Abstract
Hepatitis C virus (HCV) infection affects an estimated 185 million people worldwide, with chronic infection often leading to liver cirrhosis and hepatocellular carcinoma. Although HCV is curable, there is an unmet need for the development of effective and affordable treatment options. Through a cell-based high-throughput screen, we identified chlorcyclizine HCl (CCZ), an over-the-counter drug for allergy symptoms, as a potent inhibitor of HCV infection. CCZ inhibited HCV infection in human hepatoma cells and primary human hepatocytes. The mode of action of CCZ is mediated by inhibiting an early stage of HCV infection, probably targeting viral entry into host cells. The in vitro antiviral effect of CCZ was synergistic with other anti-HCV drugs, including ribavirin, interferon-α, telaprevir, boceprevir, sofosbuvir, daclatasvir, and cyclosporin A, without significant cytotoxicity, suggesting its potential in combination therapy of hepatitis C. In the mouse pharmacokinetic model, CCZ showed preferential liver distribution. In chimeric mice engrafted with primary human hepatocytes, CCZ significantly inhibited infection of HCV genotypes 1b and 2a, without evidence of emergence of drug resistance, during 4 and 6 weeks of treatment, respectively. With its established clinical safety profile as an allergy medication, affordability, and a simple chemical structure for optimization, CCZ represents a promising candidate for drug repurposing and further development as an effective and accessible agent for treatment of HCV infection.
Editor’s Summary
Over-the-counter allergy drug inhibits viral infection
A drug commonly used for a runny nose may now be repurposed for treating hepatitis C virus (HCV) infection––a virus that often goes undetected, but can exacerbate many liver diseases, including cirrhosis and cancer. The class of compounds, called antihistamines, which are used to relieve allergies, was uncovered by He et al. in a screen of a library of approved drugs, the NIH Chemical Genomics Center Pharmaceutical Collection. Among these, the first-generation antihistamine chlorcyclizine demonstrated high antiviral activity in cell culture and in mice with “humanized” livers, without evidence of drug resistance––a common problem with existing antivirals. Chlorcyclizine was specific for HCV, demonstrating no activity against 13 other viruses, including hepatitis B, and showed synergy with different classes of anti-HCV drugs, such as ribavirin, sofosbuvir, cyclosporin A, and interferon-α. Antihistamines are widely available, safe, and inexpensive, making them ideal for imminent translation to HCV-endemic countries in Asia and Africa.
INTRODUCTION
Hepatitis C virus (HCV) chronic infection is usually asymptomatic and many individuals are unaware of their infection. However, without treatment, HCV infection hastens the development of liver diseases, including cirrhosis, liver failure, and hepatocellular carcinoma (1). In fact, more than 50% of incident hepatocellular carcinoma is due to HCV infection, which is the fastest-growing cause of cancer-related death in the United States (2, 3). In 2012, the U.S. Centers for Disease Control and Prevention recommended screening for HCV infection among all persons born between 1945 and 1965 (4). It is anticipated that a large number of infected individuals would be identified through such a screening effort. Although HCV is spread worldwide, it is particularly prevalent in Asia and Africa, as well as in high-risk populations such as intravenous drug users. With hundreds of millions of the population affected by chronic hepatitis C infection, it is essential that effective and affordable drugs are developed or repurposed to treat the chronic infection.
A protective vaccine for HCV is not yet available (5), and pegylated interferon (PEG-IFN) and ribavirin (RBV) have been the cornerstone of HCV therapy for many years. Several direct-acting antivirals have been approved by the U.S. Food and Drug Administration (FDA) for tripletherapy regimens in combination with PEG-IFN and RBV, leading to improvement of viral clearance rate in genotype 1-infected patients (1). However, direct-acting antivirals that target viral factors are costly and have a low genetic barrier to resistance, side effects, and potential for drug-drug interaction. Recently, several all-oral, interferon (IFN)-free regimens were introduced for the treatment of chronic HCV infection. These regimens, although very effective, are associated with high costs (for example, a 12-week Sovaldi treatment costs about $84,000), which generally precludes treating the populations most affected by HCV (6). There remains a great need to improve the use of existing drugs and to develop new drugs and therapeutic targets for HCV therapy to achieve the following features: activity against all genotypes, a high genetic barrier to drug resistance, a good safety profile, oral delivery, and global affordability.
Existing pharmacopeia can be repurposed to achieve unmet therapeutic needs (7). Here, we sought to screen existing, FDA-approved drugs against HCV infection. Using our previously developed cell-based quantitative high-throughput screening (qHTS) platform (8), we screened a comprehensive library of approved drugs that was built by the National Institutes of Health (NIH) Chemical Genomics Center (NCGC), named the NCGC Pharmaceutical Collection (NPC) (9), to search for approved drugs with novel anti-HCV activity and potentially new therapeutic targets. We identified multiple H1-antihistamines with anti-HCV activity. Among these, chlorcyclizine HCl (CCZ), a first-generation antihistamine approved in the 1940s, showed high antiviral activity that was synergistic with various approved anti-HCV drugs in vitro. Further in vivo studies in primary human hepatocyte-engrafted mice confirmed its efficacy in restricting genotypes 1b and 2a HCV infection. The findings from this study, together with the established safety profile of CCZ in patients, affordability, and simple chemical structure amenable for further optimization, make CCZ a promising anti-HCV candidate for further investigation, optimization, and repurposing for use in HCV-endemic regions and populations.
RESULTS
Identification and confirmation of CCZ analogs as anti-HCV agents from high-throughput screening
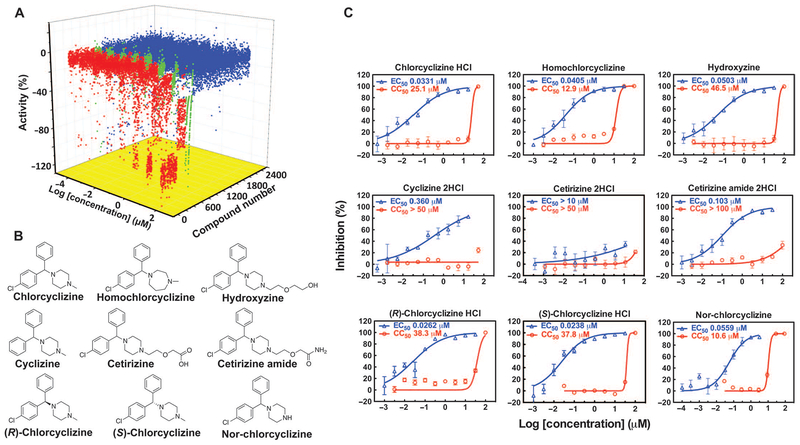
A cell-based qHTS of the NPC was carried out against HCV genotype 2a (JFH-1 strain) using the platform described previously (8). The anti-HCV activity and selectivity were further evaluated using a dose-response study with HCV-Luc (infectious HCV harboring a Renilla luciferase reporter gene) and ATPlite assays (cytotoxicity). The compound concentration that led to 50% inhibition and cytotoxicity (EC50 and CC50, respectively) was determined. From about 3800 small-molecule entities in the collection, 118 primary hits were identified on the basis of the curve classes of dose-response curves from the luciferase and ATPlite assays (curve classes defined in Materials and Methods) (8) (Fig. 1A). Out of 62 H1-antihistamines with diverse structural features in the NPC, compounds with the cyclizine moiety demonstrated potent activities in the primary screen and single-dose confirmation assay (table S1). Histamine was also included in table S1 as a control because it showed no anti-HCV activity.
Fig. 1. Activities of NPC compounds in qHTS and confirmation of CCZ analogs.
