Abstract
Pancreatic cancer ranks among the causes of cancer-related deaths. The average size of pancreatic cancer during diagnosis is about 31 mm and has not changed significantly over the past 30 years. Poor early diagnosis of a tumour has been attributed to the late-presenting symptoms. Over the years, improvement in the diagnosis of pancreatic cancer has been observed, and this can be linked to advancement in imaging techniques as well as the increasing knowledge of cancer history and genetics. Magnetic Resonance Imaging, Endoscopic Ultrasound, and Computer Topography are the approved imaging modalities utilised in the diagnosing of pancreatic cancer. Over the years, the management of patients with pancreatic cancer has seen remarkable improvement as reliable techniques can now be harnessed and implemented in determining the resectability of cancer. However, only about 10% of pancreatic adenocarcinomas are resectable at the time of diagnosis and will highly benefit from a microscopic margin-negative surgical resection. Overall, the failure of early tumour identification will result in considerable morbidity and mortality.
Keywords: Pancreatic Cancer, Epidemiology, Genetics, Diagnostic Radiology, Interventional Radiology, Pancreatic Management
Introduction
Pancreatic cancer in recent years has been one of the deadliest with an increased mortality rate of about 3% of all cancers and about 7% of all cancer death in the United States and Europe with an estimated five-year survival rate [1].
The estimated number of people expected to be diagnosed with pancreatic cancer in 2018 is about 55,440 (29,200 men and 26,240 women), and about 44,330 (23,020 men and 21,310 women) will die of pancreatic cancer [2].
Several factors have contributed to an increased risk of pancreatic cancer. Such risk factors vary from; tobacco use, overweight, obesity, workplace exposure to certain chemicals (benzene, petrochemicals, dyes, and pesticides), age, gender, race, family history, inherited genetic syndromes, diabetes, chronic pancreatitis, cirrhosis of the liver, stomach problems, diets, physical in-activities, coffee and alcohol [2].
The signs and symptoms vary due to the location and the stage of a tumour. The tumours located at the head of the pancreas cause obstructive jaundice and weight loss, which occur as a result of steatorrhea and diarrhoea. While tumours of the body and tail usually lead to abdominal pain and weight loss. Pain is also frequently associated with pancreatic cancer. The pain usually presents as a dull, deep pain, coming from the upper abdomen, radiating to the back [3].
Different case studies have shown that patients in the early stages with tumour size less than 3 cm without lymphatic metastasis have a better prognosis with a 5-year survival rate of up to 25-30% following surgical resection of a tumour. This result suggests that early detection is essential for the treatment and management of the tumour [4].
Also, advancement in diagnostic Imaging has paved the way in dictating underlining internal diseases which do not present with pain at the onset as observed in pancreatic cancers. Several imaging modalities have been used over the years for the diagnosis of different cancers [5]. This review looks at the epidemiology, genetics, screening and the management of Pancreatic cancer.
Epidemiology of Pancreatic Cancer
The epidemiological study of pancreatic cancer and the rate of its occurrence from 2005 to 2014 showed stable rates in women with a decline of approximately 2% annually in men. The cancer death rate from 2006 to 2015 had a 1.5% decrease in its annual report for both men and women. The combined cancer death rate fell continuously from 1991 to 2015 by a total of 26%, translating to approximately 2,378,600 fewer cancer deaths than would have been expected if death rates had remained at their peak [6].
Incidence
The incidence of pancreatic tumour varies from one geographical population to another. In every 10000, about 7.4 are affected by the tumor in both Western Europe and North America. Other developed countries such as New Zealand and Australia have about 6.5 per 100000 affected with the tumor. Lower incidence of approximately 1.0 in every 100000 is observed in developing countries in Africa and south-central Asia [2].
Pancreatic cancer also varies by gender in various geographical regions. The occurrence rate of pancreatic cancer among men in 2012 was 4.9 per 100000 and 3.6 per 100000 in women. The risk of developing pancreatic cancer in men was high in Armenia (11.9) and Czech Republic (11.8), Slovakia and Hungary (equally-11.5), then in Japan and Lithuania (equally-10.6). However, the risk of having pancreatic cancer in men was lowest in Guinea (0.4) and Pakistan (0.5). The incidence of pancreatic cancer in women is higher in developed countries. The risk of developing the tumour is lowest in Polynesia and central Africa (equaling-1.0), while the risk is higher in Hungary (5.9), Denmark (5.9), Finland (6.2) and Armenia (6.1) [7].
Europe and North America have 33% of the overall occurrence. This reflects on the accuracy of the diagnosis rather than the aetiology. The differences in incidence around the world have to do with quality in the data collected [6]. The incidence of pancreatic cancer around the world is summarised in Figure 1.
Figure 1.
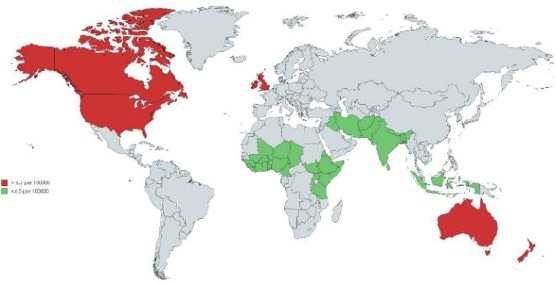
The figure showing the Incidence of pancreatic cancer around the world
The overall worldview of pancreatic cancer is showing the incidence rate. The incidence rate is more pronounced in developed countries than in the less developed countries.
