Abstract
Characterization of the microstructural properties and topography of the human corpus callosum (CC) is key to understanding interhemispheric neural communication and brain function. In this work, we tested the hypothesis that high-resolution T1 relaxometry at high field has adequate sensitivity and specificity for characterizing microstructural properties of the human CC, and elucidating the structural connectivity of the callosal fibers to the cortices of origin. The high-resolution parametric T1 images acquired from healthy subjects (N=16) at 7 Tesla clearly showed a consistent T1 distribution among individuals with substantial variation along the human CC axis, which is highly similar with the spatial patterns of myelin density and myelinated axon size based on the histology study. Compared to the anterior part of the CC, the posterior mid-body and splenium had significantly higher T1 values. In conjunction with T1-based classification method, the splenial T1 values were decoded more reliably compared to a conventional partitioning method, showing a much higher T1 value in the inferior splenium than in the middle/superior splenium. Moreover, the T1 profile of the callosal subdivision represented the topology of the fiber connectivity to the projected cortical regions: the fibers in the posterior midbody and inferior splenium with a higher T1 (inferring a larger axon size) were mainly connected to motor-sensory and visual cortical areas, respectively; in contrast, the fibers in the anterior/posterior CC with a lower T1 (inferring a smaller axon size) were primarily connected to the frontal/parietal-temporal areas. These findings indicate that high-resolution T1 relaxometry imaging could provide a complementary and robust neuroimaging tool,useful for exploring the complex tissue properties and topographic organization of the human corpus callosum.
Keywords: Parametric T1 MRI, corpus callosum, myelin density, axon size, structural connectivity, Topography
Introduction
The corpus callosum (CC) has the largest abundance of white matter fiber tracks connecting human homologous cortical regions across two hemispheres. It contains more than two hundred million fibers and numerous axonal projections essential to interhemispheric communication that is facilitated by the unique structure of myelinated axonal fibers enabling rapid and effective neural signaling and computation (Budd and Kisvarday 2012). Thus, noninvasive imaging and characterization of microstructural architectures and topographical organizations of the callosal subdivisions in human brains is of the utmost importance for providing new insights about interhemispheric structural connectivity and brain function (Myers 1959; Payne 1990; Wahl et al. 2007; Kontis et al. 2009; Sisti et al. 2012).
Quantitative analysis of the CC microstructures has relied on the transmission electron microscopy (TEM) as a gold standard method owing to its superior sensitivity and spatial resolution that make delineating the complex properties of myelinated callosal fibers with great precision and details possible. A number of microscopic studies have shown large variations in myelin density and myelinated axon size between the callosal subdivisions in the post-mortem human brain (Aboitiz et al. 1992a, b; Aboitiz and Montiel 2003) and non-human brain (Stikov et al. 2015b; Lamantia and Rakic 1990; Caminiti et al. 2013; Caminiti et al. 2009; Innocenti et al. 2010). However, TEM is not applicable for intact brain or longitudinal in vivo studies.
The advent of non-invasive MRI techniques has opened up opportunities for exploring the organization and microstructural properties of the cerebral white matter tissue in vivo (Le Bihan et al. 1986; Chenevert et al. 1990). Diffusion-based imaging methods have been used to explore callosal microstructural properties such as axon diameter and myelin thickness or contents (Assaf et al. 2008; Caminiti et al. 2013; Zhang et al. 2011; Assaf et al. 2013; Alexander et al. 2010; Tuch et al. 2002; Fabri et al. 2014; Hofer and Frahm 2006; Stikov et al. 2015a; Stikov et al. 2011; Jensen et al. 2005; Callaghan et al. 1988). The topographic information regarding the structural connectivity of callosal fibers to cortical origins has also been documented using the direct injection of axonal tracers in primate brains (Tomasi et al. 2012; Innocenti et al. 2010).
As for non-invasive approaches, there are already several geometric partitioning methods such as Witelson’s classification that have been introduced to subdivide the CC structure in the human (Witelson 1989; de Lacoste et al. 1985). Recently, diffusion tensor tractography (DTT)-based approaches have been developed to study the callosal topography (Hofer and Frahm 2006; Huang et al. 2005; Park et al. 2008; Saenz and Fine 2010). However, there have been the lack of high-resolution MRI data linking the unique spatial distribution of callosal microarchitecture to the structural connectivity with a fine spatial scale.
As one of the non-invasive and measurable magnetic resonance (MR) parameters, the longitudinal relaxation time (T1) or rate (R1 = 1/T1) has been commonly employed for brain research and clinical diagnosis due to the enhanced MRI tissue contrast and sensitivity to tissue environments such as myeloarchitectonics (Sereno et al. 2013; Sigalovsky et al. 2006). Although there is some evidence showing T1 variations of the human CC in some extent (Hofer et al. 2015; Harkins et al. 2016; Wang et al. 2016), the relatively lower spatial resolution is a limiting factor for precise assessment of the fiber microstructural properties and its relevance to the topography of human CC.
In line with our previous report (Lee et al. 2014), in this work, we aimed to test the following hypotheses: 1) high-resolution T1 relaxometry at high field could offer reliable assessment about microstructural properties of the CC and their variations in individual healthy human subjects; 2) the callosal T1 variations would be a sensitive measure to link the key features of the myelin density and myelinated axon diameter; and 3) T1 variations among the callosal subdivisions would be indicative of topography of the callosal fibers in relevance to cortical regions of origin. To test these hypotheses, high-resolution (0.5 × 1 mm3 voxel size) parametric T1 images of the human corpus callosum were acquired at a high magnetic field of 7 Tesla (T) to assess microstructure properties of the human CC with a minimal partial volume effect. Furthermore, we examined the relation between the T1 variation of the CC subdivisions and its structural connectivity to the projected cortical regions using diffusion tensor tractography. For the validation of the correlate of the in vivo T1 results with underlying microstructural properties, callosal T1 imaging results were compared to the previous histology results of the postmortem human CC specimen (Aboitiz et al. 1992a; Aboitiz and Montiel 2003).
Materials and Methods
Subjects
Sixteen healthy subjects (mean age ± SD = 29.9 ± 12.0 years, 11 male / 5 female) participated in this study, which was approved by the institutional review board committee of the University of Minnesota. The informed consent was obtained from all participants.
MR imaging protocols
All MRI studies were conducted using an actively-shielded 7.0T/90cm whole-body human MRI scanner (MAGNETOM/Siemens, Erlangen, Germany) with a 32-channel 1H receive array head coil (Nova Medical, Inc., MA, USA).
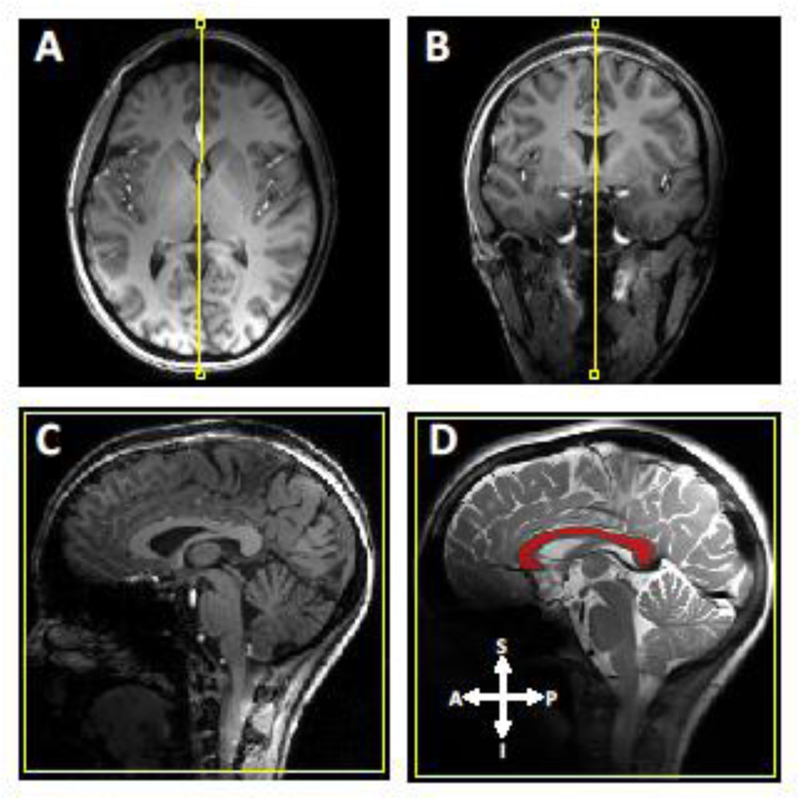
A mid-sagittal MRI slice across the central corpus callosum was selected under guidance of three-dimensional high-resolution T1-weighted (T1w) images as demonstrated in Fig. 1, which were acquired with a magnetization prepared rapid gradient echo imaging sequence (TR/TE/TI = 3000 ms / 2.4 ms / 1200 ms, radiofrequency (RF) pulse flip angle (FA) = 7°, field of view (FOV) = 256 × 256 mm2, isotropic resolution = 0.8 mm, and GRAPPA parallel imaging acceleration factor = 2).
