Abstract
Kinase fusions are rare and poorly characterized in colorectal carcinoma (CRC), yet they present unique opportunities for targeted therapy. In this study, we characterized kinase fusions from patients with advanced CRC who had MSK-IMPACT testing of their tumors between January 2014 and June 2018. Patients were analyzed for the presence of fusions, microsatellite instability (MSI), and RAS/BRAF mutations. Mismatch repair (MMR) immunohistochemistry (IHC) and promoter hypermethylation status of MLH1 (MLH1ph) in MSI-H CRC with fusions were investigated. Fusion transcripts were confirmed using a targeted RNAseq panel assay. Of 2314 CRCs with MSK-IMPACT testing, 21 harbored kinase fusions. Overall 57% (12/21) of CRC fusions were MSI-H/MMR-D. Loss of MLH1 and MLH1ph was confirmed in all 12 and all 10 cases with available material, respectively. Fusions were present in 5% of MSI-H/MMR-D CRC compared to 0.4% of MSS/MMR-P CRC (p<0.001), and 15% of MSI-H/MMR-D CRC with wild type RAS/BRAF. Of 24 total MLH1-deficient CRC with MLH1ph and wild type RAS/BRAF, 10 (42%) harbored kinase fusions. Kinase fusions in MSI-H CRC were associated with sporadic MLH1ph rather than with Lynch syndrome, and these patients may be eligible for kinase inhibitors, particularly following resistance or toxicity in response to immunotherapy. These findings identify a molecular subset of CRC with kinase fusions that may be responsive to kinase inhibitors.
Keywords: colorectal carcinoma, MLH1 hypermethylation, microsatellite instability, mismatch repair deficiency, fusion, RET, ROS1, NTRK1, NTRK3, BRAF
PRECIS
A high frequency of targetable kinase fusions in BRAF/RAS wild type, microsatelltie instability-high colorectal carcinoma offers a rationale for routine screening to identify CRC patients with kinase fusions that may be responsive to kinase inhibitors.
INTRODUCTION
Approximately 15% of colorectal carcinomas (CRCs) demonstrate mismatch repair deficiency (MMR-D)/ microsatellite instability- high (MSI-H) status. The majority of these are MLH1/ PMS2 deficient due to MLH1 promoter hypermethylation (MLH1ph). BRAF V600E mutations occur in approximately 50% of CRC with MLH1ph and have been shown to induce MLH1ph via upregulation of the transcriptional regulator MAFG (1). KRAS mutations occur in approximately 30% of MSI-H CRC MLH1ph (2), leaving 20% of CRC with MLH1ph without a known driver activating the MAPK signaling pathway. Isolated cases of MSI-H CRC with fusions have recently been reported (3–5), and we noted a similar trend in our clinical next generation sequencing (NGS) data. We provide a detailed delineation of this association, defining a previously unappreciated subset of CRC with important therapeutic implications.
MATERIALS AND METHODS
Written informed consent was obtained from patients, approval was obtained from our institutional review board, and this retrospective study was conducted in accordance with U.S. Common Rule. CRC accessioned for MSK-IMPACT (6) and/ or Archer NGS testing were assessed for kinase fusions. Patients with MSK-IMPACT testing had MSI status routinely assessed as a component of the assay (7). Archer fusion testing was clinically performed when sufficient remaining material was present for cases with WT KRAS, NRAS, and BRAF by MSK-IMPACT or a 95 gene Ampliseq-based assay, the latter performed when material was insufficient for MSK-IMPACT. Archer was also performed to confirm fusion transcripts in cases with novel DNA-level structural variants predicted to form kinase fusions. The custom Archer panel used covers fusions involving the kinase domains of the following genes: ALK, BRAF, EGFR, ERBB2, ERBB4, FGFR1, FGFR2, FGFR3, KIT, MET, NTRK1, NTRK2, NTRK3, RET, and ROS1. When tissue is available, MMR IHC is routinely clinically performed, and these data were recorded for patients with Ampliseq testing (which does not generate MSI status results).
