Abstract
Auricular therapy (AT) is a conventional therapy in traditional Chinese medicine. However, the effectiveness of perioperative AT in pain treatment after total hip arthroplasty (THA) is still controversial. Nine randomised controlled trials (RCTs) involving 605 patients who have undergone THA with or without AT from inception to March 2018 were collected and included in this study by searching more than 12 databases (e.g., PubMed, Excerpta Medica Database, and Cochrane Library). A random-effects model that pooled seven articles showed that the visual analogue scale (VAS) in the AT group was lower than that of the control group at each postoperative time point in patients after THA, except at the time points of 6 and 36 h. The intraoperative body mass-adjusted fentanyl amount in the AT group was also lower than that of the control group in two trials. The other outcomes (time to first analgesic request and incidence of postoperative nausea and vomiting, perioperative bradycardia, and transitory hypotension) showed insignificant difference. Then, subgroup analysis showed similar results to those of the total articles with the term “VAS”. Regression analysis found that the prolonged time after the operation decreased the difference in VAS between the two groups. Although all the outcomes were assessed as very low to low in the GRADE system, evidence on the effectiveness of perioperative AT in pain treatment after total hip replacement was positive.
1. Introduction
For patients who have hip joint disease, total hip arthroplasty (THA) is one of the most successful treatments for end-stage osteoarthritis and other hip diseases [1]. Generally, THA is an effective approach to relieve pain and improve function and quality of life for various hip diseases [2, 3]. Over the past decade, the incidence of THA has increased both in elderly and young individuals, and this number is estimated to reach 572,000 cases by 2030 [4, 5]. The treatments of THA can cause severe perioperative pain in patients [6]. Almost all postoperative pain is caused by tissue damage at the site of the operation. Postoperative wound pain is the most serious problem after surgery [7, 8]. In an early review, the risk of persistent pain after surgery has been described [9]. One out of three patients with THA experience moderate to severe pain during movement. Hence, medical workers and patients face the challenge of postoperative pain [10]. Postoperative pain not only hinders new exercise and rehabilitation but also affects overall recovery [11]. Acute postoperative pain management via opioid analgesics was also lower than the optimal for patients with total joint replacement because side effects, such as nausea, vomiting and, in particular, sedation, can interfere with rehabilitation [12, 13].
Complementary and alternative medicine is readily accepted by both developing and developed countries, where it is increasingly regarded as a substitute for conventional therapies and recommended to reduce the postoperative pain [14–16]. AT is defined as a method of acupuncture wherein the external surface of the ear or auricle is stimulated to alleviate pathological conditions in other parts of the body [17]. In the future, AT may become a type of therapy in multimodal pain management protocols. AT that is generally carried out by medical professionals in some Eastern countries is easy to operate, economical and a safe therapy for patients. In 1982, the World Health Organisation set up a working group to standardise the research and clinical applications of AT [18]. Auricular acupuncture and homoeopathic arnica have clinically desirable effects of decreasing analgesic administration and postoperative swelling [19].
A systematic review has indicated that acupuncture may be a feasible adjuvant therapy for pain after total hip or knee arthroplasty [20] because it can relieve postoperative pain and reduce the dosage of opioid analgesics and other related side effects [21]. However, the previous study did not provide strong evidence to prove that AT is an effective analgesic method in relieving postoperative pain [22]. Our meta-analysis may provide an objective theoretical basis for clinical decision-making to evaluate the clinical efficacy of AT on postoperative pain for patients after THA.
2. Materials and Methods
2.1. Search Methods
Electronic searching and citation snowballing were both used to locate relevant studies. A total of 12 electronic databases, including PubMed, Excerpta Medica Database (Embase), Cochrane Central Register of Controlled Trials, Web of Science, Science Direct, PsycINFO, Cumulative Index to Nursing and Allied Health Literature, Allied and Complementary Medicine, China National Knowledge Infrastructure, Chinese Biomedical Literature Database (CBM), WanFang, and Chinese Scientific Journal Database, were searched for relevant studies that were conducted from inception to March 2018. The search strategy of all available databases was determined by the principle of “PICOS” and its details were presented in the Supplemental Materials (available here). For the related inventions and patients, we used “auriculotherapy”, “acupuncture”, “ear”, “arthroplasty”, “replacement”, “hip”, and “total hip arthroplasty” as MeSH terms. For the outcomes, “pain” and “postoperative” were the MeSH terms we used.
2.2. Inclusion and Exclusion Criteria
Studies were considered eligible if they met the following criteria: (1) randomised controlled trials (RCTs) as the design type of studies; (2) patients who underwent THA regardless of gender and disease types; (3) the intervention treatment limited to AT (AT includes auricular acupuncture, auricular point buried-bean, auricular massage, auricular magnetic therapy, and auricular moxibustion); patients undergoing AT with or without conventional treatments can be regarded as the same type in our meta-analysis; (4) patients who underwent conventional treatment with or without sham–AT regarded as the control group. Clinical animal trials, case reports, and nonrandomised controlled trials were excluded.
