Abstract
BACKGROUND:
Osteoarthritis (OA) is generally considered a degenerative joint disease caused by biomechanical changes and the ageing process. In OA pathogenesis, the development of OA is thought to be regulated largely by excess matrix metalloproteinase (MMP), which contributes to the degradation of extracellular matrices such as MMP-1 and Interleukin-4.
AIM:
This study aims to prove the influence of Mesenchymal Stem Cell Wharton Jelly on decreasing MMP-1 levels and increasing IL-4 which is a specific target as a target component in cases of osteoarthritis in vivo.
MATERIAL AND METHODS:
This research is an experimental study with the design of Post-Test-Only Control Group Design. The sample consisted of 16 OA rats as a control group and 16 OA rats treated with MSC-WJ as a treatment group. OA induction is done by injection of monosodium iodoacetate (MIA) into the intra-articular right knee. Giving MSC-WJ is done in the third week after MIA induction. The serum MMP-1 and IL-4 levels were measured after 3 weeks treated with MSC-WJ using the ELISA method. The statistical test used is an independent t-test. The value of p < 0.05 was said to be statistically significant.
RESULTS:
The result showed that serum MMP-1 levels were higher in the group treated with MSC-WJ than in the control group (p < 0.05). Serum IL-4 levels were higher in the group treated with MSC-WJ than in the control group (p < 0.05).
CONCLUSION:
This study concluded that MSC-WJ increased MMP-1 levels and IL-4 levels in serum OA rats. MSC-WJ showed a negative effect on MMP-1 in the serum of OA rats.
Keywords: Matrix Metalloproteinase-1, Mesenchymal Stem Cell Wharton Jelly, Interleukin-4, Osteoarthritis
Introduction
Osteoarthritis (OA) is considered a cumulative result of mechanical and biological events caused by an imbalance between catabolic and anabolic processes in articular joint tissue [1]. At present, the development of OA is thought to be regulated largely by excess matrix metalloproteinase (MMP), which contributes to the degradation of extracellular matrices, such as MMP-1 and MMP-3 which play an important role in the development of OA by decreasing extracellular matrix [2], where this MMP is induced by inflammatory mediators, such as interleukin-1-beta (IL-1β) and tumour necrosis factor alpha (TNF-α) in tissue and OA joint fluid [3]. So far there are no drugs available to guarantee a complete cure and the possibility of recurrence from OA.
Mesenchymal stem cells (MSCs) are promising candidates for cartilage regeneration and OA therapy because they have a chondrogenic potential and the ability to form extracellular matrices [4]. Also, MSC has an immunomodulatory and trophic capacity by secreting anti-inflammatory factors and growth factors [5], which might improve the inflammatory and catabolic aspects of OA. Monosodium iodoacetate injection (MIA) to intra articular has been studied extensively as a model for OA in animals [6], [7] and is regarded as a suitable model and resembles a phenomenon observed in human OA [8].
Matrix metalloproteinase-1 is one of the protease enzymes that acts to degrade the components of the main cartilage matrix, such as collagen, aggrecan, link protein, and cartilage oligomer proteins [9], [10] while IL-4 and other cytokines are secreted in large amounts to counter the inflammatory response when Th2 dominates inflamed tissue because these immunomodulatory cytokines can reduce the production and activity of proinflammatory cytokines which are classified as inhibitors [11].
This study aims to prove the influence of Mesenchymal Stem Cell Wharton Jelly on decreasing MMP-1 levels and increasing IL-4 which is a specific target as a target component in cases of osteoarthritis in vivo.
Material and Methods
Animal and Experimental Design
Male, white rats (Rattus novergicus) with a weight ranging from 200-250 grams as experimental animals placed in clean, disinfected and pathogen-free cages and given standard food in the form of pellets and drinking in ad libitum. Trial animals adapted first for 1 week before treatment. Induction of osteoarthritis conducted with 300 μg intra-articular injection of monosodium iodoacetate (MIA) (Sigma Aldric, USA) in 50 μl of saline solution (0.9% NaCl) sterile (12) single into the right knee joint rats anaesthetized by intraperitoneal injection of xylazine 10 mg/kg and ketamine 20 mg/kg uses insulin syringe with a needle (needle) 27G. 32 osteoarthritis male, white rats (three weeks after MIA induction) were divided into 2 treatment groups (n = 16): Control group and MSC-WJ group. MSC group-WJ is given 50 μl MSC-WJ with a dose of 1 x 106 cells into the right knee joint and a control group given 50 μl complete medium after anaesthetized.