Fig 1.
A mid-sagittal slice selection of the human corpus callosum using high-resolution three-dimensional T1-weighted images. Each panel of A, B, and C represents an axial, coronal, and sagittal view, respectively. Panel D shows the masked CC (red) displayed on the T2-weighted sagittal image. The arrows shown in D represent different anatomical direction; A for anterior, P for posterior, I for inferior, and S for superior.
High-resolution diffusion-weighted images were acquired according to the protocol optimized by the Human Connectome Project (HCP) at 7T (Vu et al. 2015; Ugurbil et al. 2013) with the following acquisition parameters: TR/TE = 5700 ms / 66 ms, FA = 90 °, b = 2100 mm2/s, number of diffusion direction = 114, in-plane resolution = 1.25 × 1.25 mm2, slice thickness = 1.25 mm, GRAPPA acceleration factor = 3, and multiband factor = 2 (Moeller et al. 2010). Two diffusion image datasets with reversed phase-encode blips were collected for correcting the susceptibility-induced distortions and then combined into a single corrected one (Andersson et al. 2003), thus, a total of 228 image volumes were acquired.
A magnetization-prepared single-shot fast spin echo imaging sequence was used for the parametric T1 measurement (Li et al. 2015) with the following parameters (TR/TE = 6000 ms / 16 ms, FOV = 192 × 192 mm2, in-plane resolution = 0.5 × 0.5 mm2, slice thickness = 1 mm, GRAPPA acceleration factor = 3 with 24 reference lines, receiver bandwidth (BW) = 814 Hz/pixel, a single mid-sagittal slice image covering the entire CC structure with a perpendicular fiber orientation). Based on the reported T1 value (~1.2 s) of the human white matter at 7T (Rooney et al. 2007; Wright et al. 2008), all T1 images were acquired under a full relaxation condition (TR = 6s) with various inversion recovery times (TIs) (0.1, 0.15, 0.3, 0.5, 0.8, 1.2, and 1.6 s). The uniform RF pulse flip angles for inversion (FA = 180°) and excitation (FA = 90°) across the selected mid-sagittal CC slice were achieved by applying a slice-selective adiabatic hyperbolic secant pulse (pulse length = 20 ms, BW = 1 kHz) (Garwood and DelaBarre 2001) and variable-rate selective excitation RF pulse (Hargreaves et al. 2004), respectively. The RF transmission power was calibrated using a 2D actual flip-angle imaging (Yarnykh 2007) (TR1 / TR2 = 70 ms / 120 ms, TE = 2.5 ms, FOV = 196 196 mm2, in-plane resolution = 4 4 mm2, and average = 5). All T1 images were collected within 24 minutes under the FDA specific absorption rate limit.
T1 mapping
T1 relaxation maps were generated using the nonlinear least-square fitting algorithm using Matlab software (13.0v, Mathwork) according to the following equation (Li et al. 2015):
| [1] |
where S(TI) is the measured imaging signal intensity at a given TI, So is the proton density, Tr is the recovery time after the inversion pulse, and Cnoise is the noise-related constant.
T1 distributions of callosal subdivisions
For the regional callosal T1 analysis across subjects, a callosal T1 image of each subject was first registered to a representative subject using a linear affine transformation (Good et al. 2001; Narr et al. 2000).
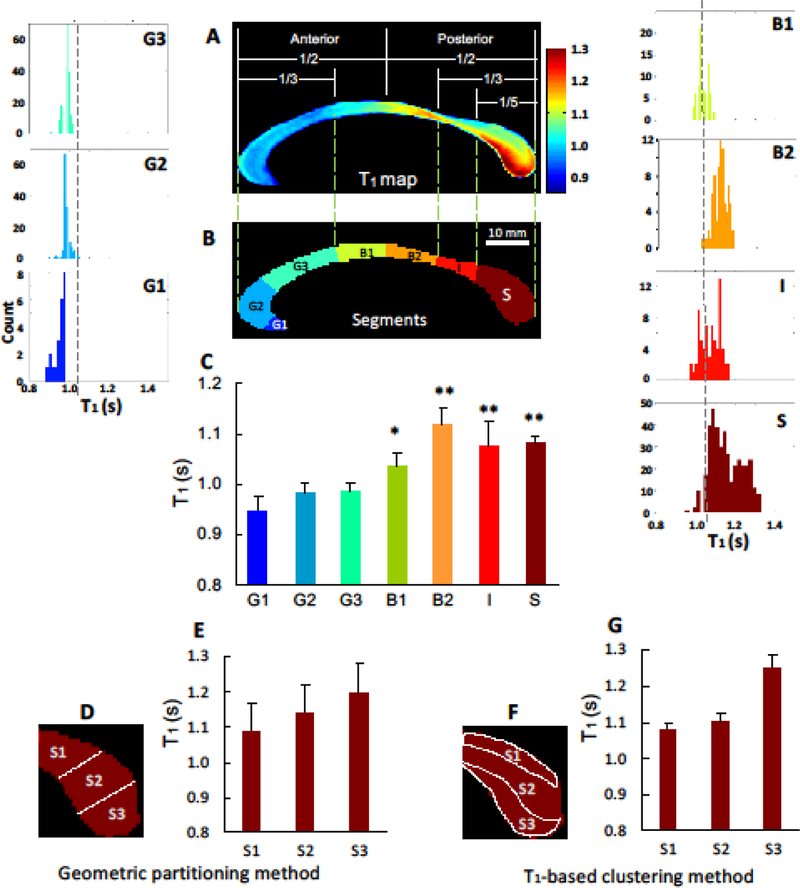
Using the registered T1 maps, histogram analysis for the T1 distribution of the callosal subdivision was performed; in conjunction with Witelson’s classification scheme (Witelson 1989), the CC was parcellated into five vertical partitions based on arithmetic fractions of the maximum anterior-posterior extent: anterior third (rostrum (G1), genu (G2), and rostral body (G3)), anterior midbody (B1), posterior midbody (B2), isthmus (I), and splenium (S). In particular, two partitioning methods were used for further classification of splenial subdivisions - superior (S1), middle (S2), and inferior (S3): an equidistant partitioning method (Aboitiz et al. 1992a; Björnholm et al. 2017; Thapaliya et al. 2017) and T1-based partitioning with a k-means method.
Myelin density and axon diameter
The relation between the callosal T1 variation and the myelinated axon size was examined based on an assumption of a linear relationship between the myelin density (or MWF: myelin water fraction) and R1 (=1/T1).