Clinicopathologic characteristics of all CRC with kinase fusions were assessed. Primary site was classified as either proximal (cecum to transverse colon) or distal (splenic flexure to rectum). Differentiation and mucinous histology were scored based on World Health Organization criteria (8). Well differentiated CRC had >95% gland formation, moderately differentiated CRC had 50–95% gland formation, poorly differentiated CRC had 0–49% gland formation. Mucinous adenocarcinoma had an extracellular mucin component of >50% while CRC with a mucinous component had extracellular mucin pools comprising <50% of the lesion.
MLH1ph was detected via ebisulfite conversion followed by either pyrosequencing or methylation array depending on specimen availability. CRC with MLH1ph and wild type (WT) for KRAS or NRAS p. G12, G13, Q61, K117, A146 and BRAF p. V600 alleles were retrospectively screened with a custom Archer targeted RNAseq-based NGS assay used for fusion and alternative isoform testing (9). Confirmatory pan-Trk IHC was performed on CRC with NTRK fusions (10).
A subset of CRC with either BRAF V600E, kinase fusions, or KRAS mutations had genomic-wide methylation profiling performed using the Illumina methylationEPIC (850k) platform (11). After excluding CpG sites from the MLH1 gene and X/Y chromosomes from the datasets, unsupervised hierarchical clustering was performed on the 10,000 most variable CpG sites (by standard deviation) using Euclidean distance and Ward’s method with R (version 3.4).
MSK-IMPACT including MSIsensor, Archer, Ampliseq MMR IHC, pan-Trk IHC, and MLH1ph assays are clinically validated assays that were performed in CLIA-accredited laboratories.
RESULTS
Prevalence and Spectrum of Kinase Fusions in CRC
We identified 2314 CRC accessioned for MSK-IMPACT and/ or Archer between January 2014 and June 2018. This dataset included 2309 CRC patients with MSK-IMPACT results of which 189 also underwent Archer targeted RNAseq testing, and 5 additional patients with insufficient material for MSK-IMPACT whose tumors underwent RAS/ BRAF testing by Ampliseq followed by Archer testing. Seventeen CRC were positive for kinase fusions via MSK-IMPACT. Four additional CRC with fusions were detected using Archer targeted RNAseq assay: 3 cases were negative by MSK-IMPACT due to lack of coverage of breakpoints (EML4-NTRK3, FGFR3-STAB1, and FGFR2-MYH15), while the fourth case (TPM3-NTRK1) identified by Archer testing alone had insufficient DNA for MSK-IMPACT and had WT KRAS/NRAS/BRAF by outside NGS testing, yielding a total of 21 CRC positive for kinase fusions.
The detected fusions included 8 NTRK fusions (6 NTRK1 and 2 NTRK3), 5 BRAF fusions 4 RET fusions, 2 FGFR fusions (1 each of FGFR2 and FGFR3), 1 ROS1 fusion, and 1 ALK fusion(Table 1, Figure 1). All detected kinase fusions were predicted to be in frame, included the kinase domain of the 3’ gene, and occurred in CRC that were BRAF/RAS WT. All 6 NTRK1 fusions and 1 of the 2 NTRK3 fusions were positive for pan-Trk IHC, with results as previously described (10).
Table 1.
Spectrum and molecular characteristics of kinase fusions in CRC.
