Abstract
BACKGROUND:
The seroconversion is a significant health concern in patients with end-stage renal disease undergoing hemodialysis particularly in high endemic zones of HBV and HCV.
PATIENTS AND METHODS:
This prospective study was conducted from January 2009 to April 2018 at Sheri Kashmir Institute of Medical Sciences, Srinagar, Kashmir. A cohort of 459 end-stage renal disease patients on hemodialysis was enrolled from four dialysis centres and followed in a longitudinal manner. Their seroconversion rates, risk factors were studied. Positive patients were treated and followed up.
RESULTS:
This study demonstrated HBV seroconversion rate of 7.4 % (n = 34) and HCV seroconversion rate of 10% (n = 46) in a cohort of 459 patients on hemodialysis attending four dialysis centres of Kashmir. Patients with diabetes mellitus outnumbered in seroconversion rates of (43.75%) followed by patients with glomerulonephritis (23.75%). Of 15 patients who had undergone renal transplantation 10 (66.67%), patients had seroconversion on hemodialysis which was statistically significant (P < 0.001). Patients who were dialysed at multiple HD centres had significant seroconversion than those who followed up at a single center. Seroconversion was associated with longer duration of dialysis (80.30 ± 30.92 vs 61 ± 9.41months, P < 0.000). HBV vaccination of the ESRD patient on hemodialysis was significantly protective against seroconversion (P = 0.000).
CONCLUSIONS:
Hepatitis B vaccination, stringent precautions in all dialysis centres could help to reduce the high seroconversion rates which have a high financial burden on ESRD patients. Intense health education to both patients and medical staff will be beneficial to lower the seroconversion rates.
Keywords: Hepatitis C, Hepatitis B Hemodialysis units, Risk Factors, Seroepidemiologic studies
Introduction
End-stage renal disease (ESRD) is an immune compromised state. Disturbed cell-mediated immunity is the hallmark of advanced renal failure. Studies have shown that patients on maintenance hemodialysis have lymphopenia and their T4 & T8 lymphocyte are low. The uremic lymphocytes are shown to have lower proliferation rates compared to normal people and thus they become particularly susceptible to viral infections [1]. Over and above when on hemodialysis (HD) these patients are prone to contract various blood-borne infections like HBV, HCV, HIV etc. as HD requires access to the bloodstream and transmission can occur between patients and staff as well. Even ESRD patients receive multiple injections predisposing them to seroconversion.
In a study by Moloughney et al., [2] authors concluded that an untreated percutaneous exposure to an infected source carries a risk of seroconversion of up to 30% for HBV. The risks for HCV and HIV even though lesser than HBV are estimated to be at 1.8% and 0.31%, respectively after inadvertent percutaneous exposure. The seroconversion in ESRD patients on HD patients is particularly high. The Turkish multicentric trial has demonstrated the seroconversion rates to be higher among HD patients than on Continuous Ambulatory Peritoneal Dialysis (CAPD), authors advocated that CAPD compared to HD provided a potential advantage to the candidates with the prospective renal transplant [3].
When patients with ESRD contract either HBV or HCV they invariably do not clear the virus and progress to chronic hepatitis. A meta-analysis of clinical studies based on 145,608 patients, anti-HCV seropositive status was a significant risk factor for death in patients on long-term dialysis [4]. Authors in the study mentioned above showed that ESRD patients with HCV positivity on dialysis are prone to have a higher cardiovascular risk making treatment of chronic HCV imperative among these patients.
Even though the treatment of HBV and HCV have become safer, better tolerated and more effective owing to the availability of direct-acting anti-virals for nearly all patients the cost factors continue to be high. Added to this chronic HBV and chronic HCV patients need regular follow up to assess the development of complications like decompensation and hepatocellular carcinoma. In a Canadian study, chronic HCV was shown to be highly burdensome to public health causing loss of productive years of life than any other infectious disease in that country [5]. Keeping in view a high prevalence of HBV and HCV in our region we were prompted to undertake this study, first of its kind, among hemodialysis patients. We estimated HBV and HCV seroconversion rates in patients undergoing maintenance HD at four dialysis centres as invariably maintenance hemodialysis is not being carried out at our tertiary care centre keeping in view limited resources and increased demand.
Patients and Methods
This study was carried out prospectively from January 2009 to April 2018. The enrolled patients gave written consent for the participation in the study and various laboratory tests were carried out at a bimonthly interval in each participant. The data were maintained on our dialysis register. Our study was conducted in full compliance with the guidelines for good clinical practice of the World Medical Assembly Declaration of Helsinki and the research guidelines of the Sheri Kashmir Institute of Medical science (SKIMS) Srinagar Kashmir, a tertiary care centre in the valley of Kashmir.