The in vivo axon diameter map was constructed from the use of g-ratio and MWF as a function of axonal diameter (da). Given the inverse relation between T1 and MWF, the T1 value can be expressed as a function of MWF (or da) according the following equations:
| [2] |
| [3] |
| [4] |
where tm is the myelin thickness, and α and β are constants. Based on the reported mean value of human axon diameter (0.69 m) (Liewald et al. 2014) and mean callosal T1 value (1.04 s) as measured in the current study, α and β were determined from the regression of Eqn. [4]. Finally, using the established relation between T1 and MWF in Eqn. [4], the axon diameter of each voxel was determined by the corresponding clustered T1 values according Eqns. [2] to [4], and then classified by the k-means method.
For a comparison purpose, another axon diameter map was scaled and constructed using a linear relation between T1 values and axon diameter (Harkins Kevin et al. 2016). The predicted axon diameter for each voxel was determined according to the corresponding clustered T1 values. This map was for visualization and comparison with the schematic draw of axon distribution of human CC based on the histology results as reported in the literature.
Structural Connectivity
The relation of T1 variations among CC subdivisions with structural connectivity to the projected cortical regions was investigated using the DTT imaging method. All diffusion data were processed according to the HCP diffusion pipeline (Sotiropoulos et al. 2013) and a probabilistic fiber tractography was applied in subject-specific native space using FSL’s FMRIB’s Diffusion Toolbox with the following parameters (number of diffusion direction = 3 / voxel, number of samples = 5000/voxel, curvature threshold = 0.2, and maximum number of step = 2000, and step length = 0.5 mm). The procedure of the probabilistic tractography is described in detail in the literature (Behrens et al. 2007). Representative regions of interest (ROIs) were first delineated on the callosal T1 map. Using Bayesian estimation, fiber directions for each ROI were inferred and then the probability of a streamline connection was calculated as the proportion of the total number of streamlines. Seeded fibers were then classified according to their proportional streamline connection probabilities to the target cortical areas. Finally, voxels with a streamline connection probability of p-value > 0.01 (50 out of 5000 streamlines) were only used for fiber tracking to reduce statistical errors due to noise.
Statistical analysis
A statistical comparison for T1 differences between discrete callosal areas was performed using a two-tailed paired t-test. The relation between in vivo 1/T1 (= R1) profile and histology analysis of fiber density was examined using Pearson correlation analysis. A P value < 0.05 was considered statistically significant after accounting for multiple comparisons using Bonferroni correction if necessary. All statistical analyses were done using the IBM SPSS software version 21.0 (IBM Corp., Armonk, NY, USA). The mean and standard deviation are shown in the form of: (mean ± SD).
Results
Callosal T1 Distribution
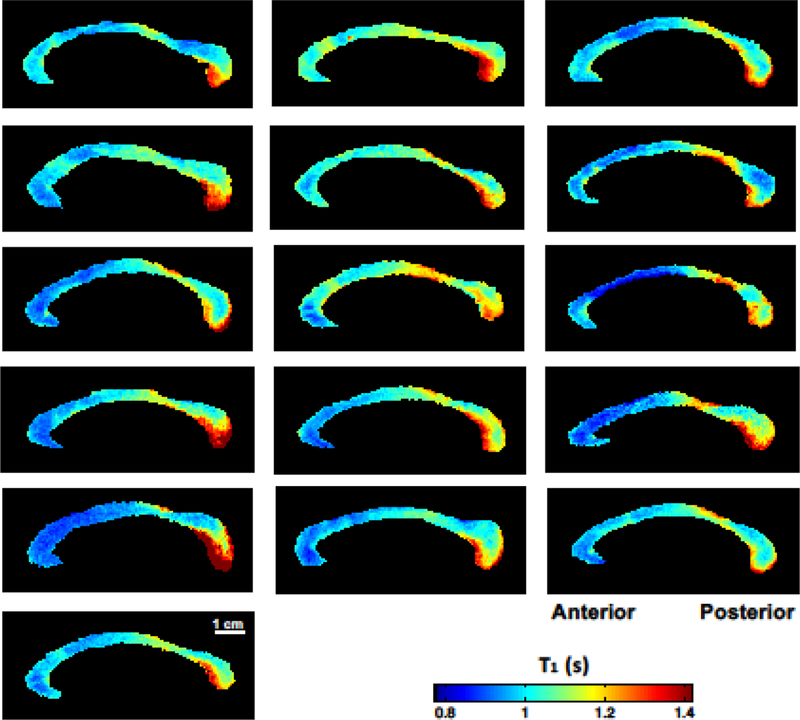
The mean of the T1 relaxation time of the entire human CC was 1.04 ± 0.04 s, which was in an agreement with the reported T1 values of the human white matter at 7T (Wright et al. 2008; Rooney et al. 2007). The T1 relaxometry map showed a clear spatial trend and widespread variation from the anterior to posterior along the CC, which was consistently observed in all subjects (Fig. 2). Histogram analysis shown in Fig. 3 reveals a significant difference in the T1 values between the anterior and posterior CC parts (P < 0.001). Higher T1 values were found in the posterior CC areas including the posterior midbody (1.12 ± 0.04 s, Fig. 3B2) and splenium (1.15 ± 0.08 s, Fig. 3S). In contrast, relatively lower T1 values were shown in the anterior CC areas (0.97 ± 0.01 s, Figs. 3G1-G3) and anterior midbody (1.04 ± 0.03 s, Fig. 3B1).
Fig 2.
High-resolution T1 relaxometry maps of the mid-sagittal corpus callosum from 16 healthy human subjects. All T1 maps were displayed in the same scale in the color bar. There was a common pattern of T1 distributions along the CC; the smaller T1 values in the anterior and higher T1 values toward the posterior areas.
Fig 3.
Histogram analysis of regional T1 distributions of the callosal subdivisions in conjunction with two partitioning methods. According to the geometric partitioning method, the mid-sagittal corpus callosum was segmented into 7 subdivisions: the anterior third including rostrum (G1), genu (G2), rostral-body (G3); midbody including anterior midbody (B1) and posterior midbody (B2); posterior-third including the isthmus (I) and splenium (S). The further segmentation for the splenial subdivisions – superior (S1), middle (S2), and inferior (S3) - were performed using the Witelson’s partitioning (D and E) and T1-based classification method (F and G). Overall, T1 map shows a heterogeneous pattern of T1 distribution from the anterior to the posterior part of the CC. Moreover, T1-based classification method provides the better separation of splenial subdivisions compared to the Witelson’s method. The vertical dashed lines in the histogram panels indicate the mean T1 value (= 1.04 s) of the entire CC structure. All brackets show statistical comparisons for regional callosal T1 compared to mean of the anterior areas (G1~G3) using 2-tailed paired t-test. *P < 0.001, ** P < 0.0001
The Whitelson’s partitioning scheme (Fig. 3D) showed large variations of T1 values (i.e., SD) between three sub-sections of the splenial areas: the superior (T1 = 1.08 ± 0.08 s, Fig. 3E-S1), the middle (T1 = 1.14 ± 0.08 s, Fig. 3E-S2), and the inferior area (T1 = 1.19 ± 0.08 s, Fig. 3E-S3). In contrast, the T1-based clustering method (Fig. 3F) showed large mean T1 differences with a much smaller SD: the superior (T1 = 1.08 ± 0.01 s, Fig. 3G-S1), the middle (T1 = 1.10 ± 0.02 s, Fig. 3G-S2), and the inferior area (T1 = 1.25 ± 0.04 s, Fig. 3G-S3). This method resulted in a better separation of these areas ( T1 = 0.17 s, Fig. 3G) compared to the Whitelson’s partitioning method ( T1 = 0.11 s, Fig. 3E).