(A) Three-axis plot of the activities of NPC compounds in the qHTS. Activity (%) was calculated by normalizing luciferase signals to the mean signal from the dimethyl sulfoxide (DMSO) control wells. Compounds were sorted according to curve classes. Red, active compounds in curve classes 1 and 2; green, weakly active compounds in curve class 3; blue, inactive compounds in curve class 4. (B and C) Chemical structures (B) and in vitro dose-response curves (C) of CCZ analogs identified in the NPC screen. The percent inhibition of the compound in the HCV-Luc assay is shown in blue triangles, and the cytotoxicity effect in the ATPlite assay of host cells is shown in red circles. EC50 and CC50 values are indicated. Data are means ± SEM (n ≥ 3 replicates). Curves are representative results from at least three independent experiments.
In a secondary confirmation assay, CCZ, homochlorcyclizine, and hydroxyzine demonstrated low EC50 values (~50 nM), with high selective indices (SI = CC50/EC50) ranging from 318 to 924 (Fig. 1, B and C). Cyclizine lacking a chlorine substitution exhibited a 10-fold lower activity than CCZ. Cetirizine was reported as a metabolite of hydroxyzine, but it was not active in inhibiting HCV infection. However, cetirizine amide still showed good activity against HCV (EC50 = 0.103 μM) (Fig. 1C). The low activity of cetirizine could be attributed to its high polarity and low permeability into cells.
The analogs discussed above—CCZ, homochlorcyclizine, hydroxyzine, cetirizine, and cetirizine amide—are racemic mixtures of (R)- and (S)-enantiomers. To identify whether the configuration affects the anti-HCV activities, we evaluated the (R)- and (S)-enantiomers of CCZ, and they exhibited no significant difference in EC50 and CC50 values on HCV infection in human hepatoma cells (Huh7.5.1 cell line) (Fig. 1, B and C, and Table 1). The primary metabolite of CCZ or cyclizine in vivo is nor-chlorcyclizine (nor-CCZ) or nor-cyclizine (Fig. 1B), which has little antihistamine activity (10, 11). The antiviral activity of nor-CCZ was comparable to that of CCZ, but with higher cytotoxicity (Fig. 1C). Under the condition of our cell-based assay, a negligible amount of CCZ was transformed to nor-CCZ (table S2).
Table 1. Anti-HCV activity, selectivity, and anti-histamine properties of CCZ analogs.
Compounds were tested in the HCV-Luc infection assay in parallel with the ATPlite assay. HCV-Luc was used to infect Huh7.5.1 cells in the presence of compound titration. Viral infection and replication were measured by luciferase signal 48 hours after treatment, and cytotoxicity was evaluated by the adenosine 5-triphosphate (ATP)-based cell viability assay. The concentration values that led to 50% and 90% viral inhibition (EC50 and EC90, respectively) and 50% cytotoxicity (CC50) were calculated with GraphPad Prism using a nonlinear regression equation. Cyclosporin A was a control. Data are means ± SEM from n ≥ 3 independent experiments. Antihistamine activity was obtained with the β-arrestin HΓhistamine receptor assay. N.D., not determined.
| Compound | HCV-Luc EC50 (μM) | HCV-Luc EC90 (μM) | ATPlite CC50 (μM) | SI | Antihistamine activity at 10 nM (%) |
|---|---|---|---|---|---|
| Racemic CCZ | 0.044 ± 0.011 | 1.40 ± 0.45 | 49.8 ± 17.2 | 994 | 72.60 |
| (R)-CCZ | 0.020 ± 0.005 | 1.09 ± 0.37 | 37.5 ± 4.15 | 1875 | 88.00 |
| (S)-CCZ | 0.024 ± 0.009 | 1.44 ± 0.43 | 33.4 ± 2.44 | 1392 | 41.70 |
| (S)-Nor-CCZ | 0.034 ± 0.012 | 0.578 ± 0.099 | 9.31 ± 0.04 | 274 | 2.24 |
| Cyclosporin A | 0.213 ± 0.044 | 0.92 ± 0.20 | N.D. | N.D. | N.D. |
Anti-HCV activities of CCZ
Racemic (R)- and (S)-CCZ were further evaluated against wild-type cell culture-derived HCV (HCVcc; genotype 2a, JFH-1 strain) infection in Huh7.5.1 cells. Intracellular and extracellular viral RNA levels were significantly reduced with the treatment of racemic, (R)-, and (S)-CCZ compared with DMSO treatment (Fig. 2A). These results further confirmed that the anti-HCV activity of CCZ analogs is independent of their configurations. However, (S)-CCZ did show less histamine receptor inhibitory effect than (R)-CCZ (Table 1); (S)-CCZ was thus chosen for further evaluation.
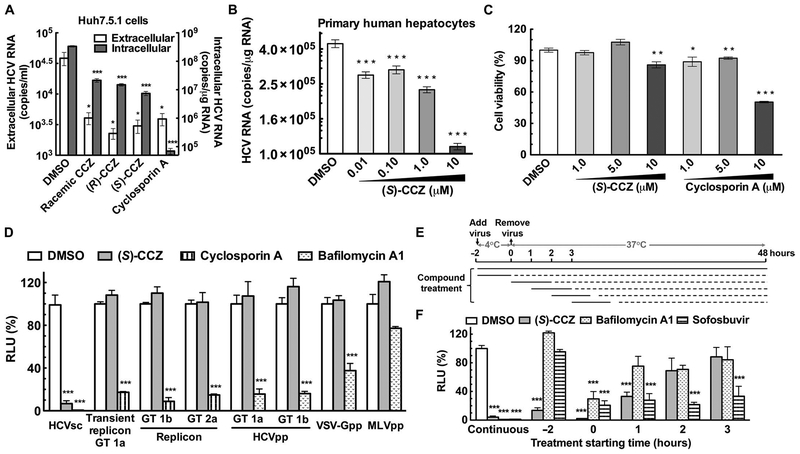
Fig. 2. Anti-HCV activities of CCZ.
(A) Human hepatocytes (Huh7.5.1 cells) were infected with wild-type HCVcc in the presence of the compounds at 10 μM overnight followed by incubation with compound treatment for an additional 48 hours. Viral RNA was evaluated by quantitative real-time polymerase chain reaction (qRT-PCR). (B) Primary human hepatocytes were infected with wild-type HCVcc in the presence of (S)-CCZ titration overnight followed by incubation with (S)-CCZ titration for an additional 48 hours. Intracellular viral RNA levels were evaluated by qRTPCR. (C) In the presence of compound treatment, Huh7.5.1 cells were passaged every 3 days for seven passages and were plated on 96-well plates 3 days before ATPlite assay to measure cell viability. (D) HCV replication cycle assays were carried out with (S)-CCZ at 10 μM. Cyclosporin A (10 μM) was a control in HCV single-cycle infection (HCVsc), transient replicon genotype 1a, and replicon genotype 1b and 2a assays. Bafilomycin A1 (10 nM) was used as a control in HCV pseudoparticle (HCVpp) genotype 1a and 1b, vesicular stomatitis virus G pseudoparticle (VSV-Gpp), and murine leukemia virus pseudoparticle (MLVpp) assays. Results were normalized to DMSO. RLU, relative luminescence units. GT, genotype. (E) At t = −2 hours, HCV-Luc was incubated with Huh7.5.1 cells at 4°C for 2 hours for attachment. At t =0 hours, the unbound virus was removed, and the plates were moved to 37°C to allow synchronous infection and incubated for 48 hours before virus load measurement. (S)-CCZ (10 μM), bafilomycin A1 (10 nM), and sofosbuvir (10 μM) were added either continuously or at the indicated time points and incubated for 2 hours. (F) Results from (E) were normalized to DMSO continuous treatment. Data are means of replicates ± SEM (n ≥ 3). *P < 0.05, **P < 0.005, ***P < 0.0001, versus DMSO (Student’s t test).