2.3. Data Extraction and Quality Assessment
Two reviewers (Ye XX and Gao YZ) independently screened the titles and abstracts of all articles and excluded those unrelated to the specified selection criteria. The data were extracted independently into a sheet that included a prespecified set of variables (articles' general information, including author names; publication year and country; patient characteristics, such as sample size, mean age, and disease types; interventions groups; main outcomes with VAS; and any other relevant findings). Data were also extracted from any author in the collaboration group. The Cochrane Collaboration Handbook Tool for the systematic reviews of interventions was used to estimate the risk of bias for each article, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting and other biases [32]. Any disagreement between the investigators was resolved by obtaining a consensus among the full review team.
2.4. Process of Auricular Therapy
The steps for the AT process or APBB by the acupuncturist are as follows: (1) selecting the specific auricular acupoints, (2) sterilising the auricular acupoints, (3) embedding the needles or vaccaria seeds (a kind of plant seed or bean) in the auricular acupoints, (4) fixing the needles or vaccaria seeds with medical adhesive tape, and (5) pressing the auricular acupoints according to patients' needs. Last, the retention time of needles or vaccaria seeds would keep in one to seven days in the process of the auricular therapy [24, 25].
2.5. Types of Outcome Measures
The primary outcome measured by VAS in our meta-analysis was postoperative pain. The secondary outcomes were body mass-adjusted fentanyl amount (μg/kg) in intraoperative time, postoperative time to first analgesic request (min) and perioperative complications (postoperative nausea and vomiting (PONV), perioperative transitory hypotension and bradycardia).
2.6. Statistical Analysis
The heterogeneity of the included studies was assessed using Q statistics and I2 index according to the suggestions of the Cochrane Collaboration. P < 0.05 with I2 index > 50% was considered to show significant heterogeneity. The estimates (standardised mean difference (SMD) or odds ratio (OR)) with 95% confidence interval (CI) were pooled with a fixed-effects model if the heterogeneity was significant. Otherwise, the estimates were pooled with a random-effects model that accounted for both within- and between-study variability. We also conducted subgroup analysis and metaregression by using the variables time group, GRADE quality, control intervention type in the control group, starting point of treatment, and other related variables to assess the impacts on outcomes. All analyses were performed using RevMan version 5.3 from the Cochrane website or STATA version 14.0 (StataCorp, College Station, Texas). P < 0.05 was considered statistically significant except otherwise specified.
2.7. GRADE Quality of Metaevidence
GRADE guidance tools, including nine RCTs, were used to assess the quality of evidence for the metaresults. The GRADE framework characterises the quality of evidence on the basis of study risk of bias, publication bias, imprecision, inconsistency, and study indirectness with the levels of high, moderate, low, and extremely low for each outcome.
3. Results
3.1. Literature Search
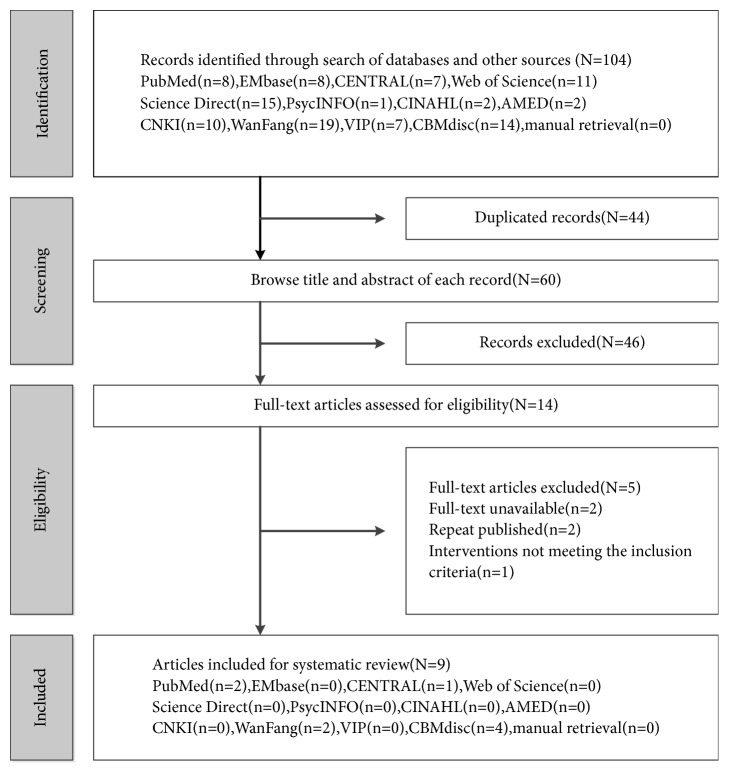
A total of 104 relevant citations were identified from the database search, and 46 potentially eligible articles were retrieved for full-text review. A total of 12 articles were excluded because of repeated publication (n = 2), absence of comparison among treatments (n = 1), and unavailability of full text (n = 2). Out of the remaining 9 RCTs, 44.4% (4/9), 22.2% (2/9), 22.2% (2/9), and 11.1%(1/9) of the articles were obtained from CBM, WANGFANG, PubMed, and Cochrane Library, respectively. Figure 1 shows the flowchart illustrating the details of the search results.
Figure 1.
Flowchart of study selection. CENTRAL: Cochrane Central Register of Controlled Trials, CNKI: China National Knowledge Infrastructure, VIP: Chinese Scientific Journal Database, and CBM: Chinese Biomedical Literature Database.