| CASE | PARTNER GENE | EXON | KINASE GENE | EXON | MMR IHC | MSI STATUS | MLH1 PROMOTER HYPERMETHYLATION | FUSION DETECTED BY |
|---|---|---|---|---|---|---|---|---|
| 1 | LMNA | 8 | NTRK1 | 12 | MMR-D (MLH1/ PMS2) | MSI-H | POSITIVE | IMPACT |
| 2 | CCDC | 8 | RET | 12 | MMR-D (MLH1/ PMS2) | MSI-H | POSITIVE | IMPACT + ARCHER |
| 3 | TPM3 | 10 | NTRK1 | 9 | MMR-D (MLH1/ PMS2) | MSI-H | POSITIVE | IMPACT |
| 4 | LMNA | 2 | NTRK1 | 11 | MMR-D (MLH1/ PMS2) | MSI-H | POSITIVE | IMPACT |
| 5 | ETV6 | 6 | NTRK3 | 15 | MMR-D (MLH1/ PMS2) | MSI-H | POSITIVE | IMPACT |
| 6 | SPTBN1 | 7 | ALK | 20 | MMR-D (MLH1/ PMS2) | MSI-H | N/A* | IMPACT |
| 7 | GEMIN5 | 24 | RET | 12 | MMR-D (MLH1/ PMS2) | MSI-H | POSITIVE | IMPACT + ARCHER |
| 8 | TPM3 | 8 | NTRK1 | 10 | MMR-D (MLH1/ PMS2) | N/A* | POSITIVE | IMPACT + ARCHER |
| 9 | AGAP3 | 10 | BRAF | 9 | MMR-D (MLH1/ PMS2) | MSI-H | POSITIVE | IMPACT |
| 10 | EML4 | 2 | NTRK3 | 14 | MMR-D (MLH1/ PMS2) | MSI-H | POSITIVE | ARCHER (IMPACT NEGATIVE) |
| 11 | TPM3 | 8 | NTRK1 | 10 | MMR-D (MLH1/ PMS2) | N/A* | N/A* | ARCHER (IMPACT INSUFFICIENT) |
| 12 | TRIM24 | 14 | BRAF | 9 | MMR-D (MLH1/ PMS2) | MSI-H | POSITIVE | IMPACT + ARCHER |
| 13 | NCOA4 | 10 | RET | 12 | MMR-P | MSS | POSITIVE | IMPACT |
| 14 | LMNA | 12 | NTRK1 | 12 | MMR-P | MSS | NEGATIVE | IMPACT + ARCHER |
| 15 | GOPC | 4 | ROS1 | 36 | MMR-P | MSS | NEGATIVE | IMPACT + ARCHER |
| 16 | NCOA4 | 8 | RET | 12 | MMR-P | MSS | NEGATIVE | IMPACT |
| 17 | CUL1 | 7 | BRAF | 9 | MMR-P | MSS | N/A* | IMPACT |
| 18 | MKRN1 | 3 | BRAF | 10 | N/A* | MSS | N/A* | IMPACT + ARCHER |
| 19 | AGAP3 | 9 | BRAF | 9 | MMR-P | MSS | N/A* | IMPACT |
| 20 | FGFR3 | 17 | STAB1 | 51 | MMR-P | MSS | N/A* | ARCHER (IMPACT NEGATIVE) |
| 21 | FGFR2 | 14 | MYH15 | 31 | MMR-P | MSS | N/A* | ARCHER (IMPACT NEGATIVE) |
Testing was not performed.
Figure 1.
