated healthcare delivery system that provides comprehensive healthcare and pharmaceutical benefits to more than 4 million members in Northern California. KPNC maintains a computerized record system with detailed information on members’ demographic characteristics, clinical visits, pharmacy dispensed medications, lab, pathology, and radiology results, and other medical services.
Study Population
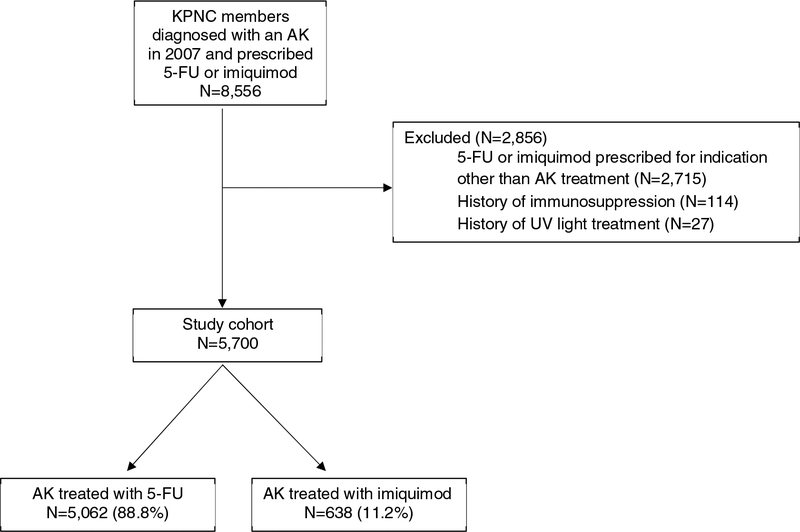
The study cohort has been described previously13 and consisted of adult KPNC members who were diagnosed with an AK (ICD-9 code 702.0) between January 1, 2007 and December 31, 2007 and received subsequent topical AK field treatment with 5-FU or imq (n=8,556) (Figure 1). The year 2007 was selected because imq was not available before 2007, and it allowed for electronic review of all outpatient ambulatory notes, which facilitated abstraction of information on treatment indication, anatomic site of application, and site of subsequent KC.
Figure 1. Study flow diagram.
Flow diagram that depicts the derivation of the study cohort.
Abbreviations: KPNC: Kaiser Permanente Northern California; AK: actinic keratosis; 5-FU: 5-fluorouracil; UV: ultraviolet
Subjects were excluded if 5-FU or imq was prescribed for any indication other than AK (such as skin cancer or warts) as determined by chart review, or if they had any of the following ever recorded in the KPNC database: (1) history of any ultraviolet light treatment; (2) infection with human immunodeficiency virus; (3) history of chronic lymphocytic leukemia; (4) history of solid organ transplant; (5) history of bone marrow transplant, due to the associated increased risk of skin cancer in those sub-populations.14–18
Exposure
Exposure was defined as pharmacy dispensation of 5-FU or imq associated with an AK diagnosis in 2007. Clinic notes and medication orders were reviewed to ensure treatment was for AK, and to abstract information on anatomic site of drug application (categorized in three anatomic groupings: 1) head and neck, including face (forehead, nose, cheek, chin, and temple), scalp, ear, neck; 2) trunk, including abdomen, back, shoulders, buttocks; and 3) extremities, including ankle, arm, calf, finger, foot, forearm, hand, knee, leg, palm, thigh, wrist.
