Abstract
BACKGROUND:
Cancer is a deadly malignant disease and is prevalent in Sub Saharan Africa. The North East part of Nigeria in particular and the country, in general, are struggling to cope with the increasing burden of cancer and other communicable and non-communicable diseases. The situation is worsened by the ongoing insurgency and terrorist activities in the area.
AIM:
The aim of this paper is to present the research findings from a cohort study aimed at the analysis of the estimation of the survivorship time of the real data of cancer patients in the North-eastern part of Nigeria and to establish if the insurgency in the region has contributed negatively to the life expectancy of its inhabitants.
MATERIAL AND METHODS:
The record of 1,090 patients from medical records departments of the University of Maiduguri Teaching Hospital (UMTH), located in Maiduguri, the capital city of Borno State in northeast Nigeria was obtained. The record showed patients that were diagnosed and died of one type of cancer or the other from 2004 to 2017. All the cancer cases included in the present study were grouped into sex, age, marital status, occupation, date admitted and date of death/discharge. Descriptive statistics and Kaplan-Meier method were used to analyse the data using SPSS version 23 while Microsoft EXCEL and Minitab 16.0 were used for data cleansing and organisation.
RESULTS:
Of the 1,090 patients analysed, 920 (84.40%) experienced the event, i.e. death, while 170 (15.60%) patients were censored. The data were analysed based on the ages and sex of the patients. 50.20% of the patients were of ages 21-50 years. The proportions of patients in this age bracket surviving past 7 days are 75%, while those between ages 80 years and above is 12 days. Others are of survival time of 5 days (ages 0-20 years) and 7 days (51-79 years). Using sex, 75% of the patients’ survival time is 7 days in the case of male and 6 days for females. It is safe to say that the survival time for cancer patients of the university the Maiduguri is 6 days and the result reflects the Northeastern part of Nigeria. This is because the hospital is one of few tertiary healthcare facilities in that area and consequently, cancer cases are often referred there.
CONCLUSION:
Cancer incidence is high, and the probability of survival reduces as the survival time increases. This is a dire situation in need of urgent intervention from the government, groups and individuals to tackle the scourge of cancer, thereby improving on the life expectancy battered by the ongoing Boko Haram insurgency in that region.
Keywords: Boko Haram, Cancer, Censoring, Cohort, Kaplan Meier, Life expectancy, Northeast Nigeria, Statistics, Survival analysis
Introduction
Sub Saharan Africa is one of the areas that plagued with the prevalence of non-communicable diseases, especially cancers which is on the increase. Cancer of any type is the most common and lethal malignancies in developing countries like Nigeria. Cancer is an unwelcome guest in every home, and it is seen as a death sentence in Nigeria. Northeast Nigeria is one of the least developed areas of the country, lagging dangerously behind in virtually every development index. The region is notoriously staggering under the weights of poverty [1], insurgency and terrorism [2], [3], [4], hunger and low life expectancy [5], polio, maternal and child mortality [6], [7] and so on. Cancer is not only prevalent in the North East, Nigeria [8], [9], but to the entire country which are battling to cope with other health challenges such as mental health [10], [11], maternal and child health [12], [13] and HIV AIDS [14]. The high case fatality rate of cancer in Nigeria is due to low level of cancer awareness and screening, late discovery, unhealthy lifestyle, superstitious beliefs, limited or poorly funded healthcare facilities, the dearth of experts in oncology and others to mention but a few.
A patient’s journey along a disease pathway can be highly complex and can be impacted by recurrences, co-morbidities, and interventions, to name a few. Cancer as one of the world’s best killer disease, for example, has killed many and left only a few to tell the tale. To disentangle the complexities, we need special kinds of survival models. These enable us to investigate diverse aspects of disease aetiology and explore the impact of risk factors at all stages. Crucially, we can then attempt to communicate risk profiles in many ways, understandable to both patient and clinician, through easily interpretable measures (such as the impact on life expectancy, postponable deaths, survival and transition probabilities). The study of survival of cancer patients will be an immense contribution to the fight against the dreaded disease.
Regard to sound statistical practice, in particular, the use of statistical approaches that provide clinically and peer-reviewed relevant information, will help maximise the potential of molecular markers for the care of cancer patients. Kaplan-Meier (K-M) method otherwise known as the product limit method is a statistical technique used to analyse cancer data. It is applied in analyzing the distribution of the patient’s survival times following their recruitment into the study. The analysis expresses this in terms of the proportion of patients still alive up to a given time t, following the recruitment or entry into the study.
This paper aims to present the research findings from a cohort study focused at the analysis of the estimation of the survivorship time of the real data of cancer patients in the North-eastern part of Nigeria and to establish if the insurgency in the region has contributed negatively to the life expectancy of its inhabitants. Also, the result that established the observed relationship between survival time and survival probability is presented. Kaplan-Meier method was used in the survival data analysis of the cancer data, and the findings were discussed extensively.
Material and Methods
Data Collection
The record of 1,090 patients from medical records departments of the University of Maiduguri Teaching Hospital (UMTH), located in Maiduguri, the capital city of Borno State in northeast Nigeria was obtained.
The record showed patients that were diagnosed and died of one type of cancer or the other from 2004 to 2017. All the cancer cases included in the present study were grouped into sex, age, marital status, occupation, date admitted and date of death/discharge.