Mesenchymal Stem Cell Wharton Jelly was obtained from the Indonesian Medical Education and Research Institute (IMERI) Faculty of Medicine, University of Indonesia. Based on the analysis of flow cytometry, MSC-WJ used for this therapy had CD73-APC cell surface expression 99.8%, CD105-PerCP-Cys5.5 95% and CD90-FITC 99.9%. Rats were sacrificed after 3 weeks of treatment. Serum and knee joint were taken and then analysed.
Analysis of Flow Cytometry
Mesenchymal Stem Cell Wharton Jelly was obtained from the Indonesian Medical Education and Research Institute (IMERI) Faculty of Medicine, University of Indonesia. Based on the analysis of flow cytometry, MSC-WJ used for this therapy had CD73-APC cell surface expression 99.8%, CD105-PerCP-Cys5.5 95% and CD90-FITC 99.9%. The photocell was taken use Nikon Ti-S microscope. Scale bar: 500 μm.
Histological Analysis
The right knee joint from the two groups and the left knee joint (normal) was cut and fixed in 4% formalin for 1 weekend calcified with formic acid (5%) for 3 days. The specimen then underwent automatic network processing for 24 hours. The tissue planted in paraffin and cutting using a microtome with a thickness of 5 μm. Ribbon cutting results were placed on the surface of warm water with a temperature of 45°C to remove folds on the ribbon due to cutting. Every ribbon was stained with Hematoxylin and Eosin (H&E).
Measurement of serum MMP-1 and IL-4 by ELISA
Blood was taken from sinus periorbital and centrifuged at 3000 rpm for 15 minutes. The collected serum was stored at -80°C until measurement. Serum MMP-1 and IL-4 levels were measured by an ELISA kit (Bioassay Technology Laboratory, China). All samples are measured in duplicate.
Examination of MMP-1 Levels (Work protocol based on rat MMP-1 ELISA Kit)
Prepare all reagents, standard solutions and samples as instructed. Bring all reagents to room temperature before use. The assay is performed at room temperature. Determine the number of strips required for the assay. Insert the strips in the framers for use. The unused strips should be stored at 2-8°C. Add 50 µL standard well. Add 40 µL sample to sample wells and then add 10 µL anti-MMP-1 antibody to sample wells, then add 50 µL streptavidin-HRP to sample wells and standard wells (Not blank control well). Mix well. Cover the plate with a shaker. Incubate 60 minutes at 37°C. Removed the sealer and wash the plate 5 times with wash buffer. Soak wells with at least 0,35 ml wash buffer for 30 seconds to minute for each wash. For automated washing, aspirate all wells and wash 5 times with wash buffer, overfilling wells with wash buffer. Blot the plate onto paper towels or other absorbent material. Add 50 µL substrate solution A to each well and then add 50 µL substrate solution B to each well. Incubate plate covered with a new sealer for 10 minutes at 37°C in the dark. Add 50 µL stop solution to each well; the blue colour will change into yellow immediately. Determine the optical density (OD value) of each well immediately using a microplate reader set a 450 nm within 30 min after adding the stop solution.
Examination of IL-4 Levels (Work protocol based on rat IL-4 ELISA Kit)
Prepare all reagents, standard solutions and samples as instructed. Bring all reagents to room temperature before use. The assay is performed at room temperature. Determine the number of strips required for the assay. Insert the strips in the framers for use. The unused strips should be stored at 2-8°C. Add 50 µL standard well. Add 40 µL sample to sample wells and then add 10 µL anti-MMP-1 antibody to sample wells, then add 50 µL streptavidin-HRP to sample wells and standard wells (Not blank control well). Mix well. Cover the plate with a shaker. Incubate 60 minutes at 37°C. Removed the sealer and wash the plate 5 times with wash buffer. Soak wells with at least 0,35 ml wash buffer for 30 seconds to minute for each wash. For automated washing, aspirate all wells and wash 5 times with wash buffer, overfilling wells with wash buffer. Blot the plate onto paper towels or other absorbent material. Add 50 µL substrate solution A to each well and then add 50 µL substrate solution B to each well. Incubate plate covered with a new sealer for 10 minutes at 37°C in the dark. Add 50 µL stop solution to each well; the blue colour will change into yellow immediately. Determine the optical density (OD value) of each well immediately using a microplate reader set a 450 nm within 30 min after adding the stop solution.