Outcome
The four outcomes were defined as time to first KC (SCC or BCC), time to first KC within the treated field, time to first BCC, and time to first SCC as identified through review of all KPNC pathology records. We abstracted tumor site in granular categories which were then grouped into categorical anatomic sites for analytic purposes to mirror the exposure categories as defined above. For each outcome definition, patients were followed-up through the earliest of outcome occurrence, health plan disenrollment, death, or the end of the study period.13
Covariates
Information on patient demographics, including age (date of birth), sex (male, female, transgender, other), and race/ethnicity (white-non-Hispanic, white-Hispanic, black, Asian, Native-American) were obtained from KPNC databases. Data were also obtained on KC risk factors, including cigarette use (current, former, never, unknown), body mass index (≤24.9 [normal], 25–29.9 [overweight], ≥30 [obese]), comorbidities (Charlson Comorbidity Index 0, 1, 2, and 3+), baseline healthcare utilization (average number of any healthcare provider emergency department or outpatient visits in the 2 years prior to baseline), baseline skin surveillance measure (average annual number of dermatology visits in the 2 years prior to baseline), prior KC history (yes/no within 5 years prior to cohort entry), prior AK (yes/no within 5 years prior to cohort entry), history of 5-FU treatment prior to cohort entry, and photosensitizing medication use within two years prior to baseline. A previously published list of photosensitizing medications was used as the basis for categorizing photosensitizing medications.19
Statistical Analysis
The comparative effectiveness of 5-FU and imq was evaluated using inverse probability weighting (IPW) to control for baseline covariates.20 We conducted an intention-to-treat analysis given that differential non-compliance or surveillance between 5-FU and imq was not expected, as their directions for application, duration of use, and side-effect profiles are similar.21 Logistic regression including all baseline variables was used to estimate the propensity score (PS). The adjusted 2-year and 5-year cumulative risk differences (RD) were estimated by linear regression using stabilized IPW weights calculated based on the PS estimates.22 To estimate the adjusted 2-year RD, a complete case analysis was performed where outcomes from patients with no subsequent KC diagnoses and with less than 2 years of follow-up were treated as missing in the regression, and missingness was assumed to occur completely at random. A similar approach was used to estimate the adjusted 5-year RD. Conservative 95% CIs were derived based on the robust variance estimator.23 The distribution of the stabilized IPW weights was examined to detect extreme weight values; the maximum IPW weight value did not warrant weight truncation.22 Results from the IPW estimation approach were compared to results from 1) crude (unadjusted) estimates of the cumulative RD derived by unweighted linear regressions; 2) crude (unadjusted) Kaplan Meier estimates of the survival curves for the two exposure groups (data not shown); and 3) IPW estimates of the hazard ratios from Cox proportional hazard marginal structural models for a single-time point intervention.23 All analyses were conducted using SAS, version 9.3. This study was approved by the Institutional Review Board, Kaiser Foundation Research Institute.
RESULTS
Among 5,700 cohort members, 5,062 were exposed to 5-FU and 638 were exposed to imq. Table 1 describes the baseline characteristics of cohort members. The average age at cohort entry was 66.6 years (standard deviation, 11.6 years). The majority of cohort members were non-Hispanic white (92.7%). Women constituted a larger proportion of those treated with imq than 5-FU (46.5% vs. 39.9%; p<0.01). Cohort members treated with imiquimod were more likely to have had a prior KC (52.2% vs. 40.9%; p<0.01) but less likely to have had prior 5-FU treatment (39.0% vs. 45.6%; p<0.01).
Table I:
Baseline characteristics of KPNC cohort with AK diagnosed in 2007 and treated with 5-FU or imiquimod
| VARIABLE | Overall (n=5,700) | 5-FU (n=5,062) | Imiquimod (n=638) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age (at index): Mean (±SD) | 66.6 (11.6) | 66.6 (10.6) | 66.6 (12.3) | 0.962 |
| Age (at index): n (%) | ||||
| Gender, n (%) | ||||
| Race and Ethnicity, n (%) | ||||
| Risk Factors for AK and KC | ||||
| Cigarette use, n (%) | ||||
| Body mass index, n (%) | ||||
| Photosensitizing drug exposure,1,2 n (%) | ||||
| Prior AK,3 n (%) | ||||
| Prior KC,3 n (%) | ||||
| Prior 5-FU treatment,4 n (%) | ||||
| Baseline Healthcare Utilization | ||||
| Charlson comorbidity score, n (%) | ||||
| Healthcare utilization: Mean (±SD) outpatient visits5 | 10.9 (9.6) | 10.9 (9.8) | 11.4 (10.1) | 0.229 |
| Surveillance measure: Mean (±SD) dermatology visits5 | 1.6 (1.8) | 1.6 (1.8) | 1.9 (1.8) | <.001 |
| Prescriber Type, n (%) |
In the two years prior to cohort entry.
