Abstract
Purpose:
To evaluate corneal nerve and immune cell alterations in Fuchs’ endothelial corneal dystrophy (FECD) and pseudophakic bullous keratopathy (PBK) by laser in vivo confocal microscopy (IVCM) as correlated to sensation and endothelial cell loss.
Design:
Prospective, cross-sectional, controlled study.
Methods:
33 eyes with FECD were compared to 13 eyes with PBK and 17 normal age-matched control eyes at a tertiary referral center. FECD was classified into early (without edema) and late stage (with edema). Corneal IVCM (HRT3/RCM) and esthesiometry (Cochet-Bonnet) were performed. Corneal nerve and immune dendritic cell (DC) alterations were evaluated and correlated to clinical parameters.
Results:
FECD and PBK eyes showed significantly (p=0.001) diminished total nerve length (11.5±1.3 and 2.9±0.7mm/mm2) and number (8.8±1.1 and 2.2±0.4 n/frame), compared to controls (23.3±8.1 and 25.9±1.3). Decreased nerves corresponded to diminished sensation in FECD (4.9±0.2cm; R=0.32; p=0.045), compared to controls (5.9±0.04). Early and late stage FECD showed significantly reduced total nerve length (13.1±1.4 and 9.9±1.2 mm/mm2, respectively) and number (8.2±2.5 and 6.5±2.1 n/frame), compared to controls (p<0.001). DC density was significantly increased in FECD (57.8±10.4 cells/mm2; p=0.01), but not in PBK (47.7±11.6; p=0.60) compared to controls (22.5±4.5). A subset of early FECD patients (7/22) demonstrated very high DC density (>100/mm2).
Conclusion:
IVCM demonstrates profound diminishment of subbasal corneal nerves in early, late stage FECD, and PBK, correlating to decreased sensation. Increased DC density in early FECD demonstrates potential subclinical inflammation. The data suggest that reduction in subbasal nerves and increased immune activation may play a role in pathophysiology of FECD.
Keywords: Fuchs Endothelial Corneal Dystrophy, Confocal Microscopy, Corneal Nerves, Pseudophakic bullous keratopathy, Dendritic Cells, Inflammation
INTRODUCTION
Fuchs’ endothelial corneal dystrophy (FECD) is one of the leading indications of corneal transplantation.1,2 Following an initial description by Ernst Fuchs in 1910,3 it is now recognized to be a bilateral degenerative disorder of the corneal endothelium.4 Genetic mutations and environmental triggers, such as oxidative stress, cause endothelial cell apoptosis in FECD.5–7 Progressive loss of the endothelial cells, following focal accumulation of abnormal collagen, thickening of the Descemet’s membrane, and formation of excrescences called guttae, may result in corneal edema and vision loss.6,8,9
Initial histopathology on excised corneas after corneal transplantation showed Descemet’s membrane thickening, guttae, and abnormal endothelium, corresponding to clinical edema.10,11 These studies only provided information on advanced disease that had progressed to the point of requiring corneal transplantation. Histopathological studies following Descemet’s stripping endothelial keratoplasty (DSEK) may be able to show endothelial changes in earlier stages of disease, but cannot demonstrate changes in the anterior corneal layers.12 However, with the advent of in vivo confocal microscopy (IVCM), it has become possible to carry out non-invasive imaging to analyze the corneal ultrastructure at a quasi-histological level.13,14 Thin optical sectioning and high magnification enable visualization of structures, which are not visible by conventional slit-lamp bio-microscopy, specifically corneal nerves15,16 and immune dendritiform cells (DCs).17
Using IVCM, decreased density of nerves has been demonstrated in FECD, which is thought to be responsible for decrease in corneal sensitivity.16,18 This corneal nerve damage has been attributed to subepithelial fibrosis, epithelial edema and bullae.16 More recently our group demonstrated in a retrospective study, significant diminishment of corneal nerves in early stages of FECD, prior to the development of epithelial edema, which inversely correlated with endothelial cell loss and stromal edema.19 Our results raised the possibly, that nerve loss in FECD may not be a consequence of corneal edema as previously thought, but may potentially play a role in the pathogenesis of the disease. Further, in a recent study on patients with infectious keratitis, we have demonstrated a strong inverse correlation between nerves loss and activation of immune cells in the cornea.17 However, to date, studies have not been conducted studying immune cellular changes in FECD. Thus the purpose of this study was to evaluate corneal nerve and immune cell alterations in patients with early and late stage FECD using laser IVCM. Herein, we demonstrate that while corneal nerve loss takes place in early FECD, it does not progress further in late FECD, and that patients with early stage FECD demonstrate immune cell changes in the cornea.
METHODS
Patients
This prospective, cross-sectional study was conducted in a controlled, single blinded fashion. Thirty-three patients with a clinical diagnosis of FECD were recruited from the Cornea Service of the Massachusetts Eye & Ear Infirmary, Boston, between 2009 and 2012. Only one eye, randomly selected, was used for image and statistical analysis to prevent statistical bias. 13 eyes of 13 patients with a diagnosis of PBK were recruited to serve as controls for corneal edema. 17 age-matched normal eyes were used as healthy controls.
