Abstract
BACKGROUND:
Cell therapies offer a promising potential in promoting bone regeneration. Stem cell therapy presents attractive care modality in treating degenerative conditions or tissue injuries. The rationale behind this is both the expansion potential of stem cells into a large cell population size and its differentiation abilities into a wide variety of tissue types, when given the proper stimuli. A progenitor stem cell is a promising source of cell therapy in regenerative medicine and bone tissue engineering.
AIM:
This study aimed to compare the osteogenic differentiation and regenerative potentials of human mesenchymal stem cells derived from human bone marrow (hBM-MSCs) or amniotic fluid (hAF-MSCs), both in vitro and in vivo studies.
SUBJECTS AND METHODS:
Human MSCs, used in this study, were successfully isolated from two human sources; the bone marrow (BM) and amniotic fluid (AF) collected at the gestational ages of second or third trimesters.
RESULTS:
The stem cells derived from amniotic fluid seemed to be the most promising type of progenitor cells for clinical applications. In a pre-clinical experiment, attempting to explore the therapeutic application of MSCs in bone regeneration, Rat lumbar spines defects were surgically created and treated with undifferentiated and osteogenically differentiated MSCs, derived from BM and second trimester AF. Cells were loaded on gel-foam scaffolds, inserted and fixed in the area of the surgical defect. X-Ray radiography follows up, and histopathological analysis was done three-four months post- operation. The transplantation of AF-MSCs or BM-MSCs into induced bony defects showed promising results. The AF-MSCs are offering a better healing effect increasing the likelihood of achieving successful spinal fusion. Some bone changes were observed in rats transplanted with osteoblasts differentiated cells but not in rats transplanted with undifferentiated MSCs. Longer observational periods are required to evaluate a true bone formation. The findings of this study suggested that the different sources; hBM-MSCs or hAF-MSCs exhibited remarkably different signature regarding the cell morphology, proliferation capacity and osteogenic differentiation potential
CONCLUSIONS:
AF-MSCs have a better performance in vivo bone healing than that of BM-MSCs. Hence, AF derived MSCs is highly recommended as an alternative source to BM-MSCs in bone regeneration and spine fusion surgeries. Moreover, the usage of gel-foam as a scaffold proved as an efficient cell carrier that showed bio-compatibility with cells, bio-degradability and osteoinductivity in vivo.
Keywords: Mesenchymal stem cells, Amniotic fluid, Bone marrow, Osteogenic differentiation, AF-MSCs, BM-MSCs
Introduction
Mesenchymal stem cells (MSCs) are multipotent stem cells that can self-renew and differentiate into different cell types including osteoblasts, chondrocytes, tenocytes, adipocytes, and hepatocytes which make them a promising tool in tissue engineering and regenerative medicine applications. MSCs can be derived from different human sources including; bone marrow tissue, fetal amniotic fluid, adipose tissue cord blood, umbilical cord, placenta and dental pulp [1], [2], [3].
MSC originating from the stroma of the bone marrow (BM) was one of the first known MSC, and are also more advanced in clinical trials. BM-MSCs generally serve as the “gold standard” against which other MSC sources are compared. It is well known that bone marrow stroma contains progenitor cells with osteogenic potential, generally referred to as mesenchymal stem cells or bone marrow stromal cells (BM-MSCs) [4]. Human BM-MSCs have been demonstrated to differentiate toward the osteoblastic lineage in-vitro and to form bone tissue upon ectopic implantation [5].
Bone marrow-derived mesenchymal stem cells (BM-MSCs) have shown a great promise in animal studies and even in a few clinical trials for skeletal tissues regeneration [6]. Harvesting BM-MSCs from a patient is an invasive and rather painful procedure. Furthermore, the number, proliferative capacity, and differentiation potential of BM-MSCs decline with age suggesting that tissue-engineering strategies based on these cells might not be feasible in older patients [7].
Fetal Amniotic Fluid Stem cells (AF-MSCs) seems a very promising type of cells and its application is rapidly growing in regenerative research. Almost ten years ago, the first suggestion of human amniotic fluid as a new putative source for stem cells was reported [8]. The first evidence for the existence of AF-MSCs was demonstrated by the discovery of a highly proliferative cell type in human amniotic fluid expressing the pluripotent stem cell marker Oct4 [9]. AF-MSCs have been applied to critically sized femoral bone defects of a nude rat in combination with biomaterial scaffold and shown the bone formation in rat femoral defect [10].
AF-MSCs cells demonstrated high potential in differentiation into hematopoietic [11], neurogenic [9], [12], [13], [14], osteogenic [13], [14], chondrogenic [14], adipogenic [13], [14], renal [15], hepatic [16], and various other lineages [9], [13]. The biological properties and markers expression pattern of AF-MSCs appears to be more similar to that of embryonic stem (ES) cells [17]. They express many but not all of the markers of embryonic stem cells (ESCs) [18]. However, they require no feeder layers for culture, they have not been observed to form teratomas in-vivo and are capable of > 300 population doublings in culture [19]. It is also possible to generate monoclonal genomically stable AF-MSC lines, harbouring high proliferative potential without raising ethical issues [20].
Both BM- and AF-derived MSCs offer a very promising and much more abundant potential cell-source for repair of bone defects, particularly the vertebral spines defects. The vertebral spine (or backbone) plays an important role in the stability of the upper body and the protection of the Spinal Cord [21]. Vertebral spines underwent pathological degeneration, or developed cancerous tumours or exposed to accidents are treated by surgical intervention, which employs autologous bone graft transplantation or substitutes for non-union defects and replacement of damaged tissue [22], [23]. However, several inconveniences are likely to occur with surgical interventions. It includes extra surgery to remove grafted bone from the patient’s body, increased potential operations’ complications [23]. Additionally, the amount of bone available for grafting might be insufficient. Moreover, the cost is very high [24].