(S)-CCZ inhibited intracellular HCV RNA level against wild-type HCVcc infection in primary human hepatocytes in a dose-dependent manner (Fig. 2B). When Huh7.5.1 cells were infected with HCV chimeric genotype viruses (1a, 1b, 2a, 2b, 3a, 4a, 5a, 6a, and 7a), the extracellular HCV RNA levels and infectivity [median tissue culture infectious dose per milliliter (TCID50/ml)] were inhibited by (S)-CCZ at 10 μM, suggesting pan-genotypic activity (table S3). Furthermore, (S)-CCZ showed no cytotoxicity at 1.0 and 5.0 μM and low cytotoxicity at 10 μM in Huh7.5.1 cells after continuous treatment for 21 days (Fig. 2C).
Mode of action of CCZ on HCV replication cycle
To better understand what stage of the viral replication cycle CCZ analogs target, we performed HCV single-cycle infection assay, HCV subgenomic replicon assays, and HCVpp assays in human hepatocytes with (S)-CCZ. For the HCV single-cycle infection assay, the core-defective, single-round infectious HCV genome (HCVsc, genotype 2a) can infect and replicate in hepatocytes but does not assemble into new virions. Thus, this assay assesses compounds with an effect on the HCV replication cycle stages before virion assembly. (S)-CCZ exhibited strong inhibitory activity in the HCVsc assay, suggesting that CCZ analogs inhibit the early stage of HCV infection (Fig. 2D).
We further performed the HCV subgenomic replicon assays to evaluate whether these compounds would target viral RNA replication. (S)-CCZ was used to treat genotype 1b and 2a HCV replicon cell lines (12) and did not exert a significant inhibitory effect (Fig. 2D). HCV replication was not inhibited with (S)-CCZ treatment in Huh7.5.1 cells transiently transfected with a genotype 1a replicon (Fig. 2D). These results indicate that CCZ analogs do not target viral RNA replication. (S)-CCZ also demonstrated no significant inhibitory effect on HCVpp (defective retroviral particles that harbor HCV envelope glycoproteins) entry into Huh7.5.1 cells with VSV-Gpp and MLVpp as control pseudoviruses (Fig. 2D).
The inhibitory kinetics of CCZ analogs on HCVcc infection was addressed in the time-of-addition experiment with (S)-CCZ, the HCV entry inhibitor bafilomycin A1, and the replication inhibitor sofosbuvir (Fig. 2E). (S)-CCZ exhibited potent inhibition when added during viral attachment at 4°C for 2 hours or during the first 2 hours of synchronous entry at 37°C, and both effects were comparable to that of continuous (S)-CCZ treatment (Fig. 2F). Less inhibitory effect was observed when (S)-CCZ was added 1 hour after synchronous entry, and no significant inhibition was detected when it was added 2 or 3 hours after entry. Bafilomycin A1, reported to modulate post-entry fusion (13), only showed significant inhibitory effect when added during the first 2 hours of synchronous entry (Fig. 2, E and F). Sofosbuvir, an RNA replication inhibitor targeting NS5B polymerase (14), inhibited HCVcc infection when added at all the time points except during the attachment stage (Fig. 2, E and F). The comparison of the kinetics of (S)-CCZ with that of bafilomycin A1 and sofosbuvir suggests that CCZ analogs inhibit viral infection possibly at a late-entry step before RNA replication.
When evaluated in an immunofluorescence assay and/or Western blotting, (S)-CCZ treatment did not alter the protein level or cellular distribution of known HCV entry factors, including CD81, claudin-1, occludin, Niemann-Pick C1-like 1 (NPC1L1), and scavenger receptor class B1, suggesting that the anti-HCV mechanism of (S)-CCZ is not directly related to affecting the expression levels or cellular distribution of these entry factors (fig. S1). Further investigation is needed to elucidate its mode of action.
Synergistic antiviral effect of CCZ in combination with anti-HCV drugs
In the treatment of chronic HCV infection, combination regimens lower the chance of developing drug-resistant viral mutations. We evaluated the anti-HCV activity of (S)-CCZ in combination with different classes of anti-HCV drugs and agents at various concentrations. The combination of (S)-CCZ and each drug led to a greater HCV inhibitory effect than either of them alone in a dose-dependent manner, without cytotoxicity (fig. S2). Using the MacSynergy II program based on the Bliss independence model (15), we generated three-dimensional surface plots (fig. S2) and calculated the log volume of synergism for each combination (Table 2). The results were also analyzed with the CalcuSyn program (16) in which the combination indices were calculated (Table 2). The antiviral effect of (S)-CCZ was highly synergistic with ribavirin, IFN-α, telaprevir, boceprevir, sofosbuvir, daclatasvir, and cyclosporin A, without significant cytotoxicity, supporting its potential usage in combination therapy with these drugs.
Table 2. Synergistic antiviral effect of CCZ in combination with anti-HCV drugs.
The level of synergy was defined in MacSynergy as follows: ++, moderate synergy (5 ≤ log volume < 9); +++, strong synergy (log volume ≥ 9). Combination indices (CI) are means ± SEM from combinations of the tested drug with (S)-CCZ at or near their EC50 values when tested alone (n ≥ 6). The level of synergy was defined by CalcuSyn as follows: ++, moderate synergy (0.7 ≤ CI < 0.85); +++, synergy (0.3 ≤ CI < 0.7).
| Program | Parameter | Ribavirin | IFN-α | Telaprevir | Boceprevir | Sofosbuvir | Daclatasvir | Cyclosporin A |
|---|---|---|---|---|---|---|---|---|
| MacSynergy | Log volume | +++ | +++ | +++ | +++ | +++ | +++ | ++ |
| CalcuSyn | CI value Synergy volume |
0.630 ± 0.106 +++ |
0.609 ± 0.128 +++ |
0.426 ± 0.138 +++ |
0.691 ± 0.114 +++ |
0.362 ± 0.075 +++ |
0.427 ± 0.142 +++ |
0.727 ± 0.187 +++ |
Lack of antiviral effect of CCZ against multiple types of viruses
Both HCV and dengue virus are members of the Flaviviridae family. (S)-CCZ demonstrated an EC50 value of 1.88 μM in the dengue reporter virus particle (RVP) assay, which is more than 70-fold higher than its EC50 value on HCV-Luc infection (fig. S3 and Table 1). The CC50 value (>31.6 μM) in this assay was consistent with the previous observation when cells were infected with HCV-Luc (fig. S3 and Table 1). Lycorine-HCl was tested as a positive control (EC50 = 0.0406 μM; CC50 > 31.6 μM).
Although CCZ has reported activity against HIV (17), (S)-CCZ had little or no antiviral activity (SI < 10 and/or EC50 > 2 μM) in the National Institute of Allergy and Infectious Diseases (NIAID) antiviral screen against 13 types of viruses: hepatitis B virus, HCV replicon, herpes simplex virus-1, human cytomegalovirus, vaccinia virus, dengue virus, influenza A (H1N1) virus, respiratory syncytial virus, SARS (severe acute respiratory syndrome) coronavirus, poliovirus 3, Rift Valley fever virus, Tacaribe virus, and Venezuelan equine encephalitis virus (table S4). It is worth noting that the NIAID panel used an infectious dengue virus in the plaque-forming assay to detect anti-dengue virus activity and therefore is more reliable.