We excluded patients with other ocular surface diseases, including dry eye disease based on abnormal Schirmer’s test, tear break-up time, corneal and conjunctival staining. We also excluded patients with history of ocular surgery, except cataract surgery in the PBK group, inflammatory and infectious eye diseases, glaucoma, contact lens use, and diabetes based on ocular history, clinical signs and symptoms. The study protocol was approved by the Institutional Review Board, complied with the Health Insurance Portability and Accountability Act (HIPAA) and adhered to the tenets of the Declaration of Helsinki. All participants provided written informed consent.
Ophthalmic Examination
All subjects underwent a detailed slit-lamp examination by a cornea specialist (P.H. and U.V. J.). Clinical grading of FECD was performed at the slit-lamp according to the following guidelines: grade 1 – non-confluent guttae; grade 2 – presence of any area of confluent guttae, but without edema or clinical thickening; grade 3 – confluent guttae with edema or clinical thickening; grade 4 – edema associated with whitening or haze. We categorized early stage FECD as grade 1 and 2 and late stage FECD as grade 3 and 4.8 The central corneal thickness (CCT) was measured 3 times in μm using ultrasound pachymetry and averaged. (AccupachVI, Accutome, Malvern, PA). We measured central corneal sensation with the Cochet-Bonnet esthesiometer (Luneau Ophthalmologie, Chartres, France) as previously described.20
In Vivo Confocal Microscopy
Laser IVCM (Heidelberg Retina Tomograph 3/Rostock Cornea Module, HRT3/RCM, Heidelberg, Germany) of the central cornea, was performed in all subjects as previously reported.17 A total of 6 to 8 sequence scans were obtained from central corneas, with particular focus on the subepithelial area, the subbasal nerve plexus as well as on the endothelial layer. The scans yielded a total of 500–600 images of the subbasal area. A minimum of 3 representative images from each layer were selected for analysis for each eye by a masker observer. Three masked observers (S.A., L.R., B.M.C) analyzed the selected images for central DC density, endothelial cell density, and corneal nerve morphology and density as previously described.20,21 The nerve analysis was done using the semi-automated tracing program NeuronJ, a plugin for ImageJ, (http://www.imagescience.org/meijering/software/neuronj/).22
IVCM images at a depth of 50 to 80 μm at the level of basal epithelial layers, basal lamina, or subbasal nerve plexus were chosen for DC analysis. DC density was assessed using the Cell Count software (HRT 3) in the manual mode. We measured endothelial cell density (ECD) in a semi-automated fashion using endothelial cell counter function in the software inbuilt in HRT3. A fixed frame of 200 × 200μm, showing endothelial cells was analyzed to obtain ECD cells/mm2. The data were expressed as density (cells/mm2) ± standard error of mean (SEM).
Statistical Analysis
Statistical analysis was performed using STATA (StataCorp LP, Texas, USA). A priori power analysis was conducted using data from our previous pilot study in FECD19 to estimate a sufficient sample size to achieve a power of 80% and alpha error of 0.05. Further, post hoc power analysis revealed that the study was adequately powered to detect a difference between FECD, PBK and controls with alpha of 0.5 and beta of at least 0.8. The magnitude of the difference in our outcome variable (total nerve density an endothelial cell counts) in the diseased and control groups, further reinforces the power of our study. Normality of data was determined using the Shapiro–Wilk normality test. The data was found to have a normal distribution and hence parametric analysis was used.
All quantitative variables were expressed by the mean ± SEM. Analysis of variance (ANOVA) and student’s t-test were used to compare differences among groups, where appropriate. Bonferroni post-hoc correction was applied for further analysis. Pearson’s correlation coefficient was used for correlations and statistical significance was considered for P values < 0.05. The intraclass correlation coefficient (ICC) was calculated to estimate the repeatability of the measurements between the observers.
RESULTS
We studied 33 eyes of 33 patients (14 males and 19 females, mean age 65.2 ± 2.3) diagnosed with FECD and compared to 13 eyes of 13 PBK patients (6 males and 7 females, mean age 70.4± 2.8), and to 17 eyes of 17 normal healthy controls (9 males and 8 females, mean age 59.4 ± 1.9). No difference was found for age and gender between the groups (p=0.45 and p=0.87, respectively). (Supplemental table 1)
Corneal sensation was significantly decreased in FECD (4.9±0.2 cm) and PBK (3.7±0.6 cm) patients, compared to controls (5.9±0.04 cm; p= 0.044 and p=0.001). CCT in FECD (597.2±10 μm) and PBK (685.7±9.4 μm) patients was significantly increased, as compared to controls (546.3±6.3μm; p=0.001 and p<0.001). A summary of the clinical and IVCM parameters is presented in Table 1.
Table 1.