Research Ethics
This study was already passed the ethics clearance and has been approved by the Ethics Committee of the Faculty of Medicine, Andalas University, Padang with registration number: 549/KEP/FK/2017.
Statistical analysis
Data is presented in mean and elementary forms. The statistical analysis used is SPSS 18.0. The statistical test used is an independent t-test. The value of p < 0.05 was said to be statistically significant.
Result
A study of 32 osteoarthritis rats induced with monosodium iodoacetate (MIA) for 3 weeks was carried out. OA rats were divided into 2 groups, namely the control group and the group treated with MSC-WJ (Figure 1). Three weeks after MSC therapy, termination was done at the expense of experimental animals. Then a histopathological examination of mouse right knee (OA) was carried out. Examination of the levels of MMP-1 and IL-4 was carried out in the serum of rats by ELISA.
Figure 1.
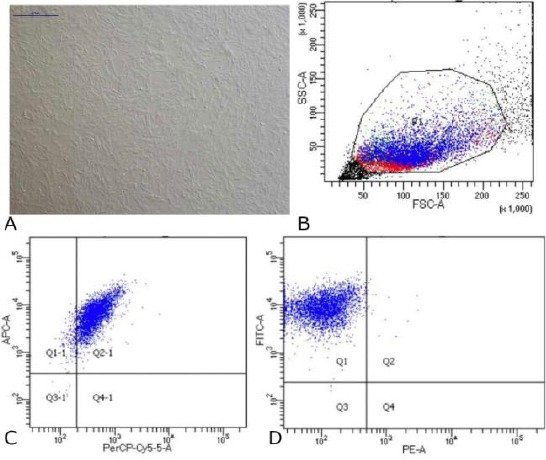
Data on Characteristics of Mesenchymal Stem Cells Wharton Jelly. (A) Cells MSC-WJ reach confluence. Scale bar: 500 µM. Photographs of cells taken using a Nikon Ti-S microscope; (B) Data flow cytometry. Forward scatter (FCS) plot&side scatter (SSC) plot. Population gated events (P1): 20,000; (C) Cell surface markers expression: CD73-APC 99.8% and CD105- PerCP-Cy5.5 95%; (D) Cell surface markers expression: CD90-FITC 99.9% and Lin (-) - PE 0.4%
Histopathology examination
After the bone portion of the rat’s knee is obtained preserved with formalin buffer, Histopathological examination was carried out using Hematoxylin-Eosin staining; the results are shown in figure 2. The results we can see in tissue reactions are changes in the thickness of cartilage and the number of chondrocytes.
Figure 2.
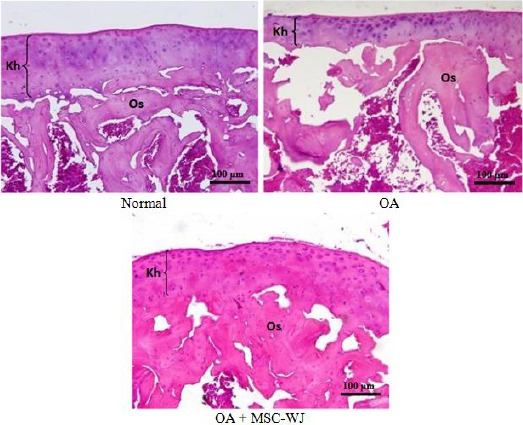
Microscopic joint tissue of experimental animals, showed joint fragility (Kh), bone tissue (Os) in normal rats, osteoarthritis (OA), and OA + MSC-WJ. Objective hematoxylin-eosin
Histopathological results show joint tissue with the surface consisting of cartilage with chondrocyte cells. In the OA group, there were areas with a thickness of cartilage that was thinner than normal animals and OA animals injected by MSC-WJ (Figure 2).