In this study, we have isolated human MSCs (hMSCs) from two sources; bone marrow and amniotic fluid to compare the proliferation and osteogenic differentiation potentials in-vitro and evaluate the ability of transplanted human fetal amniotic fluid and adult bone marrow stem cells in the enhancement of functional repair in “rats” with induced spine defect.
Subjects and Methods
The Ethics Committee of the National Research Center, Cairo, Egypt, approved the study protocol and all participants gave informed consent.
Mesenchymal stem cells were isolated from two different human sources; Bone Marrow and Amniotic fluid. Bone marrow (BM) samples were aspirated from the sternum of healthy human subjects, following their consent, at Cairo-Nasser Institute. BM aspiration was done as a clinical care procedure in the process of doing the cross-matching procedure for donor BM transplantation. The subjects age range was between 6-33 years old (n = 6). The third-trimester AF samples were collected during two deliveries of the elective cesarean section. Eight women' AF samples were collected, whereas seven samples only (n = 7) were successfully isolated. The maternal age range was from 21 to 38 years. Second trimester AF samples (collected between the 14th and 18th weeks of gestation) were obtained by amniocentesis from five women, three (n = 3) were successfully isolated. The age range was between 21 to 38 years old.
Bone marrow cells were isolated by ficoll gradient separation of mononuclear cells [25]. Second and third-trimester amniotic fluid cells were isolated by centrifugation and pelleting of cells [19]. We didn’t study the effect of the parameters; sex and age on the MSCs isolation protocol, since isolation of AF only from pregnant female but BM isolation could be from both sex.
Isolation of human Bone Marrow-derived Mesenchymal Stem Cells was done by RosetteSep® Human Mesenchymal Stem Cell Enrichment Cocktail (RosetteSep, Stem Cell Technologies, Canada) was applied to the bone marrow to get rid of the unwanted cells by negative selection. Bone marrow mononuclear cells were isolated from the bone marrow sample by Ficoll-Paque™ PLUS density gradient solution (Stem Cell Technologies, GE Healthcare, Canada). Mononuclear cells were plated at a density of 10,000 cells/cm2 in 60 mm plastic tissue culture dishes in complete culture media containing Alpha-MEM (Lonza, Belgium), 10% FBS (Lonza, Belgium), 1% glutamax (Gibco, Invitrogen, Life Technologies, USA), 1% 10,000 U/ml penicillin and 10,000 U/ml streptomycin (Pen-Strep, Lonza, Belgium), (3 ng/ml) fibroblast growth factor basic (Sigma-Aldrich, USA) and incubated in a humid 5% CO2 atmosphere at 37ºC in a CO2 incubator. Mesenchymal stem cells adhered to the culture plates, meanwhile the other non-adherent cells were discarded upon the first replaced media. Media was replaced twice a week.
Isolation of amniotic fluid-derived Mesenchymal Stem Cells was done by centrifugation and pelleting of cells [19]. Cells were cultured in α-MEM media (Lonza) containing 20% FBS (Lonza), glutamax (Invitrogen), penicillin-streptomycin (Lonza) and bFGF (Sigma). Media was exchanged two times /week and passage was done when the cells reached the confluence of about 75%.
Culture Expansions were maintained to reach about 80% confluence, and then cells were passage and reseed. Manual scraping technique using cell scraper (Corning incorporated, Costar, Mexico) and collection of the cells, followed by centrifugation and re-suspension in the complete medium then re-seeding of cells in culture plates.
Differentiation of MSCs into bone-forming cells was initiated at third passage. Cells at 70% confluence were used for osteogenic differentiation, cultured in DMEM containing 20% FBS, 100 µg/ml penicillin & streptomycin and 1% glutamax, L-ascorbic acid 2-phosphate, β-glycerol phosphate and dexamethasone [26]. A comparable control culture maintained in proliferation media was generated for each sample. Two similar simultaneous sets were created at the same time; first set: plates in differentiation media, as well as plates in proliferation media, were kept in the corresponding culture media for 14 days before being passed for characterisation protocols. The second set: plates in differentiation media and plates in proliferation media were kept in its culture media for 29 days before characterisation.
Characterisation of osteogenic differentiation was done using Alizarin Red staining. Alizarin red S (Sigma) staining for the detection of mineralized nodules in the differentiated cultures was done according to the manufacturer’s instructions.
Cultures maintained in osteogenic and control media for 14 days were used for early alizarin staining, whereas the second similar set remained in its corresponding media for 29 days were used for late alizarin staining.
Alizarin Red staining, Monolayers in the plates were washed three times with PBS and fixed in 70% ethanol at room temperature for one hour. Monolayers were then washed three times with dH2O before the addition of 1.3 g% Alizarin Red S (Sigma) (pH 4.2). The plates were incubated at 37ºC for one hour with gentle shaking. After aspiration of the unincorporated dye, the plates were washed with dH2O till colour disappears. The plates were then washed with PBS for 5 min. Distal H2O was added to the plate to prevent cell dryness, and the plates became ready for visual inspection using an inverted microscope where calcium deposits were stained orange-red.
The in vivo study was performed by transplantation of human stem cells (MSC at passage 3 and differentiated osteogenic cells after 29 days of osteogenic induction) into rat spines. Six male Sprague Dawley rats weighed 200-300 gm were divided into two groups; each group consists of three rats.
All animals experiments were conducted after animal ethics approvals with approval numbers 16/263, and all animals were treated humanely.
BM Group: this group is being transplanted with BM-derived stem cells. Transplantation of BM-MSCs was done in duplicate: Two rats labeled as Osteogenic 1 and Osteogenic 2 had received MSCs differentiated into osteogenic progenitors (experimental group). Cells used for implantation underwent osteogenic induction for 29 days. A third rat (control one), had received matched but undifferentiated BM-derived MSCs.
AF Group: this group is transplanted with second-trimester AF-derived stem cells. Two rats; Osteogenic 1 and Osteogenic 2 had received MSCs differentiated into osteogenic progenitor cells. Transplanted cells were derived from second-trimester pregnancy and completed 30 days in osteogenic induction media. The third rat (control one), had received AF-undifferentiated MSCs.