In vitro and in vivo ADME and pharmacokinetic properties of CCZ
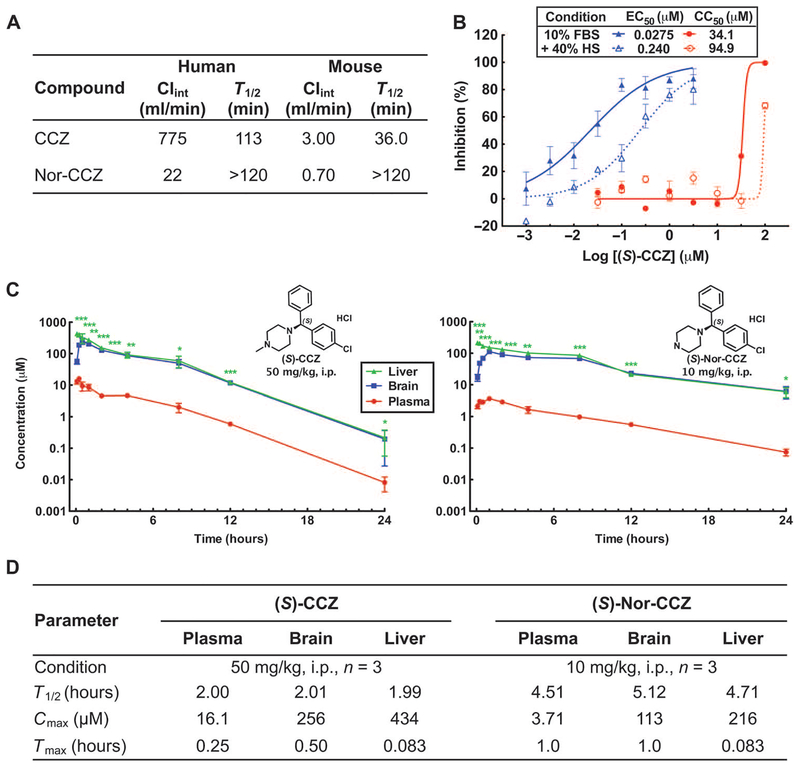
Because CCZ was developed as a first-generation antihistamine more than 50 years ago, many of its pharmacologic properties were not available. To determine its ADME (absorption, distribution, metabolism, and excretion) and pharmacokinetic properties, we evaluated CCZ and nor-CCZ in the microsomal stability assay with human and mouse microsomes. CCZ and nor-CCZ both exhibited long half-lives (T1/2) when incubated with human microsomes (T1/2 > 100 min) (Fig. 3A). However, the intrinsic clearance of CCZ was faster than that of nor-CCZ. Nor-CCZ showed more than fourfold longer T1/2 in mice than that of CCZ.
Fig. 3. In vitro and in vivo ADME and pharmacokinetics of CCZ.
(A) The microsomal stability of CCZ and nor-CCZ was measured in vitro by incubation with human or mouse microsomes. Intrinsic clearance (Clint) and T1/2 were calculated. (B) The protein binding adjusted EC50 and CC50 values of (S)-CCZ were measured in 40% of human serum (HS) with HCV-Luc and ATPlite assays. (C and D) Plasma, brain, and liver concentrations of the drug were measured over time after a single intraperitoneal dose of (S)-CCZ (50 mg/kg) or (S)-nor-CCZ (10 mg/kg). Results are means of replicates ± SEM (n = 3). Asterisks indicate statistical significance of liver concentration compared with plasma concentration by Student’s f test (*P < 0.05, **P < 0.005, ***P < 0.0001) (C). T1/2, the highest concentration after administration of compounds (Cmax), and time to reach Cmax (Tmax) are provided in (D).
In vitro activity in the presence of 40% human serum in conjunction with pharmacokinetics have been used to predict the inhibitory quotient (IQ) value of antimicrobial agents, namely, the ratio of compound exposure to microbial susceptibility. The protein binding adjusted EC50 value of (S)-CCZ in 40% human serum was 0.240 μM, which was an 8.7-fold increase relative to that in 10% fetal bovine serum (FBS), suggesting some potential for plasma protein binding (Fig. 3B).
The pharmacokinetic properties and tissue distribution of (S)-CCZ were evaluated in mice with a single intraperitoneal dose at 50 mg/kg. Depending on dosing three or two times per day (t.i.d. or b.i.d, respectively), the plasma concentration of (S)-CCZ at 8 or 12 hours after administration (2.02 or 0.592 μM, respectively) could be considered as Cmin (Fig. 3C). From Cmin and the protein binding adjusted EC50 value, the IQ values were calculated: if dosing t.i.d., IQ = Cmin/serum-adjusted EC50 value = 8.42; if dosing b.i.d., IQ = 2.47.
Because HCV infection and replication occur only in hepatocytes, it is essential for anti-HCV compounds to achieve high liver exposure. (S)-CCZ showed a significantly higher exposure in the liver than in the plasma at all time points (Fig. 3, C and D). The average concentration in the liver during 0 to 24 hours was 195 μmol/kg, whereas the average plasma concentration was 6.58 μM, resulting in a ratio of liver to plasma of 29.6 to 1 (assuming the density of the liver =1.0 g/ml). It is known that CCZ can cross the blood-brain barrier and lead to side effects, such as sedation. Thus, the brain level of (S)-CCZ was also measured (Figure 3, C and D). Brain levels were comparable to liver levels for both (S)-CCZ and (S)-nor-CCZ, suggesting central nervous system (CNS) penetration of the drugs and potential side effects that would need to be taken into consideration for future development.
(S)-Nor-CCZ, the primary metabolite of (S)-CCZ, was also tested for pharmacokinetics and tissue distribution at 10 mg/kg through the intraperitoneal route. Compared to (S)-CCZ, it showed a longer T1/2 and higher liver distribution (ratio of average concentration in liver to plasma of 60.7 to 1) (Fig. 3, C and D). The plasma concentration of (S)-nor-CCZ at 8 and 12 hours after administration was 0.890 and 0.561 μM, respectively. Although (S)-nor-CCZ was dosed at 10 mg/kg, the plasma level at 12 hours was comparable with that of (S)-CCZ dosed at 50 mg/kg. Overall, (S)-nor-CCZ showed preferable pharmacokinetic properties to (S)-CCZ in mice. These results are consistent with the results obtained in the mouse microsomal stability assay (Fig. 3A).