Clinical and confocal parameters of patients and controls.
| Fuchs’ Endothelial Corneal Dystrophy (n=33) |
Pseudophakic Bullous Keratopathy (n=13) |
Controls (n=17) |
|
|---|---|---|---|
| Central Corneal Thickness (μm) |
597.2±10.0†‡ | 685.7±9.4† | 546.3±6.3 |
| Central Corneal Sensation (cm) |
4.9±0.2†‡ | 3.7±0.6† | 5.9±0.04 |
| Number of Total Nerves |
8.8±l.l†‡ | 2.2±0.4† | 25.9±1.3 |
| Density of Total Nerves (μm/mm2) |
11,510.2±1310.3†‡ | 2,912.5±738.4† | 23,305.2±816.1 |
| Number of Main Nerves |
2.3±0.2†‡ | 0.8±0.2† | 4.1±0.2 |
| Density of Main Nerves (μm/mm2) |
5,834.5±602.1†‡ | 1,665.9±611.8† | 10,516.6±425.1 |
| Number of Nerve Branches |
6.5±0.9†‡ | 1.5±0.3† | 21.9±1.2 |
| Density of Nerve Branches (μm/mm2) |
5,675.4±776.5†‡ | l,248.2±268.0† | 12,788.6±637.8 |
| Dendritic Cell Density (cells/mm2) |
57.8±10.4†‡ | 47.7±11.6 | 22.5±4.5 |
| Endothelial Cell Density (cells/mm2) |
1,364.1 ± 119.2† | 1,114.5 ± 232.4† | 3,185.8 ± 52.6 |
(Mean ± Standard Error of Mean)
significant difference compared to controls (p<0.05);
significant difference compared to PBK (p<0.05)
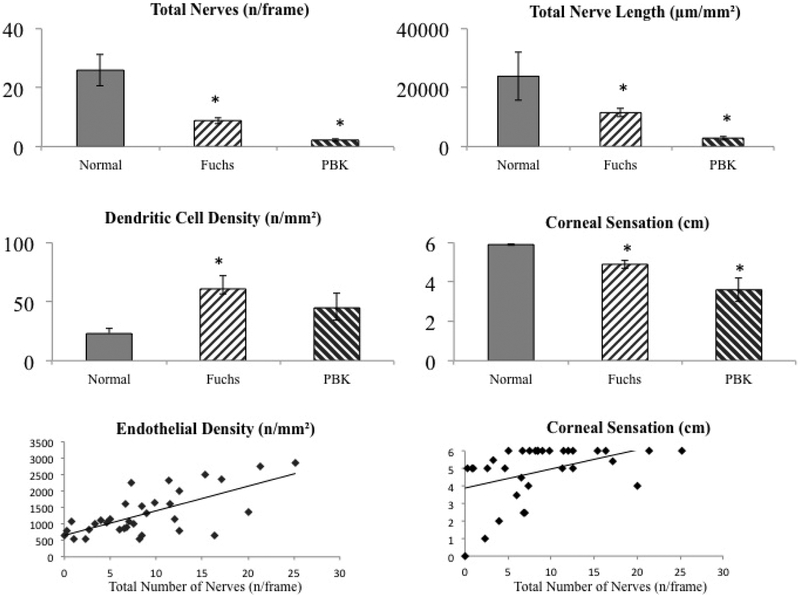
Briefly, eyes with FECD and PBK showed significantly decreased subbasal nerves, including total nerve length (11,510.2±1310.3 and 2,912.5±738.4 μm/mm2, respectively; P=0.001) and total number of nerves (8.8±1.1 and 2.2±0.4 n/frame; p=0.001), as compared to controls (23,305.2±816.1μm/mm2 and 25.9±1.3n/frame) (Fig. 1, 2). The main nerve length and the main nerve number in FECD (5,834.5±602.1 μm/mm2; 2.3±0.2 n/frame) and in PBK (1,665.9±611.8; 0.8±0.2) were significantly reduced compared to controls (10,516.6±425.1; 4.1±0.2; p<0.001 for all groups). There was a significant (p<0.001) decrease in nerve branch length and number in both FECD (5,675.4±776.5 μm/mm2; 6.5±0.9 n/frame) and PBK (1,248.2±268.0; 1.3±0.4), compared to controls (12,788.6±637.8; 21.9±1.2; p<0.001). This lower density of nerve fibres correlated significantly to the decrease in central corneal sensation in FECD (R=0.32; p=0.044 for nerve length and R=0.47; p=0.01 for nerve number). ECD was also significantly decreased in FECD (1,364.1±119.2 cells/mm2) and PBK (1,114.5±232.4 cells/mm2) as compared to controls (3,185.8±52.6 cells/mm2; p<0.001 for both, Fig. 2). The ECD showed a positive correlation with the density of total corneal subbasal nerves (R=0.66; P<0.001) (Fig. 2).
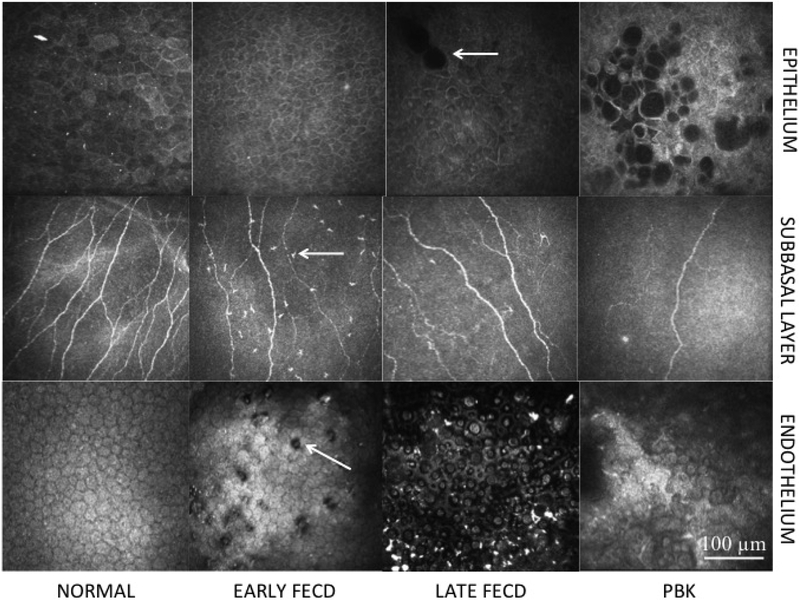
Figure 1. In vivo confocal microscopy images.