Study Population
From January 2009 to April 2018, 470, patients on hemodialysis were enrolled. Socio-demographic data were collected. These data included age, gender, duration of hemodialysis treatment, the frequency of dialysis, history of diabetes, blood transfusions, renal transplantation, surgical interventions and possible household acquirement of hepatitis infection. The medical records of the study participants were tabulated at the beginning regularly updated on follow up.
Definition of HBV and HCV Status
All patients had a baseline hemogram, liver function tests and HBsAg and anti HCV samples collected at the enrolment into the study. Then at a bimonthly interval, HBsAg detection was done by immunoassay and viral DNA (HBV DNA) Quantitative was estimated by real-time PCR (Roche Cobas Ampliprep) in HBV positive patients. Third generation ELISA test was used to detect anti HCV, and Hepatitis C viral RNA Quantitative was estimated by real-time PCR (Cobas TaqMan) in HCV positive patients.
The seroconversion was defined as a change from HCV antibody negative at the time of enrollment to HCV antibody positive status during the study period with high HCV RNA.
Exclusion criteria
Patients known to be positive for HBV and HCV at the time of enrollment were excluded.
Patients who had received a blood transfusion in the last three months at the time of enrolment.
Clinical Jaundice or high AST/ALT levels at the time of enrolment due to any reason.
Statistical Analysis
All the data were entered into Microsoft Excel. This included demographic profiles of participants, the cause of end-stage renal disease etc. We compared the two groups HCV and HBV positive with hepatitis negative patients. The analysis was focused on seroconversion, risk factors contributing to seroconversion during hemodialysis. Descriptive and analytical, methods were used throughout data analysis using SPSS version 21.
Data are presented as mean ± standard deviation or number (percentages). Chi-square test and Fisher’s exact test were used, when appropriate. P ≤ 0.05 was considered as statistically significant. Odds ratios (OR) were calculated considering the confidence interval of 95%.
Results
A total of 470 patients were enrolled during the study period. Eleven patients lost to follow up, and finally data on 459 patients on hemodialysis were evaluated. Of 459 participating subjects, the majority 275 (60%) were males. Mean age ± SD in the sero converted group was 63.09 ± 7.99 and age ranged 42-72 years. While the mean age in seronegative patients was 61.78 ± 9.411 and their age ranged 39-72 years. The duration of dialysis was 80.30 ± 30.92 (10-142 months) in sero converted patients and 61 ± 9.41(39-72) months in seronegative patients. The difference was statistically significant (p = 0.000) as shown in Table 1. Seroconversion was significantly higher in patients who had dialysis at multiple centres as shown in Table 1. Significant association of seroconversion was observed in patients with a renal transplant. Vaccination status was significantly protective in the prevention of seroconversion of viral hepatitis B (p = 0.00).
Table 1.
Clinical data of seroconverted and seronegative patients on HD
| Factors associated with seroconversion | Sero Positive N = 80 |
Sero negative N = 379 |
P value |
|---|---|---|---|
| Age in years (mean ± SD) | 63.09 ± 7.99 | 61.78 ± 9.411 | 0.407 |
| Males (No & %) | 48 (60%) | 242 (63.85%) | 0.998 |
| Mean duration of Dialysis in months | 80.3 0± 30.92 | 61 ± 9.41 | 0.000 |
| Previous blood transfusion | |||
| a. No blood transfusion | 17 (21.5%) | 96 (25.3%) | |
| b. Less than 2 units | 56 (70.9%) | 245 (64.6%) | 0.556 |
| c. Three or > 3 units | 6 (7.6%) | 38 (10%) | |
| Previous renal transplant (Median & IQR) | 10 (73.34%) | 5 (14.7%) | 0.034 |
| Erythropoietin treatment % | 55 (69.65%) | 266 (70.2%) | 0.920 |
| Visit to Multiple HD centers | 75 (94.9%) | 173 (45.6%) | 0.00 |
| HBV vaccination status | |||
| a. Fully vaccinated | 6 (7.6%) | 185 (48.8%) | |
| b. Partially vaccinated | 35 (44.3%) | 174 (45.9%) | 0.000 |
| c. Not vaccinated | 39 (48.1%) | 20 (5.3%) |
The study cohort had low haemoglobin levels, and there was no statistical difference between seroconverted or negative patients. The renal functions also showed no significant difference between the two groups. While AST and ALT levels were low in both groups but there was a significant difference between seroconverted and negative groups. Serum uric acid levels were significantly elevated in the seroconverted group compared to negative groups. There was no difference in the serum Albumin levels between the two groups Table 2.
Table 2.