In survival analysis, follow up periods are calculated from when subjects were enrolled in the study (i.e. date admitted).
Research on cancer is often very interesting because of the high fatality rate of the disease is diagnosed later and or untreated. One of the methods used in the survival analysis in oncology and epidemiology studies is the Kaplan-Meier method (K-M) [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. K-M method is usually used in conjunction with Cox proportional hazard regression, immunohisto-chemistry, lognormal, hazard ratio, Chi-squared test, log-rank test and so on.
A summary of the use of the K-M method in conjunction with other statistical methods is given in Table 1.
Table 1.
Kaplan-Meier and other statistical methods used in the survival analysis of different types of cancer
| Type of Cancer | Statistical methods | Cases investigated | Author |
|---|---|---|---|
| Breast | KM, Chi-squared test, Cox regression | 207 | [25] |
| Breast | KM, Cox regression | 300 | [26] |
| Breast | KM, Cox regression | 135 | [27] |
| Gastric | KM | 179 | [28] |
| Breast | KM | 308 | [29] |
| Breast | KM, Cox regression | 139 | [30] |
| Leukemia | KM, Cox regression | 527 | [31] |
| Ovarian | KM | 81 | [32] |
| Rectal | KM, Cox regression | 3786 | [33] |
| Liver | KM, Cox regression | 30,954 | [34] |
| Thyroid | KM, Cox regression | 12,128 | [35] |
| Gastric | KM, Chi-squared test, Cox regression | 4596 | [36] |
| Breast | KM, Cox regression | 1391 | [37] |
| Breast | KM, Hazard ratio | 10 226 | [38] |
| Prostate | KM, Cox regression | 579,608 | [39] |
| kin | KM, Cox regression, Log-rank test | 83 | [40] |
Data Preparation
In preparing the data for Kaplan-Meier survival analysis, each subject (patient) of the data component is mainly characterized by 3 variables: 1) their serial time (in days or years); 2) their status at the end of their serial time (event occurrence or censored); and 3) the study group or stage they are in.
For the computation of survival time curves and probabilities, the serial times for the patients are arranged from the shortest to the longest without regards to when they are recruited into the study as long as left censorship is not encountered. By this move, it can be ensured that all subjects within the group or stage begin the analysis at the same point and all are surviving independently until (event or censor) occurs.
Reasons for adopting the K-M method
The methodology is adopted for the following reasons. Firstly, the main target is to estimate a population survival curve from the sample obtained from the teaching hospital. Secondly, if every subject (patient) is followed until death, the curve may be estimated simply by computing the fraction surviving at each time t. Lastly, Kaplan-Meier curves have gravitated characteristics, which perhaps explains their wide applicability in medical research as they provide a pictorial depiction of all the raw data, the failure times and the censoring times; yet they also provide a mathematical estimate of the given survival model.
It is glad to note that the data presented in this article did not violate the six assumptions of the K-M method. The assumptions are stated as follows: 1) The data is composed of two mutually exclusive and exhaustive states known as an event or censored; 2) The survival time was clearly defined and accurately measured; 3) The data is right censored; 4) The censoring and the event are independent. This is vital since the efficiency of the K-M method depends on the analysis of observed data; 5) No trend was observed in the data; 6) Right censoring is similar in all the groups (sex and age).
Data Processing
Raw data are stored in MS EXCEL format using actual calendar date and time. During analysis, serial time may be automatically calculated, and this is used in curve construction and data analysis. The first step in the preparation of K-M analysis involves the construction of a table using Minitab or Excel spreadsheet or in SPSS containing the three key elements required for input. These are: 1) serial time, 2) status at serial time (1 = Alive, 0 = Death) and 3) age group.
Results
These were performed using SPSS version 23.0. Data were reported by sex, age, marital status, occupation, date of admission and date of death/discharge. A descriptive analysis of the data was carried out, and each character was described by frequencies and percentages. Kaplan-Meier analyses were conducted to mainly estimate overall survival rates of the various types of cancer. The survival time of a patient is referred to the number of months or days (duration) from the date of diagnosis of cancer to the date of the patient died or last contact (censored) or the date of the end of the study for patients who were still alive or date of loss to follow-up (censored). The differences in survival between the stages were compared by the log-rank test. A two-tailed p-value of < 0.05 was considered as statistically significant.
Descriptive Statistics
Data on 1,090 cancer cases were gathered of which 478 (43.9%) were male, while 612 (56.10%) were female, shown in Table 2.
Table 2.
The sex distribution of the patients
| Sex | Frequency | Per cent | Cumulative Percent |
|---|---|---|---|
| Male | 478 | 43.9 | 43.9 |
| Female | 612 | 56.1 | 100.0 |
| Total | 1090 | 100.0 |
From Table 3, it can be seen that 50.20% of the patients were between the ages of 21-50 years old, 17.70% were of ages below 20 years old, 29.9% were between ages 51-79 years while 1.9% were 80 years and above.
Table 3.
The age distribution of the patients
| Age | Frequency | Percent | Cumulative Percent | |
|---|---|---|---|---|
| Valid | 0-20 | 193 | 17.7 | 17.8 |
| 21-50 | 547 | 50.2 | 68.1 | |
| 51-79 | 326 | 29.9 | 98.1 | |
| 80 & Above | 21 | 1.9 | 100 | |
| Total | 1087 | 99.7 | ||
| Missing | 3 | 0.3 | ||
| Total | 1090 | 100 | ||
A group of 227 (20.8%) of the patients are single, 832 (76.3%) are married, 12 (1.1%) are widowed, and 19 (1.7%) are missing values, shown in Table 4.