Central corneal in vivo confocal microscopy images showing the epithelium (Row 1) in normal controls (Column 1); Early stage Fuchs’ endothelial corneal dystrophy (FECD) (Column 2); Late stage FECD (Column 3); pseudophakic bullous keratopathy (PBK) (Column 4); subbasal layer (Row 2) in normal controls (Column 1); Early stage FECD (Column 2); Late stage FECD (Column 3); PBK (Column 4); and endothelium (Row 3) in normal controls (Column 1); Early stage FECD (Column 2); Late stage FECD (Column 3); and PBK (Column 4). Patients with early stage FECD show decreased nerves and increased immune dendritic cells (arrow) in the subbasal layer (Row 2, Column 2) and guttae (arrow) in the endothelium (Row 3, Column 2). Patients with late stage FECD show epithelial edema (arrow) (Row 1, Column 3), decreased subbasal nerves (Row 2, Column 3) and reduced endothelial density (Row 3, Column 3). Patients with PBK show epithelial edema (Row 1, Column 4), reduced subbasal nerve density (Row 2, Column 4) and reduced endothelial cell density without guttae (Row 3, Column 4). Size bar = 100μm.
Figure 2. Confocal microscopy parameters in Fuchs’ endothelial corneal dystrophy (FECD) and pseudophakic bullous keratopathy (PBK).
Comparison of number of total nerves (top left), total nerve length (top tight); immune dendritic cell density (middle left), and corneal sensation (middle right) in normal controls, Fuchs’ endothelial corneal dystrophy (FECD) and pseudophakic bullous keratopathy (PBK). *indicates statistical significance compared to normal controls; p<0.05. Correlation of total nerves with endothelial cell density (bottom left) R=0.66; p<0.001 and with central corneal sensation (bottom right) R=0.32; p=0.045.
The DC density was significantly increased in FECD (57.8±10.4 cells/mm2; P=0.04), but not in PBK eyes (47.7±11.6; P=0.60) in comparison to controls (22.5±4.5). Interestingly, a subset of patients with early stage FECD (7/22) had very high DC density (>100 cells/mm2). DC density had a significantly negative correlations with both ECD (R= −0.32; p = 0.02) and nerve density (R= −0.31; p=0.02). Representative central corneal IVCM images are shown in Fig. 1 and the changes in various parameters have been compared graphically in Fig. 2. Interestingly, a subset of FECD patients (9/33) showed an abnormal looking cluster of cells in the subbasal layer and the stroma on confocal microscopy (Supplemental fig. 1)
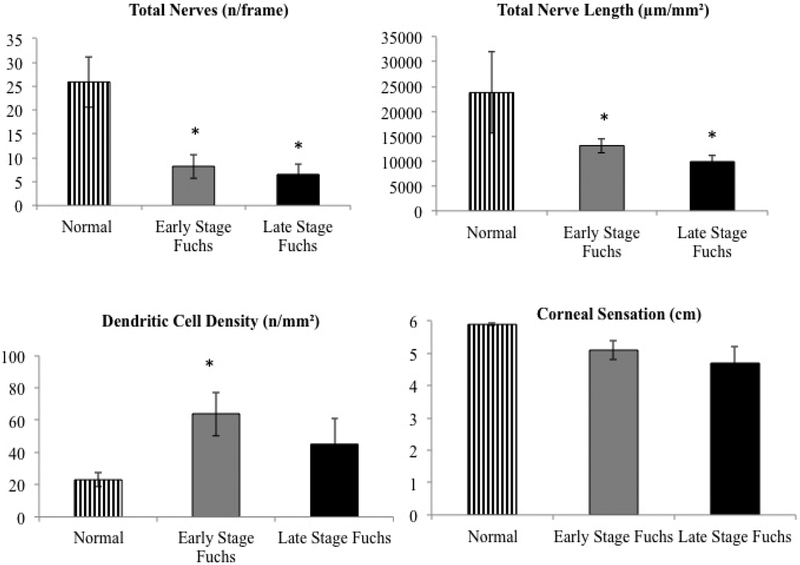
The 33 patients of FECD were further stratified by presence of corneal edema as evidenced by slit lamp exam. . The number of patients in early stage FECD was 22 and in late stage FECD was 11, respectively. Both early stage and late stage FECD showed significant reduction in total nerve length (13,156.6±1,381.3; 8,216.6±2,573.4, respectively) and number of total nerves (9.9±1.1; 6.5±2.2), compared to controls (P<0.001 for both parameters), but the decrease was not significant between the two groups (P=0.105 and P=0.350). A similar trend was seen in the main nerve length (6,823.4±617.3; 3,856.9±1,116.0) and the number of main nerves (2.6±0.2; 1.6±0.4). The main trunks fibers were significantly different compared to controls and between the groups (P=0.016 and P=0.017), but not significant amongst the groups (P=0.056 and P=0.068). Similarly, branch density (6,333.2±839.8; 4,359.7±1,578.5) and number of nerve branches (7.4±0.9; 4.9±1.8) were decreased significantly compared to controls (P=0.032), but there was no significant difference between early and late stage FECD (P=0.59). The CCT was significantly increased in late stage FECD (627.8±23.5 μm) as compared to early stage FECD (578.6±9.7; P=0.03). The ECD was also lower in late stage FECD (1,121.2±105.0 cells/mm2) than early stage FECD (1,548.4±141.6; P=0.056). However, no significant differences were observed in the central corneal sensation among early and late stage FECD (5.06±0.3 cm; 4.7±0.4; P=0.131). The DC density was 40% higher in early stage FECD (63.8±13.7) compared to both late stage FECD (45.1±15.8; p=0.043) and 100% higher than controls (P=0.038). The clinical findings and confocal parameters of early and late stage FECD have been summarized in Table 2 and Fig. 3.