Callosal Fiber Composition
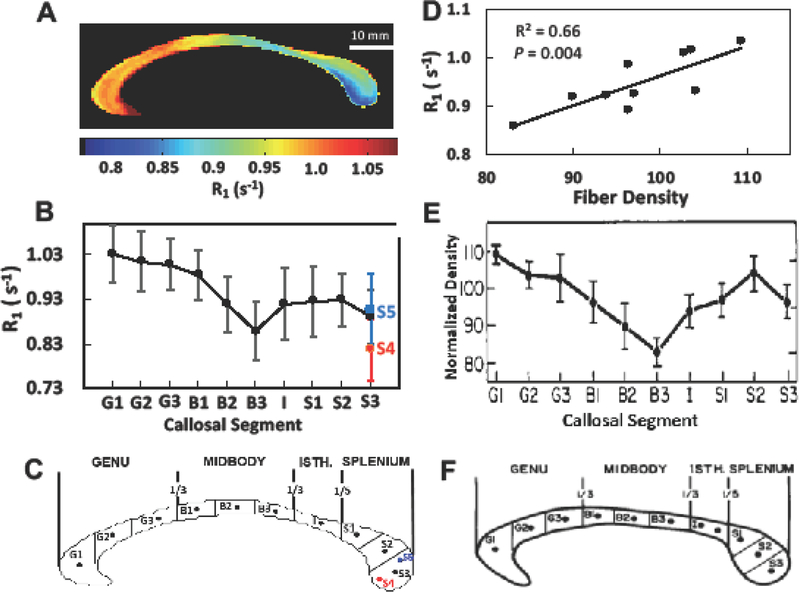
There was a strong correlation between the R1 values from MRI measurement (Figs. 4A and 4B) and the normalized fiber density from histology measurement (Fig. 4E) along the equidistant 10 sectors (Fig. 4D; regression coefficient R = 0.81, P = 0.004); the lowest R1 value in the posterior midbody (B3) corresponded to the lowest fiber density, whereas the highest R1 in the anterior parts of the CC (G1~G3) corresponded to the highest fiber density. As shown in Fig. 3, R1 in the inferior splenium (S4) was significantly lower than in the middle (S3) / superior (S5) and even lower than in B3, implying the lowest fiber density among the CC subdivisions. Note that compared to the in vivo T1 (Fig. 3A) or R1 (Fig. 4A) imaging analysis, the histology analysis in the literature did not provide full spatial distribution of the fiber density across the entire CC structure since only a very small portion of callosal sub-sections could be analyzed for each CC subdivision (Aboitiz et al. 1992a). Interestingly, a recent diffusion study showed the similar distribution of CC axon diameters along the human CC, showing the high density of larger axon size in the inferior splenium as well as the mid-body (Genc et al. 2018). Taken together, our results suggest that the high-resolution callosal R1 index or relaxometry map measured at high field highly correlates to the myelinated fiber density across the entire human CC (Hypothesis 1).
Fig 4.
Correlation study between in vivo callosal R1 (=1/T1) distribution and myelinated fiber density in the postmortem data. (A) The averaged in vivo R1 relaxometry map of the human CC from all subjects (n = 16). (B) The in vivo R1 profile along ten equidistant callosal segments: anterior third (G1~G3), midbody (B1~B3), Isthmus (I), and splenium (S1~S3) as shown in (C). (E) The normalized myelinated fiber density profile. (D) The fiber density shown in Panel E and in vivo R1 shown in Panel B show a high correlation along the 10 segments. Error bar represents a standard deviation. The panels (E and F) were reprinted with the permission from the reference (Aboitiz et al. 1992a).
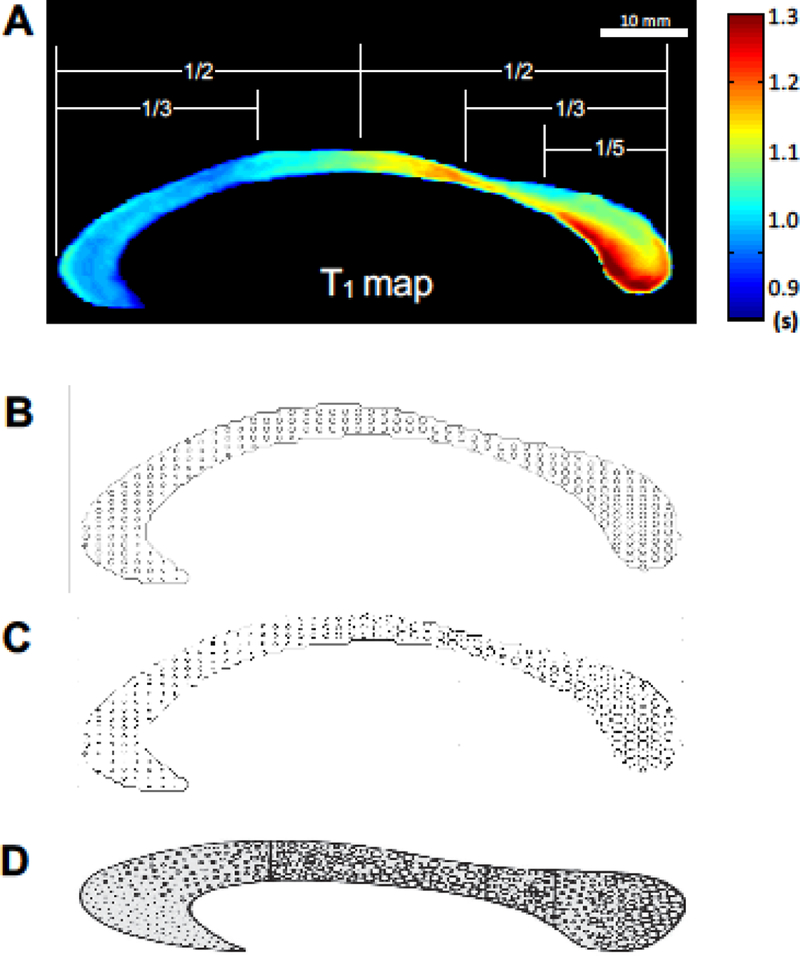
Figure 5B displays the estimated myelinated axon diameter map of the human CC (in a relative scale) based on the in vivo T1 imaging measurement, showing a heterogeneous distribution along the human CC axis. The re-scaled axon diameter map (Fig. 5C) shows a similar pattern with the schematic drawing of axon diameter from the histology study (Fig. 5D taken from the literature (Aboitiz and Montiel 2003)). The similarity suggests that the callosal T1 variation reflects different sizes of the myelinated axon in the human CC (Hypothesis 2).
Fig 5.
In vivo axon diameter maps of the human corpus callosum (A–C). Each panel represents the subject-averaged T1 relaxometry map (A), estimated in vivo axon diameter maps (B and C), and manual drawing of axon diameter map reported in the literature (Fig. 1. from the reference (Aboitiz and Montiel 2003)) (D). Panel C was drawn after rescaling the axon size distribution for matching the map shown in (D). There was a qualitatively similar distribution of axon diameter between the in vivo and postmortem estimations: large-size fibers are shown towards the posterior midbody and splenium, while small-size fibers are found in the anterior callosum. Note that all axonal diameter maps provide schematic illustrations by scaling the non-uniform fiber size across the CC, thus, they do not represent the exact ratio of diameters between fibers with varied diameter. The panel in D was reprinted with the permission from the reference (Aboitiz and Montiel 2003).