Photosensitizing drugs included tetracyclines, sulfonamide antibiotics, fluoroquinoles, thiazide diuretics, loop diuretics, calcium channel blockers, potassium-sparing diuretics, alpha-adrenergic agonists, antiarrhythmics, sulfonylureas, antimetabolites, antiestrogens, salicylic acid derivatives, proprionic acid derivatives, acetic acid derivatives, enolic acid derivatives, benzodiazepines, tricyclic antidepressants, and oral retinoids.19
In the five years prior to cohort entry.
Ever in the pharmacy records going back to 1996. There is no prior imiquimod exposure in the cohort because it was not available before 2007.
Average annual number during follow-up period.
KPNC: Kaiser Permanente Northern California; AK: actinic keratosis; 5-FU: 5-fluorouracil; KC: keratinocyte carcinoma
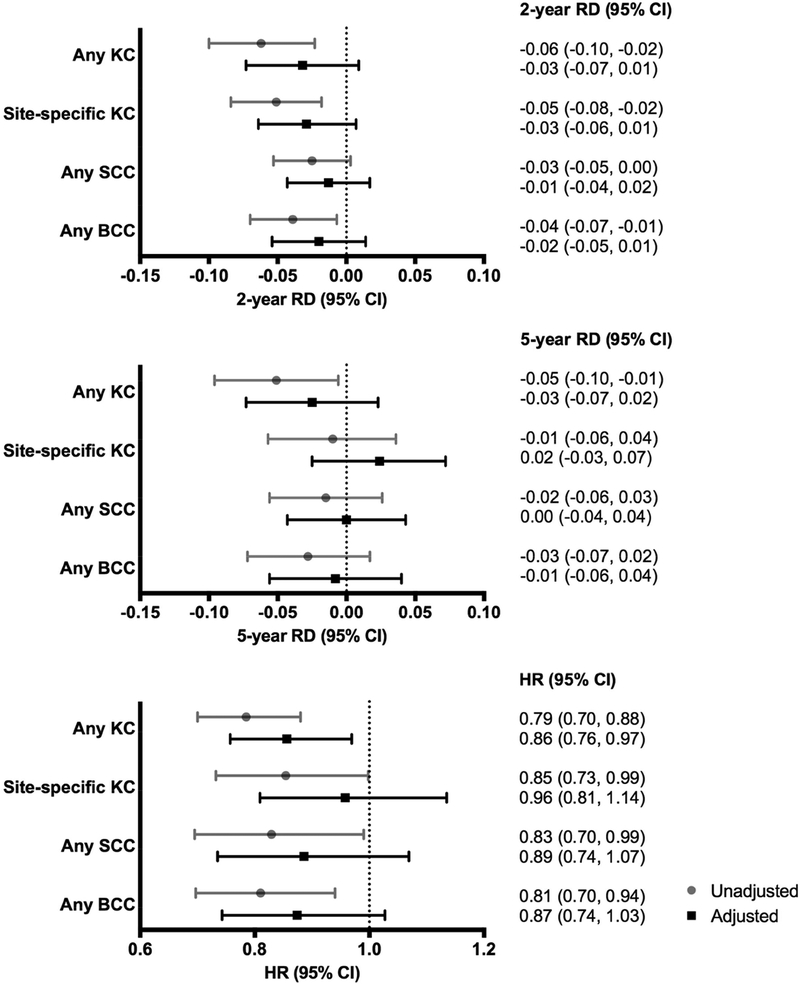
Figure 2 shows the 2- and 5-year cumulative RDs and HRs for KCs among those treated with 5-FU compared to imq. The unadjusted 2- and 5-year RDs suggested a trend toward lower cumulative KC risk following treatment with 5-FU compared to imq, with significantly lower risk observed for overall KCs at both 2-years (RD −0.06, 95% CI −0.10 to −0.02) and 5-years (RD −0.05, 95% CI −0.10 to −0.01), site-specific KCs at 2-years (RD −0.05, 95% CI −0.08 to −0.02), and BCCs at 2-years (RD −0.04, 95% CI −0.07 to −0.01). However, after adjusting for potential pre-treatment confounders, there were no significant differences in 2- or 5-year cumulative risk for subsequent KC among those treated with 5-FU and imq.