Table 2.
Clinical and confocal findings in early and late Fuchs’ endothelial corneal dystrophy.
| Early Fuchs’ Endothelial Corneal Dystrophy (n=22) |
Late Fuchs’ Endothelial Corneal Dystrophy (n=11) |
|
|---|---|---|
| Central Corneal Thickness (μm) |
578.6±9.7*† | 627.8±23.5† |
| Central Corneal Sensation (cm) |
5.±0.3 | 4.7±0.4 |
| Number of Total Nerves | 9.9±l.l† | 6.5±2.2† |
| Density of Total Nerves (μm/mm2) |
13,156.6±l,381.3† | 8,216.6±2,573.4† |
| Number of Main Nerves | 2.6±0.2† | 1.6±0.4† |
| Density of Main Nerves (μm/mm2) |
6,823.4±617.3† | 3,856.9±l,116.0† |
| Number of Nerve Branches | 7.4±0.9† | 4.9±1.8† |
| Density of Nerve Branches (μm/mm2) |
6,333.2±839.8† | 4,359.7±1,578.5† |
| Dendritic Cell Density (cells/mm2) |
63.8±13.7*† | 45.1±15.8 |
| Endothelial Cell Density (cells/mm2) |
l,548.4±141.6*† | l,121.2±105.0† |
(Mean ± Standard Error of Mean)
significant difference compared to late Fuchs’ endothelial corneal dystrophy (p<0.05);
significant difference compared to controls (p<0.05)
Figure 3. Confocal parameters in early and late stage Fuchs’ endothelial corneal dystrophy.
Reduced corneal subbasal nerve numbers (top left) and length (top right), as well as increased epithelial immune dendritic cell density (bottom left) are shown in early stage Fuchs’ endothelial corneal dystrophy (FECD) and late stage FECD. Reduced corneal sensation (bottom right) was also observed in both groups, although no statistical difference was found. *indicates statistical significance compared with normal controls; p<0.05.
DISCUSSION
IVCM has been increasingly used to study corneal dystrophies, including endothelial dystrophies.23 Since the initial IVCM study on FECD by Mustonen et al.,24 several studies have utilized various confocal microscopes to study the structural changes in the endothelium,25–27 and other corneal layers. These studies have demonstrated intraepithelial edema with bullae, subepithelial fibrosis, thickened and irregular Bowman’s layer,28,29 altered corneal nerves (including both the subbasal layer and the stroma),12,16,19,24,30 and reduced reflectivity of stromal keratocytes. These studies have confirmed previous histopathological findings, which showed that pathological changes in FECD are not limited to the endothelium alone.28,31,32 As such, several groups have previously demonstrated decreased or absent nerves in advanced FECD, and attributed nerve alterations to progressive edema.24, 16, 12 More recently, we demonstrated the decreased nerve density in early stage FECD, without corneal edema,19 findings that have now been confirmed independently by Bucher et al.30
Our current study is the first to analyze and compare subbasal nerve and immune DCs alterations, as well as corneal sensation in patients with FECD and PBK, comparing these to age-matched controls. We demonstrate decreased nerve density in FECD, with majority of nerve alterations already present in stage 1 FECD, and some additional progression with advanced disease. Further, a substantial subset of patients with early FECD demonstrated significant increase in DCs, suggesting that immune alterations may occur as part of the pathogenesis of FECD. Thus, there may be a component of immune activation or inflammation in early stages of FECD, which may influence corneal subbasal nerve density or alternatively result from loss of corneal nerves. Thus, the current study sheds light on the potential role of immune alteration in FECD and implicates potential interactions between immune cells, corneal innervation, and endothelial cell loss – which were hitherto unknown. While the difference in DC density between the FECD and PBK groups, was statistically significant, this may not be due to different underlying causes. The current study does however highlight that the immune system may play a role in patients with endothelial diseases. Nevertheless, elucidating the potential causes of DC increase and their possible effects would fall beyond the scope of this manuscript.