Laboratory data Investigations in the Cohort of ESRD on Hemodialysis
| Investigation | HBV + ve | HCV + ve | Seronegative group | P valve |
|---|---|---|---|---|
| Hemoglobin (median (IQR)) | 8.5 (1.6) | 8.6 (1.5) | 8.5 (1.5) | 0.575 |
| Platelet (median (IQR)) | 180 (19) | 188 (20) | 180 (20) | 0.361 |
| WBC (median (IQR)) | 4.9 (0.62) | 4.76 (0.74) | 4.73 (1.0) | 0.256 |
| Urea (median (IQR)) | 182 (20) | 180 (21) | 187 (25) | 0.166 |
| Creatinine (median (IQR)) | 9.0 (2.4) | 9.2 (2.1) | 9.5 (1.0) | 0.745 |
| Uric acid | 6.7 (0.5) | 6.7 (0.5) | 6.5 (1.0) | 0.008 |
| Calcium | 9.8 (1.2) | 9.4 (0.65) | 9.5 (1.0) | 0.098 |
| Phosphorus | 4.1 (0.4) | 4.1 (0.4) | 4.1 (0.4) | 0.773 |
| Bilirubin (mg/dl) | 0.8 (0.5) | 0.8 (0.4) | 0.8 (0.3) | 0.867 |
| ALT IU | 22 (8) | 22 (8) | 18 (0.5) | 0.000 |
| AST IU | 23 (7) | 22 (6) | 18 (2) | 0.000 |
| ALP(KA) | 80 (1.1) | 80 (1.1) | 80 (2.7) | 0.403 |
| T. Protein (g/dl) | 7.6 (1) | 7.7 (1.3) | 7.6 (1.2) | 0.888 |
| Albumin (g/dl) | 4.7 (.7) | 4.7 (.7) | 4.7 (.7) | 0.942 |
Primary objective: Seroconversion of Hepatitis B and Hepatitis C
Of 459 patients on hemodialysis, 80 (17.42%) patients had seroconversion. HBV seroconversion was observed among 34 (7.4%) patients and HCV in 46 (10.2%) patients. The mean interval was 6 ± 1.2 months and ranged from 4 months to 11 months in our study. None of our study cohorts had HBV and HCV combined infection. Depending upon the cause of ESRD seroconversion rates varied as under as shown in shown in Figure 1.
Figure 1.
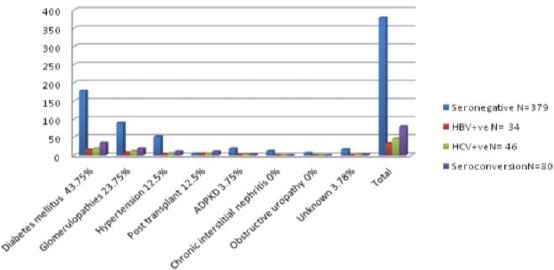
Seroconversion rates as per the aetiology of ESRD patients on HD
Diabetes mellitus: Diabetes mellitus was the most common cause of ESRD in this study. Of 213 diabetic patients on hemodialysis seroconversion was observed in 35 (16.43%) patients [HBV=16, HCV=19] corresponding to 35/80 (43.75%) of all seroconverted patients in our study cohort as shown in Figure 1.
Chronic glomerulonephritis: Of 108 patients with chronic glomerulopathies on hemodialysis seroconversion was found in 19 (17.59%) patients [HBV = 8, HCV = 11] corresponding to 19/80 (23.75%) as shown in Figure 1.
Hypertension: Of 62 patients with chronic hypertension on hemodialysis seroconversion was found in 10 (22.58%) patients [HBV = 4, HCV = 6] corresponding to 10/80 (12.5%) of our study cohort.
Post-Transplant: There were 15 patients post-transplant on hemodialysis in this study. Various reasons ascribed to their transplant failure were, chronic rejection 8 (53.34%) BK Virus 4 (26.67%) antibody rejection 3 (20%). Of 15 post renal transplant patients, seroconversion was observed in 10 (66.67%) patients [HBV = 5, HCV = 5], corresponding to 10/80 (12.5%) of our study cohort.
Adult polycystic kidney disease ADPKD: There were 22 patients with ADPKD on hemodialysis. The seroconversion was in 3 (13.63%) patients [HBV = 1, HCV = 2], 3/80 (3.75%) of our study cohort as shown in Figure 1.
Obstructive Uropathy: There were 7 patients with obstructive uropathy, and none of them underwent seroconversion.
Chronic interstitial nephritis CIN: Of 12 patients with CIN no patient underwent seroconversion.
Unknown cause of ESRD: Of 17 patients where the cause of ESRD remained unknown seroconversion was observed in 3 (17.64%) patients [HBV = 0, HCV = 3] corresponding to 3/80 (3.75%) of our study cohort as shown in Figure 1.