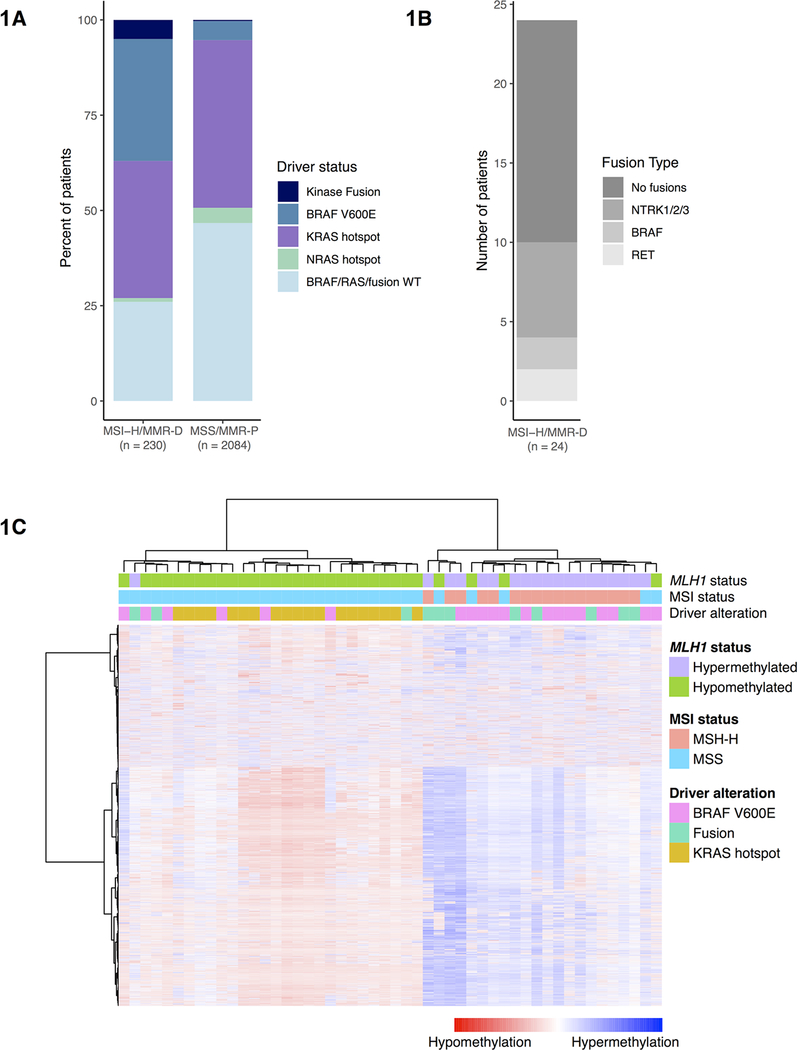
Prevalence of major MAPK driver alterations in molecular subgroups of CRC and methylation patterns. A) MSI-H (n=230) vs MSS CRC (n=2084) respectively harbored 74 (32%) vs 106 (5%) BRAF p. V600E mutations (p<0.0001), 83 (36%) vs 912 (44%) KRAS hotspot mutations (p=0.322), 2 (1%) vs 86 (4%) NRAS hotspot mutations (p=0.096), and 12 (5%) vs 9 (0.4%) kinase fusions (p<0.001). B) Of the 10 fusions detected in the group of 24 CRC with MLH1 promoter hypermethylation and WT RAS/ BRAF; there were 6 NTRK fusions, 2 BRAF fusions, and 2 RET fusions. C) Unsupervised hierarchical clustering of methylation array data using the most variable 10,000 CpG sites (excluding MLH1 loci) in a subset of BRAF p. V600E, KRAS mutant, and fusion positive CRC shows that MSI-H (MLH1 hypermethylated) BRAF p. V600E and fusion positive CRC predominantly colocalized to the hypermethylated cluster. KRAS-mutated CRCs all localized to the hypomethylated cluster.
Clinicopathologic Characteristics of Colorectal Carcinoma with Kinase Fusions
The age at diagnosis of these 21 CRC patients harboring kinase fusions ranged from 33–85 years with a median of 64 years. The majority (71%) of this cohort had CRC arising in the proximal colon. Poor differentiation (including medullary, n=2) was present in 57% of the fusion cases, while 16% of cases had a mucinous component. Looking further into the fusion cohort, 83% of MSI-H CRC had poor differentiation or were mucinous in histologic subtype while only 33% of MSS CRC with fusions had poor differentiation or a mucinous component. This data suggests that poor or mucinous differentiation may be associated with the MSI-H status rather than the presence of fusion. American Joint Committee on Cancer (AJCC) 8th edition stage at diagnosis included 6 stage II patients, 6 stage III patients, and 8 stage IV patients. Median follow up time since diagnosis was 18 months. Sixty-eight percent of patients had distant metastasis at end of follow up, and 76% of patients were alive at end of follow-up. These findings are summarized in Table 2.
Table 2.