In addition to changes in the thickness of the cartilage, histopathological results also showed an increase in chondrocyte cell density in the OA mouse group compared to normal rats. In the OA group, of rats given MSC-WJ, the density of chondrocyte was close to the mean of normal rats.
ELISA examination
The blood obtained from the centrifuged animal is then obtained serum. Serum before analysis was stored in a refrigerator temperature of -80°C. The serum obtained was analysed for MMP-1 and IL-4 levels carried out in the Biomedical laboratory FK Unand.
The measurement of MMP-1 and IL-4 levels were carried out in normal rat, and the mean levels of MMP-1 and IL-4 were 1.62 ng/ml and 34.27 ng/ml. Based on the results of the normality test the data showed that the two research variables namely MMP-1 and IL-4 were normally distributed (p > 0.05). Thus, furthermore, parametric tests (free t-test) can be carried out.
Effect of MSC-WJ on serum MMP-1 levels in OA rats treated with MSC-WJ
The measurement of MMP-1 levels by ELISA method showed that the serum MMP-1 levels of OA rat treated with MSC-WJ were higher than those not treated which can be seen in Figure 3.
Figure 3.
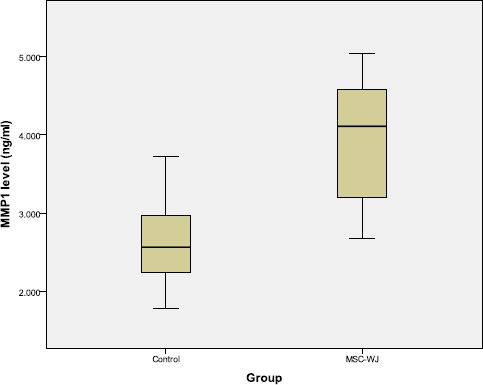
Boxplot graph of rat serum MMP-1 levels
The difference in MMP-1 levels between serum of rats treated with MSC-WJ and control can be seen in Table 1.
Table 1.
Differences in mean levels of MMP-1 by group
| Groups | MMP-1 levels (ng/ml) (Mean ± SD) | P value |
|---|---|---|
| Control | 2.63 ± 0.55 | 0.001 |
| MSC-WJ | 3.96 ± 0.81 |
Table 1 showed that there are differences in MMP-1 levels based on treatment. Increased levels of MMP-1 in the group treated with MSC-WJ from the control group. There were significant differences, between MSC-WJ with control (p < 0.05).
Effect of MSC-WJ on IL-4 levels in serum of OA rats
The results of measurement of IL-4 levels by ELISA method showed that the serum IL-4 levels of OA rats treated with MSC-WJ were higher than those not treated with bivariate tests which can be seen in Figure 4.
Figure 4.
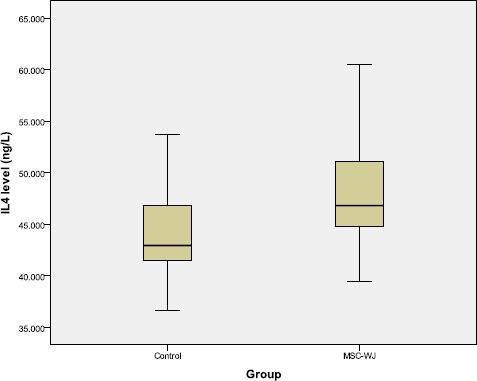
Boxplot graph of rat serum IL-4 levels
The difference in IL-4 levels between serum of rats treated with MSC-WJ and control can be seen in Table 2.
Table 2.
Mean differences in IL-4 levels by group
| Groups | IL-4 Levels (ng/l) (Mean ± SD) | P value |
|---|---|---|
| Control | 43.90 ± 4.99 | 0.027 |
| MSC-WJ | 47.95 ± 4.88 |
Table 2 showed that there are differences in IL-4 levels based on treatment. Increased IL-4 levels in the group treated with MSC-WJ from the control group. There were significant differences, between MSC-WJ with control (p < 0.05).
Discussion
The results showed that MIA induction in rats with a single dose of 300 μg after 3 weeks showed the reduced thickness of cartilage. The results of this study are also the same as those conducted by Janusz et al., (2001) regarding the effect of MIA on rat cartilage, wherein the study there was also reduced of cartilage in the surrounding area after one week of MIA induction [13].