3.2. Study Characteristics and Quality Assessment
A total of 605 patients from 9 articles with a wide sample size (range of 30–116, mean of 67) were enrolled in our meta-analysis. Most of them were strictly RCTs. A total of 33.3% (3/9) and 66.7% (6/9) of the articles were published in Germany [23–25] and China [26–31], respectively. The VAS, intraoperative application amount of fentanyl, time to the first analgesic request, nausea and vomiting, perioperative bradycardia, perioperative hypotension from 77.8% (7/9) [24, 26–31], 22.2% (2/7) [23, 25], 33.3% (3/9) [23, 24, 30], 33.3% (3/9) [23, 24, 29], 11.1% (1/9) and 11.1% (1/9) of articles [23, 25] were the outcomes in our meta-analysis, respectively. The details are shown in Table 1. On top of that, auricular acupuncture and other auricular point buried-bean were used in 33.3% (3/9) [23–25] and 66.7% (6/9) [26–31] of the articles, respectively. Intraoperative general anaesthesia (GA) was performed in 66.7%(6/9) of the known recorded articles. However, three other articles did not describe any type of anaesthesia. A total of 55.6% (5/9) and 44.4% (2/9) of the articles used preoperative and postoperative AT, respectively. Sham acupuncture (SA) also was found in 44.5%(4/9) of articles. For the conventional treatments, patient-controlled analgesia (PCA) and rehabilitation exercises were performed in most of the nine RCTs. What is more, nonsteroidal anti-inflammatory drugs such as ibuprofen and celecoxib were used in two articles of them. For the details of the treatments of nine RCTs, see Table 2.
Table 1.
The baseline characteristics of included trials.
| First Author, Year, Setting | Study Design | Participants (n) | Age, Mean(years) | Variety of Disease(n) | Main Outcomes |
|---|---|---|---|---|---|
| Sa1Wetzel, 2011, Germany [23] | Prospective randomized patient-,anesthesiologist-, evaluator-,analyst-blinded, sham-controlled study | Randomized =120; Completed = 116; AT=57; CON= 59; |
AT=66(10); CON=67(8) |
Degenerative osteoarthritis(116) | Fentanyl amount, adjusted to body mass (mg/kg); Nausea and vomiting; Bradycardia; Transitory hypotension; Time to first analgesic request |
|
| |||||
| S2Usichenko, 2005 Germany [24] | Prospective randomized patient-, anesthesiologist-, evaluator-,analyst-blinded, sham-controlled study | Randomized= 61; Complete =54; AT= 29; CON= 25; |
AT=68(10); CON =66(11) |
Degenerative osteoarthritis(54) | VAS; Nausea and vomiting; Time to first analgesic request |
|
| |||||
| S3Usichenko, 2006, Germany [25] | Prospective randomized patient-, anesthesiologist-, evaluator-, analyst- blinded, sham-controlled study | Randomized = 64; Completed = 57 AT= 30; CON= 27; |
AT=68(9); CON =67(9) |
Degenerative osteoarthritis(57) | Fentanyl amount, adjusted to body mass (mg/kg); Bradycardia Transitory hypotension |
|
| |||||
| S4Lv, 2017, China [26] | Randomized controlled trial | Randomized = 98; Completed = 98; AT= 49; CON= 49; |
AT=59.8(8.6); CON=59.5(8.7) |
Unilateral femoral intertrochanteric fracture or femoral neck fracture(98) | VAS |
|
| |||||
| S5Kong, 2010, China [27] | Randomized controlled trial | Randomized = 60; Completed = 60 AT+C= 30; CON= 30; |
NR | NR | VAS |
|
| |||||
| S6Cui, 2016, China [28] | Randomized controlled trial | Randomized = 30; Completed = 30; AT = 15; CON= 15 |
AT=71(7.8); CON=69(9) |
Avascular necrosis of the femoral head(16); Femoral intertrochanteric fracture(5); Femoral neck fracture(9) |
VAS |
|
| |||||
| S7Xu, 2014, China [29] | Prospective, randomized, sham-controlled trial | Randomized = 38; Completed = 38 AT= 19; CON=19; |
AT=60.7(8.8); CON =59.3(7.7) |
Femoral head necrosis(38) | VAS; Nausea and vomiting |
|
| |||||
| S8Shen, 2017, China [30] | Randomized controlled trial | Randomized = 80; Completed = 80; AT= 40; CON= 40 |
AT=65.8(4.6); CON=66.2,(4.7) |
Femoral neck fractures(25); old Femoral neck fractures(5); Femoral head necrosis(24); Hip arthritis(17 ); Rheumatoid arthritis(7); Ankylosing spondylitis (2) |
VAS; Time to first analgesic request |
|
| |||||
| S9Tian, 2016, China [31] | Randomized controlled trial | Randomized = 72;Completed = 72; AT = 36; CON=36 | NR | Ankylosing spondylitis(1); femoral head necrosis(43); femoral neck fracture( 28) |
VAS |
Abbreviations: AT=auricular therapy; CON=control group; GA= general anesthesia; AA=auricular acupuncture; APBB=auricular point buried-bean; PCA=patient controlled analgesia; NR=not reported; VAS=visual analogue scale; NR: not reported.
Table 2.