Figure 2. Cumulative 2- and 5-year risk differences and hazard ratios for subsequent keratinocyte carcinomas among KPNC members treated with 5-FU compared to imiquimod.
Gray circles and lines represent risk differences and hazard ratios (95% CIs) from unweighted models, while black boxes and lines represent risk differences and hazard ratios (95% CIs) from weighted models adjusted for age, gender, race/ethnicity, cigarette use, body mass index, photosensitizing drug exposure, prior actinic keratosis, prior keratinocyte carcinoma, prior 5-FU treatment, Charlson comorbidity score, healthcare utilization, surveillance measure, and prescriber type. Imiquimod was used as the reference in all analyses.
Abbreviations: RD: risk difference; HR: hazard ratio; CI: confidence interval; KC: keratinocyte carcinoma; KPNC: Kaiser Permanente Northern California; 5-FU: 5-fluorouracil
Cox proportional hazard modeling suggested a decreased risk of KCs following treatment with 5-FU compared to imq (any KC: HR 0.79, 95% CI 0.70 to 0.88; site-specific KC: HR 0.85, 95% CI 0.73 to 0.99; SCC: HR 0.83, 95% CI 0.70 to 0.99; BCC: HR 0.81, 95% CI 0.70 to 0.94). After adjusting for potential baseline confounders, 5-FU was associated with a statistically significant decreased risk of any KC compared to imq (adjusted hazard ratio [aHR] 0.86, 95% CI 0.76 to 0.97), but there were no significant differences in risk of site-specific KC (aHR 0.96, 95% CI 0.81 to 1.14), SCC (aHR 0.89, 95% CI 0.74 to 1.07), or BCC (aHR 0.87, 95% CI 0.74 to 1.03).
DISCUSSION
In this large cohort study performed within a community-based real-world setting, we did not find strong evidence that 5-FU and imq have different short- or long-term effectiveness in the prevention of subsequent BCC, SCC, or site-specific KC. Although Cox proportional hazard modeling indicated that 5-FU was associated with a statistically significant 14.4% decreased risk of any KC compared to imq, there was no significant difference in risks of site-specific KCs.
Previous studies comparing the effectiveness of 5-FU and imq for AK treatment have examined clearance of individual AK lesions as the primary outcome measure.11 To our knowledge, no prior studies comparing 5-FU and imq have examined subsequent KC as an outcome measure. However, one of the key goals of AK treatment is to prevent progression to malignancy,10,24 which makes subsequent KC a key outcome to consider when comparing the efficacy of different AK treatments. Only two small, short randomized clinical trials have directly compared the efficacy of 5-FU and imq for the treatment of AKs, and neither study examined subsequent KCs as an outcome. One small trial followed 36 subjects over 24 weeks and found that 5-FU was more effective than imq in exposing presumed subclinical AKs, reducing final AK count, and achieving complete AK clearance.25 The other small trial compared the initial and 12-month clinical and histological clearance of AKs after treatment with 5-FU (n=24), imq (n=26), and cryosurgery (n=25) and found that imq was associated with superior sustained clearance compared with 5-FU.21 A recent network meta-analysis that determined the relative efficacy of eight AK treatments based on the outcome “participant complete clearance” ranked 5-FU first, over imq, leading the authors to conclude that 5-FU should be the treatment of choice for AKs to prevent progression to KC.24 In agreement with this meta-analysis, in our previous study comparing the effectiveness of AK treatment with 5-FU and imq, we found that 5-FU was significantly more effective than imq in preventing subsequent AKs at 2 years (RD −4.54%, 95% CI −7.91% to −1.17%), though not at 5 years (RD −1.43%, 95% CI −3.43% to 0.05%).13 However, our present study revealed no differences in rates of subsequent site-specific KCs after topical field treatment with 5-FU or imq at 2 or 5 years.