This reduced cartilage occurs due to the loss of proteoglycans that build up the matrix. Also, the results of this study also showed a form of reactive chondroblast cell proliferation, so that cell densities were higher than those of OA rat cartilage that was not treated with MSC-WJ.
The results of the serum analysis using the ELISA method showed a tendency to increase serum MMP-1 and IL-4 levels compared to the serum of non-induced (normal) rat. This increase is due to chondrocyte cells and immune cells stimulated by inflammatory cytokines (IL-β and TNF-α) whose levels increase when inflammation occurs due to MIA induction.
Increased matrix-degrading enzymes and anti-inflammatory cytokines after MIA induction showed that rats experienced osteoarthritis. Induction of MSC-WJ in OA rat for 3 weeks showed a thickness of joint cartilage close to the thickness of normal cartilage and showed lower cell density closer to normal compared to OA rat. This shows the appearance of tissue repair in osteoarthritis by MSC-WJ.
Matrix metalloproteinase-1 (MMP-1)
Matrix metalloproteinase-1 is one of the protease enzymes that act to degrade the components of the main cartilage matrix, such as collagen, aggrecan, link protein, and cartilage oligomer proteins [14], [15]. This enzyme also functions to increase the proliferation and migration of MSCs [16]. MMP-1 increases production in synovial membranes, synovial fluid, and human cartilage that undergo OA [17], also in the serum of OA rat significantly [2]. The release of MMP-1 protein by human articular chondrocyte is stimulated by IL-1β [18].
This study showed that the serum MMP-1 levels of OA rat treated with MSC-WJ were higher than those not treated. Research by Saulnier et al., (2015) showed that the administration of MSC in rabbits OA had was not effective in reducing the expression of MMP-1 after 2 weeks of injection with MSC, but effectively reduced MMP-1 expression after 8 weeks of injection [19].
The results of this study indicate that MSC-WJ therapy has not been effective in reducing MMP-1 levels within 3 weeks after injection. Possibly in this period, MSC-WJ was still synthesising and releasing MMP-1 which is needed for the apoptosis of chondrocyte and synovial cells [20], the process of MSC migration and proliferation [16] and differentiation. Ho et al., (2009) showed that MMP-1 plays an important role in the MSC migration function, which operates through MMP1-PAR1 axis signalling [21]. According to Voronkina et al., (2017) that the involvement of MMP-1 is in the process of MSC differentiation, namely the increase in MMP-1 activity during the differentiation process [22].
The high levels of MMP-1 in OA mice treated with MSC-WJ were compared with OA mice that were not treated because in this period MSC expressed MMP-1 constitutively [23]. It is possible that MSC-WJ requires MMP-1 in repairing cell damage because it involves the process of apoptosis, migration and MSC differentiation and proliferation.
Interleukin-4 (IL-4)
Interleukin-4 (IL-4) is an anti-inflammatory cytokine that plays a role in stimulating the proliferation of B cells and T cells and encourages differentiation of CD4 + T cells into Th2. IL-4 also plays a key role in regulating humoral and adaptive immunity. IL-4 and other cytokines are secreted in large amounts to counter the inflammatory response when Th2 dominates inflamed tissue. Because these immunomodulatory cytokines can reduce the production and activity of proinflammatory cytokines which are classified as inhibitors [11].
Research by Hui et al., (2005) found that IL-4 was reported to play a role in the regulation of ADAMTS-4 in chondrocyte, although the exact mechanism has not been explained [24]. The proteins secreted by rat MSC and humans are chemokines, cytokines, growth factors and protease inhibitors [25], including IL-4, IL-10, and IL-13 which are anti-inflammatory cytokines [26].
In this study, it was found that IL-4 levels were higher in the serum of OA rat treated with MSC-WJ compared with those not treated. The same thing was found in the study of Kay et al., (2017) which showed that IL-4 expression was higher in arthritis rats given MSC compared to those not given [27]. Whereas Yan et al., (2017) research found that serum IL-4 levels of arthritis rats treated with MSC were compared with serum of non-treated arthritis rats, but the difference was not significant [28]. Chai et al., (2016) also found the same thing that UC-MSC can increase IL-4 in liver fibrosis both in vitro and in vivo [29].