An absorbable gelatin sponge, gel foam (Cutanplast®, Italy), was used as a scaffold to load the cells on.
The rats were anesthetized by being injected intraperitoneally with 200 µl (10 mg) freshly prepared Thiopental Sodium and 200 µl (0.093 mg) Xyla-Ject® (23.3 mg/ml Xylazine Hydrochloride).
Induction of rat spine bone defects in both AF-MSCs and BM-MSCs groups: Spines “decortication” defects have been created in the transverse processes of spines at the lumbar region, consists of 5 vertebrae that have a larger bed to work on.
Scaffold Implantation loaded with approximately 2 million cells, Cell-loaded gel foams, were applied and fitted in the decorticated areas of the lumbar spines in the backbone of the rats.
The X-ray photographing of the rat’s backbone was done for both groups at different time intervals to follow up the healing process in the osteogenic versus control rats.
In the BM transplanted group: X-ray filming was done after 4, 9, 13 and 17 weeks from the first day of cells transplantation. For the AF transplanted group: X-ray filming was done after 4, 8 and 12 weeks (as this AF group had started later than the BM group).
Histological analysis was performed for qualitative evaluation of new bone formation in the defected lumber spine. Three-four months post-implantation rats were sacrificed according to the recommendation of the ethical committee of the National Research Centre for Animal Experiment. The specimens of rat defected lumber spines were cut in blocks 0.5-1 cm apart from the defect, decalcified in 4% formic acid at room temperature and routinely prepared to be embedded in paraffin blocks. Lateromedial 5 µm thick sections were obtained and stained with hematoxylin and eosin to be examined by a light microscope [26].
Results
Proliferative potential and morphological criteria of BM-MSCs and AF-MSCs, human Mesenchymal Stem Cells (hMSCs) were compared. The primary BM-MSCs and AF-MSCs cultured cells took a similar period (10-14 days) to reach confluence and showed comparable plating efficiency (Figure 1A and 1E), respectively. The BM-MSCs showed a higher proliferation rate than the second-trimester AF-MSCs at the first Passage (Figure 1B) and (Figure 1F) respectively. After subsequent passages (the 2nd and 3rd passages), both BM-MSCs and AF-MSCs showed spindle-shaped fibroblast morphology (Figure 1C and 1G) (Figure 1D and 1H), respectively. The proliferation capacity of BM samples differed from one sample to another, but generally, the younger age derived sample showed a higher proliferation rate and more typical spindle cell morphology than the older age sample (data not shown).
Figure 1.
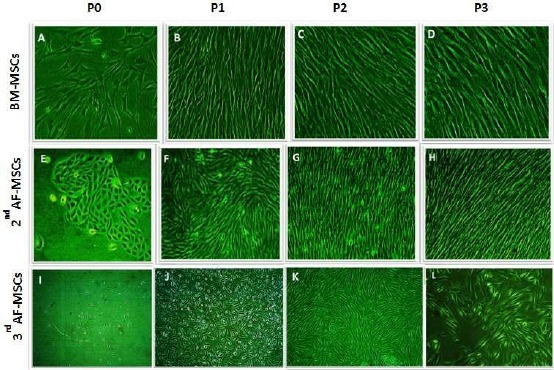
Proliferation and culture expansion of BM-MSCs, 2nd trimester AF- MSCs & 3rd trimester AF-MSCs; A-D) represented Bone marrow (BM) Proliferation & culture expansion at different passage; A) P0, B) P1, C) P2, D) P3 by x10 magnification; E-H) showed 2nd trimester AF-MSCs Proliferation & culture expansion at different passage; E) P0; F) P1; G) P2; H) P3 by x 10 magnification; I-L) represented 3rd trimester AF-MSCs Proliferation & culture expansion at different passage; I) P0; J) P1; K) P2; L) P3 by x 10 magnification
Proliferative potential and morphological criteria of 2nd and 3rd trimester AF: The cells can form colonies (clonogenic ability) at the primary culture that appeared mostly in the 2nd trimester AF cultures (Figure 1E). However, the adherent cells of 3rd-trimester cultures appeared in a scattered manner with the detection of a few aggregates of no more than ten cells (Figure 1I).
The primary AF cell cultures of 2nd and 3rd trimesters had a heterogeneous morphology consisting of fibroblastoid-like morphology and small rounded resembling epithelial cells (Figure 1E) and (Figure 1I), respectively. During the subsequent passages, the cultures exhibited only typical fibroblastic cells. The epithelioid ones had no longer observed in cases of 2nd trimester (Figure 1F). While in some of 3rd trimester AF cultures both morphologies, the fibroblastic and rounded epithelial, were shown (Figure 1J). Cultures at P3 in all AF samples had a homogenous typical fibroblast-like MSCs morphology (Figure 1H and Figure 1L).
The cells acquire senesce-like morphology (Figure 1L). The culture period in osteogenic media was 17 days from the third passage to osteogenic induction. The cell morphology showed elongated flattened cells with widened inter-cellular spaces (Figure 1L).
The BM-MSCs, 2nd and 3rd-trimester AF-MSCs after the first 14 days in osteogenic media are shown in Figure 2. There were three sets of plates, one set, in which cells continue in proliferation growth media (control), two sets in which, cells cultured in osteogenic media extended for two-time intervals, 14 and 29 days.
Figure 2.