Table 4.
Marital status of the patients
| Marital status | Frequency | Per cent |
|---|---|---|
| Single | 227 | 20.8 |
| Married | 832 | 76.3 |
| Widow/Widower | 12 | 1.1 |
| Missing | 19 | 1.7 |
| Total | 1090 | 100 |
Of the 478 females, 40.1% were housewives. 10.8% of the patients were children while 13.9% were civil servants. The details of the other occupation are given in Table 5.
Table 5.
the Recorded occupation of the patients
| Occupation | Frequency | Percent | Cumulative Percent | |
|---|---|---|---|---|
| Valid | Applicant | 12 | 1.1 | 1.1 |
| Soldier | 4 | 0.4 | 1.5 | |
| Banker | 1 | 0.1 | 1.6 | |
| Bricklayer | 1 | 0.1 | 1.7 | |
| Business Man | 71 | 6.5 | 8.4 | |
| Butcher | 1 | 0.1 | 8.5 | |
| Civil Servant | 151 | 13.9 | 22.7 | |
| Caterer | 1 | 0.1 | 22.7 | |
| Cattle Rearer | 5 | 0.5 | 23.2 | |
| Child | 118 | 10.8 | 34.3 | |
| Clergy | 1 | 0.1 | 34.4 | |
| Contractor | 1 | 0.1 | 34.5 | |
| Police Officer | 4 | 0.4 | 34.9 | |
| Driver | 11 | 1 | 35.9 | |
| Politician | 3 | 0.3 | 36.2 | |
| Farmer | 97 | 8.9 | 45.3 | |
| Retired | 19 | 1.7 | 47.1 | |
| Fisherman | 2 | 0.2 | 47.3 | |
| Scholar | 5 | 0.5 | 47.7 | |
| House Wife | 437 | 40.1 | 88.8 | |
| Security Man | 4 | 0.4 | 89.2 | |
| Mechanic | 2 | 0.2 | 89.4 | |
| Student | 85 | 7.8 | 97.4 | |
| Tailor | 5 | 0.5 | 97.8 | |
| Teacher | 8 | 0.7 | 98.6 | |
| Technician | 2 | 0.2 | 98.8 | |
| Widow/Widower | 13 | 1.2 | 100 | |
| Total | 1064 | 97.6 | ||
| Missing | System | 26 | 2.4 | |
| Total | 1090 | 100 |
The weird occupations seen in Table 5 can be attributed to high illiteracy of the patients and poor record keeping by the staff of the hospital. Finally, it can be seen from Table 6, that 170 (15.6%) were alive and 920 (84.4%) were dead after admission in the hospital.
Table 6.
End status of the patients
| Status | Frequency | Per cent | Cumulative Percent |
|---|---|---|---|
| Dead | 920 | 84.4 | 84.4 |
| Alive | 170 | 15.6 | 100 |
| Total | 1090 | 100 |
Kaplan-Meier Results
The data presented in this subsection are from the K-M analysis. These are given as: case summary for age (Table 7), case summary for sex (Table 8), means and medians for survival time for age (Table 9), means and medians for survival time for sex (Table 10), overall comparison tests for the age (Table 11) and overall comparison tests for the age (Table 12).
Table 7.
Case processing summary for age
| Age | Total N | N of Events | Censored | |
|---|---|---|---|---|
| N | Per cent | |||
| 0-20 | 193 | 150 | 43 | 22.30% |
| 21-50 | 547 | 473 | 74 | 13.50% |
| 51-79 | 326 | 274 | 52 | 16.00% |
| 80 & Above | 21 | 20 | 1 | 4.80% |
| Overall | 1087 | 917 | 170 | 15.60% |
Table 8.
Case processing summary for sex
| Sex | Total N | N of Events | Censored | |
|---|---|---|---|---|
| N | Per cent | |||
| Male | 478 | 401 | 77 | 16.10% |
| Female | 612 | 519 | 93 | 15.20% |
| Overall | 1090 | 920 | 170 | 15.60% |
Table 9.
Means and medians for survival time for age
| AGE | Mean | Median | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| 0-20 | 26.035 | 3.225 | 19.715 | 32.356 | 14 | 1.949 | 10.18 | 17.82 |
| 21-50 | 30.89 | 2.414 | 26.159 | 35.622 | 16 | 1.488 | 13.084 | 18.916 |
| 51-79 | 35.808 | 4.234 | 27.509 | 44.107 | 19 | 1.182 | 16.683 | 21.317 |
| 80 & Above | 29.651 | 6.487 | 16.936 | 42.366 | 20 | 4.577 | 11.028 | 28.972 |
| Overall | 31.41 | 1.839 | 27.807 | 35.014 | 17 | 0.864 | 15.306 | 18.694 |
Table 10.
Means and medians for survival time for sex
| Sex | Mean | Median | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| Male | 32.947 | 2.984 | 27.099 | 38.795 | 19 | 1.236 | 16.578 | 21.422 |
| Female | 30.184 | 2.286 | 25.703 | 34.665 | 16 | 1.33 | 13.394 | 18.606 |
| Overall | 31.355 | 1.833 | 27.762 | 34.948 | 17 | 0.852 | 15.331 | 18.669 |
Table 11.