5-FU and imq have distinct mechanisms of action that account for their efficacy in AK field treatment. 5-FU, which inhibits thymidylate synthetase, interferes with DNA and RNA synthesis and thus decreases cellular proliferation and induces cell death.11 Imq, which acts as an immune modulator through activation toll-like receptors, enhances both innate and acquired immune responses that have antitumor activity.11 Our previous study showing that 5-FU is more effective than imq at preventing subsequent AKs at 2 years suggested that 5-FU’s inhibitory effect on DNA and RNA synthesis may persist longer than imq’s immunomodulatory effects.13 This distinct mechanism of action could lead to differential responses at non-treated AK sites and could explain the 14.4% decreased risk any KC among those treated with 5-FU compared to imq.
Strengths of this study include KPNC’s closed, pre-paid, integrated healthcare system, which enabled a real-world comparison of the efficacy of 5-FU and imq in preventing KCs within a stable, well-characterized population. KPNC’s computerized health system contains detailed records of the comprehensive care patients receive, which allowed us to accurately assess potential pre-treatment confounders, prescription details, and subsequent KC diagnoses. Limitations include to inability to control for unmeasured confounding, including physicians’ perception of drug tolerability and differential physician counseling based on experience with each medication. Furthermore, we were unable to control for several known KC risk factors, including skin type, hair and eye color, and sun exposure history. However, by limiting the cohort to members who were prescribed field-based therapy for their actinic damage, we sought to make AK severity as similar as possible across the treatment groups, minimizing potential differences in skin type or sun exposure history between the two treatment groups. We did not account for subsequent exposure to 5-FU or imq during the follow-up period; patients may have been prescribed multiple rounds of field treatment during the follow-up period, which would have affected KC risk. Prescriptions filled were used as a surrogate for medication use and exposure was inferred from directions at dispensing, which may not reflect patients’ true exposure. However, this reflects a real-world practice setting and enables measurement of effectiveness, rather than efficacy like in a clinical trial. Because treatment with 5-FU and imq spans several weeks, there were many opportunities for non-compliance. Differential non-compliance between the two treatments was not expected because their directions for use, duration of application, and side-effect profiles are very similar.21 Finally, because the study cohort consisted of insured adults in Northern California, the results may not be completely generalizable to the uninsured or other healthcare or geographic settings.
In summary, we found no difference in the effectiveness of 5-FU and imq in preventing subsequent site-specific KCs in the short- or long-term in a real-life clinical setting, though we did see a statistically significant decreased risk of any (not site-specific) KC with 5-FU compared to imq. Given the burden AKs pose to the healthcare system with their high prevalence, significant cost, and potential for malignant progression, dermatologists should be aware of how available treatments compare in their effectiveness in preventing KCs.
CAPSULE SUMMARY.
5-fluorouracil and imiquimod are frequently prescribed actinic keratosis treatments, but no studies have compared their effectiveness at preventing keratinocyte carcinoma in a real-world setting.
Whereas 5-fluorouracil was more effective in reducing keratinocyte carcinoma risk overall, we found no differences in the short- or long-term risk of site-specific keratinocyte carcinoma.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Arthritis Musculoskeletal and Skin Diseases (grant numbers R03AR064014 and K24AR069760 to MA). Dr. Asgari has research contracts with Pfizer Inc. and Valeant Pharmaceuticals, which are not relevant to the contents of this manuscript.
Abbreviations:
- AK
actinic keratosis
- KC
keratinocyte carcinoma
- 5-FU
5-fluorouracil
- Imq
imiquimod
- KPNC
Kaiser Permanente Northern California
- ICD-9
International Statistical Classification of Diseases and Related Health Problems, 9th revision
- RD
risk difference
- HR
hazard ratio
- CI
confidence interval
- IPW
inverse probability weighting
- PS
propensity score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Disclosure:
Dr. Asgari has research contracts with Pfizer Inc. and Valeant Pharmaceuticals, which are not relevant to the contents of this manuscript.
This study was approved by the Institutional Review Board, Kaiser Foundation Research Institute.