The reduction in mortality incidence, when compared to past years, has been due to the improvement in medical care. Improved medical care showered significant positive effects on cancer treatment and management yet leaving some loopholes for some cancers such as pancreatic cancer [7]. Table 1 relates pancreatic cancer to other common cancers in the United States.
Table 1.
Relation of pancreatic cancer to the common types of cancers that affect the American population with the estimated new cases and deaths for 2018
| Rank | Common Types of Cancer | Estimated New Cases 2018 | Estimated Deaths 2018 |
|---|---|---|---|
| 1. | Breast Cancer (Female) | 266,120 | 40,920 |
| 2. | Lung and Bronchus Cancer | 234,030 | 154,050 |
| 3. | Prostate Cancer | 164,690 | 29,430 |
| 4. | Colorectal Cancer | 140,250 | 50,630 |
| 5. | Melanoma of the Skin | 91,270 | 9,320 |
| 6. | Bladder Cancer | 81,190 | 17,240 |
| 7. | Non-Hodgkin Lymphoma | 74,680 | 19,910 |
| 8. | Kidney and Renal Pelvis Cancer | 65,340 | 14,970 |
| 9. | Uterine Cancer | 63,230 | 11,350 |
| 10. | Leukemia | 60,300 | 24,370 |
| 11. | Pancreatic Cancer | 55,440 | 44,330 |
The table shows the relation of pancreatic cancer to the common types of cancers that affect the American population with the estimated new cases and deaths for 2018 [6].
Despite the improvement in the treatments of cancers in general, pancreatic cancer remains one of the deadliest cancers to date with high mortality as shown in Figure 2. For 2018 projection, new cases were estimated at 55,440 (3.2%) and 44,330 (7.3%) estimated deaths. The most predominant types of cancers are more common in comparison to pancreatic cancer. However, the late discovery of pancreatic cancer makes treatment and management challenging [6].
Figure 2.
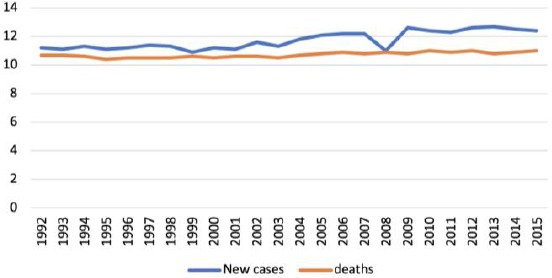
New incidence of pancreatic cancer was at 12.6 per 100,000 men and women annually. The number of mortality was 10.9 per 100,000 men and women annually. These rates are age-adjusted and based on 2011-2015 incidence and mortality
Survival Rate
Survival rate helps to generally estimate life expectancy after diagnosing cancer from the available data. It aids the comparison of patients diagnosed with cancer and the survival of people in the general population within the same age, sex, and race who have not been diagnosed with cancer.
It’s also important to note that survival statistics are based on large groups of people which obstruct the use of it to predict an individual status. No two patients are entirely alike, and treatment and responses to treatment can vary greatly [6]. The 5-year relative survival rate about the cancer staging is shown in Figure 3.
Figure 3.
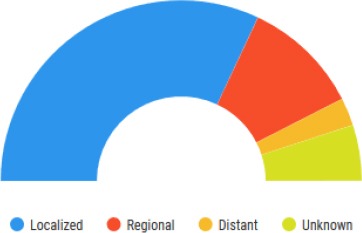
Five Years Relative Survival. The figure is showing a 5-year relative survival rate about the staging of cancer. The staging is broken down into Localized, Regional, Distant and Unknown.
Cases by Stage
Cancer staging during diagnosis refers to the extent of metastasis in the body. This helps to determine treatment options and life expectancy. When cancers do not undergo metastasis, it is known as localised cancer (stage 1). The spread of cancer to part of the body makes it regional or distant (stage II-IV).
The earlier a pancreatic cancer is identified, the higher the chance of survival rate for the five-year interval after being diagnosed. Only about 10.0% of pancreatic cancers are diagnosed at the local stage, and the 5-year survival rate for localised pancreatic cancer is 34.3% [6]. Figure 4 shows the staging of pancreatic cancers at the time of diagnosis.
Figure 4.
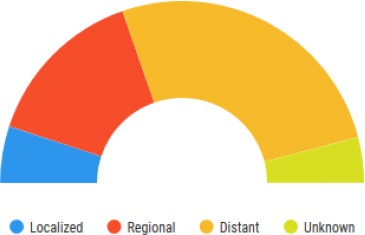
Percent of Case by Stage. The figure shows the staging of pancreatic cancer in the American population from 2008-2014 with all races, and both exes indicated
Prevalence in Gender, Race, and Ethnicity
The risk of Pancreatic cancer increases with age and more common in men than women. The number of new cases of pancreatic cancer was 12.6 per 100,000 for men and women annually based on 2011-2015 cases. Future predictions of pancreatic cancer at some point in some adults stands at 1.6 per cent according to the 2013-2015 data. In 2015, 68,615 people were estimated to be living with pancreatic cancer in the United States [6].