The detailed treatments in both groups of included trials.
| First Author, Year, Setting | Intraoperative Anesthesia | Starting Time of AT | AT Group | Control Group | Details of Sham Acupuncture | Preoperative or Postoperative Analgesics |
Details of Conventional Treatments | |
|---|---|---|---|---|---|---|---|---|
| NSAIDs | Narcotics | |||||||
| Sa1Wetzel, 2011, Germany [23] | GA | Preoperative | AA+CT | SA+CT | Three nonacupuncture points | Yes, but no name | NR | Preoperative medication such as NSAIDs, Anticonvulsants, Beta-blocking agents and Antihypertensive agents |
|
| ||||||||
| S2Usichenko, 2005 Germany [24] | GA | Preoperative | AA+CT | SA + CT | Four nonacupuncture points | Ibuprofen | PCA pump | PCA pump with piritramide in 48h; Oral Ibuprofen with 400–800 mg twice a day in 36 hours after the operation |
|
| ||||||||
| S3Usichenko, 2006, Germany [25] | GA | Preoperative | AA+CT | SA+CT | Four nonacupuncture points | NR | NR | NR |
|
| ||||||||
| S4Lv, 2017, China [26] | NR | Preoperative | APBB+ CT | CT | NR | NR | Opioid analgesics | Postoperative per two hours for the assessment; Opioid analgesics for those sever pain patients; Ice bag cold compress in pain area; other rehabilitation exercise |
|
| ||||||||
| S6Cui, 2016, China [28] | NR | Postoperative | APBB+ CT | CT | NR | NR | PCA pump | PCA pump in 48 hours; Rehabilitation exercise begin 6 hours after operation |
|
| ||||||||
| S5Kong, 2010, China [27] | NR | Postoperative | APBB+ CT | CT | NR | NR | PCA pump | PCA pump with fentanyl and Lappaconitine in 48 hours; Rehabilitation exercise: Isometric contraction training of quadriceps femoris after 6 hours and Isometric contraction training of gluteus maximus and gluteus medius after 24 hours; Active motion of knee joint after 2 or 3 days |
|
| ||||||||
| S7Xu, 2014, China [29] | GA ∗ | Preoperative | APBB+ CT | SA + CT | Nonacupuncture points | Celecoxib | PCA pump | PCA pump with Fentanyl, Tramadol, Tropisetron in 48 hours; Celecoxib 200mg was given 3 days before operation, per 12 hours |
|
| ||||||||
| S8Shen,2017, China [30] | GA | Preoperative | APBB+ CT | CT | NR | NR | PCA pump | PCA pump with sufentanil and tropisetron in 24 hours; health education |
|
| ||||||||
| S9Tian, 2016,China [31] | GA ∗ | Postoperative | APBB+ CT | CT | NR | NR | PCA pump | PCA pump and rehabilitation guidance for every patients |
Abbreviations: AT=auricular therapy; PCA=patient-controlled analgesia; GA= general anesthesia AA=auricular acupuncture; APBB=auricular point buried-bean; SA=sham acupuncture; CT= conventional treatments; RCT=randomized controlled trial; NSAIDs = nonsteroidal anti-inflammatory drugs; NR= not reported. ∗information from the authors' email.
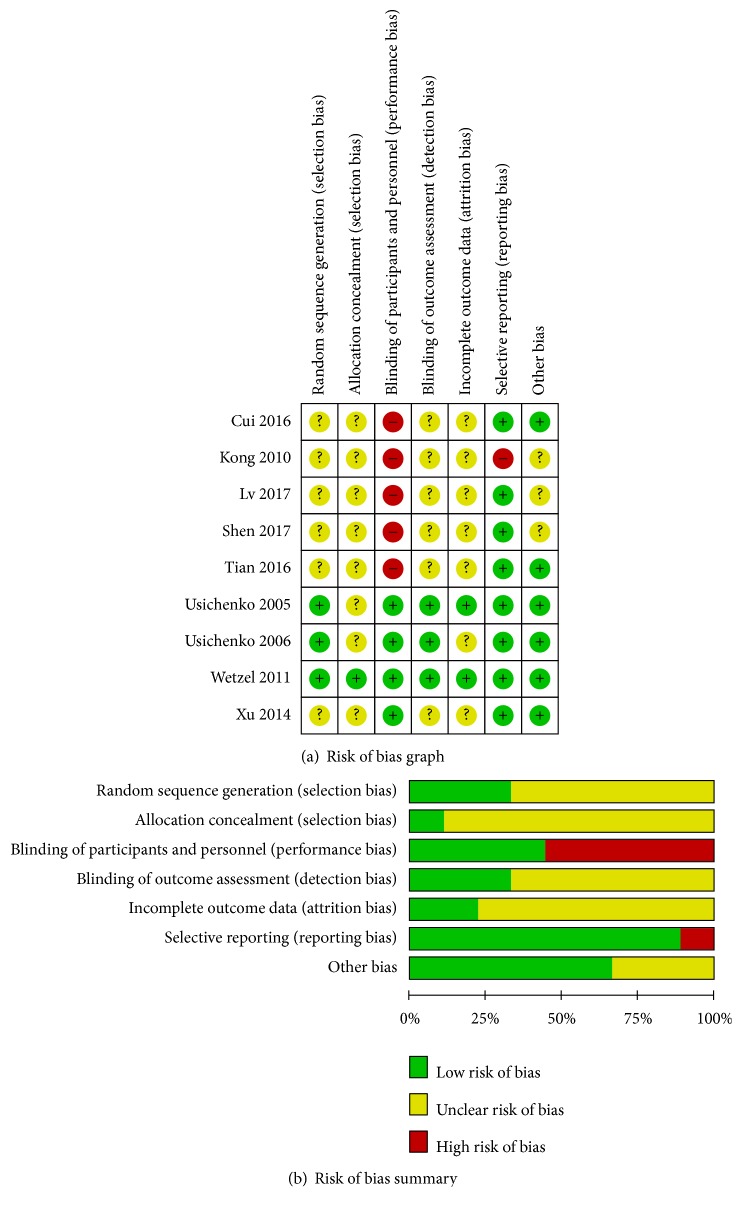
The methodological quality and risk of bias for the included studies are shown in Figure 2. Among the nine RCTs, three used random number method [23–25], one exhibited appropriate allocation concealment [23], four performed participant and personnel blinding [23–25, 29], two showed incomplete outcome data [23, 24], and eight displayed selective reporting [23–26, 28–31]. The total quality of each included article was generally assessed from A to C.