Clinicopathologic features of colorectal carcinoma patients harboring kinase fusions.
| CASE | AGE AT DIAGNOSIS | SEX | PRIMARY SITE | SPECIMEN TESTED | HISTOLOGY/ DIFFERENTIATION | STAGE AT DIAGNOSIS | DISTANT METASTASES (AT END OF FOLLOW-UP) | FOLLOW-UP (MONTHS) | VITAL STATUS |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 33 | F | Distal | Primary | Poor, Mucinous Component | IV | Liver | 195 | Alive |
| 2 | 85 | M | Proximal | Primary | Poor | III | Liver | 9 | Deceased |
| 3 | 60 | F | Proximal | Primary | Poor | II | None | 120 | Alive |
| 4 | 72 | M | Proximal | Metastasis (Adrenal) | Poor | III | Adrenal | 71 | Alive |
| 5 | 61 | F | Proximal | Primary | Poor | IV | Liver, Lungs | 6 | Deceased |
| 6 | 57 | M | Distal | Primary | Poor | II | None | 18 | Alive |
| 7 | 70 | F | Proximal | Primary | Moderate | III | None | 15 | Alive |
| 8 | 58 | F | Proximal | Primary | Poor (medullary) | II | None | 11 | Alive |
| 9 | 83 | M | Proximal | Primary | Mucinous adenocarcinoma | II | None | 18 | Alive |
| 10 | 69 | F | Distal | Primary | Poor, Mucinous Component | IV | Liver, Stomach | 30 | Alive |
| 11 | 70 | F | Proximal | Metastasis (Neck) | Moderate | IV | Liver, Lung, Retroperitoneum, Neck | 15 | Alive |
| 12 | 83 | F | Proximal | Primary | Poor (medullary) | II | None | 6 | Alive |
| 13 | 66 | F | Proximal | Primary | Poor, Mucinous Component | IV | Apical Lymph Node | 26 | Deceased |
| 14 | 52 | F | Proximal | Primary, Metastasis x2 (Right abdomen, Liver) | Moderate | II | Abdominal wall, Liver | 58 | Alive |
| 15 | 36 | F | Distal | Primary | Moderate | III | None | 16 | Alive |
| 16 | 65 | M | Distal | Primary | Poor | III | Lung | 18 | Alive |
| 17 | 64 | F | Proximal | Primary | Poor | IV | Omentum, Peritoneum | 8 | Deceased |
| 18 | 64 | M | Proximal | Metastasis (Cerebullum) | Moderate | IV | Cerebellum, Lung | 80 | Alive |
| 19 | 63 | F | Proximal | Primary | Moderate | IV | Liver, Retroperitoneum, Lung, Spleen, Adrenals | 18 | Deceased |
| 20 | 52 | M | Distal | Primary | Moderate | 4 | Liver | 17 | Alive |
| 21 | 58 | F | Proximal | Primary | Moderate | 3 | Liver | 24 | Alive |
Relationship of MSI to the Presence of Kinase Fusions.
Of the 2314 total CRC, 230 were MSI-H/ MMR-D and 2084 were MSS/ MMR-P. The presence of kinase fusions was mutually exclusive with BRAF V600 and RAS hotspot mutations. The MSI-H/ MMR-D and MSS/MMR-P cohorts respectively harbored 74 (32%) vs 106 (5%) BRAF V600E mutations (p<0.001), 83 (36%) vs 912 (44%) KRAS hotspot mutations (p=0.322), 2 (1%) vs 86 (4%) NRAS hotspot mutations (p=0.096), and 12 (5%) vs 9 (0.4%) kinase fusions (p<0.001) (Figure 1). Fifteen percent of MSI-H/ MMR-D and 0.9% of MSS/ MMR-P CRC that were RAS/BRAF WT harbored kinase fusions.