Overall Comparison Tests for the age
| Test of equality | Chi-Square | Df | Sig. |
|---|---|---|---|
| Log Rank (Mantel-Cox) | 3.812 | 3 | 0.283 |
| Breslow (Generalized Wilcoxon) | 6.845 | 3 | 0.077 |
| Tarone-Ware | 5.622 | 3 | 0.131 |
Table 12.
Overall Comparison Tests for the sex
| Test of equality | Chi-Square | Df | Sig. |
|---|---|---|---|
| Log Rank (Mantel-Cox) | 1.440 | 1 | 0.230 |
| Breslow (Generalized Wilcoxon) | 3.573 | 1 | 0.059 |
| Tarone-Ware | 2.814 | 1 | 0.093 |
Table 7 displays the age of the cancer patients (categorised into age groups from 0-20 years, 21-50 years, 51-79 years and 80 years and above), the total number of patients in each age group, number of patients experienced events, and censored patients. Age 0-20 years has a total number of 193 patients with 150 been number of events and 43 (22.3%) as censored patients, age 21-50 has a total number of 547 patients with 473 patients that have experienced the event and 74 (13.5%) as censored patients, age 51-79 has a total number of 326 patients with 274 patients that have experienced the event and 52 (16.0%) as censored patients and age 80 and above has a total number of 21 patients with 20 total number of events and 1 (4.8%). It was noted that age group 21-50 has the highest number of events followed by 51-79 and age group 80 and above with the lowest number of events.
Table 8 displays the sex of patients diagnosed with cancer, the total number of patients for each sex group, number of patients experienced events, and censored patients. Four hundred seventy-eight male patients were admitted with 401 been number of events and 77 (16.1%) as censored patients while there were a total of 612 females with 519 patients that have experienced the event and 93 (15.2%) as censored patients Clearly, the male patients have the lowest number of events.
Table 9 gives a quick quantitative comparison of the typical survival times to effect for each of the age groups. The median survival time is calculated as the smallest amount of survival time for which the survivor function is less than or equal to 0.5.
The overall median survival time (i.e. the time at which the survival probability is 50% or 0.5) is 31 days. In other words, for cancer patients in the North, the chance of living beyond 31 days is 50%. The median survival time for ages 0-20 was 26 days, 30 days for ages 21-50, 35 days for age 51-79 and ages 80 and above was 29 days. These clearly show that the chance of living beyond 26 days, 30, 35 and 29 for ages 0-20, 21-50, 51-70 and above 80 years respectively after being admitted/diagnosed with the disease is 50%.
It can be seen that the higher the estimated mean time, the greater the chances of survival. This goes to show that ages 51-79 has the highest chance of survival while ages 0-20 years has the least chance of survival.
The log-rank test can be applied if the confidence limits do not overlap between the given levels. Clearly, from Table 9, there is no overlap between ages in the confidence intervals; hence differences in effect on time to an event can be inferred using the log-rank test.
From Table 10, it can be seen that the estimated mean time until death is 32 days for male, 30 days for females, which shows that females have a slightly increased chance of survival than males.
The median survival time for a male is 19 days while that of females is 16 days. These clearly show that the chance of living beyond 19 and 16 days for males and females, respectively for cancer patients in the Northeastern part of Nigeria is 0.5 after being diagnosed with the disease.
Clearly, from Table 10, there is a lot of overlap in the confidence intervals; hence it is unlikely that there is much difference in the average survival time.
Table 11 is the test of equality of survival distributions for the different levels of age. The significance values of the tests (0.283, 0.077, 0.131) are all greater than 0.05. The interpretation of this is the acceptance of the null hypothesis means that there is no significant evidence of a difference in the observed survival times for the categories of age considered.
Table 12 is the test of equality of survival distributions for the different levels of sex. It shows that the significance values of the tests (0.230, 0.059 and 0.093) are all greater than 0.05. The interpretation of this is the acceptance of the null hypothesis implies that there is no significant evidence of a difference in the observed survival times for the categories of age considered.
Although, females have a slightly increased chance of survival even though, as inferred from the log-rank test, sex is not a barrier to death.
Plots of the Survival Functions and survival probability estimation
The survival curves (Figures 1 and 2) give a visual depiction of the life tables, the horizontal axis represents the time to the event, and the vertical axis shows the estimated probability of survival.
Figure 1.
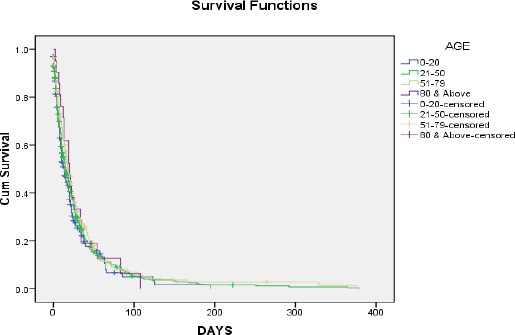
Kaplan-Meier survival plot for the ages
Figure 2.