Callosal Fiber Connectivity
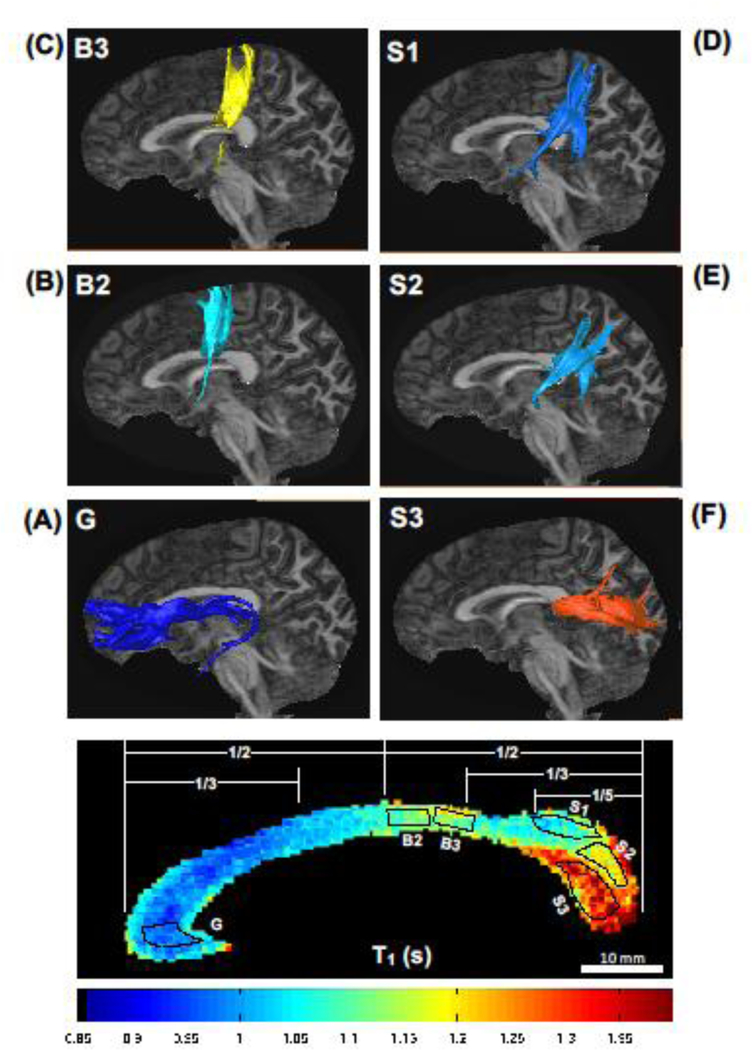
Six representative ROIs were defined (i.e., ROI G-S3 as shown in Fig. 6 from a representative subject) for DTT Fiber tractography based on callosal regional T1 profiles. DTT Fiber tractography analysis showed that the callosal fiber bundles located in the genu with the smallest T1 value (ROI G, T1 = 0.98 ± 0.02 s) were primarily connected to the prefrontal cortex (Fig. 6A). The fiber bundles from two ROIs located in the posterior midbody of B2 (ROI B2, T1 = 1.09 ± 0.02 s; ROI B3, T1 = 1.13 ± 0.03 s) were connected to the primary motor cortex (Fig. 6B) and the somatosensory cortex (Fig. 6C), respectively. The fiber bundles in the superior (ROI S1, T1 = 1.07 ± 0.03 s) and middle part of the splenium (ROI S2, T1 = 1.14 ± 0.05 s) were mainly connected to the parietal lobe (Fig. 6D) and temporal lobe (Fig. 6E), respectively. In contrast, the ROI located in the inferior part of splenium with the highest T1 value (ROI S3, T1 = 1.31 ± 0.06 s) projected the callosal axons to the visual cortex (Fig. 6F). These results support the reasoning that the regional T1 profile of CC could reflect the key topographic features of callosal subdivisions in the human brain (Hypothesis 3).
Fig 6.
Diffusion tractography for the human callosal fibers. The callosal fibers from each seeding region of interest (ROI, the dotted areas), which was delineated from the callosal T1 relaxometry map (panel at the bottom), were connected to the structurally projected cortical areas: (A) ROI G to prefrontal lobe; (B) ROI B2 to motor cortex; (C) ROI B3 to sensory cortex; (D-E) ROIs S1 and S2 to parietal-temporal lobes; and (F) ROI S3 to occipital lobe.
Discussion
In this study, we demonstrate that the high-resolution T1 relaxometry at 7T showed greater sensitivity and specificity for detecting the large degree of T1 heterogeneity of the human CC in a fine spatial scale. In addition, such regional T1 variation was relevant to the topographical organization of the fiber connectivity of origin, which was not shown in the previous MR relaxometry studies. Based on a tight correlation of T1 (or R1) to the myelinated axon size and myelin density, the callosal T1 variation may reflect the biological variation, diversity, and structural/functional specialization of the callosal microstructure. Thus, our in vivo results support a key scientific notion: interhemispheric communication for sensorimotor/auditory/visual processing through the CC could be facilitated by the larger-diameter myelinated axons with a faster conduction velocity (Aboitiz et al. 1992a; Caminiti et al. 2013), which was associated with a higher T1 value or lower myelin density in the connected CC subdivisions. In contrast, the relatively slower communication between higher-order neural processing cortical areas encompassing the frontal and parietal lobes could be facilitated by relatively higher fiber density and smaller-diameter axons with a low conduction velocity (Berlucchi 1972; Aboitiz and Montiel 2003). Therefore, the high-resolution T1 relaxometry could provide a valuable and robust surrogate reflecting the callosal fiber and axon microarchitecture and neural communication functionality.
To date, several new classification methods for the CC partitions have been proposed to revise the classic viewpoint of human CC topology. For instance, DTT-based parcellation methods have indicated that most fibers in the primary motor cortex were projected toward the posterior midbody of the human CC (Hofer and Frahm 2006). Moreover, fibers from the primary visual cortex were projected toward the inferior part of the splenium (Huang et al. 2005; Putnam et al. 2010; Saenz and Fine 2010). Notably, these findings are strikingly consistent with our observation of regional callosal T1 distributions (Fig. 3) and the concomitant fiber connectivity to the cortex (Fig. 6). Thus, the high-resolution T1 map could be useful as a hallmark underlying topographic organization of the callosal subdivisions. Furthermore, it could provide a feature of the fiber connectivity of individual subjects and account for the inter-subject variability of callosal topography (Aboitiz et al. 1992b; Dougherty et al. 2005a).
Changes in T1 or R1 values in the CC are largely attributed to the degree of the myelinated fiber density or myelin-bound water fraction. R1 relaxivity in the brain tissue can be described as the following (Rooney et al. 2007; Stuber et al. 2014):
| [5] |
Where R1s is the relaxivity of a pure saline solution (at physiological temperature) that can be treated as a constant; R1M and R1Fe are the T1 relaxivities of macromolecule and iron, respectively. dM is the macromolecular density and [Fe] is the iron concentration in the tissue. Since macromolecular composition is rich in the myelin sheath in the human CC tissue, R1M and dM could represent the relaxivity of myelin and myelin density, respectively, in Eqn. [5]. The R1 variation ( R1) within the CC structure can be approximated by the following equation:
| [6] |
Interestingly, the contribution from the second term in the CC is negligible in considering several factors; i) very low [Fe] in the white matter (WM) compared to the sub-cortical grey matter tissues (Haacke et al. 2005; Langkammer et al. 2010; Hallgren and Sourander 1958); ii) less R1 dependence on [Fe] compared to the R2 dependence in WM (Haacke et al. 2005); and iii) a weak correlation between WM R1 and [Fe] across the brain compared to the relation of R2 or R * with [Fe] (Gelman et al. 2001; Gelman et al. 1999). Thus, the contribution of the second term in Eqn. [6] becomes negligible and the first term dominates the spatial R1 variation in the human CC tissue and the equation can be simplified to:
| [7] |
Therefore, the parametric R1 change mainly results from the underlying microstructural properties of myelinated CC tissue and is directly proportional to the myelin density (dM ).