CCZ inhibits HCV infection in vivo without evidence of drug resistance
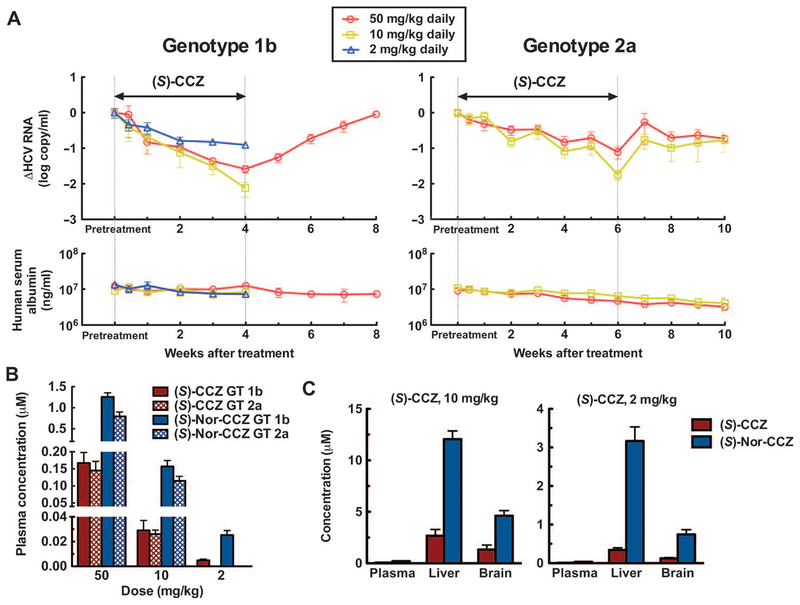
(S)-CCZ was tested in an albumin-urokinase plasminogen activator/severe combined immunodeficient (Alb-uPA/SCID) chimeric mouse model infected with HCV genotype 1b or 2a. On the basis of the pharmacokinetic data above, doses of 50 and 10 mg/kg daily (for 4 weeks in genotype 1b infection and for 6 weeks in genotype 2a infection) were used and led to a time-dependent reduction of HCV titers from the pretreatment baselines in mice infected with HCV (2 log in genotype 1b and 1.5 log in genotype 2a, respectively) (Fig. 4A). Doses as low as 2 mg/kg daily for 4 weeks also caused a decrease of genotype 1b virus titer (about 1 log). A rebound of virus titer after stopping treatment was observed in both genotype infections. However, HCV titers continued to decline during the treatment period without rebound, suggesting the absence of emergence of drug-resistant virus.
Fig. 4. In vivo efficacy and pharmacokinetics of CCZ in mice infected with HCV genotype 1b or 2a.
Alb-uPA/SCID mice were engrafted with primary human hepatocytes and then infected with HCV serum samples of genotype 1 b or 2a. The mice were monitored for serum HCV RNA and human albumin for 4 to 6 weeks before treatment. Pretreatment HCV RNA values were determined by averaging HCV RNA levels at weeks −2, −1, and 0. (A) Changes in the genotype 1b and 2a HCV titers from pretreatment baseline over time. For 1b, over a period of 4 weeks during (S)-CCZ treatment and 4 weeks of follow-up with or without treatment. For 2a, over a period of 6 weeks of (S)-CCZ treatment and 4 weeks of follow-up without treatment in both groups. The result at each last dosing time point (4 and 6 weeks for 1 b and 2a, respectively) was compared to the corresponding pretreatment level using the Mann-Whitney test after the Shapiro-Wilk normality test. Significant difference in HCV RNA was obtained with all the treatment conditions (P < 0.05). (B) The concentrations of (S)-CCZ and (S)-nor-CCZ were measured in plasma samples collected at weeks 1, 2,3, and 4 in genotype 1b-infected mice and at weeks 1, 2, 3, 4, 5, and 6 in genotype 2a-infected animals. The weekly concentrations were averaged for each dosing group and shown. (C) The concentrations of (S)-CCZ and (S)-nor-CCZ in plasma, liver, and brain samples collected at day 28 in genotype 1b-infected mice were measured by liquid chromatography-mass spectrometry (LC-MS). Data are means of mice in each group ± SEM (for genotype 1b, n = 5 in the 50 mg/kg group, n = 4 in the 10 mg/kg group, and n = 5 in the 2 mg/kg group; for genotype 2a, n = 8 in the 50 mg/kg group and n = 5 in the 10 mg/kg group).
Nor-CCZ was found to be similarly active in vitro against HCV infection as CCZ but demonstrated a preferable pharmacokinetic profile in the mouse model. It is possible that part of the antiviral activity observed on (S)-CCZ in vivo was due to the conversion to (S)-nor-CCZ. To detect the metabolism of (S)-CCZ to (S)-nor-CCZ in an Alb-uPA/SCID chimeric mouse model and to confirm the pharmacokinetic properties and tissue distribution of these two analogs, we evaluated the concentrations of (S)-CCZ and (S)-nor-CCZ in serum, liver, and brain samples from these chimeric mice during the in vivo efficacy experiment. There was no significant difference in the concentration of (S)-CCZ in mice infected with HCV of different genotypes (n ≥ 14; P > 0.5, two-sided Student’s t test), and a slightly higher concentration of (S)-nor-CCZ was observed in the genotype 1b group (Fig. 4B).
(S)-Nor-CCZ was detected in plasma from all dosing conditions with about 5- to 10-fold higher concentration than that of (S)-CCZ. After 28 days of treatment, (S)-nor-CCZ was more concentrated than (S)-CCZ in all tissues (Fig. 4C). The liver to plasma ratios of (S)-CCZ were 41 and 38 (in the 10 and 2 mg/kg groups, respectively), which is consistent with what has been observed in the CD-1 pharmacokinetic mouse model (Fig.3, C and D). At a dose of 10 mg/kg, the liver concentrations of (S)-CCZ and (S)-nor-CCZ (2.66 and 12.1 μM respectively) were higher than their in vitro EC90 values (1.44 and 0.578 μM, respectively). At a dose of 2 mg/kg, although the liver concentration of (S)-CCZ (0.342 μM) was below its EC90 value, the (S)-nor-CCZ level (3.16 μM) was still above its EC90 value (Fig. 1C), which might contribute to the observed minor decrease of HCV titers.
DISCUSSION
Despite the recent approval of direct-acting antivirals and multiple drug candidates in the pipeline for the treatment of HCV infection, an affordable yet efficacious treatment for this viral infection is an unmet clinical need. The repurposing or repositioning of existing drugs for diseases other than what the drugs were approved for may streamline and facilitate pharmaceutical development (7). It is well known that polypharmacology is common for most drugs and often explains their side effect profiles in human application. Many studies have reported drugs for other purposes having antiviral activity against various viral infections, but none of them have proceeded to potential clinical application (18–20). To this end, we performed a qHTS of approved drugs in the NPC, with the aim of identifying new antivirals for HCV treatment. Here, we described the discovery and preclinical characterization of CCZ as an anti-HCV agent. Its potent anti-HCV activity both in vitro and in vivo and high liver distribution makes CCZ a promising candidate for the treatment of HCV infection.
Cyclizine and phenothiazine were the two most potent series of H1 antihistamines with anti-HCV activity that we identified from the qHTS of the NPC (table S1). One of the antihistamines, clemizole, was shown previously to have anti-HCV activity by interfering with the nonstructural protein 4B functions (21), but our screen indicated a much less potent activity of clemizole as compared to CCZ. The anti-HCV properties of cyclizines and phenothiazines have been reported previously and are consistent with our results (22–26). Compared to the phenothiazines in table S1, CCZ analogs were chosen for further studies on the basis of the following aspects: (i) higher potency and selectivity against HCV than phenothiazines, (ii) fewer CNS-related side effects than phenothiazines, and (iii) potential for further structure modification to reduce side effects and increase anti-HCV activity.
Cyclizine H1-antihistamines bind to the H1-histamine receptor and inhibit the binding of histamine as inverse agonists (27). (R)-CCZ is more active as an antihistamine than (S)-CCZ, whereas both enantiomers are equally active in inhibiting HCV infection. Nor-CCZ, which is a metabolite of CCZ but has little antihistamine activity, has a similarly potent anti-HCV activity. Histamine also has no effect on HCV infection (table S1). Therefore, the anti-HCV activity of CCZ analogs is unlikely to be attributed to their action on the H1-histamine receptor.