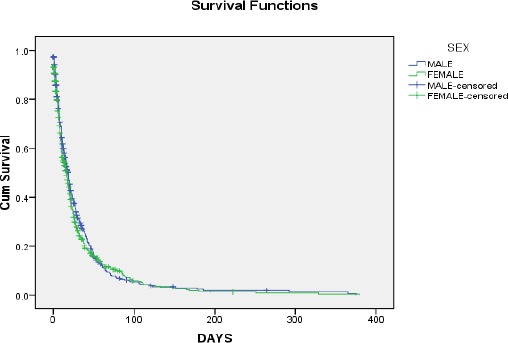
Kaplan-Meier survival plot for the sex
In this plot, drops in the survival curve occur whenever the patients experience the event
Also, the summary of survival probability estimate with the survival time for the age and sex are presented in Table 13 and 14 respectively.
Table 13.
Survival probability estimate with the survival time for age
| Age | 25 | 50 | 75 | |||
|---|---|---|---|---|---|---|
| Estimate | Std. Error | Estimate | Std. Error | Estimate | Std. Error | |
| 0-20 | 32 | 4.755 | 14 | 1.949 | 5 | 0.915 |
| 21-50 | 35 | 2.56 | 16 | 1.488 | 6 | 0.561 |
| 51-79 | 40 | 3.279 | 19 | 1.182 | 7 | 0.97 |
| 80 & Above | 34 | 4.799 | 20 | 4.577 | 12 | 2.588 |
| Overall | 35 | 1.815 | 17 | 0.864 | 6 | 0.443 |
Note; Survival time is given in days (D). This can easily be seen in Figure 1.
Table 14.
Survival probability estimate with the survival time for sex
| Sex | 25% Estimate | 50% Estimate | 75% Estimate | |||
|---|---|---|---|---|---|---|
| Estimate (days) | Std. Error | Estimate (days) | Std. Error | Estimate (days) | Std. Error | |
| Male | 39 | 2.69 | 19 | 1.236 | 7 | 0.829 |
| Female | 32 | 2.048 | 16 | 1.33 | 6 | 0.52 |
| Overall | 35 | 1.816 | 17 | 0.852 | 6 | 0.44 |
Note; Survival time is given in days (D). This can easily be seen in Figure 2.
From Table 13, the proportion of subjects or patients surviving past 5 days is 75%, the proportion of subjects or patients surviving past 14 days is 50% and the proportion of subjects or patients surviving past 32 days is 25% for age 0-20 years.
The proportion of subjects or patients surviving past 6days is 75% and the proportion of subjects or patients surviving past 16 days (which is the median survival time) is 50% and the proportion of subjects or patients surviving past 35 days is 25% for age 21-50 years.
The proportion of subjects or patients surviving past 7 days is 75%, the proportion of subjects or patients’ surviving past 19 days is 50% and the proportion of subjects or patients surviving past 40 days is 25% for age 51-79 years.
The proportion of subjects or patients surviving past 12 days is 75%, the proportion of subjects or patients surviving past 20 days is 50% and the proportion of subjects or patients surviving past 34 days is 25% for ages 80 years and above.
From this analysis, the probability of survival reduces as the survival time increases.
From Table 14, the proportion of subjects or patients surviving past 7 days is 75%, the proportion of subjects or patients’ surviving past 19 days is 50% and the proportion of subjects or patients surviving past 39 days is 25% for Males.
The proportion of subjects or patients surviving past 6days is 75% and the proportion of subjects or patients surviving past 16 days (which is the median survival time) is 50% and the proportion of subjects surviving past 32 days is 25% for Females.
Also, from this analysis, the probability of survival reduces as the survival time increases.
Discussion
A good glance at the results shows most patients died the same day or few days to date of admission. Hence, it was not a surprise that the analysis shows overall survival time of 6 days for 75% of patients with cancer at the University Teaching Hospital, Maiduguri. This is a far-cry to survival data obtained in another part of the country. The study shows that over 84% of the cancer cases died. We have more of the cases for women than for men. Over 40% of the cases for female were those who reported their occupation as housewives. Over 50% of the cases were age 21-50 years old. Only 1.9% of the cases were of ages 80years and above.
Following the result of the research, it is obvious that we have a major problem with the management of cancer in the region under review and Nigeria in general. The reasons for this might not be a difference of the below points.
The Northeast part of Nigeria is one of the poorest parts of Nigeria. Years of corruption and lack of investment in the health facilities have contributed to a near collapse of the health facilities of the region in particular and Nigeria in general. Poverty, illiteracy and superstition are contributory factors why diseases like cancer are endemic in the area. This is exacerbated by the ongoing Boko Haram insurgency and herdsman attacks. The insurgency has added to the already strain on the few available health facilities in the area and drastically reduction to access to health care. The effect is not limited to cancer, but other illness such as HIV AIDS epidemic, a cholera outbreak and others. It can be noted that the University of Maiduguri teaching is the only major tertiary health facility that covers Borno, Yobe, Gombe and Taraba states, an area and population that is greater than London, United Kingdom.
The precarious political and social factors are drivers to the culture of patients reporting of their cases very late. Most cancers have different stages, one, two, three and four. While stage one and two can achieve a cure, stage three and four are usually advanced where you no longer talk about radical treatment but palliative treatment to see how you can prolong the patient’s life and improve the quality of life. The data for this research did not include the stage of cancer upon admission at the hospital, we suggest most cases to be at stage three and four going by the time to the event.
Most patients do not go for screening; they mostly consult roadside chemists, extreme cases among the people approach churches, Muslim clerics and herbalist.
Acknowledgement
The authors appreciate the efforts of the anonymous reviewers toward this publication. The financial support from Covenant University, Nigeria is also deeply appreciated.