The diminishment of subbasal nerve plexus in FECD patients significantly correlated to decreased central corneal sensation in our patients. Similarly, Ahuja et al.16 showed qualitative morphological abnormalities of subbasal nerves and decreased sensitivity in patients with FECD. A number of studies have shown a correlation in corneal sensitivity and density of corneal subbasal nerve plexus in normal eyes33 and diseased states, such as in keratoconus,34 herpes keratitis,20,21 dry eye disease,35 after refractive surgery,36 and diabetes.37
Recently, Alomar et al.12 studied the histological and confocal microscopical changes in chronic corneal edema, including 11 patients with FECD and 5 patients with PBK. They showed that 30% of their cases had absent nerves, while the other 70% had decreased nerve density. Our findings of decreased nerve density in PBK patients in the current study and similar decreased nerve density observed by Al-Aqaba et al.38 previously, suggest that corneal edema may play a role in the reduction of corneal nerves. However, the fact that early FECD patients without corneal edema have significant loss of subbasal nerves, suggests that it is not cornea edema that results in nerve loss in patients with FECD. While there is further reduction in subbasal corneal nerves in more advanced stages of FECD, this reduction is not significant in later stages as compared to early FECD.
Strong positive correlation between the decrease in corneal nerve density and ECD suggests a potential interaction between corneal nerves and endothelium. A concomitant decrease in ECD and/or function has been reported in various conditions that lead to decreased corneal nerve density such as dry eye disease,39,40 contact lens wear41 and diabetes.42 This interaction between corneal nerves and endothelium has been the focus of recent studies. Lambiase et al.43 have shown decreased ECD in surgically induced neurotrophic keratitis. We have found a similar significant decrease in ECD in herpes simplex neurotrophic keratitis with a strong correlation between decreased nerve density and endothelial cell loss.44 These findings implicate that corneal nerves play a role in maintaining endothelial homeostasis. Koh et al.45–47 have found that autocrine signaling by the neuropeptide vasoactive intestinal peptide (VIP) is critical in regulating apoptosis and survival of endothelial cells. Moreover, in recent preclinical studies our finding of decreased VIP and subsequent endothelial loss following trigeminal axotomy and prevention of endothelial loss with exogenous VIP administration further confirm the link between corneal innervation and corneal endothelial homeostasis.48
FECD has been associated with the variation in alleles in the transcription factor 4 gene (TCF4), which encodes the transcription factor E2–2. E2–2 is expressed in the developing corneal endothelium,49 regulates ZEB1 and represses E-cadherin with loss of cell polarity, cell to cell contact, and regulation of ZEB1. Baratz et al. proposed that alteration in the activity of E2–2 and ZEB1 could lead to loss of corneal endothelial cells and extracellular matrix deposition leading to FECD, in patients with variant TCF4.49 Interestingly, E2–2 is also an essential and specific regulator in the development of plasmacytoid dendritic immune cells (pDCs).50 The shared transcription factor E2–2 between the corneal endothelium and pDCs may suggest a potential inter-dependence between the immune system and endothelial cell loss observed in the development and progression of FECD.
Collectively, our findings suggest that there may be a hitherto unknown pathogenic mechanism which leads to immune activation, decreased nerve density early in FECD, and may act independently or potentially in concert with endothelial cell loss and progressive corneal edema, leading to further decrease in nerve density during the course of disease. Based on the genetic variations, triggers for development of FECD and pathologic changes, Zhu et al. have drawn several parallels between FECD and other neurodegenerative diseases and suggest a possibility that FECD may be a neurodegenerative condition.51 We acknowledge that it is not possible to determine the exact nature and role of these interactions without further studies and more evidence, but the current data warrants additional investigations in this field.
A limitation of the present study is that a single frame captures 400 × 400 μm of the cornea, although our recent study comparing 3 representative images to composite wide field images have not shown significant differences.52 Further, contact esthesiometry is not an absolute measure of corneal sensitivity, as it only measures the mechanoreceptor function.53 The division of FECD into early and late stages was based on clinically evident edema on slit lamp examination, hence there is a possibility that cases of mild edema were mis-classified. Finally, we present the quantitative changes in the endothelium but we cannot address the endothelial functional capacity.
In conclusion, the current prospective study demonstrates a decrease in the subbasal corneal nerve density in FECD, which is present at the earliest stage of FECD. The concurrent increase in immune DC density may suggest that immune activation, and loss of nerves may potentially play a role in the pathogenesis of FECD and interact with the endothelial cell density, function and ultimately in the development of corneal edema. Further studies are needed to determine the exact nature of these seemingly interdependent pathways.
Supplementary Material
Supplemental figure 1.
In vivo confocal microscopy images in Fuchs' endothelial corneal dystrophy
Central corneal IVCM images (right, middle, and left) showing an abnormal cluster of cells in three patients of Fuchs' endothelial corneal dystrophy (FECD). The arrows show the nuclei of the cells. Size bar = 100μm.
ACKNOWLEDGEMENT:
a. Funding/Support: Funding: NIH K08-EY020575, Research to Prevent Blindness Career Development Award, Falk Medical Research Trust, New England Corneal Transplant Research Fund, MEEI Foundation. The funding organizations had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
b. Financial Disclosures: No financial disclosures for any authors
REFERENCES
- 1.EBAA 2012. Statistical Report. http://www.restoresight.org/wp-content/uploads/2014/04/2013_Statistical_Report-FINAL.pdf.