Instead, CCZ may act through a novel mode of action by inhibiting late-stage HCV entry but not affecting viral replication or assembly/secretion. CCZ exhibited no inhibitory effect on the entry of HCVpp. There is growing evidence of HCV entry inhibitors with no activity against HCVpp, such as the NPC1L1 antagonist ezetimibe and human apolipoprotein E peptides (13, 28). Therefore, the lack of an effect in the HCVpp system by an agent does not necessarily exclude entry as a target. The agent could be targeting an entry step of HCV infection that is not otherwise captured by the HCVpp system. When HCVcc was used to evaluate its inhibitory kinetics, the inhibitory effect of (S)-CCZ on viral level during the 2-hour viral attachment and the first 2 hours of synchronous entry was comparable to that of continuous (S)-CCZ treatment for 48 hours, although no significant inhibition was detected when (S)-CCZ was added 2 or 3 hours after entry. Conversely, treatment with bafilomycin A1 and sofosbuvir during the attachment step had no effect on viral level. The sustained activity of CCZ when incubated during the attachment step could be explained by a longer off rate from its target, whereas bafilomycin may have a rapid off rate and the sofosbuvir’s target has not been formed yet during the viral attachment step. Overall, these data suggest that (S)-CCZ inhibits HCV late-entry step before RNA replication. On the basis of the kinetics and other virologic assays, (S)-CCZ probably functions at a step somewhat later than where bafilomycin A1 acts to block viral fusion, suggesting a novel target of CCZ in HCV infection that needs to be explored further. An HCVcc “fusion” assay has been developed previously, but the assay does not measure fusion directly because inhibitors of any of the viral entry steps before fusion would score positive in this assay (28).
Various drugs and anti-HCV agents with distinct mechanisms of action were tested in combination with (S)-CCZ against HCV infection in vitro. The mechanism of action of ribavirin and IFN-α is mediated through host antiviral response. Telaprevir and boceprevir are NS3/4A serine protease inhibitors, daclatasvir inhibits HCV NS5A, and sofosbuvir is an NS5B polymerase inhibitor (2,14,29). Cyclosporin A targets viral RNA replication (30). The strong synergism of (S)-CCZ with all these agents not only supports its use in combination therapy but also indicates that (S)-CCZ inhibits HCV infection through a different mechanism, such as targeting viral entry.
(S)-Nor-CCZ demonstrated better pharmacokinetic properties than those of (S)-CCZ, namely, a longer T1/2 and higher liver distribution. Consistent results were observed in the mouse microsomal stability assay and pharmacokinetic studies during the efficacy evaluation in HCV-infected chimeric mice. These observations in mice may not reflect the ADME in humans. Indeed, CCZ and nor-CCZ showed less of a difference in T1/2 in the human microsomal stability assay. On the other hand, nor-CCZ was less selective in vitro than CCZ (SI = 188 and 758, respectively). Overall, CCZ is preferable for further development, particularly because its safety profile is established in humans. One potential drawback of using CCZ to treat HCV infection is its brain distribution—the level of which is comparable to its liver distribution in mouse pharmacokinetic studies. Reducing blood-brain barrier penetration would thus be important for the future development of this class of drugs.
Entry inhibitors typically should not affect established infection when used alone in vivo. However, the in vivo antiviral effect of CCZ on preexisting steady-state infection in mice is similar to that of IFN-α (31). It is known that in vivo, circulating HCV has a very short T1/2 (32), HCV-infected hepatocytes may turnover quickly, and the newly produced virus probably continues to infect uninfected hepatocytes (33). If viral reinfection and spread can be effectively prevented in the infected liver, a reduction in viremia can be achieved. In addition, recent data suggested that cell-to-cell spread may be an important pathway for viral dissemination in vivo (34). It is possible that CCZ might be particularly effective in blocking this pathway. Finally, the kinetics of decline in viremia in CCZ-treated mice is a steady and linear decline, which is quite different from that of direct-acting antivirals, which typically have a rapid first and then a slow second-phase decline. Studies have shown similar reduction of HCV titer in clinical trials as that of the efficacy study using the SCID/uPA mouse model, suggesting the applicability of this mouse model in predicting antiviral activity in humans (35).
A dose response of anti-HCV activities against both genotype 1 and 2 by CCZ treatment was observed in the SCID/uPA mouse model. The anti-HCV activity of CCZ was initially discovered using the infectious HCV genotype 2a system; however, (S)-CCZ showed even higher efficacy on genotype 1b infection in the mouse model. This finding suggests that (S)-CCZ may have pan-genotypic in vivo activity, which is consistent with the pan-genotypic in vitro activity shown in table S1.
Several FDA-approved drugs have shown anti-HCV activities and can potentially be repurposed for the clinical treatment of HCV, such as erlotinib and dasatinib (anticancer drugs), ezetimibe (cholesterol drug), and ferroquine (antimalarial) (28, 36, 37). Here, CCZ showed more compelling in vitro and in vivo activity against HCV infection than these drugs. Although in vitro activity cannot adequately be compared between different assays with different strains or reported virus, it is worth noting that the above drugs do not have in vitro EC50 values comparable to that of CCZ (~50 nM). Some of the drugs, like erlotinib and dasatinib, also have substantial side effects. CCZ is also advantageous because of its established safety profile in patients for a long period of time (25 mg per tablet by mouth every 6 to 8 hours, not to exceed three tablets in 24 hours) and cost-effectiveness ($0.55 per tablet).
In summary, this study provides compelling evidence that CCZ, an over-the-counter allergy drug, has a strong anti-HCV activity in vitro and in vivo in a mouse model of HCV infection. On the basis of these results, a clinical assessment of CCZ alone and in combination with other anti-HCV drugs is warranted. The repurposing or repositioning of CCZ in HCV treatment may provide a more affordable alternative to the current costly options, especially in low-resource settings where chronic HCV infection is endemic. This study also lays the foundation for further structure modification of CCZ to discover more optimal analogs for the treatment of HCV infection.
MATERIALS AND METHODS
Study design
The objective of this study was to identify anti-HCV compounds from existing, FDA-approved drugs through qHTS of the NPC library (9) and to repurpose candidate drugs for the treatment of HCV infection. The identified drugs with potent anti-HCV activity were characterized in vitro, with a focus on confirming antiviral properties, target identification in the HCV replication cycle, and activity in combination with other clinically used anti-HCV drugs. In vivo studies were carried out to evaluate candidate drug pharmacokinetic properties and efficacy in an Alb-uPA/SCID mouse model, which has been successfully used for translational drug development, including for HCV infection (35, 38). This study conducted controlled laboratory experiments with cell culture and animals. The animal study protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of NIH for the mouse pharmacokinetic study and by the IACUC of Hiroshima University, Japan, for the mouse efficacy study. Power analysis was not performed, and replication conditions for each experiment are defined and described in the figure legends. The animals were randomized, but the investigators were not blinded to the experimental conditions.
Primary qHTS and secondary confirmation assays
Human hepatocytes and viruses were obtained and maintained as described in Supplementary Materials and Methods. The suppliers of the compounds in the NPC were published previously (9). In the primary screen using the cell-based HCV infection assay, the qHTS was performed as described previously using a fully automated robotic screening system (8). Dose-response curves and EC50 and CC50 values were generated through a seven-concentration titration for each compound from the NPC. Curve classes were defined on the basis of the quality of the curve fitting derived from the Hill equation (39). Primary hits were selected with the dose-response curves in class 1 or 2 in the anti-HCV luciferase assay and class 3 or 4 in the ATPlite assay (cytotoxicity). A two-part HCV infection assay, as described before, was carried out to confirm the primary hits.