MMR Deficiency and Relationship of MLH1 Hypermethylation Status to the Presence of Kinase Fusions
Twelve (57%) of 21 CRC with kinase fusions were MMR-D/ MSI-H. All MSI-H/MMR-D CRC with available material had MLH1/ PMS2 loss (n=12) and MLH1ph (n=10). Looking further into the 71 MSI-H CRC that were RAS/ BRAF WT, 47 were MLH1/ PMS2 deficient by IHC. Twenty-four of 37 of these MLH1/PMS2 deficient CRC with WT RAS/BRAF had MLH1ph data available were positive for MLH1ph. Of these 24 cases with MLH1 promoter hypermethylation, 10 harbored kinase fusions. Therefore, the incidence of fusions in MLH1 deficient CRC with MLH1ph and WT RAS/ BRAF was 42% (Figure 1).
Methylation Array Results
Due to the similarity of our findings relating fusions and MLH1ph to those of BRAF V600E and MLH1ph (1), we performed unsupervised hierarchical clustering of Illumina 850k methylation array data on both MSS and MSI-H CRC samples with fusions, BRAF V600E, and KRAS mutations after exclusion of MLH1 loci. Clear separation of hypermethylated and hypomethylated groups was evident. The hypermethylated group was composed of 2 predominant sub-clusters, suggesting CIMP-H and CIMP-L subgroupings. Eight out of 11 (73%) fusion-driven and fourteen out of twenty (70%) of BRAF V600E CRCs localized to the hypermethylated group. All 19 (100%) KRAS mutants segregated to the hypomethylated group. Interestingly, 2 MSS CRC (1 fusion and 1 BRAF V600E) harbored MLH1ph.
DISCUSSION
In recent years, cancers bearing kinase fusions have shown some of the most dramatic and durable responses to kinase inhibitors (12–13). For instance, larotrectinib has shown a response rate of 75% in adult patients with NTRK fusions, with 71% of responses ongoing and 55% of patients being progression-free at 1 year of treatment (12). While such targetable fusions are rare in CRC overall, the present study shows that approximately 15% of advanced MSI-H/ MMR-D CRC which are WT for BRAF/ KRAS/ NRAS harbor kinase fusions, and that all of the detected kinase fusions in MSI-H CRC occurred specifically in non-Lynch Syndrome cases with MLH1 deficiency associated with MLH1ph. Further, fusions were present in almost half of MLH1 deficient CRC with WT KRAS/ NRAS/ BRAF with MLH1ph.
A mechanistic basis for the relationship between BRAF V600E, genome-wide hypermethylation, and MSI has been proposed by Fang et al, who showed that BRAF V600E mutations in CRC induce CpG island hypermethylation including MLH1ph via upregulation of ERK and MAFG, resulting in deficient MMR (1). The strong relationship between kinase fusions and MLH1ph suggests fusions may induce a similar phenomenon. Results from our methylation array studies show that BRAF p. V600E mutant and kinase fusion positive CRC have similar genomic CpG methylation patterns even after exclusion of data from the MLH1 promoter CpG loci. Functional studies elucidating the mechanistic relationship between kinase fusions and MLH1ph are warranted.
Our study does have several limitations. These include the rarity of kinase fusions in CRC and resulting relatively small cohort, the fact that none of the MSI-H/ MMR-D CRC with fusions received a tyrosine kinase inhibitor and had available response data, and limited material on several of these cases, precluding MMR IHC, MSI testing, or MLH1ph.