The period of 2014-2015 however, showed a decline in the occurrence of cancer in comparison to previous years. In 2015, the cancer mortality rate increased by 14% in non-Hispanic African Americans than non-Hispanic Caucasians overall. The mortality rate ratio was 1.14; with a 95% confidence interval. But the racial disparity was much larger for individuals aged < 65 years with the mortality rate ratio as 1.31 and the confidence interval at 95%, compared with those aged ≥ 65 years with mortality rate ratio as 1.07 and the confidence interval at 95%, 1.06-1.09 [6]. The comparison between different races and gender about new incidence and mortality has been summarised in Table 2 below.
Table 2.
The table compares different race and gender about new incidence and mortality
| MALE | FEMALE | |||
|---|---|---|---|---|
| Incidence | Deaths | Incidence | Deaths | |
| All Race | 14.4 | 12.6 | 11.2 | 9.5 |
| Caucasian | 14.4 | 12.6 | 11.1 | 9.4 |
| African descent | 16.9 | 14.8 | 14.3 | 12.2 |
| Asian/Pacific Islander | 11.0 | 8.3 | 9.2 | 7.3 |
| American Indian/Alaska Native | 11.3 | 9.7 | 7.8 | 8.0 |
| Hispanic | 12.0 | 9.5 | 10.5 | 7.7 |
| Non-Hispanic | 14.7 | 12.9 | 11.3 | 9.7 |
This shows every new case and death per 100,000 persons in the population at the time of the census [6].
Genetics of Pancreatic Cancer
Genetics has become a vital aspect in the early detection of pancreatic cancer. The genetics of pancreatic cancer is classified into two major origins, the exocrine pancreas, and the neuroendocrine pancreas. Among these two origins, 85% of cases seen is from the exocrine pancreatic origin which is the pancreatic ductal adenocarcinoma [3].
Genes like Kras, CDKN2A, TP53, SMAD4 have been implicated in most cases of pancreatic cancer [3], the understanding of this main genes has given insight into the diagnosis and treatment of pancreatic cancer. However, the main driver genes for pancreatic tumour; KRAS (90%), CDKN2A (90%), TP53 (70%), SMAD4 (55%) undergo different mutations that give rise to carcinogenesis of a pancreatic tumour [1].
Furthermore, Roboslit pathway (5%), Notch signalling (5%), WNT (10%), chromatin (20%), DNA repair (17%), cell cycle processing (15%) are the minor pathways implicated in pancreatic cancer [8].
Kras gene is responsible for 90% of most pancreatic cancer cases. RAS protein is responsible for cell differentiation and proliferation by sending the signals for cell differentiation. The RAS protein binds to GTP in G coupled receptor and gives the signal for the hydrolyses of GTP to GDP resulting in other downstream signals for uncontrolled proliferation and growth. The mutation in RAS gene makes the gene bind to GTP simultaneously, and signals are given at the cellular level for uncontrolled proliferation [9].
TP53 is a tumour suppressor gene involved in cell cycle, the inactivation of this gene by point mutation causes several changes in the cell cycle. This causes several cell cycle check points to be bypassed, thus inducing gene mutations and hence cancer formation. P53 also plays important roles in apoptosis by mostly arresting cells in the G1-S phase [10].
The normal function of the p53 gene is to bind to other genes like miRNA34a which codes for p21 [4]. P21 is a protein that acts as a signal for the shutdown of DNA replication. Hence, mutation to p53 results in the inactivation of the p21 gene and results in uncontrolled growth and proliferation [1].
CDKN2A is a tumour suppressor gene for regulating G1-S phase of the cell cycle in a pancreatic tumour. When the CDKN2A gene undergoes inactivation, it leads to unregulated and uncontrolled growth and differentiation [1].
SMAD4 is a tumour suppressor gene which activates the attachments of TGFb immediately to cell surface receptors. This sends signals into the nucleus to turn on the SMAD4 gene to attach themselves to other protein to regulate and control the growth and proliferation in specific areas of the DNA. Mutation in this gene causes uncontrolled proliferation and growth which gives rise to pancreatic cancer [1].
Studies have shown that targeting the Kras axis eliminates cancer cells and pancreatic tumour formation, so the RAS gene is the major contributing factor to pancreatic tumour formation [10].
A recent study has shown that CCAT2 gene which is a long non-coding RNA is the oncogene in the development of pancreatic ductal adenocarcinoma (PDAC). A total of 80 human PDAC tissues and 3 PDAC cell lines were assayed, and it was shown that there was more CCAT2 expression in the PDAC cell lines compared to the normal pancreatic tissues [8].
Studies have also shown that out of all the genes that are responsible for pancreatic cancer, the SMAD4/DPC4 gene is a good marker of metastasis [11]. The study showed that 641 patients showed DPC4/SMAD4 correlation with overall survival and recurrence patterns. The inactivation or loss of this gene has caused uncontrolled differentiation and metastatic development seen in pancreatic cancer [11].
Epigenetics
There are other genetic mechanisms different from the Kras mutation or the tumour suppressor deletion; these other mechanisms are also useful in the therapeutic management of PDAC [9].