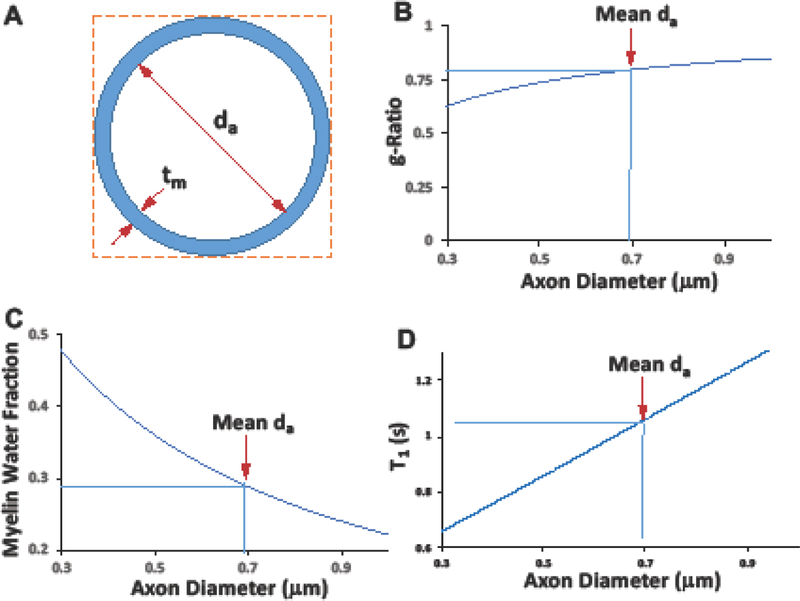
The tissue properties of myelinated axons and distribution have been documented with great details by the histology studies. The majority of human CC axons (> 75%) have a diameter range of 0.3 – 1.0 m (mean = 0.69 m) (Liewald et al. 2014). Very few myelinated axons in the human brain CC have a small diameter (≤ 0.3 m) (Waxman and Bennett 1972) and less than 1% of axons have a large diameter (3 m) (Aboitiz et al. 1992a). In addition, the thickness of myelin sheath (tm ~ 0.09 m) is relatively uniform for the axon population with a diameter range of < 1.0 m (Aboitiz et al. 1992a; Liewald et al. 2014). Given these factors and parameters reported in the literature, we proposed a simplified model to estimate the relation between axon diameter (da) and the MWF (Fig. 7C) or the T1 parameter (Fig. 7D) for the human CC. The predicted T1 dependence on the axon diameter is consistent with the reports of histological studies for a similar range of axon diameter (Waxman and Bennett 1972; Ford et al. 2015; Wang et al. 2016). As shown in Fig. 7C, an increase in the axon diameter decreases the MWF (or myelin density) in the human CC, resulting in a lower R1 (or a longer T1) value according to Eqn. [7] (Harkins et al. 2016; Wang et al. 2016). Recent in vivo MRI study showed that the MWF in the brain tends to increase in the anterior part of callosum and decrease in the mid-body and splenium (Stikov et al. 2015a). In line with our observation of the high correlate of the callosal R1 with the fiber density (Fig. 4D) and T1 dependence on axon diameter (Fig. 7D), it is likely that the high population of small-size axons in the anterior callosum links high myelin contents and/or increase myelin-water interface, consequentially leading to a short T1 relaxation time. In contrast, the higher population of the large axons located in the posterior midbody and inferior splenium have relatively lower myelin contents and/or reduced myelin-water interfaces, resulting in a long T1 value (Saenz and Fine 2010; Hofer and Frahm 2006; Dougherty et al. 2005b).
Fig 7.
In vivo model for myelinated axonal diameter. (A) A simplified model of myelinated axon diameter under assumption of an approximation of constant myelin sheath thickness (tm ~ 0.09 m). (B) The estimated microstructure g-ratio , where da is the axon diameter, and its mean value is 0.69 m (pointed by the red arrow) in the human CC (Aboitiz et al. 1992b; Liewald et al. 2014)). (C) The estimated myelin water fraction ( or as a function of axon diameter. (D) The estimated relation between T1 and MWF (T1 = 0.291/MWF +0.05).
We postulate that the mean of callosal T1 value (~1.04 s) over the entire mid-sagittal CC slice should correspond to the mean value of the human CC axon diameter (~ 0.69 m (Liewald et al. 2014)). Given the T1-based model for axon diameter as depicted in Fig. 7D, the estimated diameter of the superior splenium (S1 in Fig. 3, T1 = 1.08 s) and the inferior splenium (S3 in Fig. 3, T1 = 1.25 s) were 0.72 m and 0.89 m, respectively, resulting in up to 19% difference within the splenium. On the other hand, the genu (Segment G in Fig. 3, T1 = 0.97 s) has the smallest mean axon diameter of 0.61 m, resulting in 31% difference compared to the inferior splenium.
Despite the similar pattern of in vivo T1 relaxometry data with the human CC fiber composition of post-mortem histology analysis, there are still limited factors to be considered for potential application and interpretation of the T1 relaxometry data for human brain. First, the current image resolution of T1 relaxometry is not sufficient to discriminate the populations of various fiber size down to a much fine spatial scale. Instead, we assumed that the T1 value measured from a single MRI voxel represents an averaged quantity from all types of fiber or myelinated axon size. Under this assumption, we aimed to understand the key characteristics of CC subdivisions as investigated in this study. Further improved resolution of callosal T1 image is needed to provide a more precise estimation of the fine fiber composition and distinct features of the human CC in healthy subjects. Secondly, the proposed model for axon diameter was used to visualize the key feature of callosal fiber diameter under the assumption of major contribution of MWF to the T1 relaxation changes. Considering the complexity of fiber structure of the CC, this simplified model might underestimate the other mechanisms responsible for T1 changes via the interactions with intracellular and extracellular water contents.
Conclusions
In summary, this study suggests that the CC characterization using the high-resolution T1 relaxometry mapping method has the potential to robustly assess and quantify the microstructural properties, which links its functional connectivity in the human brain. Therefore, this T1 imaging approach could be a highly useful neuroimaging tool for studying basic brain function and development, neural plasticity and aging process in healthy population.
Funding acknowledgement
This work was supported by NIH Grants RO1 NS070839 and MH111413; R24 MH106049, S10 RR026783, U01 EB026978, P41 EB015894, and P30NS076408 and the W.M. Keck Foundation.