Treatment of HCV, HBV and follow up
All patients with HBV were treated with Tab. Entecavir 0.5mg once every 5 days. There was a progressive fall in HBV DNA levels in all patients. Patients with HCV were initially treated with Interferon-based therapy at the start of the study and later with oral drugs when oral drugs became available. Both HBV and HCV seroconverted patients were followed by ultrasound liver examination every six month, and AFP levels were monitored. There was no evidence of HCC in any of the study participants during the study period.
Discussion
This study demonstrated an HBV seroconversion rate of 7.2% and HCV seroconversion rate of 10% in a cohort of 459 ESRD patients on maintenance hemodialysis (HD). The mean time from the start of HD to seroconversion was 6 ± 1.2 months, and it ranged from 6 months to 11 months. As summarized in Table 3 the prevalence of HBV and HCV in various HD units varies from country to country across the globe and the prevalence in the Indian HD centers continues to be high [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17].
Table 3.
Global data of seroprevalence of HBV & HCV in HD centres
| Virus | Place | Author | Year | Prevalence |
|---|---|---|---|---|
| HBV | Western Europe Japan &USA | Burdick et al [6] | 2003 | 0-6.6% |
| Middle East: Saudi Arabia & Bahrain | Almawi WY et al [7] | 2004 | 11.8% & 3.7% | |
| Turkey | Yakaryilmaz et al [8] | 2006 | 13.3% | |
| Brazil | Ferreira et al [9] | 2006 | 2.4-10% | |
| Asia & Pacific countries | Johnson et al [10] | 2009 | 1.3% & 14.6% | |
| North Indian data | Malhotra R et al [11] | 2016 | 1.5-33.5% | |
| HCV | United Kingdom | Wreghitt et al. [12]. | 1999 | 4% |
| Saudi Arabia | Souqiyyeh et al [13] | 2001 | 50% | |
| Germany | Hinrichsen H et al [14] | 2002 | 6% | |
| Slovenia | Buturovic-Ponikvar et al [15] | 2003 | 1.9% | |
| Casablanca | Boulaajaj K et al [16] | 2005 | 76% | |
| Egypt | ZahranAM [17] | 2014 | 49.6% | |
The risk of seroconversion in ESRD on HD is directly proportional to the prevalence of viral infection in a given society and the quality of dialysis centres. In this study, as shown in Figure 1 the patients with Diabetes mellitus had higher seroconversion rates than the non-diabetic population which has been demonstrated by other researchers as well [18], [19]. The seroconversion was proportional to duration of dialysis in years possibly due to increased nosocomial infection rates in HD population as demonstrated by Carneiro et al., [20]. Patients who were dialysed at multiple dialysis centres had more seropositivity rates compared to those who followed in a single centre as shown in Table 1 supported by Petrosillo et al., [21].
There was no marked elevation of AST and ALT levels in the seroconverted group. Nevertheless the difference between seroconverted and negative patients on HD was statistically significant (Table 2). Patients with the end-stage renal disease do not show a marked rise of AST and ALT levels following seroconversion warranting a high clinical suspicion as the actual liver damage may be profound irrespective of AST levels. As such, it has been suggested by Wong et al., [22] that in a dialysis patient with chronic HBV infection an unexplained elevation in serum ALT level persistently above 30 IU/L or just 0.75 times upper limit of normal (ULN), liver biopsy should be considered to rule out significant hepatic inflammation if the clinical evidence of progressive liver disease is high.
Our study demonstrated a significant elevation of serum uric acid (UA) levels in the seroconverted group compared to the seronegative group as shown in Table 1. In a study by Afzali A et al., [23] the data on 5518 participants during a mean follow of 12 years showed that the high serum uric acid levels strongly predicted the progression of liver disease. In their study serum UA level was associated with the development of cirrhosis. There was no significant association observed in hemogram, serum calcium, phosphorous, serum proteins, albumin levels between the two groups as shown in Table 2.
Prevention seems to be the cornerstone in declining seroconversion rates of HBV and HCV in HD patients. As per the guidelines of CDC endorsed by Kidney Disease: Improving Global Outcomes (KDIGO) [24], stringent precautions in dialysis units must be observed to decline seroconversion rates. These include wearing and changing of gloves after a clinical encounter with HD patient, isolation of positive patients, water-proof gowns between patients, systematic decontamination of the equipment circuit and surfaces after each patient’s treatment. CDC also refrains sharing of various instruments like tourniquets, stethoscope, blood pressure cuff and use of multi-use vials of heparin between HD patients [25]. Apart from stringent precautions the use of recombinant erythropoietin (EPO) therapy seems to be the plausible solution in preventing the seroconversion among HD patients. However, blood transfusions cannot be avoided entirely in end-stage renal disease (ESRD). There are situations when ESRD patients need to be transfused exposing them to the risk of seroconversion especially when they develop EPO resistance due to suboptimal dialysis [26]. However, blood transfusion rates didn’t affect sero positivity in our study as shown in Table 1.