Increased levels of IL-4 in the serum of OA mice show that MSC-WJ can increase the immunosuppressive activity of Th2 cells. MSC increases anti-inflammatory cytokines such as IL-4 which have anti-inflammatory effects through inhibition of the NF-κB cascade, which contributes to the regulation of proinflammatory cytokines [30]. MSC plays an important role in cartilage repair through direct differentiation into chondrocyte and paracrine effects [31], [32].
Information about the increase in expression in the form of serum IL-4 proteins in this study is important for the development of MSC-WJ as a therapy for osteoarthritis. The development of MSC-WJ as a therapy for osteoarthritis is quite promising. Differences in IL-4 levels between serum of rats treated and those not treated with MSC-WJ proved that MSC-WJ had a therapeutic effect. Thus, the information obtained encourages further studies of MSC-WJ as a treatment for OA.
Acknowledgements
Thank you to the Indonesian Medical Education and Research Institute (IMERI), Faculty of Medicine, University of Indonesia.
Footnotes
Funding: This research was funded by DIPA PNBP Medical Faculty of Andalas University, Ministry of Research, Technology and Higher Education with Research Contract Number: 90/BBPT/PNP/FK-UNAND-2018 Budget Year 2018
Competing Interests: The authors have declared that no competing interests exist
Reference
- 1.Poole A.R. Cartilage in health and disease. In: Koopman W.J, editor. Arthritis and Allied Conditions:A Textbook of rheumatology. 14th Edition. Baltimore: Williams & Wilkins; 2000. [Google Scholar]
- 2.Yan Z, Xiong J, Zhao C, Qin C, He 2015. Decreasing cartilage damage in a rat model of osteoarthritis by intra-articular injection of deoxycholic acid. Int J Clin Exp Med. 2015;8(6):9038–9045. PMid:26309557 PMCid:PMC4538145. [PMC free article] [PubMed] [Google Scholar]
- 3.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis research & therapy. 2009;11(3):224. doi: 10.1186/ar2592. https://doi.org/10.1186/ar2592 PMid:19519926 PMCid:PMC2714092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. https://doi.org/10.1126/science.284.5411.143 PMid:10102814. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS one. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. https://doi.org/10.1371/journal.pone.0001886 PMid:18382669 PMCid:PMC2270908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis and cartilage. 2003;11(11):821–30. doi: 10.1016/s1063-4584(03)00163-8. https://doi.org/10.1016/S1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 7.Kelly S, Dobson KL, Harris J. Spinal nociceptive reflexes are sensitized in the monosodium iodoacetate model of osteoarthritis pain in the rat. Osteoarthritis and cartilage. 2013;21(9):1327–35. doi: 10.1016/j.joca.2013.07.002. https://doi.org/10.1016/j.joca.2013.07.002 PMid:23973147. [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, Kroin JS, Buvanendran A, Li X, Van Wijnen AJ, Tuman KJ, Im HJ. Characterization of a new animal model for evaluation and treatment of back pain due to lumbar facet joint osteoarthritis. Arthritis & Rheumatism. 2011;63(10):2966–73. doi: 10.1002/art.30487. https://doi.org/10.1002/art.30487 PMid:21953085 PMCid:PMC3187574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stracke JO, Fosang AJ, Last K, Mercuri FA, Pendás AM, Llano E, Perris R, Di Cesare PE, Murphy G, Knäuper V. Matrix metalloproteinases 19 and 20 cleave aggrecan and cartilage oligomeric matrix protein (COMP) FEBS letters. 2000;478(1-2):52–6. doi: 10.1016/s0014-5793(00)01819-6. https://doi.org/10.1016/S0014-5793(00)01819-6. [DOI] [PubMed] [Google Scholar]