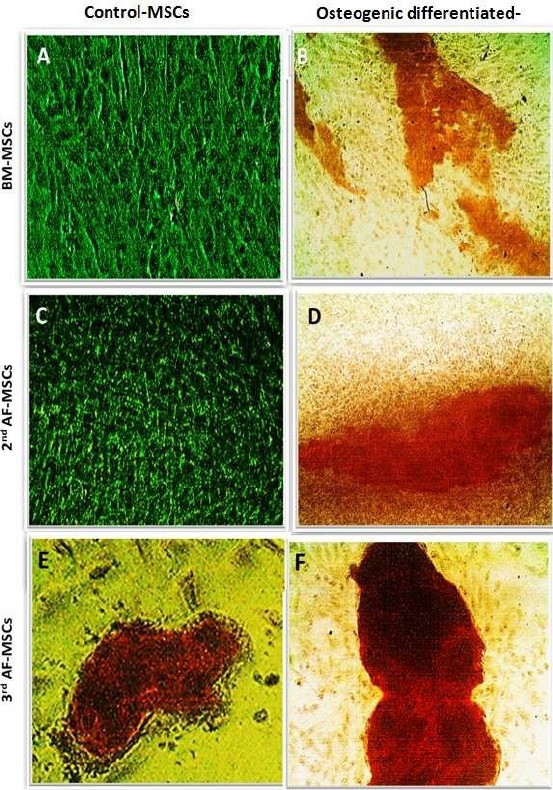
Osteogenic differentiation of BM-MSCs, 2nd trimester AF-MSCs and 3rd trimester AF-MSCs after 14 days; Osteogenic differentiation of BM cells on day 14; A) Control; B) Osteogenic differentiation stained by Alizarin; Osteogenic differentiation of 2nd trimester AF cells; C) Control; D) Osteogenic differentiation stained by Alizarin after 14 days; Osteogenic differentiation of 3rd trimester AF cells; E) Control; F) Osteogenic differentiation stained by Alizarin after 14 days
The BM-MSCs after 14 days in osteogenic medium were stained by Alizarin Red stain. The control plate showed a negative Alizarin stain (Figure 2A), while the osteogenic plate showed weak to moderate staining intensity (Figure 2B).
The 2nd-trimester AF-MSCs plates after 14 days in osteogenic medium and staining by Alizarin Red stain showed no calcium stained spots across the control plate (Figure 2C). The osteogenic plate showed multiple orange-red spots scattered all over the plate, including the periphery (Figure 2D).
The 3rd-trimester AF-MSCs on day 14th of osteogenic induction: the control plate showed multiple stained patches of variable sizes (Figure 2E) and the osteogenic plate showed multiple stained spots and patches that were of more enhanced when compared to the control plate (Figure 2F).
Cultured plates after the 28 days: BM-MSCs, 2nd & 3rd-trimester AF-MSCs in osteogenic media are shown in Figure 3. BM-MSCs cultures in the osteogenic medium stained with by Alizarin Red stain: The control plate, showed negative Alizarin stain (Figure 3A), while the osteogenic plate showed strong red staining intensity spots all over the plate (Figure 3B).
Figure 3.
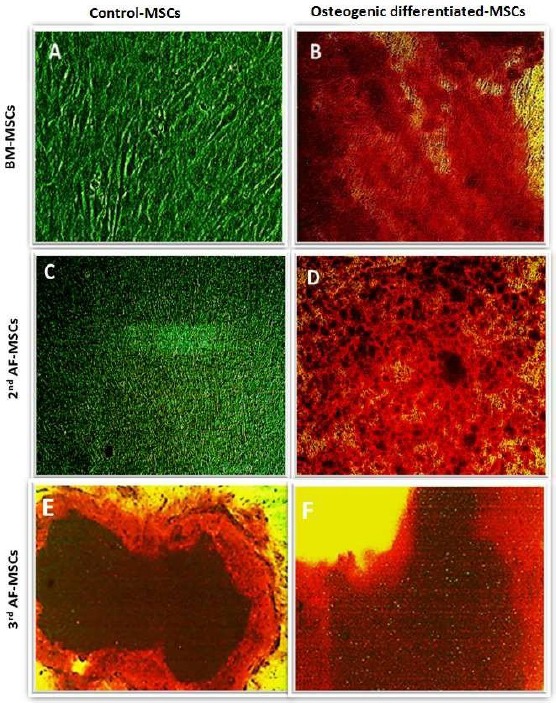
Osteogenic differentiation of BM-MSCs, 2nd AF-MSCs & 3rd AF-MSCs at 28 days; Osteogenic differentiation of BM cells on day 28th; A) Control; B) Osteogenic differentiation stained by Alizarin; Osteogenic differentiation of 2nd trimester AF cells; C) Control; D) Osteogenic differentiation stained by Alizarin; Osteogenic differentiation of 3rd AF cells; E) Control; F) osteogenic differentiation stained by Alizarin
The 2nd-trimester AF-MSCs in osteogenic medium stained by Alizarin Red stain: The Control plate showed no stained spots across the plate (Figure 3C), while the osteogenic plate showed multiple orange-red stained spots (Figure 3D).
The 3rd-trimester AF-MSCs in osteogenic medium stained by Alizarin Red stain: Control plate showed red-orange stained spots in few areas of the plate (Figure 3E), while the osteogenic plate showed strong red stained spots in multiple different parts across the plate (Figure 3F).
In this study, we suggested that MSCs cultured in osteogenic differentiation medium may induce new bone formation in experimental spinal fusion rats. In pre- clinical experiments testing the therapeutic application of MSCs in bone regeneration, rat lumbar spines defects were surgically created and treated with undifferentiated and osteogenic differentiated MSCs, derived from BM and 2nd trimester AF. Cells were loaded on gel-foam scaffolds. X-ray radiography follows up, and histopathological analysis showed that AF-MSCs offer better healing effect and have a higher potential of achieving successful spinal fusion.
The BM–rats group, after four months, the control rat, transplanted with undifferentiated MSCs showed no change at the lumbar spine, while one of the two osteogenic rats, who transplanted with osteogenic differentiated MSCs, showed bone mottling, and a shadow area at the lumbar spine (Figure 4).
Figure 4.