The occult HBV remains another potential risk of seroconversion and its prevalence as demonstrated by Gutiérrez-García et al., [27] is parallel with the prevalence of apparent HBV infection prevalence. This poses a significant risk of seroconversion not only during transfusion but also during the disease among ESRD patients on HD. Various studies have shown that the prevalence of occult hepatitis B in dialysis patients ranges between 0% and 58% [28], [29]. Thus whether the higher seroconversion rates observed in this study were due to activation of occult hepatitis B or due to some other reasons remained elusive. The enigma could have been solved had we checked HBV DNA in all patients undergoing hemodialysis but due to financial constraints, such practice may not be feasible everywhere. Having said this, keeping in view a high prevalence of HBV in our region whether testing for Anti-HBc antibody & HBV DNA levels both in a given blood donor and the ESRD patient before enrolment to hemodialysis will help to reduce further transmission needs to be studied. This has particular public health importance in our region as the number of dialysis units is limited, and the ESRD patient population on HD is on rise due to the shortage of renal transplantation facilities.
With the advent of Hepatitis B vaccination there has been a progressive decline in new conversion rates all over the globe. There was a significant association between vaccination status and seroconversion as shown in Table 1. Since there is no vaccine against HCV, aseptic precautions in various dialysis centres can decline the incidence of HCV seroconversion. Way back in 1977 when CDC guidelines [30] were laid Dinits-Pensy et al., [31] demonstrated a decline of new HBV among HD patients from 6.2 to 1% among US dialysis centres a few years later.
In another study, the prevalence of HBV infection in HD patients in the United States of America progressively fell from 7.8% to 1.0% between 1976 and 2002. Similarly, the prevalence of HCV infection fell from 10.4% to 7.8% from 1995 to 2002 [32]. Hemodialysis environment proves to be the vehicles of transmission of these viruses and renal transplant seems to be another modality in circumventing this vicious cycle. The Iranian data showed a significant fall of seroconversion after renal transplantation in ESRD patients [33].
There was a higher HCV seroconversion (10%) than HBV seroconversion rates (7.2%) possibly due to a higher prevalence of HCV in our region as demonstrated by Jasuja et al., [34]. We utilized utilised the third generation of enzyme-linked immunosorbent assay (ELISA) for the detection of anti HCV. Various studies have shown that the frequency of HCV RNA-positive among anti-HCV-negative patients undergoing HD varies from 0% to 12% [35], [36]. Nevertheless, routine screening of HCV RNA in our patient population with limited resources and no insurance coverage may not be feasible and serological testing, preferably by the third-generation enzyme-linked immunosorbent assay (ELISA) has been recommended for routine screening of HD patients [37]. Recently occult HCV has been demonstrated in the liver tissue and peripheral blood mononuclear cells of HCV RNA negative patients on HD connoting that despite negative HCV RNA and serology patients can have HCV infection and potentially transfer via dialysis units or get activated in the given patient [38].
The high percentage of seroconversion in this study could be partly attributed to the shortage of nursing staff, in an environment of a high prevalence of HBV and HCV positivity in our region. Further, invariably our HD units remain crowded units due to limited resources. Last but not least there are inadequate infection control policies and procedures in this part of the globe.
The drawback of our study, we believe, is that we did not calculate fibrosis scores in our cohort of seroconverted patients. However, all patients were Child Turcot Pugh class A and no patient had decompensation on follow up. The seroconverted patients were treated and regularly followed with Alfa-fetoprotein (AFP) levels and six-monthly ultrasound of the liver. None of our study participants developed Hepatocellular carcinoma during the study period. In a study by Cheng et al., [39], while comparing the outcome of hepatic resection in patients with CRF and normal kidney functions tests, it was observed that even though ESRD patients had high creatinine levels and low haemoglobin, a similar outcome in both ESRD & controls groups was observed by the authors. Their study highlights that patients with seroconversion must be followed up for HCC and operated as per the standard protocol.
We conclude that a high prevalence of viral hepatitis seroconversion among ESRD patients on hemodialysis was observed. All our efforts must be to prevent seroconversion of ESRD patients on HD by adequate HBV vaccination (40 µg HBV vaccine at 0, 1, 2 and six months) and unpermissive precautions in hemodialysis centres. Nevertheless, after an unfortunate seroconversion, ESRD patients must be treated with standard therapy which is safe and effective.