- 10.DeGroot J, Verzijl N, Marion JG, Wenting-Van Wijk, Bank RA, Lafeber FP, Bijlsma WJ, TeKoppele JM. Age-Related Decrease in Susceptibility of Human Articular Cartilage to Matrix Metalloproteinase–Mediated Degradation. Arthritis Rheum. 2001;44(11):2562–2571. doi: 10.1002/1529-0131(200111)44:11<2562::aid-art437>3.0.co;2-1. https://doi.org/10.1002/1529-0131(200111)44:11<2562::AID-ART437>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis & Rheumatism:Official Journal of the American College of Rheumatology. 2000;43(9):1916–26. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. https://doi.org/10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.van Buul GM, Siebelt M, Leijs MJ, Bos PK, Waarsing JH, Kops N, Weinans H, Verhaar JA, Bernsen MR, van Osch GJ. Mesenchymal stem cells reduce pain but not degenerative changes in a mono-iodoacetate rat model of osteoarthritis. Journal of Orthopaedic Research. 2014;32(9):1167–74. doi: 10.1002/jor.22650. https://doi.org/10.1002/jor.22650 PMid:24839120. [DOI] [PubMed] [Google Scholar]
- 13.Janusz MJ, Hookfin EB, Heitmeyer SA, Woessner JF, Freemont AJ, Hoyland JA, Brown KK, Hsieh LC, Almstead NG, De B, Natchus MG. Moderation of iodoacetate-induced experimental osteoarthritis in rats by matrix metalloproteinase inhibitors. Osteoarthritis and Cartilage. 2001;9(8):751–60. doi: 10.1053/joca.2001.0472. https://doi.org/10.1053/joca.2001.0472 PMid:11795995. [DOI] [PubMed] [Google Scholar]
- 14.Stracke JO, Fosang AJ, Last K, Mercuri FA, Pendás AM, Llano E, Perris R, Di Cesare PE, Murphy G, Knäuper V. Matrix metalloproteinases 19 and 20 cleave aggrecan and cartilage oligomeric matrix protein (COMP) FEBS letters. 2000;478(1-2):52–6. doi: 10.1016/s0014-5793(00)01819-6. https://doi.org/10.1016/S0014-5793(00)01819-6. [DOI] [PubMed] [Google Scholar]
- 15.DeGroot J, Verzijl N, Marion J. G, Wenting-Van Wijk, Bank RA, Lafeber FP, Bijlsma WJ, TeKoppele JM. Age-Related Decrease in Susceptibility of Human Articular Cartilage to Matrix Metalloproteinase–Mediated Degradation. Arthritis Rheum. 2001;44(11):2562–2571. doi: 10.1002/1529-0131(200111)44:11<2562::aid-art437>3.0.co;2-1. https://doi.org/10.1002/1529-0131(200111)44:11<2562::AID-ART437>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Almalki SG, Agrawal DK. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem cell research & therapy. 2016;7(1):129. doi: 10.1186/s13287-016-0393-1. https://doi.org/10.1186/s13287-016-0393-1 PMid:27612636 PMCid:PMC5016871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahmi H, Pelletier J.-P, Di Battista JA, CheungÜ H. S, Fernandes J. C, Martel-Pelletier J. Peroxisome proliferator-activated receptor gamma activators inhibit MMP-1 production in human synovial fibroblasts likely by reducing the binding of the activator protein 1. J. OsteoArthritis Research Society Int. 2002;10(2):100–108. doi: 10.1053/joca.2001.0485. https://doi.org/10.1053/joca.2001.0485 PMid:11869069. [DOI] [PubMed] [Google Scholar]
- 18.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage:associations with degenerative changes. Arthritis & Rheumatism. 2001;44(3):585–94. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. https://doi.org/10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Saulnier N, Viguier E, Perrier-Groult E, Chenu C, Pillet E, Roger T, Maddens S, Boulocher C. Intra-articular administration of xenogeneic neonatal mesenchymal stromal cells early after meniscal injury down-regulates metalloproteinase gene expression in synovium and prevents cartilage degradation in a rabbit model of osteoarthritis. Osteoarthritis and cartilage. 2015;23(1):122–33. doi: 10.1016/j.joca.2014.09.007. https://doi.org/10.1016/j.joca.2014.09.007 PMid:25219668. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S, Zhao Y, Guo J, Fei C, Zheng Q, Li X, Chang C. Downregulation of MMP1 in MDS-derived mesenchymal stromal cells reduces the capacity to restrict MDS cell proliferation. Scientific reports. 