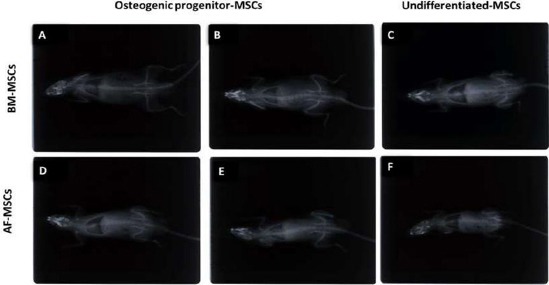
X-ray for AF-MSCs and BM-MSCs transplantation in rats. AF- and BM-derived undifferentiated MSCs, and differentiated osteogenic cells transplanted on decorticated lumber rat-spine beds. Among the BM group, after 4 months, the control rat, transplanted with undifferentiated MSCs showed no change at the lumbar spine. While, one of the two osteogenic rats transplanted with osteogenic differentiated MSCs, showed bone mottling, a shadow area at the lumbar spine. For the AF– group, after 3 months, the control rat (undifferentiated MSCs) showed no bone changes. However, the two osteogenic rats (transplanted with osteoblastic 2nd trimester AF- derived MSCs) showed bone mottling
The AF–rats group, after three months, the control rat (undifferentiated MSCs) showed no bone changes, whereas the two osteogenic rats (transplanted with osteoblastic 2nd trimester AF- derived MSCs showed bone mottling (Figure 4).
Histopathological examination of spinal-decorticated rat lumber specimens that were transplanted with different grafts has been performed. Grafts used in this experiment were the osteogenic differentiated BM-MSCs versus undifferentiated BM-MSCs as well as undifferentiated AF-MSCs versus osteogenic differentiated AF-MSCs (Figure 5).
Figure 5.
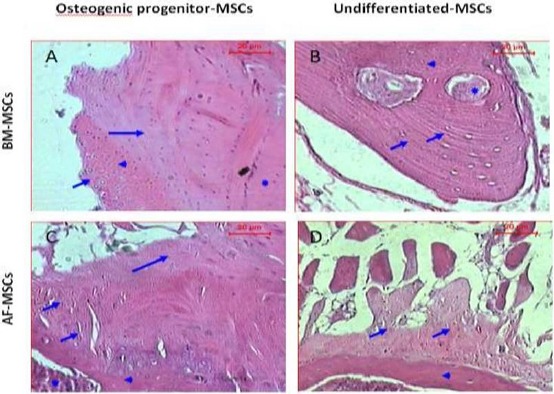
Histopathological examination of sections of experimental and control rats, 4 months after transplantation with (BM-MSCs) & 3 months after transplantation with (AF-MSCs) stained with hematoxylin and eosin dye. (A & B) Are sections of rat spinal defect transplanted with BM-MSCs, A) section of rat spinal defect transplanted with BM- MSCs differentiated into osteogenic progenitors shows cartilage area (short arrow), calcified cartilage (arrowhead) and formed new bone (long arrow) and host bone (asterisk). B) Section of rat spinal defect transplanted with undifferentiated BM-MSCs shows irregular calcified areas (short arrows) that associated with few osteocytes (arrowhead) and blood vessels (asterisk). (C & D) Represented sections of rat spinal injury transplanted with AF-MSCs, C) Section of rat spinal defect transplanted with AF-MSCs differentiated into osteogenic progenitors shows calcified cartilage (short arrow), newly formed bone (long arrow), host bone (arrowhead) and bone marrow (asterisk). D) Section of rat spinal defect transplanted with undifferentiated AF-MSCs shows calcified area with few osteogenic (short arrow) on the top of the host bone (arrowhead) (H&E, Scale bar: 20 µm)
At four months post-transplantation, histopathological examination of sections of the rat transplanted with BM-MSCs differentiated into osteogenic progenitors showed the peripheral cartilage area that was followed by calcified cartilage and area of newly formed bone in contact with the host bone (Figure 5A). While the control rat received undifferentiated BM-MSCs exhibited irregular calcified areas that associated with few osteocytes and blood vessels (Figure 5B).
At three months post-transplantation, microscopic examination of the defect section of the rat transplanted with matched AF-MSCs differentiated into osteogenic progenitor cells, showed calcified cartilage, newly formed bone, host bone and bone marrow (Figure 5C), while the control rat received matched undifferentiated AF-MSCs displayed calcified areas with few osteogenic foci at the top of the host bone (Figure 5D).
Discussion
MSCs have a powerful ability to differentiate into a range of multi-cell types, including osteoblasts, adipocytes, chondrocytes, myoblasts, and neurons [27], [28]. The findings of Wang et al., (2018) reveal that the tumour antigen 15-leucine-rich repeat containing membrane protein (LRRC15) is an essential regulator for osteogenesis of MSCs through modulating p65 cytoplasmic/nuclear translocation [29].
The classic method of osteogenic differentiation of MSCs in vitro involves incubating a confluent monolayer of MSCs with ascorbic acid, beta-glycerophosphate, and dexamethasone for 2-3 weeks. The MSCs form aggregates or nodules and increase their expression of alkaline phosphatase, and calcium accumulation can be seen over time. These bone nodules stain positively by alizarin red [30]. In our experiments, the standard osteogenic differentiation media and protocol worked very effectively and osteogenic differentiation indicated by alizarin nodules was obviously detected in cell cultures applying osteogenic differentiation media, whereas, no mineralized nodules were seen in comparable cultures with normal proliferation media except in plates of 3rd trimester AF, which might indicate the presence of shaded differentiated cells at that advanced gestational age.
BM-MSCs generally serve as the „gold standard against‟ which other MSCs sources are compared. Previous studies demonstrated that populations of bone marrow-derived MSCs from human, canine, rabbit, rat, and mouse can develop into terminally differentiated mesenchymal phenotypes both in vitro and in vivo, including bone and cartilage [31], [32].