Footnotes
Funding: The investigation was financially covered by the Covenant University
Competing Interests: The authors have declared that no competing interests exist
Reference
- 1.Khan A, Cheri L. An Examination of Poverty as the Foundation of Crisis in Northern Nigeria. Insight Afr. 2016;8(2):59–71. https://doi.org/10.1177/0975087815612283. [Google Scholar]
- 2.Omole O, Welye H, Abimbola S. Boko Haram insurgency:Implications for Public Health. The Lancet. 2015;385(9972):941. doi: 10.1016/S0140-6736(15)60207-0. https://doi.org/10.1016/S0140-6736(15)60207-0. [DOI] [PubMed] [Google Scholar]
- 3.Dunn G. The impact of the Boko Haram insurgency in Northeast Nigeria on childhood wasting:a double-difference study. Conflict and health. 2018;12(1):6. doi: 10.1186/s13031-018-0136-2. https://doi.org/10.1186/s13031-018-0136-2 PMid:29410702 PMCid:PMC5782364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amusan L, Ejoke UP. The psychological trauma inflicted by Boko Haram insurgency in the North Eastern Nigeria. Aggression and violent behavior. 2017;36:52–9. https://doi.org/10.1016/j.avb.2017.07.001. [Google Scholar]
- 5.Roberts L. Nigeria's Invisible Crisis:Hunger Amplifies Infectious Diseases for Millions fleeing the Violence of Boko Haram. Sci. 2017;356(6333):18–23. doi: 10.1126/science.356.6333.18. https://doi.org/10.1126/science.356.6333.18 PMid:28385968. [DOI] [PubMed] [Google Scholar]
- 6.Cumber SN, Jaila S, Nancy B, Tsoka-Gwegweni JM. Under five malnutrition crises in the Boko Haram area of Cameroon. South African Journal of Clinical Nutrition. 2017;30(2):41–2. https://doi.org/10.1080/16070658.2016.1251685. [Google Scholar]
- 7.Bigna JJR. Polio Eradication Efforts in Regions of Geopolitical Strife:The Boko Haram Threat to Efforts in Sub-Saharan Africa. Afr. Health Sci. 2016;16(2):584–7. doi: 10.4314/ahs.v16i2.28. https://doi.org/10.4314/ahs.v16i2.28 PMid:27605975 PMCid:PMC4994566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oguntunde PE, Adejumo AO, Okagbue HI. Breast Cancer Patients in Nigeria:Data exploration approach. Data in Brief. 2017;15:47–57. doi: 10.1016/j.dib.2017.08.038. https://doi.org/10.1016/j.dib.2017.08.038 PMid:28971122 PMCid:PMC5612794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamu PI, Oguntunde PE, Okagbue HI, Agboola OO. Statistical data analysis of cancer incidences in insurgency affected states in Nigeria. Data in Brief. 2018;18:2029–2046. doi: 10.1016/j.dib.2018.04.135. https://doi.org/10.1016/j.dib.2018.04.135 PMid:29904711 PMCid:PMC5998707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adejumo AO, Ikoba NA, Suleiman EA, Okagbue HI, Oguntunde PE, Odetunmibi OA, Job O. Quantitative Exploration of Factors influencing Psychotic Disorder Ailments in Nigeria. Data in Brief. 2017;14:175–85. doi: 10.1016/j.dib.2017.07.046. https://doi.org/10.1016/j.dib.2017.07.046 PMid:28795095 PMCid:PMC5537424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olawande TI, Okagbue HI, Jegede AS, Edewor PA, Fasasi LT. Survey datasets on patterns of utilization of mental healthcare services among people living with mental illness. Data in Brief. 2018;19:2095–2103. doi: 10.1016/j.dib.2018.06.086. https://doi.org/10.1016/j.dib.2018.06.086 PMid:30229086 PMCid:PMC6141371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamu PI, Adamu MO, Okagbue HI. Data in support of high rate of pregnancy related deaths in Maiduguri, Borno State, Northeast Nigeria. Data in Brief. 2018;18:409–414. doi: 10.1016/j.dib.2018.03.038. https://doi.org/10.1016/j.dib.2018.03.038 PMid:29900198 PMCid:PMC5996266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adejumo AO, Suleiman EA, Okagbue HI, Oguntunde PE, Odetunmibi OA. Quantitative Evaluation of Pregnant Women Delivery Status'Records in Akure, Nigeria. Data in Brief. 2018;16:127–34. doi: 10.1016/j.dib.2017.11.041. https://doi.org/10.1016/j.dib.2017.11.041 PMid:29201979 PMCid:PMC5699871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamu PI, Oguntunde PE, Okagbue HI, Agboola OO. On the Epidemiology and Statistical Analysis of HIV/AIDS Patients in the Insurgency Affected States of Nigeria. Open Access Macedonian Journal of Medical Sciences. 2018;6(7):1315–1321. doi: 10.3889/oamjms.2018.229. https://doi.org/10.3889/oamjms.2018.229 PMid:30087744 PMCid:PMC6062286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand M, Gaylard P, Ramos J. Colorectal cancer in South Africa:An assessment of disease presentation, treatment pathways and 5-year survival. South African Medical Journal. 2018;108(2):118–122. doi: 10.7196/SAMJ.2017.v108i2.12338. https://doi.org/10.7196/SAMJ.2018.v108i2.12338 PMid:29429443. [DOI] [PubMed] [Google Scholar]