DNA methylation is one of the mechanisms that inactivates suppressor genes. These genes do not undergo any mutation, but the cellular level methyl groups are added to carbon 5 of the pyrimidine ring which silences the gene [10]. Recent studies have shown that multiple genes are silenced or methylated in 45 pancreatic carcinomas. It was analysed that RARb, p16, CACNAIG, TIMP-3, Ecad, THBSI, Hmlh1, DAPkinase, MINT31 are genes seen in pancreatic cancer [12]. Overexpression of EGF, EGF-R, HER-2/neu, and p185 has also been found to be common in pancreatic tumours of advanced stages [13].
It has been observed that some micro-RNAs are deregulated in some pancreatic ductal adenocarcinomas. MiR-21, for instance, is overexpressed in 20 pancreatic carcinoma tissues and cell lines compared to normal tissue or cell lines [14].
Most pancreatic neuroendocrine tumours show great phenotypic and genotypic heterogenicity, they also occur sporadically or as familial tumours in association with other familial diseases like multiple endocrine neoplasia types 1 (MEN1), Von Hippel Lindau disease (VHL) or tuberous sclerosis [15], [16].
In a research carried out by Yuchen Jiao et al., in determining the genetic basis of PANnet resulted in the discovery that 44% of the tumours had somatic mutations in the MEN1 gene, 43% had mutation in the DAXX (death domain-associated protein) and ATRX gene; they also found a 14% mutation in the mTOR gene [17].
PHLDA3b which is a tumour suppressor has also been implicated in the formation of PANnet, the loss of heterozygosity at high frequency has been showed to lead to the development of PANnet, methylation of this gene has been implicated in the generation of PANnet [18].
Aberrant hypermethylation of 11 tumour suppressor genes were detected in PANnet, this gene includes RASSFIA (75%), ink4a/p16 (40%), OMGMT (40%), O-MGMT (40%), RAR-B (25%), hMLHI (23%), TIMP3, GSTπ, E-cadherin, P14ARF, APC, the aberrant hypermethylation of this gene has been associated with advanced tumor stage of pancreatic neuroendocrine tumors [19].
Screening Modalities
Several types of modalities exist in Diagnostic Imaging. These modalities act as a benchmark in diagnosing pancreatic cancer. The different types of imaging vary from computed tomography (CT), magnetic resonance imaging (MRI), Positron-emission tomography (PET), Ultrasound and Nuclear scans [5].
Computed tomography is the combination of several x-ray pictures taking at the same time from different angles to produce a 3-dimensional image of the region been exposed to the CT machine. This creates an all-around view of the internal organs and structures for better diagnosis and analysis [20].
Based on available literature, CT has mostly been used in determining the staging of pancreatic cancer. However, it has been observed to underestimate the spread of cancer which results in the need for invasive surgeries or other imaging techniques to determine the extent of cancer. The combination of other imagines solutions have been proven to be more efficient when used together with the CT scan [21].
The most used combined imaging technique in the diagnosis of pancreatic cancer is the CT Scan and Positron Emission Tomography (PET Scan). The PET Scan utilises nuclear medicine in observing the metabolic processes in the body [22].
This system utilises gamma rays emitted indirectly by a positron-emitting radionuclide (tracer). The biologically active molecules used during PET scan examination aid in the visualisation of interesting areas in 3-dimension by reconstructing the image with computer analysis [23].
The molecule mostly used clinically is Fludeoxyglucose (FDG), an analogue of glucose. The concentration of this biomarker helps in showing the metabolic rate of tissues which in most cases indicates high metastasis in the presence of cancer [24].
Another imaging modality which is safer than the CT and PET scan relation to radiation emitted during the scanning process is Magnetic Resonance Imaging (MRI). It is used in conditions where the data for nonrigid motion characterised as tumour and organs will be at risk of radiation therapy. It also helps in identifying soft tissues such as blood vessels about tumour growth. The image produced is also in 4 dimensions like that of CT scan with some structural changes [25].
In an observation carried out in Karolinska University Hospital between 2010 and 2013, using an MRI procedure. All patients with the genetic risk associated with pancreatic mutations were checked [25]. This gave a clearer understanding of the MRI potential in identifying precancerous or early cancers in individuals at risk for pancreatic cancer. Based on the study it showed how effective the procedure and protocol used was in the early dictation of cancer [25].
Endoscopic Ultrasound (EUS) is the most accurate form of ultrasound useful in the diagnosis of pancreatic cancer. This imaging modality is achieved by a small ultrasound probe on the tip of an endoscope, which is a thin and flexible tube used in looking inside the digestive tract [26].
This procedure can be done in place of having a large opened incision to explore the extent of cancer. The procedure involves the probe being passed through the mouth down to the first part of the small intestine. It is then pointed towards the pancreas to view the extent of the tumour and take a biopsy for confirmation [26].
Factors that Affects Screening
Pancreatic cancer screening mainly focuses on people with an increased risk of developing the disease. Some of these risk factors include; smoking, diet, diabetes mellitus, obesity [2]. Individuals in the population with a family history of pancreatic cancer are also at risk; some genetic syndromes also pose risk factors of pancreatic cancer [2].