HCVcc infection assay
Huh7.5.1 cells were seeded in 12-well plates (1 × 105 cells per well) and cultured overnight. Wild-type HCV genotype 2a and chimeric genotype 1a, 1b, 2b, 3a, 4a, 5a, 6a, or 7a was used to infect the cells with the treatment of compounds. Virus-containing medium was removed after overnight incubation, and compound treatment was added back followed by incubation for an additional 48 hours. Cyclosporin A (Sigma-Aldrich) was used as a positive control. Intracellular and extracellular viral RNA levels were evaluated by qRT-PCR. The same methods were used with primary human hepatocytes in 24-well plates. For TCID50/ml determination, medium collected was used to infect naïve Huh7.5.1 cells attached in 96-well plates in serial dilutions. TCID50/ml was calculated as described on the basis of the dilution at which 50% of the wells were positive for HCV (40).
In vitro combination evaluation
Huh7.5.1 cells were seeded in 96-well plates (1 × 104 cells per well) and cultured overnight. In the presence of (S)-CCZ and the agent of interest titrated in vertical and horizontal, respectively, HCV-Luc infection assay was carried out in parallel with ATPlite assay. The anti-HCV effect of (S)-CCZ in combination with each agent was analyzed using two independent mathematical models, the Bliss independence model and the Loewe additivity model (15,16), to predict the theoretical additive, synergistic, or antagonistic effect (Supplementary Materials and Methods).
ADME and pharmacokinetics
In vitro pharmacokinetic studies are described in Supplementary Materials and Methods. The in vivo pharmacokinetic properties of the compounds were measured in the plasma, brain, and liver of male CD-1 mice after a single intraperitoneal administration. The plasma and tissue concentrations of the compounds at various time points up to 24 hours after administration were determined by LC-MS analysis.
In vivo efficacy studies in chimeric mouse model
Alb-uPA/SCID mice were engrafted with primary human hepatocytes and then infected with HCV serum samples of genotype 1b or 2a. The mice were monitored for serum HCV RNA and human albumin for 4 to 6 weeks before treatment. Genotype 1b HCV titers were monitored in HCV-infected chimeric mice over a 4-week period of (S)-CCZ treatment. A 4-week follow-up monitoring without treatment was carried out only in the group that received the 50 mg/kg dose. The means of changes in serum HCV RNA level in each group were calculated (n = 5 in the 50 mg/kg daily group, n = 4 in the 10 mg/kg daily group, and n = 5 in the 2 mg/kg daily group). Genotype 2a HCV titers were monitored over a period of 10 weeks with 6 weeks of (S)-CCZ treatment and 4 weeks of follow-up without treatment in both groups in HCV-infected chimeric mice. The means of changes in serum HCV RNA level in each group were calculated (n = 8 in the 50 mg/kg daily group and n = 5 in the 10 mg/kg daily group). Human serum albumin was measured in parallel for control.
Statistical analysis
Statistical significance was assessed with GraphPad Prism 5.0 software. Data are presented as means ± SEM (n ≥ 3). Student’s t test was used to determine whether the means of two groups are significantly different on the basis of one continuous variable when normal distribution was assumed in a small sample size. Normal distribution was examined by the Shapiro-Wilk normality test. In case of nonparametric distribution, the Mann-Whitney test was used. In all analyses, two-sided P values were used, and P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments:
We thank B. Zhang for technical assistance; S. Michael and M. Balcom for assistance in robotic control in qHTS; and P. Shinn, M. Itkin, and D. van Leer for help with compound management.
Funding: The Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases and Molecular Libraries funding.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/7/282/282ra49/DC1
Materials and Methods
Fig. S1. CCZ does not affect the expression levels or cellular distribution of HCV entry factors.
Fig. S2. Synergistic antiviral effects of CCZ in combination with anti-HCV drugs.
Fig. S3. Antiviral activity of CCZ against dengue virus.
Table S1. Structure, anti-HCV activity, and cytotoxicity of H1-antihistamine compounds from the NPC library.
Table S2. Negligible amount of transformation of (S)-CCZ to (S)-nor-CCZ in vitro.
Table S3. Antiviral activity of CCZ against HCV genotypes 1 to 7.
Table S4. NIAID antiviral screen of CCZ against 13 viruses.
Competing interests: TJ.L., M.F., S.H., X.H., Z.H., JJ.M., J.X., and W.Z. are named as inventors on a patent related to antihistamines and heterocyclic compounds for the treatment of HCV.The other authors declare that they have no competing interests.
Data and materials availability: Primary human hepatocytes were provided by the NIH-funded Liver Tissue Procurement and Cell Distribution System (N01-DK-7–0004/HHSN26700700004C; principal investigator, S. Strom, University of Pittsburgh). The plasmids (pNL4-3.Luc.R-E- and pHEF-VSVG) were obtained from the NIH AIDS Reagent Program (Division of AIDS, NIAID, NIH). pNL4-3.Luc.R-E- was originally from N. Landau. pHEF-VSVG was from L.-J. Chang. Plasmids encoding HCV genotype 1a envelope proteins were provided by S. Ray (Johns Hopkins University). HCV genotypes 1 to 7 were obtained from J. Bukh (Copenhagen University Hospital).
REFERENCES AND NOTES
- 1.Liang TJ, Ghany MG, Current and future therapies for hepatitis C virus infection. N. Engl. J. Med 368, 1907–1917 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas DL, Global control of hepatitis C: Where challenge meets opportunity. Nat. Med 19, 850–858 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH, Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann. Intern. Med 132, 296–305 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Ward JW, Hepatitis C virus testing of persons born during 1945–1965: Recommendations from the Centers for Disease Control and Prevention. Ann. Intern. Med 157, 817–822 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang TJ, Current progress in development of hepatitis C virus vaccines. Nat. Med 19, 869–878 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaway E, Hepatitis C drugs not reaching poor. Nature 508, 295–296 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Collins FS, Mining for therapeutic gold. Nat. Rev. Drug Discov 10, 397 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Z, Lan K-H, He S, Swaroop M, Hu X, Southall N, Zheng W, Liang TJ, Novel cell-based hepatitis C virus infection assay for quantitative high-throughput screening of anti-hepatitis C virus compounds. Antimicrob. Agents Chemother 58, 995–1004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang R, Southall N, Wang Y, Yasgar A, Shinn P, Jadhav A, Dac-Trung N, Austin CP, The NCGC Pharmaceutical Collection: A comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci. Transl. Med 3, 80ps16 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaertner HJ, Breyer U, Liomin G, Chronic administration of chlorcyclizine and meclizine to rats: Accumulation of a metabolite formed by piperazine ring cleavage. J. Pharmacol. Exp. Ther 185, 195–201 (1973). [PubMed] [Google Scholar]
- 11.Dumasia MC, Grainger L, Houghton E, Biotransformation of cyclizine in greyhounds. 1: Identification and analysis of cyclizine and some basic metabolites in canine urine by gas chromatography-mass spectrometry. Xenobiotica 32, 795–807 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Horwitz JA, Dorner M,Friling T, Donovan BM,Vogt A,Loureiro J,Oh T, Rice CM, Ploss A, Expression of heterologous proteins flanked by NS3–4A cleavage sites within the hepatitis C virus polyprotein. Virology 439, 23–33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, McCormick KD, Zhao W, Zhao T, Fan D, Wang T, Human apolipoprotein E peptides inhibit hepatitis C virus entry by blocking virus binding. Hepatology 56, 484–491 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sofia MJ, Bao D, Chang W, Du J, Nagarathnam D, Rachakonda S, Reddy PG, Ross BS, Wang P, Zhang HR, Bansal S, Espiritu C, Keilman M, Lam AM, Steuer HM, Niu C, Otto MJ, Furman PA, Discovery of a β-d-2′-deoxy-2′-α-fluoro-2′-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J. Med. Chem 53, 7202–7218 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Prichard MN, Shipman C Jr., A three-dimensional model to analyze drug-drug interactions. AntiviralRes 14, 181–205 (1990). [DOI] [PubMed] [Google Scholar]