Figure 2.
Risk of bias graph and summary of the included 9 RCTs.
3.3. Meta-Analysis Results
3.3.1. Postoperative VAS
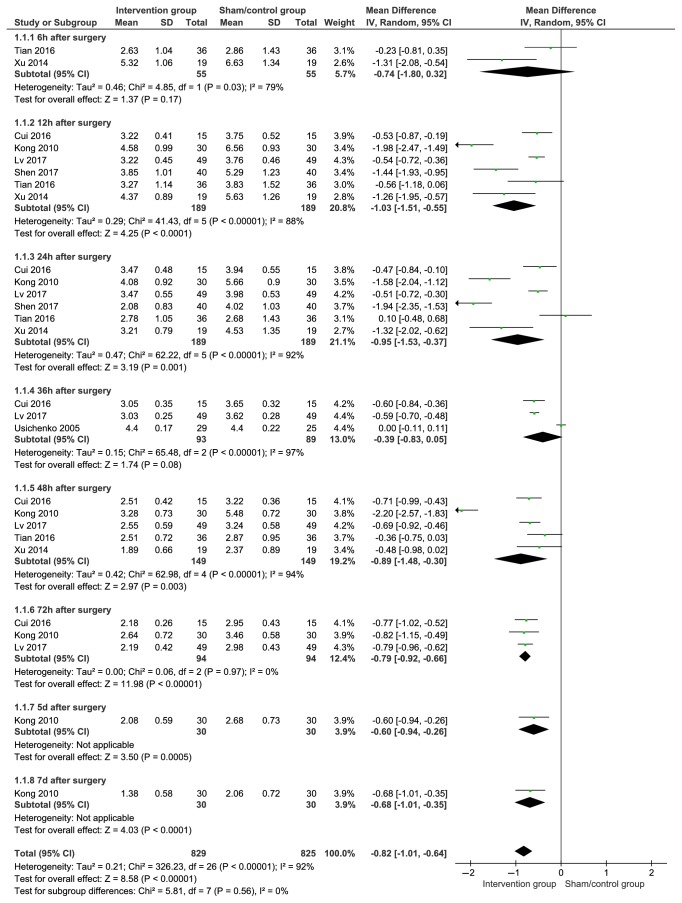
VAS was used at different time points (postoperative 6 h, 12 h, 24 h, 36 h, 48 h, 72 h, 5 and 7 days) on patients after hip arthroplasty in the total of seven articles. The results were pooled using a randomised effect model because of the high heterogeneity. On the subgroup analysis, the observation time points of postoperative 12 h (SMD with 95%CI=−1.03(−1.51,−0.55), P<0.001), postoperative 24 h (SMD with 95%CI=−0.95 (−1.53, −0.37), P=0.001), P=0.08), postoperative 48 h (SMD with 95%CI=−0.89 (−1.48, −0.30), P=0.003), postoperative 72 h (SMD with 95%CI=−0.79 (−0.92, −0.66), P<0.001), postoperative 5 days (SMD with 95%CI=−0.60 (−0.94, −0.26), P<0.001), and postoperative 7 days (SMD with 95%CI=−0.68 (−1.01, −0.35), P<0.001) were found such that the pooled results of VAS of the AT group were lower than that of the control group, but not the time points of postoperative 6 h (SMD with 95%CI=−0.74 (−1.80, 0.32), P=0.17) and postoperative 36 h (SMD with 95%CI=−0.39 (−0.83, 0.05). The details are shown in Figure 3.
Figure 3.
Pooled VAS pain score results in patients after auricular therapy and total hip arthroplasty.
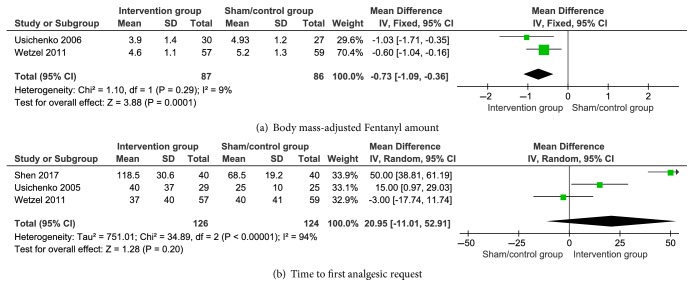
3.3.2. Body Mass-Adjusted Fentanyl Amount and Time to First Analgesic Request
Body mass-adjusted fentanyl amount was pooled using a fixed-effects model with low heterogeneity (P = 0.29, I2 = 9%). Figure 4 shows that the experimental group had lower values than the control group (SMD with 95% CI = −0.73 (−1.09, −0.36), P = 0.0001). The time to first analgesic request also showed insignificant difference between the two groups (SMD with 95% CI = 20.95 (−11.01, 52.91), P = 0.20). The details are shown in Figure 4.