Our study emphasises enforcement of the quality control in various dialysis centres by healthcare authorities across the state, including periodic serological testing of dialysis staff. The practice guidelines must be laid, and rigorous infection control policies must be adopted by all the dialysis centres in the region to prevent the longterm consequences of seroconversion among ESRD patients on hemodialysis.
Acknowledgements
Authors wish to thank all the study participants, senior residents, residents and paramedical staff of the Dept. of Nephrology SKIMS, Srinagar, for their support during the study period.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
Reference
- 1.Kurz P, Köhler H, Meuer S, Hütteroth T, Meyer zum Büschenfelde KH. Impaired cellular immune responses in chronic renal failure:evidence for a T cell defect. Kidney Int. 1986;29(6):1209–14. doi: 10.1038/ki.1986.129. https://doi.org/10.1038/ki.1986.129 PMid:3489122. [DOI] [PubMed] [Google Scholar]
- 2.Moloughney BW. Transmission and postexposure management of bloodborne virus infections in the health care setting:Where are we now?CMAJ. 2001; 165:445–51. PMid:11531058 PMCid:PMC81374. [PMC free article] [PubMed] [Google Scholar]
- 3.Akpolat T, Dilek M, Yavuz M, Utas C, et al. Turkish Multicenter PD Study Group. Low seroconversion rates in CAPD patients compared to hemodialysis patients:potential advantages for transplant candidates. Perit Dial Int. 2002;22(4):520–3. PMid:12322827. [PubMed] [Google Scholar]
- 4.Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients:a link with cardiovascular mortality? J Viral Hepat. 2012;19(9):601–7. doi: 10.1111/j.1365-2893.2012.01633.x. https://doi.org/10.1111/j.1365-2893.2012.01633.x PMid:22863263. [DOI] [PubMed] [Google Scholar]
- 5.Myers RP, Krajden M, Bilodeau M, et al. Burden of disease and cost of chronic hepatitis C infection in Canada. Can J Gastroenterol Hepatol. 2014;28:243–50. doi: 10.1155/2014/317623. https://doi.org/10.1155/2014/317623 PMid:24839620 PMCid:PMC4049256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdick RA, Bragg-Gresham JL, Woods JD, et al. Patterns of hepatitis B prevalence and seroconversion in hemodialysis units from three continents:The DOPPS. Kidney Int. 2003;63:2222–9. doi: 10.1046/j.1523-1755.2003.00017.x. https://doi.org/10.1046/j.1523-1755.2003.00017.x PMid:12753311. [DOI] [PubMed] [Google Scholar]
- 7.Almawi WY, Qadi AA, Tamim H, Ameen G, Bu-Ali A, Arrayid S, et al. Seroprevalence of hepatitis C virus and hepatitis B virus among dialysis patients in Bahrain and Saudi Arabia, Transplant Proc. 2004;36:1824–6. doi: 10.1016/j.transproceed.2004.07.019. https://doi.org/10.1016/j.transproceed.2004.07.019 PMid:15350487. [DOI] [PubMed] [Google Scholar]
- 8.Yakaryilmaz F, Gurbuz OA, Guliter S, Mert A, Songur Y, Karakan T, et al. Prevalence of occult hepatitis B and hepatitis C virus infections in Turkish hemodialysis patients. Ren Fail. 2006;28:729–35. doi: 10.1080/08860220600925602. https://doi.org/10.1080/08860220600925602 PMid:17162434. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira RC, Teles SA, Dias MA, Tavares VR, Silva SA, Gomes SA, et al. Hepatitis B virus infection profile in hemodialysis patients in Central Brazil:Prevalence, risk factors, and genotypes. Mem Inst Oswaldo Cruz. 2006;101:689–92. doi: 10.1590/s0074-02762006000600019. https://doi.org/10.1590/S0074-02762006000600019 PMid:17072485. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DW, Dent H, Yao Q, Tranaeus A, Huang CC, Han DS, et al. Frequencies of hepatitis B and C infections among haemodialysis and peritoneal dialysis patients in Asia-Pacific countries:Analysis of registry data. Nephrol Dial Transplant. 2009;24:1598–603. doi: 10.1093/ndt/gfn684. https://doi.org/10.1093/ndt/gfn684 PMid:19096083. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra R, Soin D, Grover P, Galhotra S, Khutan H, Kaur N. Hepatitis B virus and hepatitis C virus co-infection in hemodialysis patients:A retrospective study from a tertiary care hospital of North India. J Nat Sci Biol Med. 2016;7(1):72–4. doi: 10.4103/0976-9668.175076. https://doi.org/10.4103/0976-9668.175076 PMid:27003974 PMCid:PMC4780172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wreghitt TG. Blood-borne virus infections in dialysis units —A review. Rev Med Virol. 1999;9:101–9. doi: 10.1002/(sici)1099-1654(199904/06)9:2<101::aid-rmv234>3.0.co;2-u. https://doi.org/10.1002/(SICI)1099-1654(199904/06)9:2<101::AID-RMV234>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 13.Souqiyyeh MZ, Al-Attar MB, Zakaria H, Shaheen FA. Dialysis centers in the kingdom of saudi arabia. Saudi J Kidney Dis Transpl. 2001;12(3):293–304. PMid:18209376. [PubMed] [Google Scholar]
- 14.Hinrichsen H, Leimenstoll G, Stegen G, Schrader H, Folsch UR, Schmidt WE. Prevalence and risk factors of hepatitis C virus infection in haemodialysis patients:a multicentre study in 2796 patients. Gut. 2002;51(3):429–433. doi: 10.1136/gut.51.3.429. https://doi.org/10.1136/gut.51.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buturović-Ponikvar J. Renal replacement therapy in Slovenia:annual report 2001. Nephrology Dialysis Transplantation. 2003;18(Suppl 5):53–5. doi: 10.1093/ndt/gfg1048. https://doi.org/10.1093/ndt/gfg1048 PMid:12817072. [DOI] [PubMed] [Google Scholar]
- 16.Boulaajaj K, Elomari Y, Elmaliki B, Madkouri B, Zaid D, Benchemsi N. Prevalence of hepatitis C, hepatitis B and HIV infection among haemodialysis patients in Ibn-rochd university hospital, casablanca. Nephrol Ther. 2005;1(5):274–284. doi: 10.1016/j.nephro.2005.06.012. https://doi.org/10.1016/j.nephro.2005.06.012 PMid:16895696. [DOI] [PubMed] [Google Scholar]
- 17.Zahran AM. Prevalence of seroconversion of hepatitis C virus among hemodialysis patients in Menoufia Governorate, Egypt. Arab J Nephrol Transplant. 2014;7(2):133–5. PMid:25366511. [PubMed] [Google Scholar]
- 18.Saxena AK, Panhotra BR. The susceptibility of patients with type-2 diabetes to hepatitis C virus infection during long-term haemodialysis. Swiss Med Wkly. 2003;133:611–8. doi: 10.4414/smw.2003.10402. PMid:14745667. [DOI] [PubMed] [Google Scholar]
- 19.Ocak S, Duran N, Kaya H, Emir I. Seroprevalence of hepatitis C in patients with type 2 diabetes mellitus and non-diabetic on haemodialysis. Int J Clin Pract. 2006;60:670–4. doi: 10.1111/j.1368-5031.2006.00738.x. https://doi.org/10.1111/j.1368-5031.2006.00738.x PMid:16805751. [DOI] [PubMed] [Google Scholar]
- 20.Carneiro MA, Martins RM, Teles SA, Silva SA, Lopes CL, Cardoso DD, et al. Hepatitis C prevalence and risk factors in hemodialysis patients in Central Brazil:A survey by polymerase chain reaction and serological methods. Mem Inst Oswaldo Cruz. 2001;96:765–9. doi: 10.1590/s0074-02762001000600003. https://doi.org/10.1590/S0074-02762001000600003 PMid:11562698. [DOI] [PubMed] [Google Scholar]
- 21.Petrosillo N, Gilli P, Serraino D, Dentico P, Mele A, Ragni P, et al. Prevalence of infected patients and understaffing have a role in hepatitis C virus transmission in dialysis. Am J Kidney Dis. 2001;37:1004–10. doi: 10.1016/s0272-6386(05)80017-4. https://doi.org/10.1016/S0272-6386(05)80017-4. [DOI] [PubMed] [Google Scholar]
- 22.Wong PN, Fung TT, Mak SK, Lo KY, Tong GM, Wong Y, et al. Hepatitis B virus infection in dialysis patients. J Gastroenterol Hepatol. 2005;20:1641–51. doi: 10.1111/j.1440-1746.2005.03837.x. https://doi.org/10.1111/j.1440-1746.2005.03837.x PMid:16246180. [DOI] [PubMed] [Google Scholar]
- 23.Afzali A, Weiss NS, Boyko EJ, Ioannou GN. Association between serum uric acid level and chronic liver disease in the United States. Hepatology. 2010;52(2):578–89. doi: 10.1002/hep.23717. https://doi.org/10.1002/hep.23717 PMid:20683957. [DOI] [PubMed] [Google Scholar]
- 24.Kidney Disease:Improving Global Outcomes (KDIGO) KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int. 2008;109(Suppl):S1–99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR. 2001;50:1–43. [PubMed] [Google Scholar]