Footnotes
Compliance with ethical standards
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aboitiz F, Montiel J (2003) One hundred million years of interhemispheric communication: the history of the corpus callosum. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al. ] 36 (4):409–420 [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E (1992a) Fiber composition of the human corpus callosum. Brain Res 598 (1–2):143–153 [DOI] [PubMed] [Google Scholar]
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E (1992b) Individual differences in brain asymmetries and fiber composition in the human corpus callosum. Brain Res 598 (1–2):154–161 [DOI] [PubMed] [Google Scholar]
- Alexander DC, Hubbard PL, Hall MG, Moore EA, Ptito M, Parker GJ, Dyrby TB (2010) Orientationally invariant indices of axon diameter and density from diffusion MRI. Neuroimage 52 (4):1374–1389. doi: 10.1016/j.neuroimage.2010.05.043 [DOI] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20 (2):870–888. doi: 10.1016/S1053-8119(03)00336-7 [DOI] [PubMed] [Google Scholar]
- Assaf Y, Alexander DC, Jones DK, Bizzi A, Behrens TE, Clark CA, Cohen Y, Dyrby TB, Huppi PS, Knoesche TR, Lebihan D, Parker GJ, Poupon C, consortium C, Anaby D, Anwander A, Bar L, Barazany D, Blumenfeld-Katzir T, De-Santis S, Duclap D, Figini M, Fischi E, Guevara P, Hubbard P, Hofstetter S, Jbabdi S, Kunz N, Lazeyras F, Lebois A, Liptrot MG, Lundell H, Mangin JF, Dominguez DM, Morozov D, Schreiber J, Seunarine K, Nava S, Poupon C, Riffert T, Sasson E, Schmitt B, Shemesh N, Sotiropoulos SN, Tavor I, Zhang HG, Zhou FL (2013) The CONNECT project: Combining macro- and micro-structure. Neuroimage 80:273–282. doi: 10.1016/j.neuroimage.2013.05.055 [DOI] [PubMed] [Google Scholar]
- Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ (2008) AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magn Reson Med 59 (6):1347–1354. doi: 10.1002/mrm.21577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34 (1):144–155. doi: 10.1016/j.neuroimage.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlucchi G (1972) Anatomical and physiological aspects of visual functions of corpus callosum. Brain Res 37 (2):371–392 [DOI] [PubMed] [Google Scholar]
- Björnholm L, Nikkinen J, Kiviniemi V, Nordström T, Niemelä S, Drakesmith M, Evans JC, Pike GB, Veijola J, Paus T (2017) Structural properties of the human corpus callosum: Multimodal assessment and sex differences. Neuroimage 152:108–118. doi: 10.1016/j.neuroimage.2017.02.056 [DOI] [PubMed] [Google Scholar]
- Budd JM, Kisvarday ZF (2012) Communication and wiring in the cortical connectome. Front Neuroanat 6:42. doi: 10.3389/fnana.2012.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan PT, Eccles CD, Xia Y (1988) NMR microscopy of dynamic displacements: k-space and q-space imaging. Journal of Physics E: Scientific Instruments 21 (8):820 [Google Scholar]
- Caminiti R, Carducci F, Piervincenzi C, Battaglia-Mayer A, Confalone G, Visco-Comandini F, Pantano P, Innocenti GM (2013) Diameter, length, speed, and conduction delay of callosal axons in macaque monkeys and humans: comparing data from histology and magnetic resonance imaging diffusion tractography. J Neurosci 33 (36):14501–14511. doi: 10.1523/JNEUROSCI.0761-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminiti R, Ghaziri H, Galuske R, Hof PR, Innocenti GM (2009) Evolution amplified processing with temporally dispersed slow neuronal connectivity in primates. Proc Natl Acad Sci U S A 106 (46):19551–19556. doi: 10.1073/pnas.0907655106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenevert TL, Brunberg JA, Pipe JG (1990) Anisotropic diffusion in human white matter: demonstration with MR techniques in vivo. Radiology 177 (2):401–405. doi: 10.1148/radiology.177.2.2217776 [DOI] [PubMed] [Google Scholar]
- de Lacoste MC, Kirkpatrick JB, Ross ED (1985) Topography of the human corpus callosum. J Neuropathol Exp Neurol 44 (6):578–591 [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Bammer R, Brewer AA, Wandell BA (2005a) Functional organization of human occipital-callosal fiber tracts. Proc Natl Acad Sci U S A 102 (20):7350–7355. doi: 10.1073/pnas.0500003102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch G, Potanina P, Bammer R, Wandell BA (2005b) Occipital-callosal pathways in children: Validation and atlas development. Ann N Y Acad Sci 1064:98–112. doi: 10.1196/annals.1340.017 [DOI] [PubMed] [Google Scholar]
- Fabri M, Pierpaoli C, Barbaresi P, Polonara G (2014) Functional topography of the corpus callosum investigated by DTI and fMRI. World J Radiol 6 (12):895–906. doi: 10.4329/wjr.v6.i12.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, Kopp-Scheinpflug C, Pecka M, Attwell D, Grothe B (2015) Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat Commun 6:8073. doi: 10.1038/ncomms9073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood M, DelaBarre L (2001) The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson 153 (2):155–177. doi: 10.1006/jmre.2001.2340 [DOI] [PubMed] [Google Scholar]
- Gelman N, Ewing JR, Gorell JM, Spickler EM, Solomon EG (2001) Interregional variation of longitudinal relaxation rates in human brain at 3.0 T: relation to estimated iron and water contents. Magn Reson Med 45 (1):71–79 [DOI] [PubMed] [Google Scholar]
- Gelman N, Gorell JM, Barker PB, Savage RM, Spickler EM, Windham JP, Knight RA (1999) MR imaging of human brain at 3.0 T: preliminary report on transverse relaxation rates and relation to estimated iron content. Radiology 210 (3):759–767. doi: 10.1148/radiology.210.3.r99fe41759 [DOI] [PubMed] [Google Scholar]
- Genc S, Malpas CB, Ball G, Silk TJ, Seal ML (2018) Age, sex, and puberty related development of the corpus callosum: a multi-technique diffusion MRI study. Brain Struct Funct 223 (6):2753–2765. doi: 10.1007/s00429-018-1658-5 [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14 (1 Pt 1):21–36 [DOI] [PubMed] [Google Scholar]
- Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23 (1):1–25. doi: 10.1016/j.mri.2004.10.001 [DOI] [PubMed] [Google Scholar]
- Hallgren B, Sourander P (1958) The Effect of Age on the Non-Haemin Iron in the Human Brain. J Neurochem 3 (1):41–51. doi:DOI 10.1111/j.1471-4159.1958.tb12607.x [DOI] [PubMed] [Google Scholar]
- Hargreaves BA, Cunningham CH, Nishimura DG, Conolly SM (2004) Variable-rate selective excitation for rapid MRI sequences. Magn Reson Med 52 (3):590–597. doi: 10.1002/mrm.20168 [DOI] [PubMed] [Google Scholar]
- Harkins KD, Xu J, Dula AN, Li K, Valentine WM, Gochberg DF, Gore JC, Does MD (2016) The microstructural correlates of T1 in white matter. Magn Reson Med 75 (3):1341–1345. doi: 10.1002/mrm.25709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins Kevin D, Xu J, Dula Adrienne N, Li K, Valentine William M, Gochberg Daniel F, Gore John C, Does Mark D (2016) The microstructural correlates of T1 in white matter. Magn Reson Med 75 (3):1341–1345. doi:doi: 10.1002/mrm.25709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Frahm J (2006) Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32 (3):989–994. doi: 10.1016/j.neuroimage.2006.05.044 [DOI] [PubMed] [Google Scholar]
- Hofer S, Wang X, Roeloffs V, Frahm J (2015) Single-shot T1 mapping of the corpus callosum: a rapid characterization of fiber bundle anatomy. Front Neuroanat 9:57. doi: 10.3389/fnana.2015.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, van Zijl PC, Hillis AE, Wytik R, Mori S (2005) DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage 26 (1):195–205. doi: 10.1016/j.neuroimage.2005.01.019 [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Caminiti R, Hof PR (2010) Fiber composition in the planum temporale sector of the corpus callosum in chimpanzee and human. Brain Struct Funct 215 (2):123–128. doi: 10.1007/s00429-010-0274-9 [DOI] [PubMed] [Google Scholar]
- Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53 (6):1432–1440. doi: 10.1002/mrm.20508 [DOI] [PubMed] [Google Scholar]
- Kontis D, Catani M, Cuddy M, Walshe M, Nosarti C, Jones D, Wyatt J, Rifkin L, Murray R, Allin M (2009) Diffusion tensor MRI of the corpus callosum and cognitive function in adults born preterm. Neuroreport 20 (4):424–428. doi: 10.1097/WNR.0b013e328325a8f9 [DOI] [PubMed] [Google Scholar]