Figure 4.
Pooled body mass-adjusted Fentanyl amount and time to first analgesic request results in the patients after auricular therapy and total hip arthroplasty.
3.3.3. Perioperative Complications
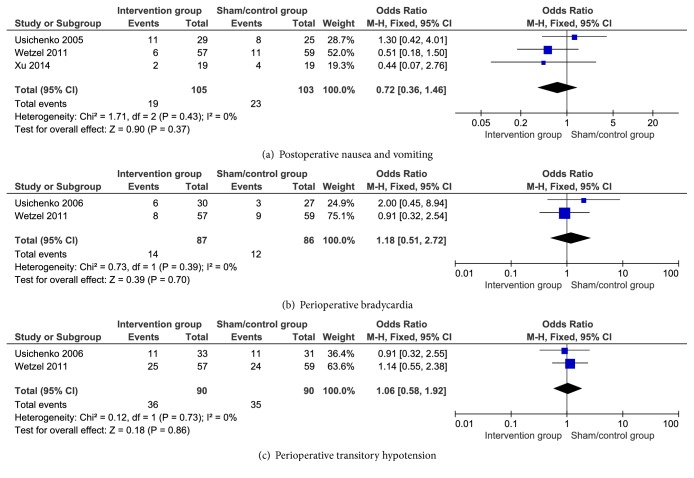
The incidences of PONV (OR, 95% CI = 0.72 (0.36, 1.46), P=0.37), perioperative bradycardia (OR, 95%CI=1.18(0.51, 2.72), P=0.70), and perioperative transitory hypotension (OR, 95% CI =1.06 (0.58, 1.92), P=0.86) that were pooled using a fixed-effects model with low heterogeneity (all I2 = 0%) showed insignificant difference between the two groups. The details are shown in Figure 5.
Figure 5.
Postoperative nausea and vomiting, perioperative transitory hypotension, and bradycardia results in the patients after auricular therapy and total hip arthroplasty.
3.4. Subgroup and Regression Analysis
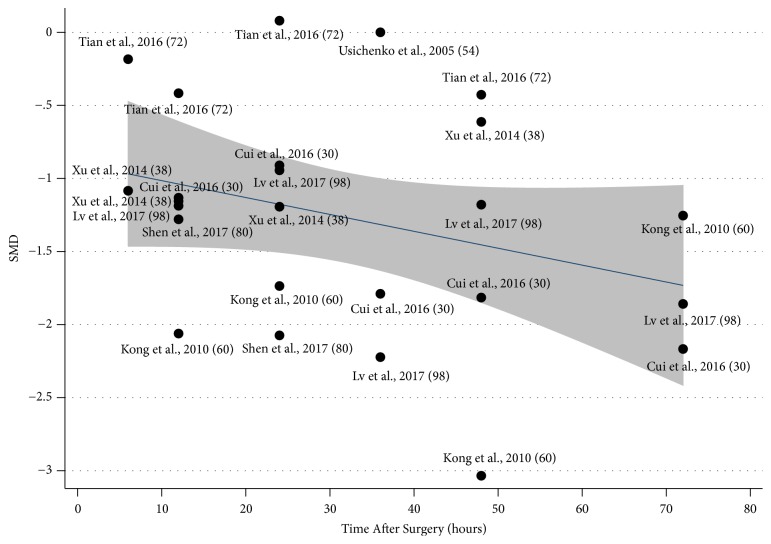
Most results of subgroup analysis in the different variables such as the threshold of observation time (<24h, SMD95%CI= -1.076(-1.426, -0.726), P<0.001; >24h, SMD95%CI=-1.375(-1.813, -0.938), P<0.001); <=48h, SMD95%CI=-1.174(-1.496, -0.853), P<0.001; >48h, SMD95%CI -1.394(-1.832,-0.955), P=0.021), GRADE quality (Grade C, SMD95%CI= -1.311(-1.62, -1.001), P<0.001), the type of intraoperative anaesthesia (intraoperative GA SMD95%CI= -1.111(-1.479,-0.743), P<0.001), the type of control treatment (SA+CT type SMD95%CI= -1.311(-1.620,-1.001), P<0.001; Just CT type, SMD95%CI=-0.780(-1.270, -0.290), P=0.022), Intraoperative General Analgesics (General NASIDs, SMD95%CI= -0.772(-0.172,-0.292), P=0.001; No general NASIDs, SMD95%CI=-1.217(-1.492,-0.941), P=0.002) and the starting time of AT (Preoperative, SMD95%CI= -1.241(-1.071,-1.207), P<0.001; Postoperative, SMD95%CI= -1.114(-1.221, -1.008), P<0.001) were consistent with the original results with full articles (total, SMD95%CI=-0.82(-1.012,-0.642), P<0.001), except in B-GRADE articles (SMD95%CI= -1.000 (-1.339,-0.660), P=0.591). The details are shown in Table 3. In addition, the trend of SMD with long time after THA declined was calculated by regression analysis without statistical significance (P=0.108), as shown in Figure 6.