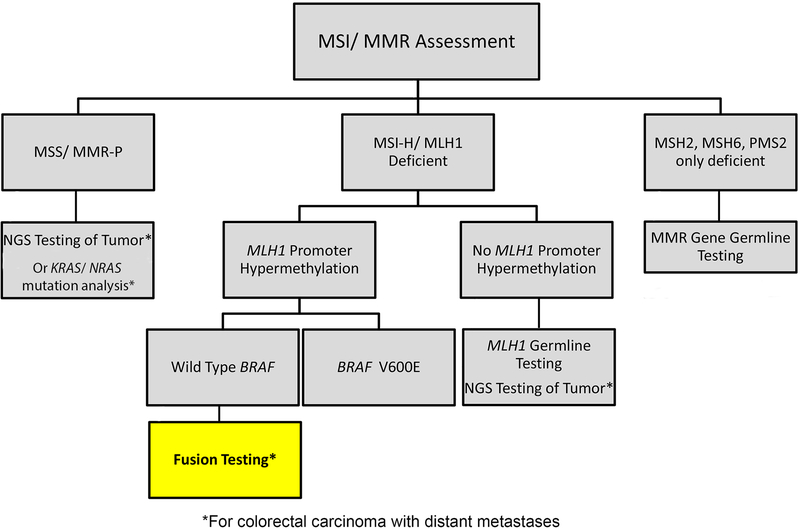
To our knowledge, this study is the first to establish the relationship between kinase fusions and MSI-H CRC, specifically with MLH1ph. Given the rarity of fusions and the fact that fusion testing is not routinely performed on CRC, it is important to identify subtypes that are more likely to carry these fusions. Thus, testing for kinase fusions is warranted in advanced CRC with MLH1ph and WT BRAF/ RAS and the present findings inform an updated proposed molecular testing workflow for CRC (Figure 2). This proposed updated workflow begins with universal MSI or MMR IHC as recommended by the NCCN (14). CRC patients with MSS/ MMR-P tumors should undergo NGS testing if available or KRAS/ NRAS mutation analysis for eligibility for anti-EGFR therapy. Patients with MSI-H/ MLH1 deficient CRC should undergo MLH1ph testing as part of the work-up for Lynch Syndrome. If MLH1ph is not detected, MLH1 germline testing to rule out Lynch Syndrome may be performed. For CRC patients with deficiency of MSH2, MSH6, and/ or PMS2 but not MLH1, germline testing of the deficient MMR gene is recommended due to the potential presence of Lynch Syndrome. If MLH1ph is present, the patient has distant metastases, and the tumor is negative for BRAF p. V600E mutation, fusion testing may be performed due to the high likelihood of finding a kinase fusion with potential therapeutic implications.
Figure 2.
Workflow for molecular testing in colorectal carcinoma. Testing for MSI/ MMR status should be performed universally in CRC. Patients with metastatic MSS/ MMR-P CRC should undergo next generation sequencing or RAS/ BRAF mutation testing. Patients with MLH1 deficiency of MSI-H results without available MMR IHC should undergo MLH1 promoter hypermethylation testing. If MLH1 promoter hypermethylation is detected in metastatic CRC and the tumor is negative for BRAF p. V600E, fusion testing should be performed. Patients with MMR-D of MSH2, MSH6, or PMS2 should receive germline testing.
Immune checkpoint inhibition produces response rates of 20%−50% of MSI-H CRC (15–16), and the presence of a kinase fusion would create a window of opportunity for treatment with kinase inhibitors when resistance or toxicity occurs after immune checkpoint inhibition therapy.
To conclude, while kinase fusions are rare in CRC overall (0.9%), 57% of kinase fusions in CRC occur in MMR-D/ MSI-H CRC. These cases have MLH1ph and WT BRAF/ RAS. Almost half of CRC with MLH1ph and WT RAS/ BRAF harbor kinase fusions. This subset of advanced CRC may benefit from screening for oncogenic kinase fusions.
ACKNOWLEDGMENTS
This study was funded by the National Cancer Institute (NCI) under the MSK Cancer Center Support Grant/Core Grant (P30 CA008748) and the R01CA226864 (to MS and AD).
Archer testing was supported in part by a grant from LOXO Oncology (to M.L.).
M.S. acknowledges Cycle for Survival.
E.C. acknowledges MSK society for a scholar prize.
Footnotes
This study was approved by the institutional review board at Memorial Sloan Kettering Cancer Center. The data in this manuscript have not been presented previously.
The authors have the following potential conflicts of interest to disclose:
J.F.H. has received honoraria from Medscape, the European Society of Medical Oncology, and Axiom Biotechnologies as well as research funding from Bayer.