- 16.Bassit L, Grier J, Bennett M, Schinazi RF, Combinations of 2′-C-methylcytidine analogues with interferon-α2b and triple combination with ribavirin in the hepatitis C virus replicon system. Antivir. Chem. Chemother 19, 25–31 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakrabarty AN, Mookerjee M, Dastidar SG, Screening for anti-HIV drugs that can combine virucidal and virustatic activities synergistically. Int. J. Antimicrob. Agents 14, 215–220 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Simanjuntak Y, Liang JJ, Lee YL, Lin YL, Repurposing of prochlorperazine for use against dengue virus infection. J. Infect. Dis 211, 394–404 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Boonyasuppayakorn S, Reichert ED, Manzano M, Nagarajan K, Padmanabhan R, Amodiaquine, an antimalarial drug, inhibits dengue virus type 2 replication and infectivity. Antiviral Res 106, 125–134 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clouser CL, Patterson SE, Mansky LM, Exploiting drug repositioning for discovery of a novel HIV combination therapy. J. Virol 84, 9301–9309 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Einav S, Gerber D, Bryson PD, Sklan EH, Elazar M, Maerkl SJ, Glenn JS, Quake SR, Discovery of a hepatitis C target and its pharmacological inhibitors by microfluidic affinity analysis. Nat. Biotechnol 26, 1019–1027 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chockalingam K, Simeon RL, Rice CM, Chen Z, A cell protection screen reveals potent inhibitors of multiple stages of the hepatitis C virus life cycle. Proc. Natl. Acad. Sci. U.S.A 107, 3764–3769 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gastaminza P, Whitten-Bauer C, Chisari FV, Unbiased probing of the entire hepatitis C virus life cycle identifies clinical compounds that target multiple aspects of the infection. Proc. Natl. Acad. Sci. U.S.A 107, 291–296 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu X, Sainz B Jr., Petukhov PA, Uprichard SL, Identification of hepatitis C virus inhibitors targeting different aspects of infection using a cell-based assay. Antimicrob. Agents Chemother 56, 6109–6120 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chamoun-Emanuelli AM, Pecheur EI, Simeon RL, Huang D, Cremer PS, Chen Z, Phenothiazines inhibit hepatitis C virus entry, likely by increasing the fluidity of cholesterol-rich membranes. Antimicrob. Agents Chemother 57, 2571–2581 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamoun-Emanuelli AM, Pécheur EI, Chen Z, Benzhydrylpiperazine compounds inhibit cholesterol-dependent cellular entry of hepatitis C virus. Antiviral Res 109, 141–148 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Simons FE, Simons KJ, Histamine and H1-antihistamines: Celebrating a century of progress. J. Allergy Clin. Immunol 128, 1139–1150.e4 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Sainz B Jr., Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, Marsh KA, Yu X, Chayama K, Alrefai WA, Uprichard SL, Identification of the Niemann-Pick C1 -like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat. Med 18, 281–285 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheel TKH, Rice CM, Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat. Med 19, 837–849 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watashi K, Hijikata M, Hosaka M, Yamaji M, Shimotohno K, Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology 38, 1282–1288 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Abe H, Imamura M, Hiraga N, Tsuge M, Mitsui F,Kawaoka T, Takahashi S, Ochi H,Maekawa T, Hayes CN, Tateno C, Yoshizato K, Murakami S, Yamashita N, Matsuhira T, Asai K, Chayama K, ME3738 enhancesthe effect of interferon and inhibits hepatitis C virus replication both in vitro and in vivo. J. Hepatol 55, 11–18 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Guedj J, Dahari H, Rong L, Sansone ND, Nettles RE, Cotler SJ,Layden TJ, Uprichard SL, Perelson AS, Modeling shows that the NS5A inhibitor daclatasvir has two modes of action and yields a shorter estimate of the hepatitis C virus half-life. Proc. Natl. Acad. Sci. U.SA 110, 3991–3996 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS, Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-α therapy. Science 282, 103–107 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Barretto N, Sainz B Jr., Hussain S, Uprichard SL, Determining the involvement and therapeutic implications of host cellular factors in hepatitis C virus cell-to-cell spread. J. Virol 88, 5050–5061 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kneteman NM, Weiner AJ, O’Connell J, Collett M, Gao T, Aukerman L, Kovelsky R, Ni ZJ, Zhu Q, Hashash A, Kline J, Hsi B, Schiller D, Douglas D, Tyrrell DL, Mercer DF, Anti-HCV therapies in chimeric scid-Alb/uPA mice parallel outcomes in human clinical application. Hepatology 43, 1346–1353 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, Royer C, Fischer B, Zahid MN, Lavillette D, Fresquet J, Cosset FL, Rothenberg SM, Pietschmann T, Patel AH, Pessaux P, Doffoël M, Raffelsberger W, Poch O, McKeating JA, Brino L, Baumert TF, EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat. Med 17, 589–595 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vausselin T, Calland N, Belouzard S, Descamps V, Douam F, Helle F, François C, Lavillette D, Duverlie G, Wahid A, Fénéant L, Cocquerel L, Guérardel Y, Wychowski C, Biot C, Dubuisson J, The antimalarial ferroquine is an inhibitor of hepatitis C virus. Hepatology 58, 86–97 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barth H, Robinet E, Liang TJ, Baumert TF, Mouse models for the study of HCV infection and virus-host interactions. J. Hepatol 49, 134–142 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP, Quantitative high-throughput screening: A titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc. Natl. Acad. Sci. USA 103, 11473–11478 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Pène V, Krishnamurthy S, Cha H, Liang TJ, Hepatitis C virus infection activates an innate pathway involving IKK-α in lipogenesis and viral assembly. Nat. Med 19, 722–729 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones CT, Murray CL, Eastman DK, Tassello J, Rice CM, Hepatitis C virus p7 and NS2 proteins are essential for production of infectious virus. J. Virol 81, 8374–8383 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottwein JM, Scheel TK, Jensen TB, Lademann JB, Prentoe JC, Knudsen ML, Hoegh AM, Bukh J, Development and characterization of hepatitis C virus genotype 1–7 cell culture systems: Role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology 49, 364–377 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Masaki T, Suzuki R, Saeed M, Mori K, Matsuda M, Aizaki H, Ishii K, Maki N, Miyamura T, Matsuura Y, Wakita T, Suzuki T, Production of infectious hepatitis C virus by using RNA polymerase I-mediated transcription. J. Virol 84, 5824–5835 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA, Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. U.S.A 100, 7271–7276 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR, Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol 69, 6705–6711 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang LJ, Urlacher V, Iwakuma T, Cui Y, Zucali J, Efficacy and safety analyses of a recombinant human immunodeficiency virus type 1 derived vector system. Gene Ther 6, 715–728 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Wang P, Li LF, Wang QY, Shang L-Q, Shi PY, Yin Z, Anti-dengue-virus activity and structure-activity relationship studies of lycorine derivatives. ChemMedChem 9,1522–1533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naritomi Y, Terashita S, Kimura S, Suzuki A, Kagayama A, Sugiyama Y, Prediction of human hepatic clearance from in vivo animal experiments and in vitro metabolic studies with liver microsomes from animals and humans. Drug Mefab. Dispos 29, 1316–1324 (2001). [PubMed] [Google Scholar]
- 49.Obach RS, Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Mefab. Dispos 27, 1350–1359 (1999). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.