- 16.Okwor VC, Fagbamigbe AF, Fawole OI. Survivorship of patients with head and neck cancer receiving care in a tertiary health facility in Nigeria. Cancer Management and Research. 2017;9:331–338. doi: 10.2147/CMAR.S133108. https://doi.org/10.2147/CMAR.S133108 PMid:28790865 PMCid:PMC5531721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gizaw M, Addissie A, Getachew S, Ayele W, Mitiku I, Moelle U, Yusuf T, Begoihn M, Assefa M, Jemal A, Kantelhardt EJ. Cervical cancer patients presentation and survival in the only oncology referral hospital, Ethiopia:A retrospective cohort study. Infectious Agents and Cancer. 2017;12(1):61. doi: 10.1186/s13027-017-0171-4. https://doi.org/10.1186/s13027-017-0171-4 PMid:29213299 PMCid:PMC5708091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butt JL, Botha MH. Vulvar cancer is not a disease of the elderly:Treatment and outcome at a tertiary referral centre in South Africa. South African Medical Journal. 2017;107(11):1000–1004. doi: 10.7196/SAMJ.2017.v107i11.12497. https://doi.org/10.7196/SAMJ.2017.v107i11.12497 PMid:29262943. [DOI] [PubMed] [Google Scholar]
- 19.Sengayi MM, Kielkowski D, Egger M, Dreosti L, Bohlius J. Survival of patients with Kaposi's sarcoma in the South African antiretroviral treatment era:A retrospective cohort study. South African Medical Journal. 2017;107(10):871–876. doi: 10.7196/SAMJ.2017.v107i10.12362. https://doi.org/10.7196/SAMJ.2017.v107i10.12362 PMid:29022531 PMCid:PMC5913753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou G-X, Liu P, Yang J, Wen S. Mining expression and prognosis of topoisomerase isoforms in non-small-cell lung cancer by using Oncomine and Kaplan-Meier plotter. PLoS ONE. 2017;12(3):e0174515. doi: 10.1371/journal.pone.0174515. https://doi.org/10.1371/journal.pone.0174515 PMid:28355294 PMCid:PMC5371362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Y, Lu X, Wang G. STAT3 inhibitor enhances chemotherapy drug efficacy by modulating mucin 1 expression in non-small cell lung carcinoma. Tropical Journal of Pharmaceutical Research. 2017;16(7):1513–1521. https://doi.org/10.4314/tjpr.v16i7.8. [Google Scholar]
- 22.Oyekunle AA, Durosinmi MA, Bolarinwa RA, Owojuyigbe T, Salawu L, Akinola NO. Chronic myeloid leukemia in Nigerian patients:Anemia is an independent predictor of overall survival. Clinical Medicine Insights:Blood Disorders. 2016;9:9–13. doi: 10.4137/CMBD.S31562. https://doi.org/10.4137/CMBD.S31562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Català A, Garces JR, Alegre M, Gich IJ, Puig L. Mohs micrographic surgery for basal cell carcinomas:Results of a Spanish retrospective study and Kaplan-Meier survival analysis of tumour recurrence. Journal of the European Academy of Dermatology and Venereology. 2014;28(10):1363–1369. doi: 10.1111/jdv.12293. https://doi.org/10.1111/jdv.12293 PMid:25383396. [DOI] [PubMed] [Google Scholar]
- 24.Cao N, Zhao A, Zhao G, Wang X, Han B, Lin R, Zhao Y, Yang J. Survival analysis of 272 patients with pancreatic cancer undergoing combined treatment. Integrative Cancer Therapies. 2015;14(2):133–139. doi: 10.1177/1534735414564185. https://doi.org/10.1177/1534735414564185 PMid:25567328. [DOI] [PubMed] [Google Scholar]
- 25.Ebili HO, Iyawe VO, Adeleke KR, Salami BA, Banjo AA, Nolan C, Rakha E, Ellis I, Green A, Agboola AOJ. Checkpoint Kinase 1 Expression Predicts Poor Prognosis in Nigerian Breast Cancer Patients. Molecular Diagnosis and Therapy. 2018;22(1):79–90. doi: 10.1007/s40291-017-0302-z. https://doi.org/10.1007/s40291-017-0302-z PMid:29075961. [DOI] [PubMed] [Google Scholar]
- 26.Ikpatt OF, Kuopio T, Collan Y, Ndoma-Egba R. Tubular differentiation in African breast cancer. Advances in clinical pathology:the official journal of Adriatic Society of Pathology. 2003;7(1):27–32. [PubMed] [Google Scholar]
- 27.Buhmeida A, Al-Maghrabi J, Merdad A, Al-Thubaity F, Chaudhary A, Gari M, Abuzenadah A, Collan Y, Syrjänen K, Al-Qahtani M. Nuclear morphometry in prognostication of breast cancer in Saudi Arabian patients:Comparison with European and African breast cancer. Anticancer Research. 2010;30(6):2185–2191. PMid:20651368. [PubMed] [Google Scholar]
- 28.Ahmed A, Ukwenya AY, Makama JG, Mohammad I. Management and outcome of gastric carcinoma in Zaria, Nigeria. African Health Sciences. 2011;11(3):353–361. PMid:22275924 PMCid:PMC3261017. [PMC free article] [PubMed] [Google Scholar]