- 2.Ghosheh FR, Cremona FA, Rapuano CJ, et al. Trends in penetrating keratoplasty in the United States 1980–2005. Int Ophthalmol. 2008;28(3):147–153. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs E Dystrophia epithelias corneae. Graefes Arch Klin Exp Ophthalmol. 1910;76:478–508. [Google Scholar]
- 4.Moller HU, Weiss JS. IC3D classification of corneal dystrophies. Dev Ophthalmol. 2011;48:1–8. [DOI] [PubMed] [Google Scholar]
- 5.Elhalis H, Azizi B, Jurkunas UV. Fuchs endothelial corneal dystrophy. Ocul Surf. 2010;8(4):173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamill CE, Schmedt T, Jurkunas U. Fuchs endothelial cornea dystrophy: a review of the genetics behind disease development. Semin Ophthalmol. 2013;28(5–6):281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmedt T, Silva MM, Ziaei A, Jurkunas U. Molecular bases of corneal endothelial dystrophies. Exp Eye Res. 2012;95(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38(2):149–168. [DOI] [PubMed] [Google Scholar]
- 9.Wilson SE, Bourne WM. Fuchs’ dystrophy. Cornea. 1988;7(1):2–18. [PubMed] [Google Scholar]
- 10.Kayes J, Holmberg A. The Fine Structure of the Cornea in Fuchs’ Endothelial Dystrophy. Invest Ophthalmol. 1964;3:47–67. [PubMed] [Google Scholar]
- 11.Rodrigues MM, Krachmer JH, Hackett J, Gaskins R, Halkias A. Fuchs’ corneal dystrophy. A clinicopathologic study of the variation in corneal edema. Ophthalmology. 1986;93(6):789–796. [PubMed] [Google Scholar]
- 12.Alomar TS, Al-Aqaba M, Gray T, Lowe J, Dua HS. Histological and confocal microscopy changes in chronic corneal edema: implications for endothelial transplantation. Invest Ophthalmol Vis Sci. 2011;52(11):8193–8207. [DOI] [PubMed] [Google Scholar]
- 13.Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea - a major review. Clin Experiment Ophthalmol. 2009;37(1):100–117. [DOI] [PubMed] [Google Scholar]
- 14.Niederer RL, McGhee CN. Clinical in vivo confocal microscopy of the human cornea in health and disease. Prog Retin Eye Res. 2010;29(1):30–58. [DOI] [PubMed] [Google Scholar]
- 15.Muller LJ, Pels L, Vrensen GF. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. 1996;37(4):476–488. [PubMed] [Google Scholar]
- 16.Ahuja Y, Baratz KH, McLaren JW, Bourne WM, Patel SV. Decreased Corneal Sensitivity and Abnormal Corneal Nerves in Fuchs Endothelial Dystrophy. Cornea. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52(8):5136–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klien BA. Fuchs’ epithelial dystrophy of the cornea; a clinical and histopathologic study. Am J Ophthalmol. 1958;46(3 Part 1):297–304. [DOI] [PubMed] [Google Scholar]
- 19.Schrems-Hoesl LM, Schrems WA, Cruzat A, et al. Cellular and subbasal nerve alterations in early stage Fuchs’ endothelial corneal dystrophy: an in vivo confocal microscopy study. Eye (Lond). 2013;27(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117(10):1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamrah P, Cruzat A, Dastjerdi MH, et al. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58(2):167–176. [DOI] [PubMed] [Google Scholar]
- 23.Shukla AN, Cruzat A, Hamrah P. Confocal microscopy of corneal dystrophies. Semin Ophthalmol. 2012;27(5–6):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mustonen RK, McDonald MB, Srivannaboon S, Tan AL, Doubrava MW, Kim CK. In vivo confocal microscopy of Fuchs’ endothelial dystrophy. Cornea. 1998;17(5):493–503. [DOI] [PubMed] [Google Scholar]
- 25.Chiou AG, Kaufman SC, Beuerman RW, Ohta T, Soliman H, Kaufman HE. Confocal microscopy in cornea guttata and Fuchs’ endothelial dystrophy. Br J Ophthalmol. 1999;83(2):185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hara M, Morishige N, Chikama T, Nishida T. Comparison of confocal biomicroscopy and noncontact specular microscopy for evaluation of the corneal endothelium. Cornea. 2003;22(6):512–515. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman SC, Beuerman RW, Kaufman HE. Diagnosis of advanced Fuchs’ endothelial dystrophy with the confocal microscope. Am J Ophthalmol. 1993;116(5):652–653. [DOI] [PubMed] [Google Scholar]
- 28.Alomar TS, Nubile M, Lowe J, Dua HS. Corneal intraepithelial neoplasia: in vivo confocal microscopic study with histopathologic correlation. Am J Ophthalmol. 2011;151(2):238–247. [DOI] [PubMed] [Google Scholar]
- 29.Amin SR, Baratz KH, McLaren JW, Patel SV. Corneal Abnormalities Early in the Course of Fuchs’ Endothelial Dystrophy. Ophthalmology. 2014;121(12):2325–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucher F, Adler W, Lehmann HC, et al. Corneal nerve alterations in different stages of Fuchs’ endothelial corneal dystrophy: an in vivo confocal microscopy study. Graefes Arch Clin Exp Ophthalmol. 2014;252(7):1119–1126. [DOI] [PubMed] [Google Scholar]