2016;7:43849. doi: 10.1038/srep43849. https://doi.org/10.1038/srep43849 PMid:28262842 PMCid:PMC5338350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho IA, Chan KY, Ng WH, Guo CM, Hui KM, Cheang P, Lam PY. Matrix metalloproteinase 1 is necessary for the migration of human bone marrow-derived mesenchymal stem cells toward human glioma. Stem Cells. 2009;27(6):1366–75. doi: 10.1002/stem.50. https://doi.org/10.1002/stem.50 PMid:19489099 PMCid:PMC2771102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voronkina IV, Smagina LV, Krylova TA, Musorina AS, Poljanskaya GG. Analysis of matrix metalloproteinase activity during differentiation of mesenchymal stem cells isolated from different tissues of one donor. Cell and Tissue Biology. 2017;11(2):95–103. https://doi.org/10.1134/S1990519X17020092. [Google Scholar]
- 23.Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells:comparison of chemokine and growth factor chemotactic activities. Stem cells. 2007;25(7):1737–45. doi: 10.1634/stemcells.2007-0054. https://doi.org/10.1634/stemcells.2007-0054 PMid:17395768. [DOI] [PubMed] [Google Scholar]
- 24.Hui W, Barksby HE, Young DA, Cawston TE, Mckie N, Rowan AD. Oncostatin M in combination with tumour necrosis factor αinduces a chondrocyte membrane associated aggrecanase that is distinct from ADAMTS aggrecanase-1 or -2. Annals of the rheumatic diseases. 2005;64(11):1624–32. doi: 10.1136/ard.2004.028191. https://doi.org/10.1136/ard.2004.028191 PMid:15883123 PMCid:PMC1755260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sze SK, de Kleijn DP, Lai RC, Tan EK, Zhao H, Yeo KS, Low TY, Lian Q, Lee CN, Mitchell W, El Oakley RM. Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Molecular & Cellular Proteomics. 2007;6(10):1680–9. doi: 10.1074/mcp.M600393-MCP200. https://doi.org/10.1074/mcp.M600393-MCP200 PMid:17565974. [DOI] [PubMed] [Google Scholar]
- 26.Skalnikova H, Motlik J, Gadher SJ, Kovarova H. Mapping of the secretome of primary isolates of mammalian cells, stem cells and derived cell lines. Proteomics. 2011;11(4):691–708. doi: 10.1002/pmic.201000402. https://doi.org/10.1002/pmic.201000402 PMid:21241017. [DOI] [PubMed] [Google Scholar]
- 27.Kay AG, Long G, Tyler G, Stefan A, Broadfoot SJ, Piccinini AM, Middleton J, Kehoe O. Mesenchymal stem cell-conditioned medium reduces disease severity and immune responses in inflammatory arthritis. Scientific reports. 2017;7(1):18019. doi: 10.1038/s41598-017-18144-w. https://doi.org/10.1038/s41598-017-18144-w PMid:29269885 PMCid:PMC5740178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan M, Liu X, Dang Q, Huang H, Yang F, Li Y. Intra-articular injection of human synovial membrane-derived mesenchymal stem cells in murine collagen-induced arthritis:assessment of immunomodulatory capacity in vivo. Stem cells international. 2017;2017 doi: 10.1155/2017/9198328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chai NL, Zhang XB, Chen SW, Fan KX, Linghu EQ. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World journal of gastroenterology. 2016;22(26):6036–6048. doi: 10.3748/wjg.v22.i26.6036. https://doi.org/10.3748/wjg.v22.i26.6036 PMid:27468195 PMCid:PMC4948270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;141:1–10. doi: 10.1038/nrrheum.2013.141. https://doi.org/10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 31.Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee:a proof-of-concept clinical trial. Stem cells. 2014;32(5):1254–66. doi: 10.1002/stem.1634. https://doi.org/10.1002/stem.1634 PMid:24449146. [DOI] [PubMed] [Google Scholar]
- 32.Chung JY, Song M, Ha CW, Kim JA, Lee CH, Park YB. Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model. Stem cell research & therapy. 2014;5(2):39. doi: 10.1186/scrt427. https://doi.org/10.1186/scrt427 PMid:24646697 PMCid:PMC4055114. [DOI] [PMC free article] [PubMed] [Google Scholar]