- 29.Agboola AJ, Musa AA, Wanangwa N, Abdel-Fatah T, Nolan CC, Ayoade BA, Oyebadejo TY, Banjo AA, Deji-Agboola AM, Rakha EA, Green AR, Ellis IO. Molecular characteristics and prognostic features of breast cancer in Nigerian compared with UK women. Breast Cancer Research and Treatment. 2012;135(2):555–569. doi: 10.1007/s10549-012-2173-7. https://doi.org/10.1007/s10549-012-2173-7 PMid:22842985. [DOI] [PubMed] [Google Scholar]
- 30.Ayoade BA, Agboola AJ, Olatunji AA, Tade AO, Salami BA, Adekoya AO. Clinical characteristics and survival outcome of breast cancer in Southwest Nigerian women. Journal Africain du Cancer. 2014;6(2):79–84. https://doi.org/10.1007/s12558-014-0311-8. [Google Scholar]
- 31.Oyekunle AA, Durosinmi MA, Bolarinwa RA, Owojuyigbe T, Salawu L, Akinola NO. Chronic myeloid leukemia in Nigerian patients:Anemia is an independent predictor of overall survival. Clinical Medicine Insights:Blood Disorders. 2016;9:9–13. doi: 10.4137/CMBD.S31562. https://doi.org/10.4137/CMBD.S31562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aluloski I, Tanturovski M, Jovanovic R, Kostadinova-Kunovska S, Petrusevska G, Stojkovski I, Petreska B. Survival of Advanced Stage High-Grade Serous Ovarian Cancer Patients in the Republic of Macedonia. Open access Macedonian journal of medical sciences. 2017;5(7):904–908. doi: 10.3889/oamjms.2017.215. https://doi.org/10.3889/oamjms.2017.215 PMid:29362616 PMCid:PMC5771292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XJ, Chi P, Zhang YY, Lin HM, Lu XR, Huang Y, Xu ZB, Ghareeb WM, Huang SH, Sun YW, Ye DX. Survival outcome of adjuvant radiotherapy after local excision for T2 early rectal cancer:An analysis based on the surveillance, epidemiology, and end result registry database. European Journal of Surgical Oncology. 2018;44(12):1865–72. doi: 10.1016/j.ejso.2018.08.024. https://doi.org/10.1016/j.ejso.2018.08.024 PMid:30262325. [DOI] [PubMed] [Google Scholar]
- 34.Ren F, Zhang J, Gao Z, Zhu H, Chen X, Liu W, Xue Z, Gao W, Wu R, Lv Y, Hu L. Racial disparities in the survival time of patients with hepatocellular carcinoma and intrahepatic cholangiocarcinoma between Chinese patients and patients of other racial groups:A population-based study from 2004 to 2013. Oncology Letters. 2018;16(6):7102–7116. doi: 10.3892/ol.2018.9550. https://doi.org/10.3892/ol.2018.9550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, Gong J, Guo B, Shang J, Cheng Y, Xu H. Association of adjuvant radioactive iodine therapy with survival in node-positive papillary cancer. Oral Oncology. 2018;87:152–157. doi: 10.1016/j.oraloncology.2018.10.041. https://doi.org/10.1016/j.oraloncology.2018.10.041 PMid:30527231. [DOI] [PubMed] [Google Scholar]
- 36.Ren J, Niu G, Wang X, Song T, Hu Z, Ke C. Effect of Age on Prognosis of Gastric Signet-Ring Cell Carcinoma:A SEER Database Analysis. Medical science monitor:international medical journal of experimental and clinical research. 2018;24:8524–8532. doi: 10.12659/MSM.911766. https://doi.org/10.12659/MSM.911766 PMid:30473583 PMCid:PMC6278247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu TJ, Liu YY, Hu X, Di GH. Survival following breast-conserving therapy is equal to that following mastectomy in young women with early-stage invasive lobular carcinoma. European Journal of Surgical Oncology. 2018;44(11):1703–7. doi: 10.1016/j.ejso.2018.06.026. https://doi.org/10.1016/j.ejso.2018.06.026 PMid:30029824. [DOI] [PubMed] [Google Scholar]
- 38.Yu TJ, Liu YY, Hu X, Di GH. No survival improvement of contralateral prophylactic mastectomy among women with invasive lobular carcinoma. Journal of surgical oncology. 2018;118(6):928–35. doi: 10.1002/jso.25221. https://doi.org/10.1002/jso.25221 PMid:30311653. [DOI] [PubMed] [Google Scholar]
- 39.Krasnow RE, Rodríguez D, Nagle RT, Mossanen M, Kibel AS, Chang SL. InUrologic Oncology:Seminars and Original Investigations. 11. Vol. 36. Elsevier; 2018. The impact of age at the time of radiotherapy for localized prostate cancer on the development of second primary malignancies; pp. 500–e11. https://doi.org/10.1016/j.urolonc.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Oranges CM, Sisti G, Nasioudis D, Tremp M, Di Summa PG, Kalbermatten DF, Largo RD, Schaefer DJ. Hard palate melanoma:A population-based analysis of epidemiology and survival outcomes. Anticancer Research. 2018;38(10):5811–5817. doi: 10.21873/anticanres.12921. https://doi.org/10.21873/anticanres.12921 PMid:30275204. [DOI] [PubMed] [Google Scholar]