Table 3.
Subgroup analysis for the VAS in the patients after THA by Random-Effect Model.
| Variables | Number of points# | Pooled SMD | 95%CI | P-value | I-squared | Tau-squared |
|---|---|---|---|---|---|---|
| Total | 27 | -0.82 | (-1.012,-0.642) | <0.001 | 92.30% | 0.2100 |
| the threshold of Observation Time | ||||||
| 24 hours | ||||||
| <24 hours | 14 | -1.076 | (-1.426,-0.726) | <0.001 | 82.30% | 0.3582 |
| >=24 hours | 13 | -1.375 | (-1.813,-0.938) | <0.001 | 86.40% | 0.5446 |
| 48 hours | ||||||
| <=48 hours | 22 | -1.174 | ( -1.496, -0.853) | <0.001 | 86.10% | 0.4969 |
| >48 hours | 5 | -1.394 | (-1.832,-0.955) | 0.021 | 65.30% | 0.1593 |
| the Grade of quality | ||||||
| A | 1 | 0.000 | (-0.535,0.535 ) | - | - | - |
| B | 4 | -1.000 | (-1.339,-0.660 ) | 0.591 | 0% | 0.0000 |
| C | 22 | -1.311 | (-1.62,-1.001) | <0.001 | 85.70% | 0.4583 |
| the type of Intraoperative anesthesia | ||||||
| GA | 18 | -1.111 | (-1.479,-0.743) | <0.001 | 86.7% | 0.5421 |
| Not reported | 9 | -1.436 | (-1.492,-0.941) | 0.002 | 66.7% | 0.1567 |
| the type of control treatment | ||||||
| SA +CT | 5 | -1.311 | (-1.620,-1.001) | <0.001 | 85.70% | 0.4583 |
| Just CT | 22 | -0.780 | (-1.270,-0.290) | 0.022 | 65.10% | 0.2022 |
| General Analgesics (NASIDs) | ||||||
| Yes | 5 | -0.772 | (-0.172,-0.292) | 0.001 | 65.8% | 0.2045 |
| No | 22 | -1.217 | (-1.492,-0.941) | 0.002 | 85.2% | 0.4583 |
| the starting time of AT | ||||||
| Preoperative | 14 | -1.241 | (-1.071,-1.207 ) | <0.001 | 86.2% | 0.2922 |
| Postoperative | 13 | -1.114 | (-1.221,-1.008) | <0.001 | 83.5% | 0.5877 |
#: The number of points was including the different observation time for the patients after THA. SA=sham acupuncture; CT= conventional treatments; GA= general anesthesia; SMD= standardised mean difference; CI= confidence interval.
Figure 6.
Metaregression for the VAS pain score in the patients after auricular therapy and total hip arthroplasty (P=0.108).
3.5. GRADE Quality of the Main Outcomes
The GRADE tool was used to evaluate the evidence in the results that showed extremely low to low values for each main outcome. The details of this meta-analysis in terms of evidence quality are presented in Table 4.
Table 4.
The GRADE tool for the pooled results of different period in the patients after total hip arthroplasty.
| Outcomes | No. of studies | Quality assessment | Summary of results | Importance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | No. of patients | 95%CI | Quality | ||||
| Auricular therapy | Control | ||||||||||
| Postoperative-results | |||||||||||
| Pain intensity (VAS-10) | |||||||||||
| At 6h | 2 | Serious(1) | Serious(2) | no | very serious(4)(5) | no | 55 | 55 | SMD -0.74 (-1.80,0.32) | VERY LOW | CRITICAL |
| At 12h | 6 | serious(1) | serious(2) | no | serious(4) | no | 189 | 189 | SMD -1.03 (-1.51,-0.55) | VERY LOW | CRITICAL |
| At 24h | 6 | serious(1) | serious(2) | no | Serious(4) | no | 189 | 189 | SMD -0.95 (-1.53,-0.37) | VERY LOW | CRITICAL |
| At 36h | 3 | serious(1) | serious(2) | no | very serious(4)(5) | no | 93 | 89 | SMD -0.39 (-0.83,0.05) | VERY LOW | CRITICAL |
| At 48h | 5 | serious(1) | serious(2) | no | serious(4) | no | 149 | 149 | SMD -0.89 (-1.48,-0.30) | VERY LOW | CRITICAL |
| At 72h | 3 | serious(1) | no | no | serious(4) | no | 94 | 94 | SMD -0.79 (-0.92,-0.66) | LOW | CRITICAL |
| At 5d | 1 | serious(1) | no | no | serious(4) | no | 30 | 30 | SMD -0.60 (-0.94,-0.26) | LOW | CRITICAL |
| At 7d | 1 | serious(1) | no | no | serious(4) | no | 30 | 30 | SMD -0.68 (-1.01,-0.35) | LOW | CRITICAL |
| Time to first analgesic request (min) | 3 | no | serious(2) | serious(3) | very serious(4)(5) | no | 126 | 124 | SMD20.95. (-11.01,52.9) | VERY LOW | IMPORTANT |
| Intraoperative results | |||||||||||
| Fentanyl amount, adjusted to body mass (μg/kg) | 2 | no | no | serious(3) | serious(4) | no | 87 | 86 | SMD -0.73 (-1.09,-0.36) | LOW | IMPORTANT |
| Perioperative complications | |||||||||||
| Nausea and vomiting | 3 | no | no | serious(3) | very serious(4)(5) | no | 19/105 | 23/103 | OR 0.72 (0.36,1.46) | VERY LOW | IMPORTANT |
| Bradycardia | 2 | no | no | serious(3) | very serious(4)(5) | no | 14/87 | 12/86 | OR 1.18 (0.51,2.72) | VERY LOW | IMPORTANT |
| Transitory hypotension | 2 | no | no | serious(3) | very serious(4)(5) | no | 36/90 | 35/90 | OR 1.06 (0.58,1.92) | VERY LOW | IMPORTANT |
Note: (1) allocation sequence concealment and blinding are missing, (2) I2>50%, P<0.1, (3) indirectness, (4) insufficient sample size, (5) confidence interval spanning invalid lines.