Screening is recommended for individuals considered to be at high risk of developing pancreatic cancer, individuals with > 5% lifetime risk [27]. The family history is the main tool used to determine pancreatic cancer risk; the number of affected family members and the relationships among the individuals (especially first-degree relatives) at risk is used as the basis for risk assessment [28]. The incidence of pancreatic cancer as relating to the number of affected first-degree relatives is summarised in Table 3.
Table 3.
Table shows the incidence of pancreatic cancer by some affected first-degree relatives [33]
| First degree relatives | Incidence ratio | Incidence per 104 |
|---|---|---|
| 1 | 4.5 x | 41 |
| 2 | 6.4 x | 58 |
| > 3 | 32.0 x | 288 |
The chances of occurrence of pancreatic cancer in an individual increase with the number of family members with pancreatic cancer. Familial pancreatic cancer (FPC) is defined as having 2 or more of first-degree relatives with pancreatic cancer that does not meet the criteria of other hereditary cancer syndromes [29]. Familial pancreatic cancer accounts for at least 4-10% of pancreatic cancer. According to Matsubayashi H et al., European countries have been reported in FPC families, also seen in other hereditary syndromes; occurrence at a younger age and the worse prognosis are seen in the late years [30]. The resected pancreases of FPC relative often show multiple pancreatic intraepithelial neoplasia (PanIN) foci [31].
Individuals with mutations in the BRCA2, PALB2, p16, STK11, ATM, PRSS1, and HNPCC genes are associated with significantly increased risk for Pancreatic cancer and need to be screened [32]. These gene mutations are responsible for 10% of the familial susceptibility to pancreatic cancer [27]. Patients with Peutz Jegher syndrome also have an increased risk of pancreatic cancer [29].
Management of Pancreatic Cancer
Like various tumours or malignancies in the human body, it requires precise staging to determine if the tumor is respectable. The resectable nature of the tumour indicates the extent of its metastasis. This knowledge helps in choosing the best course of treatment and management [6].
The criteria for resectable pancreatic cancer are determined by the borderline of the tumour in contact with the superior mesenteric artery, a small segment of the celiac artery and the whole common hepatic artery [33]. However, the obstruction of the superior mesenteric artery and portal vein confluences caused by tumor growth is fixable by minimally inversive surgery [34].
Staging can only be very effective if the imaging of the malignancy is done with utmost accuracy. Most of the imaging techniques have one disadvantage or the other, but in cases where the advantage outweighs the disadvantage, they deemed fit for investigations [27].
However, there are some hurdles during the management which include;
Most patients are in the age group of the late sixties (70%) which undeniably predisposes them to multiple morbidities.
Some chemotherapeutic agents used during therapy present with other symptoms which affect the patient’s functionality.
In addition to the reduced potency of chemotherapy; pancreatic cancer does not comply well with chemotherapy making treatment options restricted and cumbersome. The assessment of the effect of chemotherapy has been challenging due to the dense desmoplastic reaction (this refers to the growth of dense fibrous tissue around the tumour stimulated by various factors especially TGF-β) exhibited by pancreatic malignancies.
Obtaining biopsy samples from the tumor is difficult. However, chemotherapy has the capability of targeting specific cancerous cells [27].
Following the prognostic classification of pancreatic cancer, surgical intervention is only applicable to resectable tumours [10], [16]. Neoadjuvant therapy for resectable pancreatic cancer has shown varying results due to limited sample size and different patient responses to the therapies. The different means of classification has also led to the different types of therapy being used in the pre, peri and postoperatively state [16]. However, the data supporting its benefits justify its use [31].
Gemcitabine, a well-known medication has significantly increased the survival rate of patients with pancreatic cancer. Although, this comes with a heavy price in terms of negative side effects. Therefore, various combination therapies are being used to reduce side effects and be of more benefit than harm [31], [35].
Preoperatively Cisplatin in combination with gemcitabine significantly increases the resection rate by 70% which is almost doubled the rate of gemcitabine alone at 38% [33]. Studies were done by Adamska et al., (2017) also shows an overall survival rate of 21% alive and pancreatic cancer free with this combination therapy [31]. Gemcitabine in combination with capecitabine, oxaliplatin and docetaxel improve resection rates in comparison with Gemcitabine alone [31], [33]. Borderline resectable patients treated with Xeloda, Taxotere, Gemzar, and radiation showed that 55 patients were able to have microscopically margin-negative resection out of 57 who were treated [1].
Neoadjuvant therapy for locally advanced and metastatic pancreatic cancer is more restricted due to the advanced state of cancer. The area concerned is more widespread and would involve lymph nodes in the case of metastasis. The effect of the drugs on other parts of the body can vary per individual. Nab-paclitaxel and Gemcitabine accompanied by Folfirinox therapy showed tumour regression and microscopically margin-negative resection of the tumour [16]. Other combinations which are also used for resectable and borderline resectable pancreatic cancer like Gemcitabine and cisplatin, gemcitabine and oxaliplatin, gemcitabine and capecitabine, PDXG (docetaxel, gemcitabine, capecitabine, and cisplatin) have also shown fairly good results encouraging the use of neoadjuvant in such patients [31].