- 26.de Oliveira Júnior WV, Sabino Ade P, Figueiredo RC, Rios DR. Inflammation and poor response to treatment with erythropoietin in chronic kidney disease. J Bras Nefrol. 2015;37(2):255–63. doi: 10.5935/0101-2800.20150039. https://doi.org/10.5935/0101-2800.20150039 PMid:26154647. [DOI] [PubMed] [Google Scholar]
- 27.Finelli L, Miller JT, Tokars JI, et al. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18:52–61. doi: 10.1111/j.1525-139X.2005.18108.x. https://doi.org/10.1111/j.1525-139X.2005.18108.x PMid:15663766. [DOI] [PubMed] [Google Scholar]
- 28.Fabrizi F, Messa PG, Lunghi G, Aucella F, Bisegna S, Mangano S, et al. Occult hepatitis B virus infection in dialysis patients:A multicentre survey. Aliment Pharmacol Ther. 2005;21:1341–7. doi: 10.1111/j.1365-2036.2005.02501.x. https://doi.org/10.1111/j.1365-2036.2005.02501.x PMid:15932364. [DOI] [PubMed] [Google Scholar]
- 29.Minuk GY, Sun DF, Greenberg R, Zhang M, Hawkins K, Uhanova J, et al. Occult hepatitis B virus infection in a North American adult hemodialysis patient population. Hepatology. 2004;40:1072–7. doi: 10.1002/hep.20435. https://doi.org/10.1002/hep.20435 PMid:15486926. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control. Hepatitis:control measures for hepatitis B in dialysis centers. HEW publication no. (CDC) 1977:78–8358. [Google Scholar]
- 31.Dinits-Pensy M, Forrest GN, Cross AS, Hise MK. The use of vaccines in adult patients with renal disease. Am J Kidney Dis. 2005;46:997–1011. doi: 10.1053/j.ajkd.2005.08.032. https://doi.org/10.1053/j.ajkd.2005.08.032 PMid:16310566. [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez-García ML, Fernandez-Rodriguez CM, Lledo-Navarro JL, Buhigas-Garcia I. Prevalence of occult hepatitis B virus infection. World J Gastroenterol. 2011;17:1538–42. doi: 10.3748/wjg.v17.i12.1538. https://doi.org/10.3748/wjg.v17.i12.1538 PMid:21472117 PMCid:PMC3070122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Somi MH, Etemadi J, Ghojazadeh M, et al. Risk factors of HCV seroconversion in hemodialysis patients in tabriz, iran. Hepat Mon. 2014;14(6):e17417. doi: 10.5812/hepatmon.17417. https://doi.org/10.5812/hepatmon.17417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jasuja S, Gupta AK, Choudhry R, Kher V, et al. Prevalence and associations of hepatitis C viremia in hemodialysis patients at a tertiary care hospital. Indian J Nephrol. 2009;19(2):62–7. doi: 10.4103/0971-4065.53324. https://doi.org/10.4103/0971-4065.53324 PMid:20368926 PMCid:PMC2847810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yen T, Keeffe EB, Ahmed A. The epidemiology of hepatitis C virus infection. J Clin Gastroenterol. 2003;36:47–53. doi: 10.1097/00004836-200301000-00015. https://doi.org/10.1097/00004836-200301000-00015 PMid:12488709. [DOI] [PubMed] [Google Scholar]
- 36.Strader DB, Wright T, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases:Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–71. doi: 10.1002/hep.20119. https://doi.org/10.1002/hep.20119 PMid:15057920. [DOI] [PubMed] [Google Scholar]
- 37.Fabrizi F, Messa PG, Lunghi G, Aucella F, Bisegna S, Mangano S, et al. Occult hepatitis B virus infection in dialysis patients:A multicentre survey. Aliment Pharmacol Ther. 2005;21:1341–7. doi: 10.1111/j.1365-2036.2005.02501.x. https://doi.org/10.1111/j.1365-2036.2005.02501.x PMid:15932364. [DOI] [PubMed] [Google Scholar]
- 38.Abdelmoemen G, Khodeir SA, Abou-Saif S, Kobtan A, Abd-Elsalam S. Prevalence of occult hepatitis C virus among hemodialysis patients in Tanta university hospitals:a single-center study. Environ Sci Pollut Res Int. 2018;25(6):5459–5464. doi: 10.1007/s11356-017-0897-y. https://doi.org/10.1007/s11356-017-0897-y PMid:29214477. [DOI] [PubMed] [Google Scholar]
- 39.Cheng SB, Wu CC, Shu KH, Ho WL, Chen JT, Yeh DC, Liu TJ, P'eng FK. Liver resection for hepatocellular carcinoma in patients with end-stage renal failure. J Surg Oncol. 2001;78(4):241–6. doi: 10.1002/jso.1160. https://doi.org/10.1002/jso.1160 PMid:11745817. [DOI] [PubMed] [Google Scholar]