- 31.Hecker LA, McLaren JW, Bachman LA, Patel SV. Anterior keratocyte depletion in fuchs endothelial dystrophy. Arch Ophthalmol. 2011;129(5):555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li QJ, Ashraf MF, Shen DF, et al. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Arch Ophthalmol. 2001;119(11):1597–1604. [DOI] [PubMed] [Google Scholar]
- 33.Patel DV, Tavakoli M, Craig JP, Efron N, McGhee CN. Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea. 2009;28(7):735–740. [DOI] [PubMed] [Google Scholar]
- 34.Patel DV, Ku JY, Johnson R, McGhee CN. Laser scanning in vivo confocal microscopy and quantitative aesthesiometry reveal decreased corneal innervation and sensation in keratoconus. Eye (Lond). 2009;23(3):586–592. [DOI] [PubMed] [Google Scholar]
- 35.Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48(1):173–181. [DOI] [PubMed] [Google Scholar]
- 36.Stachs O, Zhivov A, Kraak R, Hovakimyan M, Wree A, Guthoff R. Structural-functional correlations of corneal innervation after LASIK and penetrating keratoplasty. J Refract Surg. 2010;26(3):159–167. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg ME, Tervo TM, Immonen IJ, Muller LJ, Gronhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41(10):2915–2921. [PubMed] [Google Scholar]
- 38.Al-Aqaba M, Alomar T, Lowe J, Dua HS. Corneal nerve aberrations in bullous keratopathy. Am J Ophthalmol. 2011;151(5):840–849 e841. [DOI] [PubMed] [Google Scholar]
- 39.Kheirkhah A, Saboo US, Abud TB, et al. Reduced Corneal Endothelial Cell Density in Patients With Dry Eye Disease. Am J Ophthalmol. 2015;159(6):1022–1026 e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol. 2012;27(5–6):138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel SV, McLaren JW, Hodge DO, Bourne WM. Confocal microscopy in vivo in corneas of long-term contact lens wearers. Invest Ophthalmol Vis Sci. 2002;43(4):995–1003. [PubMed] [Google Scholar]
- 42.Sudhir RR, Raman R, Sharma T. Changes in the corneal endothelial cell density and morphology in patients with type 2 diabetes mellitus: a population-based study, Sankara Nethralaya Diabetic Retinopathy and Molecular Genetics Study (SN-DREAMS, Report 23). Cornea. 2012;31(10):1119–1122. [DOI] [PubMed] [Google Scholar]
- 43.Lambiase A, Sacchetti M, Mastropasqua A, Bonini S. Corneal changes in neurosurgically induced neurotrophic keratitis. JAMA Ophthalmol. 2013;131(12):1547–1553. [DOI] [PubMed] [Google Scholar]
- 44.Muller RT, Pourmirzaie R, Pavan-Langston D, et al. In Vivo Confocal Microscopy Demonstrates Bilateral Loss of Endothelial Cells in Unilateral Herpes Simplex Keratitis. Invest Ophthalmol Vis Sci. 2015;56(8):4899–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koh SW. Corneal endothelial autocrine trophic factor VIP in a mechanism-based strategy to enhance human donor cornea preservation for transplantation. Exp Eye Res. 2012;95(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koh SW, Chandrasekara K, Abbondandolo CJ, Coll TJ, Rutzen AR. VIP and VIP gene silencing modulation of differentiation marker N-cadherin and cell shape of corneal endothelium in human corneas ex vivo. Invest Ophthalmol Vis Sci. 2008;49(8):3491–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh SW, Guo Y, Bernstein SL, Waschek JA, Liu X, Symes AJ. Vasoactive intestinal peptide induction by ciliary neurotrophic factor in donor human corneal endothelium in situ. Neurosci Lett. 2007;423(2):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi T, Ziaei A, Cavalcanti BM, Harris D, Andrian U, Jurkunas UV et al. Neurogenic Homeostasis of Corneal Endothelial Cells: Peripheral Innervation Maintains Endothelial Cell Survival Through Vasoactive Intestinal Peptide. [ARVO abstract 2077] Invest Ophthalmol Vis Sci 2014;55: [Google Scholar]
- 49.Baratz KH, Tosakulwong N, Ryu E, et al. E2–2 protein and Fuchs’s corneal dystrophy. N Engl J Med. 2010;363(11):1016–1024. [DOI] [PubMed] [Google Scholar]
- 50.Cisse B, Caton ML, Lehner M, et al. Transcription factor E2–2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu AY, Eberhart CG, Jun AS. Fuchs endothelial corneal dystrophy: a neurodegenerative disorder? JAMA Ophthalmol. 2014;132(4):377–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kheirkhah A, Muller R, Mikolajczak J, et al. Comparison of Standard Versus Wide-Field Composite Images of the Corneal Subbasal Layer by In Vivo Confocal Microscopy. Invest Ophthalmol Vis Sci. 2015;56(10):5801–5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golebiowski B, Papas E, Stapleton F. Assessing the sensory function of the ocular surface: implications of use of a non-contact air jet aesthesiometer versus the Cochet-Bonnet aesthesiometer. Exp Eye Res. 2011;92(5):408–413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1.
In vivo confocal microscopy images in Fuchs' endothelial corneal dystrophy
Central corneal IVCM images (right, middle, and left) showing an abnormal cluster of cells in three patients of Fuchs' endothelial corneal dystrophy (FECD). The arrows show the nuclei of the cells. Size bar = 100μm.