M.S. is in the Advisory Board of Bioscience Institute, received research funds from Puma Biotechnoloy, Daiichi-Sankio and Menarini Ricerche, is a co-founder of Medendi Medical Travel and in the past two years he received honoraria from Menarini Ricerche and ADC Pharma. M.S. acknowledges Cycle for Survival.
D.M.H. has received personal fees from Atara Biotherapeutics, personal fees from Chugai Pharma, personal fees from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from Pfizer, personal fees from Bayer, personal fees from Debiopharm Group, personal fees from Genetech, grants from AstraZeneca, grants from Puma Biotechnology, grants from Loxo Oncology, outside the submitted work.
A.D. has honoraria from Medscape,OncLive, PeerVoice, Physician Education Resources, Tyra Biosciences, Targeted Oncology, MORE Health,Research to Practice, Foundation Medicine, PeerView, AstraZeneca, Genentech/ Roche, Bayer; consulting roles at Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Gnenetech/ Roche, Takeda, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, and Bayer.
M.L. has received honoraria for ad hoc advisory board participation from Astra-Zeneca, Bristol Myers Squibb, Takeda, and Bayer, and research support from LOXO Oncology (for expanded Archer targeted RNAseq testing) and Helsinn Therapeutics.
REFERENCES
- 1.Fang M, Ou J, Hutchinson L, Green MR. The BRAF oncoprotein functions through the transcriptional repressor MAFG to mediate the CpG Island Methylator phenotype. Mol Cell. 2014;55(6):904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farchoukh L, Kuan SF, Dudley B, Brand R, Nikiforova M, Pai RK. MLH1-deficient colorectal carcinoma with wild-type BRAF and MLH1 promoter hypermethylation harbor KRAS mutations and arise from conventional adenomas. Am J Surg Pathol. 2016;40(10):1390–1399. [DOI] [PubMed] [Google Scholar]
- 3.Pietrantonio F, Di Nicolantonio F, Schrock AB, et al. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann Oncol. 2018. ;29(6):1394–1401. [DOI] [PubMed] [Google Scholar]
- 4.Yakirevich E, Resnick MB, Mangray S, et al. Oncogenic ALK Fusion in Rare and Aggressive Subtype of Colorectal Adenocarcinoma as a Potential Therapeutic Target. Clin Cancer Res. 2016;22(15):3831–3840. [DOI] [PubMed] [Google Scholar]
- 5.Hechtman JF, Zehir A, Yaeger R, Wang L, Middha S, Zheng T, et al. Identification of targetable kinase alterations in patients with colorectal carcinoma that are preferentially associated with wild-type RAS/RAF. Mol Cancer Res. 2016;14(3):296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middha S, Zhang L , Nafa K, et al. Reliable Pan-Cancer Microsatellite Instability Assessment by Using Targeted Next-Generation Sequencing Data. DOI: 10.1200/PO.17.00084 JCO Precision Oncology - published online October 3, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosman FT, Carneiro F, Hruban R H, Theise N. WHO classification of tumours of the digestive system, fourth edition France: IARC; 2010 [cited 2018. July 10] Available from: http://www.ncbi.nlm.nih.gov/nlmcatalog/101553728 [Google Scholar]
- 9.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20(12):1479–84. [DOI] [PubMed] [Google Scholar]
- 10.Hechtman JF, Benayed R, Hyman DM, Drilon A, Zehir A, Frosina D, Arcila ME, Dogan S, Klimstra DS, Ladanyi M, Jungbluth AA. Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions. Am J Surg Pathol. 2017;41(11):1547–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran S, Arribas C, Esteller M. Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences. Epigenomics .2016;8(3):389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of Larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007. December;6(12 Pt 1):3314–22. [DOI] [PubMed] [Google Scholar]
- 14.Benson AB 3rd, Venook AP, Cederquist L,et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15(3):370–398. [DOI] [PubMed] [Google Scholar]
- 15.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]