REFERENCES
- 1.Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1 Pt 2):4–7. [DOI] [PubMed] [Google Scholar]
- 2.Warino L, Tusa M, Camacho F, Teuschler H, Fleischer AB, Feldman SR. Frequency and cost of actinic keratosis treatment. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2006;32(8):1045–1049. doi: 10.1111/j.1524-4725.2006.32228.x [DOI] [PubMed] [Google Scholar]
- 3.Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55(3):490–500. doi: 10.1016/j.jaad.2006.05.048 [DOI] [PubMed] [Google Scholar]
- 4.Feldman SR, Fleischer AB, McConnell RC. Most common dermatologic problems identified by internists, 1990–1994. Arch Intern Med. 1998;158(7):726–730. [DOI] [PubMed] [Google Scholar]
- 5.Gupta AK, Cooper EA, Feldman SR, Fleischer AB. A survey of office visits for actinic keratosis as reported by NAMCS, 1990–1999. National Ambulatory Medical Care Survey. Cutis. 2002;70(2 Suppl):8–13. [PubMed] [Google Scholar]
- 6.Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115(11):2523–2530. doi: 10.1002/cncr.24284 [DOI] [PubMed] [Google Scholar]
- 7.Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1 Pt 2):23–24. doi:a103339 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Lebwohl M Actinic keratosis: epidemiology and progression to squamous cell carcinoma. Br J Dermatol. 2003;149 Suppl 66:31–33. [DOI] [PubMed] [Google Scholar]
- 9.Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet Lond Engl. 1988;1(8589):795–797. [DOI] [PubMed] [Google Scholar]
- 10.Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. August 2016. doi: 10.1111/bjd.14852 [DOI] [PubMed] [Google Scholar]
- 11.Gupta AK, Paquet M, Villanueva E, Brintnell W. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12:CD004415. doi: 10.1002/14651858.CD004415.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of Basal and Squamous Cell Carcinoma With a Single Course of Fluorouracil, 5%, Cream: A Randomized Clinical Trial. JAMA Dermatol. 2018;154(2):167–174. doi: 10.1001/jamadermatol.2017.3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neugebauer R, Levandoski KA, Zhu Z, et al. A real-world, community-based cohort study comparing the effectiveness of topical fluoruracil vs. topical imiquimod for the treatment of actinic keratoses. J Am Acad Dermatol. 2017;Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nijsten TEC, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen+ultraviolet A: a cohort study. J Invest Dermatol. 2003;121(2):252–258. doi: 10.1046/j.1523-1747.2003.12350.x [DOI] [PubMed] [Google Scholar]
- 15.Silverberg MJ, Leyden W, Warton EM, Quesenberry CP, Engels EA, Asgari MM. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105(5):350–360. doi: 10.1093/jnci/djs529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi F, Randimbison L, Te VC, La Vecchia C. Non-Hodgkin’s lymphomas, chronic lymphocytic leukaemias and skin cancers. Br J Cancer. 1996;74(11):1847–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47(1):1–17; quiz 18–20. [DOI] [PubMed] [Google Scholar]
- 18.Kolb HJ, Socié G, Duell T, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med. 1999;131(10):738–744. [DOI] [PubMed] [Google Scholar]
- 19.Robinson SN, Zens MS, Perry AE, Spencer SK, Duell EJ, Karagas MR. Photosensitizing agents and the risk of non-melanoma skin cancer: a population-based case-control study. J Invest Dermatol. 2013;133(8):1950–1955. doi: 10.1038/jid.2013.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578–586. doi: 10.1136/jech.2004.029496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krawtchenko N, Roewert-Huber J, Ulrich M, Mann I, Sterry W, Stockfleth E. A randomised study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157 Suppl 2:34–40. doi: 10.1111/j.1365-2133.2007.08271.x [DOI] [PubMed] [Google Scholar]
- 22.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiol Camb Mass. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 24.Gupta AK, Paquet M. Network meta-analysis of the outcome “participant complete clearance” in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169(2):250–259. doi: 10.1111/bjd.12343 [DOI] [PubMed] [Google Scholar]
- 25.Tanghetti E, Werschler P. Comparison of 5% 5-fluorouracil cream and 5% imiquimod cream in the management of actinic keratoses on the face and scalp. J Drugs Dermatol JDD. 2007;6(2):144–147. [PubMed] [Google Scholar]