Amniotic fluid (AF) of second-trimester pregnancy has been used, many years ago, for prenatal diagnosis of genetic and chromosomal disorders. Research reports have shown that AF contains in addition to committed and differentiated cells a subpopulation with stem cell characteristics of multi-lineage differentiation, and expression of stem cell markers [33].
Amniotic fluid cells are heterogeneous, including cells shed from embryonic and extra- embryonic tissues. Ectodermal, mesodermal and endodermal cells can be found in amniotic fluid. Placental amnion may be derived from the epiblast as early as eight days after fertilisation. Thus, amnion epithelial cells are thought to retain the pluripotent properties of early epiblast cells, and these found in amniotic fluid and may serve as a source for mesenchymal stem cells [34]. The osteogenic commitment of AF-MSCs could be enhanced by using appropriate osteoconductive scaffolds and osteoinductive growth factors [35].
Amniotic fluid-derived stem cells (AF-MSCs) display high self-renewal capacity and plasticity, the second trimester derived cells, in particular, are rapidly expanded cells. Age of females and the stage of gestations constitute very important contributing factors marking AF properties. AF-MSCs showed the capacity to engraft and to contribute lineage-specific progeny in animal models. Therefore, AF-MSCs show great promise for use in cell-based therapeutic application in regenerative medicine. In this study, there was a significant difference in both the proliferation rate and osteogenic differentiation potentials of MSCs derived from the AF at different gestational ages a higher proliferation capacity and osteogenic potential compared to that of BM-derived cells. In a previous study, BM-MSCs showed a stronger osteogenic differentiation potential compared to adipose tissue-derived MSCs (AT-MSCs) [36].
Other investigators concluded similar findings are informing that the osteogenic differentiation potential of AF-MSCs is highly promising at both 2nd and 3rd trimester when incubated in osteogenic media for 2 or 4 weeks [31]. The striking finding in our study was detecting osteoblastic progenitor cells in the cellular content of 3rd trimester AF-MSCs, as clearly shown by detection of orange-red stained mineralised patches in control plates in which MSCs were continuously fed with MSCs proliferation media only.
In general, cell attachment depends on the intrinsic characteristics of the cells and is modulated by choice of medium and supplements, the type of culture surface used as well as the interaction with heterotypic cell types. The combination of these factors influences the activation of the required signal transduction pathways leading to isolation and proliferation of the desired cell type [37].
In our in vivo study, transplantation of both osteogenic differentiated and undifferentiated MSCs, derived from bone marrow or AF (2nd trimester) loaded on gel-foam and directly applied to the decorticated spines into rat-spines showed potential promising results. The x-rays, 3-4 months from the day of transplantation, showed a shadow (described as potential bone mottling) in one of the BM-groups, the rats transplanted with osteoblastic cells. In the AF-MSCs group, the two rats transplanted with osteoblastic cells showed potential bone mottling on X-ray filming. Additional 3-4 months may be required to confirm that this mottling is a true bone-forming tissue.
The use of AF-MSCs in cell therapy and tissue repair applications is yet in its beginning; however, several studies indicated that human amniocytes obtained by amniocentesis could be largely expanded and have the capacity to attach and proliferate on biodegradable scaffolds [38]. Osteogenically differentiated human CD117+ AFS (isolated by FACS separation) gave rise to tissue-engineered bone grafts after subcutaneous transplantation into immune-deficient mice [19]. The therapeutic potential of human CD117+ AFS was also shown after subcutaneous implantation in athymic nude rats [38]. They produced mineralized structures and supported the functional repair of large bone defects. Unsorted human AFS, as in our case, were cultured under osteoblastic differentiation condition. They formed osteoblasts with detectable mineralization on titanium surfaces indicating a possible application in osteosynthesis.
In this study, we suggested that MSCs cultured in osteogenic differentiation medium may induce the formation of new bone in experimental spinal fusion rats. X-ray radiography follows up, and histopathological analysis done three months post-operation showed that AF-MSCs have a better healing effect and increasing the future likelihood of achieving successful spinal fusion.
The present histopathological analysis was performed at three months post-cells transplantation of spinal-decorticated rat lumber specimens transplanted with AF-MSCs and at four months post-cells transplanted with BM-MSCs grafts. Results observed represented some differences in bone formation between the AF-MSCs and BM-MSCs rat groups. The experimental rats transplanted with osteogenically differentiated BM-MSCs showed cartilage area, calcified cartilage (ossified) and newly formed bone, while the control rats that received matched undifferentiated BM-MSCs exhibited irregular calcified areas that associated with few osteocytes and blood vessels representing the beginning of bone repair healing phase. The experimental rats transplanted with osteogenically differentiated AF-MSCs showed a more advanced stage than that of BM-MSCs rats involving calcified area with new bone structures contained osteocyte-like cells. The control rat received matched undifferentiated AF-MSCs showed calcified area with little osteogenic formed bone at the top of the host bone, which presents a more advanced stage of healing compared to that of BM-MSCs control rats.
In conclusion, there was a significant difference noted between the 2nd-trimester AF-MSCs which have a greater proliferation capacity and typical fibroblast morphology compared to that of the 3rd-trimester AF-MSCs which showed limited proliferation capacity in vitro. However, assessment of osteogenic differentiation of the AF-MSCs at early pregnancy 2nd trimester, versus late pregnancy 3rd trimester, by Alizarin Red Staining has shown that the 3rd trimester AF-MSCs contains osteoblast committed progenitor cells at both 14th and 28th days of osteogenic differentiation induction. However, both 2nd and 3rd-trimester AF-MSCs have greater proliferation capacity and osteogenic differentiation potentials in vitro compared to that of BM-MSCs. The in vitro osteogenic induction of MSCs derived from the two human sources; BM or 2nd trimester AF before they’re in vivo implantation, promotes their osteogenic/bone forming capacity and raising their healing effect. AF-MSCs, have a higher potential of inducing bone healing in vivo than that of BM-MSCs. Hence, they can be used as an alternative source to BM-MSCs in bone regeneration and spine fusion surgeries. Our findings suggested AF-MSCs a promising and safe alternative to BM-MSCs for cell therapy of bone defects and injury.