The prognosis of resectable, borderline resectable and some locally advanced pancreatic cancer has a better probability for survival if managed properly. However, metastatic pancreatic cancer survival is solely based on increased therapies and palliative care [31].
Side Effects and Benefits of Therapy
The common side effects of some of the medications are neutropenia, thrombocytopenia. Examples of drugs which cause this side effect include 5-FU and gemcitabine, gemcitabine and erlotinib, gemcitabine and cisplatin, gemcitabine and nab-paclitaxel, FOLIFIRINOX which is a combination of fluorouracil, leucovorin, irinotecan, and oxaliplatin [31], [35].
The benefits of the monotherapy of gemcitabine include improved disease induced symptoms and increased survival response. However, it comes with several side effect except for haematological problems [35].
Benefits of novel agents include a reduction in pain, increased survival rate. Common side effects are haematological effects, musculoskeletal toxicities [35]. Examples include molecular targeting (olaparib), mitochondrial targeting (mFOLFIRINOX), microenvironment targeting tumor-associated-macrophages (CCR2 selective inhibitors), RAS inhibitors (Tipifarnib), metalloproteinase inhibitors (marimastat, BAY12-9566), epidermal growth factor receptor antagonist (erlotinib, trastuzumab, ZD1839), Antiangiogenics (thalidomide, paclitaxel, bevacizumab, combretastatin) [31].
Post-surgical patients are given adjuvant therapy, preferably gemcitabine or gemcitabine and capecitabine, although other regimens could be used. The use of other regimens is dependent on the response of the patients [35].
Discussion
Pancreatic cancer has been shown to have poor prognosis over the years. The disease falls within the most common causes of cancer-related deaths yet has a very low occurrence in comparison to the leading causes of cancer-related deaths. The availability of various diagnostic tools, treatment, and management have helped to contain the mortality of the tumour [1].
The study of pancreatic cancer from 2014 to 2015 showed a decline in mortality in men and a stable state in a woman. However, the decline in men is not significant enough to be considered clinically relevant [2]. The phenomenon seen between men and women is however not clear. Although, the decline in mortality for men can be correlated with the improvement in the health sector.
Research has shown that pancreatic cancers show good survival rates when diagnosed early [5]. Statistically, an increase of about 14% in mortality rate is seen in African Americans [7].
Genes like Kras, CDKN2A, TP53, SMAD4 have been the point of interest in most cases of pancreatic cancer. The breakthrough in diagnostic techniques has paved the way for further examination to conclude early enough if an individual is at risk or has the tumour and guide early treatment or management.
Available research on pancreatic cancer indicates that genetics plays a vital role in detecting patients prone to developing the tumour [9]. Certain genes in the genetic line-up act as precursors for pancreatic cancer [16]. This understanding aids in further examination such as diagnostic imaging to confirm the state of the pancreas.
As shown by the literature, diagnostic imaging has over the years made some tremendous strides in aiding early detection of pancreatic cancer. Research has shown that MRI and EUS diagnostic technique is one of the best means of diagnosing pancreatic cancer without having to expose the patients to radioactive rays [25] further.
Various factors have been shown to cause pancreatic cancer. These factors mostly affect those who are genetically prone to having cancer. For instance, smoking acts as a carcinogen when used by genetically prone individuals [2]. The extreme exposure to some of the imagine radiations such as from x-rays and CT scans can escalate the dormant state [25].
The treatment and management of pancreatic cancer have improved between the 90s to date showing a decrease in mortality [35]. These improvements are all due to the advancement in technologies and medications.
The technological improvement includes the use of procedures such as interventional radiology. This improvement has aided in the precise removal of tumours with quick recovery time [5]. The medications Cisplatin and gemcitabine are helpful in tumour resectability. Radiation therapy with the combination of Taxotere and Gemzar also showed improvement in tumour resectability. Nab-paclitaxel and gemcitabine accompanied by folfirinox therapy significantly have been shown to help in the regression of the pancreatic tumour [16]. The medications also improve the disease-induced symptoms and increase survival response. However, the use of these medications is not devoid of side effects such as haematological and musculoskeletal toxicities [35].