- Lamantia AS, Rakic P (1990) Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey. J Comp Neurol 291 (4):520–537. doi: 10.1002/cne.902910404 [DOI] [PubMed] [Google Scholar]
- Langkammer C, Krebs N, Goessler W, Scheurer E, Ebner F, Yen K, Fazekas F, Ropele S (2010) Quantitative MR imaging of brain iron: a postmortem validation study. Radiology 257 (2):455–462. doi: 10.1148/radiol.10100495 [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161 (2):401–407. doi: 10.1148/radiology.161.2.3763909 [DOI] [PubMed] [Google Scholar]
- Lee B-Y, Zhu X-H, Li X, Chen W (2014) Quantitative Assessment of Microstructure Properties of Human Corpus Callosum using Parametric T1 and Myelin imaging. In: Proc Intl Soc Mag Reson Med:p 3236
- Li X, Bolan PJ, Ugurbil K, Metzger GJ (2015) Measuring renal tissue relaxation times at 7 T. NMR Biomed 28 (1):63–69. doi: 10.1002/nbm.3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liewald D, Miller R, Logothetis N, Wagner HJ, Schuz A (2014) Distribution of axon diameters in cortical white matter: an electron-microscopic study on three human brains and a macaque. Biol Cybern 108 (5):541–557. doi: 10.1007/s00422-014-0626-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Uğurbil K (2010) Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med 63 (5):1144–1153. doi: 10.1002/mrm.22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RE (1959) Localization of function in the corpus callosum. Visual gnostic transfer. Arch Neurol 1:74–77 [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Sharma T, Moussai J, Cannestra AF, Toga AW (2000) Mapping morphology of the corpus callosum in schizophrenia. Cereb Cortex 10 (1):40–49 [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, Lee JD (2008) Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum Brain Mapp 29 (5):503–516. doi: 10.1002/hbm.20314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR (1990) Function of the corpus callosum in the representation of the visual field in cat visual cortex. Vis Neurosci 5 (2):205–211 [DOI] [PubMed] [Google Scholar]
- Putnam MC, Steven MS, Doron KW, Riggall AC, Gazzaniga MS (2010) Cortical projection topography of the human splenium: hemispheric asymmetry and individual differences. J Cogn Neurosci 22 (8):1662–1669. doi: 10.1162/jocn.2009.21290 [DOI] [PubMed] [Google Scholar]
- Rooney WD, Johnson G, Li X, Cohen ER, Kim SG, Ugurbil K, Springer CS Jr. (2007) Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med 57 (2):308–318. doi: 10.1002/mrm.21122 [DOI] [PubMed] [Google Scholar]
- Saenz M, Fine I (2010) Topographic organization of V1 projections through the corpus callosum in humans. Neuroimage 52 (4):1224–1229. doi: 10.1016/j.neuroimage.2010.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Lutti A, Weiskopf N, Dick F (2013) Mapping the human cortical surface by combining quantitative T(1) with retinotopy. Cereb Cortex 23 (9):2261–2268. doi: 10.1093/cercor/bhs213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigalovsky IS, Fischl B, Melcher JR (2006) Mapping an intrinsic MR property of gray matter in auditory cortex of living humans: a possible marker for primary cortex and hemispheric differences. Neuroimage 32 (4):1524–1537. doi: 10.1016/j.neuroimage.2006.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisti HM, Geurts M, Gooijers J, Heitger MH, Caeyenberghs K, Beets IA, Serbruyns L, Leemans A, Swinnen SP (2012) Microstructural organization of corpus callosum projections to prefrontal cortex predicts bimanual motor learning. Learn Mem 19 (8):351–357. doi: 10.1101/lm.026534.112 [DOI] [PubMed] [Google Scholar]
- Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, Glasser MF, Hernandez M, Sapiro G, Jenkinson M, Feinberg DA, Yacoub E, Lenglet C, Van Essen DC, Ugurbil K, Behrens TE, Consortium WU-MH (2013) Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage 80:125–143. doi: 10.1016/j.neuroimage.2013.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stikov N, Campbell JS, Stroh T, Lavelee M, Frey S, Novek J, Nuara S, Ho MK, Bedell BJ, Dougherty RF, Leppert IR, Boudreau M, Narayanan S, Duval T, Cohen-Adad J, Picard PA, Gasecka A, Cote D, Pike GB (2015a) In vivo histology of the myelin g-ratio with magnetic resonance imaging. Neuroimage 118:397–405. doi: 10.1016/j.neuroimage.2015.05.023 [DOI] [PubMed] [Google Scholar]
- Stikov N, Campbell JS, Stroh T, Lavelee M, Frey S, Novek J, Nuara S, Ho MK, Bedell BJ, Dougherty RF, Leppert IR, Boudreau M, Narayanan S, Duval T, Cohen-Adad J, Picard PA, Gasecka A, Cote D, Pike GB (2015b) Quantitative analysis of the myelin g-ratio from electron microscopy images of the macaque corpus callosum. Data Brief 4:368–373. doi: 10.1016/j.dib.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stikov N, Perry LM, Mezer A, Rykhlevskaia E, Wandell BA, Pauly JM, Dougherty RF (2011) Bound pool fractions complement diffusion measures to describe white matter micro and macrostructure. Neuroimage 54 (2):1112–1121. doi: 10.1016/j.neuroimage.2010.08.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber C, Morawski M, Schafer A, Labadie C, Wahnert M, Leuze C, Streicher M, Barapatre N, Reimann K, Geyer S, Spemann D, Turner R (2014) Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. Neuroimage 93 Pt 1:95–106. doi: 10.1016/j.neuroimage.2014.02.026 [DOI] [PubMed] [Google Scholar]
- Thapaliya K, Vegh V, Bollmann S, Barth M (2017) Assessment of microstructural signal compartments across the corpus callosum using multi-echo gradient recalled echo at 7 T. Neuroimage doi: 10.1016/j.neuroimage.2017.11.029 [DOI] [PubMed]
- Tomasi S, Caminiti R, Innocenti GM (2012) Areal differences in diameter and length of corticofugal projections. Cereb Cortex 22 (6):1463–1472. doi: 10.1093/cercor/bhs011 [DOI] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ (2002) High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 48 (4):577–582. doi: 10.1002/mrm.10268 [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, Lenglet C, Wu X, Schmitter S, Van de Moortele PF, Strupp J, Sapiro G, De Martino F, Wang D, Harel N, Garwood M, Chen L, Feinberg DA, Smith SM, Miller KL, Sotiropoulos SN, Jbabdi S, Andersson JL, Behrens TE, Glasser MF, Van Essen DC, Yacoub E, Consortium WU-MH (2013) Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. Neuroimage 80:80–104. doi: 10.1016/j.neuroimage.2013.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu AT, Auerbach E, Lenglet C, Moeller S, Sotiropoulos SN, Jbabdi S, Andersson J, Yacoub E, Ugurbil K (2015) High resolution whole brain diffusion imaging at 7T for the Human Connectome Project. Neuroimage 122:318–331. doi: 10.1016/j.neuroimage.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U (2007) Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci 27 (45):12132–12138. doi: 10.1523/JNEUROSCI.2320-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhu XH, Zhang Y, Chen W In vivo Parametric T1/R1 Imaging Correlation with Myelin Density and Microstructure Properties of Rat Corpus Callosum. In: Proc. Intl. Soc. Mag. Reson. Med, Singapore, 2016. p 1465 [Google Scholar]
- Waxman SG, Bennett MV (1972) Relative conduction velocities of small myelinated and non-myelinated fibres in the central nervous system. Nat New Biol 238 (85):217–219 [DOI] [PubMed] [Google Scholar]
- Witelson SF (1989) Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain 112 (Pt 3):799–835 [DOI] [PubMed] [Google Scholar]
- Wright PJ, Mougin OE, Totman JJ, Peters AM, Brookes MJ, Coxon R, Morris PE, Clemence M, Francis ST, Bowtell RW, Gowland PA (2008) Water proton T1 measurements in brain tissue at 7, 3, and 1.5 T using IR-EPI, IR-TSE, and MPRAGE: results and optimization. MAGMA 21 (1–2):121–130. doi: 10.1007/s10334-008-0104-8 [DOI] [PubMed] [Google Scholar]
- Yarnykh VL (2007) Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med 57 (1):192–200. doi: 10.1002/mrm.21120 [DOI] [PubMed] [Google Scholar]
- Zhang H, Hubbard PL, Parker GJ, Alexander DC (2011) Axon diameter mapping in the presence of orientation dispersion with diffusion MRI. Neuroimage 56 (3):1301–1315. doi: 10.1016/j.neuroimage.2011.01.084 [DOI] [PubMed] [Google Scholar]