Acknowledgements
All authors acknowledge the National Research Centre for funding this project. We thank the participant for providing their samples.
Footnotes
Funding: This research was partially funded by the National Research Centre
Competing Interests: The authors have declared that no competing interests exist
Reference
- 1.Barry FP, Murphy JM. Mesenchymal stem cells:clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36(4):568–584. doi: 10.1016/j.biocel.2003.11.001. https://doi.org/10.1016/j.biocel.2003.11.001 PMid:15010324. [DOI] [PubMed] [Google Scholar]
- 2.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources:their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806. doi: 10.1177/0022034509340867. https://doi.org/10.1177/0022034509340867 PMid:19767575 PMCid:PMC2830488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. https://doi.org/10.1634/stemcells.2005-0342 PMid:16410387. [DOI] [PubMed] [Google Scholar]
- 4.Klingemann H, Matzilevich D, Marchand J. Mesenchymal Stem Cells - Sources and Clinical Applications. Transfus Med Hemother. 2008;35(4):272–277. doi: 10.1159/000142333. https://doi.org/10.1159/000142333 PMid:21512642 PMCid:PMC3076359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaquiéry C, Schaeren S, Farhadi J, Mainil-Varlet P, Kunz C, Zeilhofer HF, Heberer M, Martin I. In vitro osteogenic differentiation and in vivo bone-forming capacity of human isogenic jaw periosteal cells and bone marrow stromal cells. Annals of Surgery. 2005;242(6):859–867. doi: 10.1097/01.sla.0000189572.02554.2c. https://doi.org/10.1097/01.sla.0000189572.02554.2c PMid:16327496 PMCid:PMC1409890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seong JM, Kim BC, Park JH, Kwon IK, Mantalaris A, Hwang YS. Stem cells in bone tissue engineering. Biomedical materials. 2010;5(6):062001. doi: 10.1088/1748-6041/5/6/062001. https://doi.org/10.1088/1748-6041/5/6/062001 PMid:20924139. [DOI] [PubMed] [Google Scholar]
- 7.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, LeBoff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging cell. 2008;7(3):335–43. doi: 10.1111/j.1474-9726.2008.00377.x. https://doi.org/10.1111/j.1474-9726.2008.00377.x PMid:18248663 PMCid:PMC2398731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cananzi M, Atala A, De Coppi P. Stem cells derived from amniotic fluid:new potentials in regenerative medicine. Reproductive biomedicine online. 2009;18:17–27. doi: 10.1016/s1472-6483(10)60111-3. https://doi.org/10.1016/S1472-6483(10)60111-3. [DOI] [PubMed] [Google Scholar]
- 9.Rosner M, Mikula M, Preitschopf A, Feichtinger M, Schipany K, Hengstschläger M. Neurogenic differentiation of amniotic fluid stem cells. Amino Acids. 2012;42(5):1591–6. doi: 10.1007/s00726-011-0929-8. https://doi.org/10.1007/s00726-011-0929-8 PMid:21573873. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama K, Chen C, Gronthos S, Shi S. Lineage differentiation of mesenchymal stem cells from dental pulp, apical papilla, and periodontal ligament. Methods Mol Biol. 2012;887:111–121. doi: 10.1007/978-1-61779-860-3_11. https://doi.org/10.1007/978-1-61779-860-3_11 PMid:22566051. [DOI] [PubMed] [Google Scholar]
- 11.Torricelli F, Brizzi L, Bernabei PA, Gheri G, Di SL, Nutini L, Lisi E, Di MT, Cariati E. Identification of hematopoietic progenitor cells in human amniotic fluid before the 12th week of gestation. Italian journal of anatomy and embryology=Archivio italiano di anatomia ed embriologia. 1993;98(2):119–26. [PubMed] [Google Scholar]
- 12.Prusa AR, Marton E, Rosner M, Bettelheim D, Lubec G, Pollack A, Bernaschek G, Hengstschläger M. Neurogenic cells in human amniotic fluid. American journal of obstetrics and gynecology. 2004;191(1):309–14. doi: 10.1016/j.ajog.2003.12.014. https://doi.org/10.1016/j.ajog.2003.12.014 PMid:15295384. [DOI] [PubMed] [Google Scholar]
- 13.Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19(6):1450–1456. doi: 10.1093/humrep/deh279. https://doi.org/10.1093/humrep/deh279 PMid:15105397. [DOI] [PubMed] [Google Scholar]
- 14.Savickiene J, Treigyte G, Baronaite S, Valiuliene G, Kaupinis A, Valius M, Arlauskiene A, Navakauskiene R. Human amniotic fluid mesenchymal stem cells from second-and third-trimester amniocentesis:differentiation potential, molecular signature, and proteome analysis. Stem cells international. 2015;2015 doi: 10.1155/2015/319238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perin L, Sedrakyan S, Giuliani S, Da Sacco S, Carraro G, Shiri L, Lemley KV, Rosol M, Wu S, Atala A, et al. Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis. PLoS One. 2010;5(2):e9357. doi: 10.1371/journal.pone.0009357. https://doi.org/10.1371/journal.pone.0009357 PMid:20195358 PMCid:PMC2827539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Coppi P. C-kit/CD117 cells from amniotic fluid and membranes and their cardiomyogenic potential. Circ Res. 2010;107(6):e11. doi: 10.1161/CIRCRESAHA.110.227322. author reply e12. [DOI] [PubMed] [Google Scholar]
- 17.Rosner M, Schipany K, Shanmugasundaram B, Lubec G, Hengstschläger M. Amniotic fluid stem cells:future perspectives. Stem cells international. 2012;2012 doi: 10.1155/2012/741810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME. Isolation of amniotic stem cell lines with potential for therapy. Nature biotechnology. 2007;25(1):100–106. doi: 10.1038/nbt1274. https://doi.org/10.1038/nbt1274 PMid:17206138. [DOI] [PubMed] [Google Scholar]
- 19.Dupont KM, Sharma K, Stevens HY, Boerckel JD, García AJ, Guldberg RE. Human stem cell delivery for treatment of large segmental bone defects. Proceedings of the National Academy of Sciences. 2010;107(8):3305–10. doi: 10.1073/pnas.0905444107. https://doi.org/10.1073/pnas.0905444107 PMid:20133731 PMCid:PMC2840521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. http://www.priorityconsult.com/mh_spine.php .