Conclusion
Pancreatic cancer has had a history of poor prognosis because of its late detection. A family history of pancreatic cancer closely followed up with a genetic screen has the potential to predict the likely incidence, early detection and possible management of pancreatic cancers. Also, further screening modalities and investigations using imaging techniques and interventional radiology have also helped to improve the early diagnosis and management of pancreatic cancer.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
Reference
- 1.Xu Y-P. Advancement in treatment and diagnosis of pancreatic cancer with radiopharmaceuticals. World J Gastrointest Oncol. 2016;8(2):165. doi: 10.4251/wjgo.v8.i2.165. https://doi.org/10.4251/wjgo.v8.i2.165 PMid:26909131 PMCid:PMC4753167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2017. Genes Dev. 2017;21(20):2525–38. [Google Scholar]
- 3.Dragovich T, Espat J, Erickson R. Pancreatic Cancer Clinical Presentation:History, Physical Examination. Medscape; 2017. [Google Scholar]
- 4.Puleo F. New challenges in perioperative management of pancreatic cancer. World J Gastroenterol. 2015;21(8):2281. doi: 10.3748/wjg.v21.i8.2281. https://doi.org/10.3748/wjg.v21.i8.2281 PMid:25741134 PMCid:PMC4342903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer:A state-of-the-art review. World J Gastroenterol. 2014;20(24):7864–77. doi: 10.3748/wjg.v20.i24.7864. https://doi.org/10.3748/wjg.v20.i24.7864 PMid:24976723 PMCid:PMC4069314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. https://doi.org/10.3322/caac.21442 PMid:29313949. [DOI] [PubMed] [Google Scholar]
- 7.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide:Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. https://doi.org/10.1002/ijc.29210 PMid:25220842. [DOI] [PubMed] [Google Scholar]
- 8.Cai Y, Li X, Shen P, Zhang D. CCAT2 is an oncogenic long non-coding RNA in pancreatic ductal adenocarcinoma. Biol Res. 2018;51(1):1–9. doi: 10.1186/s40659-017-0149-0. https://doi.org/10.1186/s40659-017-0149-0 PMid:29298720 PMCid:PMC5751927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iovanna J, Mallmann MC, Gonçalves A, Turrini O, Dagorn J-C. Current Knowledge on Pancreatic Cancer. Front Oncol. 2012;2:1–24. doi: 10.3389/fonc.2012.00006. https://doi.org/10.3389/fonc.2012.00006 PMid:22655256 PMCid:PMC3356035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada M, Shibuya K, Sato A, Seino S, Suzuki S, Seino M, et al. Targeting the K-Ras--JNK axis eliminates cancer stem-like cells and prevents pancreatic tumor formation. Oncotarget. 2014;5(13):5100–12. doi: 10.18632/oncotarget.2087. https://doi.org/10.18632/oncotarget.2087 PMid:24947996 PMCid:PMC4148125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin SH, Kim HJ, Hwang DW, Lee JH, Song KB, Jun E, et al. The DPC4/SMAD4 genetic status determines recurrence patterns and treatment outcomes in resected pancreatic ductal adenocarcinoma:A prospective cohort study. Oncotarget. 2017;8(11):17945–59. doi: 10.18632/oncotarget.14901. https://doi.org/10.18632/oncotarget.14901 PMid:28160547 PMCid:PMC5392299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueki T, Toyota M, Sohn T, Yeo CJ, Issa JJ, Hruban RH, et al. Hypermethylation of Multiple Genes in Pancreatic Adenocarcinoma Hypermethylation of Multiple Genes in Pancreatic Adenocarcinoma 1. Cancer Res. 2000;60(410):1835–9. PMid:10766168. [PubMed] [Google Scholar]
- 13.Talar-Wojnarowska R, Malecka-Panas E. Molecular pathogenesis of pancreatic adenocarcinoma:potential clinical implications. Med Sci Monit. 2006;12(9):RA186–93. PMid:16940943. [PubMed] [Google Scholar]
- 14.Pelosi E, Castelli G, Testa U. Pancreatic Cancer:Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Biomedicines. 2017;5(4):65. doi: 10.3390/biomedicines5040065. https://doi.org/10.3390/biomedicines5040065 PMid:29156578 PMCid:PMC5744089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sipos B, Sperveslage J, Anlauf M, Hoffmeister M, Henopp T, Buch S, et al. Glucagon cell hyperplasia and neoplasia with and without glucagon receptor mutations. J Clin Endocrinol Metab. 2015;100(5):E783–8. doi: 10.1210/jc.2014-4405. https://doi.org/10.1210/jc.2014-4405 PMid:25695890. [DOI] [PubMed] [Google Scholar]
- 16.Hackeng WM, Hruban RH, Offerhaus GJA, Brosens LAA. Surgical and molecular pathology of pancreatic neoplasms. Diagn Pathol. 2016;11(1):1–17. doi: 10.1186/s13000-016-0497-z. https://doi.org/10.1186/s13000-016-0497-z PMid:27267993 PMCid:PMC4897815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiao Y, Shi C, Edil BH, Wilde RF De, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1 and mTOR Pathway Genes are Frequently Altered in Pancreatic Neuroendocrine Tumors. Science (80) 2011;331(6021):1199–203. doi: 10.1126/science.1200609. https://doi.org/10.1126/science.1200609 PMid:21252315 PMCid:PMC3144496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohki R, Saito K, Chen Y, Kawase T, Hiraoka N, Saigawa R, et al. PHLDA3 is a novel tumor suppressor of pancreatic neuroendocrine tumors. Proc Natl Acad Sci. 2014;111(23):E2404–13. doi: 10.1073/pnas.1319962111. https://doi.org/10.1073/pnas.1319962111 PMid:24912192 PMCid:PMC4060701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.House MG, Herman JG, Guo MZ, Hooker CM, Schulick RD, Lillemoe KD, et al. Aberrant Hypermethylation of Tumor Suppressor Genes in Pancreatic Endocrine Neoplasms. Trans. Meet Am Surg Assoc. 2003;121(3):117–26. doi: 10.1097/01.sla.0000086659.49569.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayo Clinic CT scan [Internet] Mayo Foundation for Education and Research. 2015 [Google Scholar]