- 21.Henkel J, Woodruff MA, Epari DR, Steck R, Glatt V, Dickinson IC, Choong PF, Schuetz MA, Hutmacher DW. Bone Regeneration Based on Tissue Engineering Conceptions - A 21st Century Perspective. Bone Res. 2013;1(3):216–248. doi: 10.4248/BR201303002. https://doi.org/10.4248/BR201303002 PMid:26273505 PMCid:PMC4472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giannoudis PV, Faour O, Goff T, Kanakaris N, Dimitriou R. Masquelet technique for the treatment of bone defects:tips-tricks and future directions. Injury. 2011;42(6):591–598. doi: 10.1016/j.injury.2011.03.036. https://doi.org/10.1016/j.injury.2011.03.036 PMid:21543068. [DOI] [PubMed] [Google Scholar]
- 23.Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics. the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8(4):114–124. doi: 10.4161/org.23306. https://doi.org/10.4161/org.23306 PMid:23247591 PMCid:PMC3562252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittenger GL, Malik RA, Burcus N, Boulton AJ, Vinik AI. Specific fiber deficits in sensorimotor diabetic polyneuropathy correspond to cytotoxicity against neuroblastoma cells of sera from patients with diabetes. Diabetes Care. 1999;22(11):1839–1844. doi: 10.2337/diacare.22.11.1839. https://doi.org/10.2337/diacare.22.11.1839 PMid:10546017. [DOI] [PubMed] [Google Scholar]
- 25.Carleton HM, Drury RA, Wallington EA. Carleton's histological technique. USA: Oxford University Press; 1980. PMCid:PMC1162107. [Google Scholar]
- 26.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. https://doi.org/10.1126/science.284.5411.143 PMid:10102814. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41. doi: 10.1038/nature00870. https://doi.org/10.1038/nature00870 PMid:12077603. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Liu Y, Zhang M, Lv L, Zhang X, Zhang P, Zhou Y. LRRC15 promotes osteogenic differentiation of mesenchymal stem cells by modulating p65 cytoplasmic/nuclear translocation. Stem cell research & therapy. 2018;9(1):65. doi: 10.1186/s13287-018-0809-1. https://doi.org/10.1186/s13287-018-0809-1 PMid:29523191 PMCid:PMC5845373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review:mesenchymal stem cells:their phenotype, differentiation capacity, immunological features, and potential for homing. Stem cells. 2007;25(11):2739–49. doi: 10.1634/stemcells.2007-0197. https://doi.org/10.1634/stemcells.2007-0197 PMid:17656645. [DOI] [PubMed] [Google Scholar]
- 30.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells:characterization, differentiation, and application in cell and gene therapy. Journal of cellular and molecular medicine. 2004;8(3):301–16. doi: 10.1111/j.1582-4934.2004.tb00320.x. https://doi.org/10.1111/j.1582-4934.2004.tb00320.x PMid:15491506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC cell biology. 2006;7(1):14. doi: 10.1186/1471-2121-7-14. https://doi.org/10.1186/1471-2121-7-14 PMid:16529651 PMCid:PMC1435883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Wang X, Chen Z, Liu G, Chen Z. Semi-quantitative RT-PCR analysis of LIM mineralization protein 1 and its associated molecules in cultured human dental pulp cells. archives of oral biology. 2007;52(8):720–6. doi: 10.1016/j.archoralbio.2007.02.005. https://doi.org/10.1016/j.archoralbio.2007.02.005 PMid:17368558. [DOI] [PubMed] [Google Scholar]
- 33.Peng HH, Wang TH, Chao AS, Chang SD. Isolation and differentiation of human mesenchymal stem cells obtained from second trimester amniotic fluid;experiments at Chang Gung Memorial Hospital. Chang Gung Med J. 2007;30(5):402–407. PMid:18062170. [PubMed] [Google Scholar]
- 34.Rodrigues MT, Lee SJ, Gomes ME, Reis RL, Atala A, Yoo JJ. Amniotic fluid-derived stem cells as a cell source for bone tissue engineering. Tissue Engineering Part A. 2012;18(23-24):2518–27. doi: 10.1089/ten.tea.2011.0672. https://doi.org/10.1089/ten.tea.2011.0672 PMid:22891759. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Liu Y, Sun Y, Wang B, Xiong Y, Lin W, Wei Q, Wang H, He W, Li G. Tissue source determines the differentiation potentials of mesenchymal stem cells:a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem cell research & therapy. 2017;8(1):275. doi: 10.1186/s13287-017-0716-x. https://doi.org/10.1186/s13287-017-0716-x PMid:29208029 PMCid:PMC5718061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ibrahim A, Mahmoud M, Ismail T, Gaber K, Abel Aleem A. Isolation, propagation and osteogenic differentiation of amniotic fluid derived mesenchymal stem cells. Journal of Biotechnology Research. 2016;6(16):7–27. [Google Scholar]
- 37.Peister A, Deutsch ER, Kolambkar Y, Hutmacher DW, Guldberg RE. Amniotic fluid stem cells produce robust mineral deposits on biodegradable scaffolds. Tissue Engineering Part A. 2009;15(10):3129–38. doi: 10.1089/ten.tea.2008.0536. https://doi.org/10.1089/ten.tea.2008.0536 PMid:19344289 PMCid:PMC2792053. [DOI] [PMC free article] [PubMed] [Google Scholar]