- 21.Allen VB DB. Cochrane Database of Systematic Reviews Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer (Review) Diagnostic accuracy of laparoscopy. Cochrane Database Syst Rev Art. 2016;(7):11–20. doi: 10.1002/14651858.CD009323.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson JM, Mukherjee S, Brunner TB, Partridge M, Hawkins MA. Correlation of18F-Fluorodeoxyglucose Positron Emission Tomography Parameters with Patterns of Disease Progression in Locally Advanced Pancreatic Cancer after Definitive Chemoradiotherapy. Clin Oncol. 2017;29(6):370–7. doi: 10.1016/j.clon.2017.01.038. https://doi.org/10.1016/j.clon.2017.01.038 PMid:28190636 PMCid:PMC5429392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson JM, Mukherjee S, Brunner TB, Partridge M, Hawkins MA. Correlation of18F-Fluorodeoxyglucose Positron Emission Tomography Parameters with Patterns of Disease Progression in Locally Advanced Pancreatic Cancer after Definitive Chemoradiotherapy. Clin Oncol. 2017;29(6):370–7. doi: 10.1016/j.clon.2017.01.038. https://doi.org/10.1016/j.clon.2017.01.038 PMid:28190636 PMCid:PMC5429392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung W, Jang JY, Kang MJ, Chang YR, Shin YC, Chang J, et al. The clinical usefulness of 18F-fluorodeoxyglucose positron emission tomography–computed tomography (PET–CT) in follow-up of curatively resected pancreatic cancer patients. Hpb. 2016;18(1):57–64. doi: 10.1016/j.hpb.2015.06.001. https://doi.org/10.1016/j.hpb.2015.06.001 PMid:26776852 PMCid:PMC4750231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stemkens B, Tijssen RH, de Senneville BD, Heerkens HD, van Vulpen M, Lagendijk JJ, et al. Optimizing 4-dimensional magnetic resonance imaging data sampling for respiratory motion analysis of pancreatic tumors. Int J Radiat Oncol Biol Phys. 2015;91(3):571–8. doi: 10.1016/j.ijrobp.2014.10.050. https://doi.org/10.1016/j.ijrobp.2014.10.050 PMid:25596109. [DOI] [PubMed] [Google Scholar]
- 26.Dababou S, Marrocchio C, Rosenberg J, Bitton R, Pauly KB, Napoli A, et al. A meta-analysis of palliative treatment of pancreatic cancer with high intensity focused ultrasound. J Ther Ultrasound. 2017;5(1):9. doi: 10.1186/s40349-017-0080-4. https://doi.org/10.1186/s40349-017-0080-4 PMid:28373906 PMCid:PMC5376281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, et al. International cancer of the pancreas screening (CAPS) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62(3):339–47. doi: 10.1136/gutjnl-2012-303108. https://doi.org/10.1136/gutjnl-2012-303108 PMid:23135763 PMCid:PMC3585492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Chen S, Brune KA, Hruban RH, Parmigiani G, Klein AP. Risk assessment for individuals with a family history of pancreatic cancer. J Clin Oncol. 2008;25(11):1417–22. doi: 10.1200/JCO.2006.09.2452. https://doi.org/10.1200/JCO.2006.09.2452 PMid:17416862 PMCid:PMC2267288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsubayashi H, Takaori K, Morizane C, Maguchi H, Mizuma M, Takahashi H, et al. Familial pancreatic cancer:Concept, management and issues. World J Gastroenterol. 2017;23(6):935–48. doi: 10.3748/wjg.v23.i6.935. https://doi.org/10.3748/wjg.v23.i6.935 PMid:28246467 PMCid:PMC5311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64(7):2634–8. doi: 10.1158/0008-5472.can-03-3823. https://doi.org/10.1158/0008-5472.CAN-03-3823 PMid:15059921. [DOI] [PubMed] [Google Scholar]
- 31.Adamska A, Domenichini A, Falasca M. Pancreatic ductal adenocarcinoma:Current and evolving therapies. Int J Mol Sci. 2017;18(7) doi: 10.3390/ijms18071338. https://doi.org/10.3390/ijms18071338 PMid:28640192 PMCid:PMC5535831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang Wu, Yang, et al. Pancreatic cancer screening in different risk individuals with family history of pancreatic cancer-a prospective cohort study in Taiwan. Am J Cancer Res. 2017;7(2):357–69. PMid:28337383 PMCid:PMC5336508. [PMC free article] [PubMed] [Google Scholar]
- 33.Hammel P, Huguet F, Van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib the LAP07 randomized clinical trial. JAMA - J Am Med Assoc. 2016;315(17):1844–53. doi: 10.1001/jama.2016.4324. https://doi.org/10.1001/jama.2016.4324 PMid:27139057. [DOI] [PubMed] [Google Scholar]
- 34.Chang J, Schomer D, Dragovich T. Anatomical, physiological, and molecular imaging for pancreatic cancer:Current clinical use and future implications. Biomed Res Int. 2015;2015:1–7. doi: 10.1155/2015/269641. https://doi.org/10.1155/2015/269641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mckenna S EM. The Medical Management of Pancreatic Cancer:A Review. Oncologist. 2003;2003(8):149–60. doi: 10.1634/theoncologist.8-2-149. https://doi.org/10.1634/theoncologist.8-2-149. [DOI] [PubMed] [Google Scholar]