4. Discussion
Gan et al. [33] concluded that patients mostly suffer from moderate or severe pain after THA. Subsequently, Guay et al. [34] believed that pain is associated with the increase in postoperative bleeding. PONV which is mainly caused by anaesthesia inhalation and opioid analgesics is also regarded as a common complication after anaesthesia [35]. The incidence of PONV after THA is in the range of 20%–83% which significantly affects postoperative quality of life [36, 37]. Postoperative pain, nausea, and vomiting lead to discomfort, decreased surgical satisfaction, and prolonged hospital stay [38]. Therefore, considering these serious postoperative problems, postoperative pain and perioperative complications should be decreased.
A total of 9 RCTs including 605 patients were included in our systematic review. Our results showed that the perioperative VAS value of the intervention group was significantly lower than that of the control group at different time points in patients after THA. The typical period of maximal postoperative pain after THA is 2–3 days [24]. Therefore, we conducted subgroup analysis using the variable of time points from 6 h to 7 days after THA. Fortunately, we achieved the same conclusion at different time points for patients after THA, except those at 6 and 36 h. The analgesic mechanism of AT was still unclear for patients after THA. The analgesic effect of AT can be blocked by opioid antagonists which can be used to explain the role of the endorphin system in the analgesic mechanism of AT [39]. Krause [40, 41] also found that AT could improve the pain threshold in the local area of the patients. The auricular point of Shen Men is the most used to generate analgesic, sedative and anti-inflammatory effects [42] and increased endorphin secretion and serotonin production, thereby suppressing the transmission of pain messages and pain perception [43]. In our study, the auricular point of Shen Men was selected in all articles, and hip was used as the secondary auricular point in all the patients after THA.
To explore other impact factors of the postoperative VAS results in our meta-analysis, we conducted other subgroup analyses according to some features of the articles in patients after THA. For the risk of bias, the statistical significance of postoperative VAS was only shown in C-level literature but not in the A- and B-level ones. Sham acupuncture is physiological and not inert [44]. It can produce the measurable clinical effects for patients by providing the analgesic effect in 40%–50% of the patients, but it obtained 60% for true acupuncture [45]. However, when we conducted subgroup analysis, we obtained consistent results for patients who underwent THA and were treated with or without sham acupuncture. Although, there was no difference between the subgroup of the starting points of the AT in whole time, we found that there was a difference between the two groups at six hours after surgery in our meta-analysis [29, 31]. It means that the different onset time of adjuvant analgesic may affect the postoperative VAS results.
We found that the body mass-adjusted fentanyl amount under intraoperative time for the patients after THA was lower than those of the AA and control groups. It is similar with the research obtained results of Wetzel et al. [25]. The difference in the required fentanyl between the two groups prompted the treatment of AA and can support the analgesic effect for patients with chronic and acute postoperative pain [46, 47]. We also explored whether AT can prolong the time to first analgesic request for the patient after THA. However, the result showed insignificant differences between the two groups (Figure 4(b)). Postoperative analgesic requirements were controlled by the medical staff and directly affected by the surgery type and patient's economic condition [46]. Thus, time to the first analgesic request is a particularly unreliable outcome in assessing the effects of AT.
Multimodal pain management protocols, which usually involved different analgesic treatments such as nonsteroidal anti-inflammatory drugs, opioid drugs, and perioperative regional anaesthesia/analgesia, were becoming more and more popular in recent studies [48]. However, due to the lack of effective data analysis, we cannot reasonably evaluate the effectiveness of these protocols. The treatment of PCA (e.g., fentanyl) is the most involved in our research. Although some of the common side effects of the drug are hypotension, hypertension, bradycardia, tachycardia, hypoxemia, nausea, vomiting, and inhalation, and these adverse effects can be observed during anaesthesia induction [24], we found the incidence of postoperative nausea and vomiting, perioperative transitory hypotension and bradycardia had insignificant differences between the two groups of patients after THA in our meta-analysis (Figure 5). We believe that the results may be influenced by the small sample. Therefore, further studies and analysis must be performed in the future.
Usichenko et al. [21] only conducted a systematic review of AT for postoperative pain and not a meta-analysis due to the low quality and heterogeneity of the included trials. However, new studies that have been conducted in recent years were included, and we conducted various analyses in decreasing the heterogeneity of the results in our meta-analysis. The present evidence of this meta-analysis showed that AT can decrease postoperative VAS pain scores and intraoperative body mass-adjusted fentanyl amount but not the incidence of complications in the patients after THA. Nonetheless, among the available evidence, the GRADE system evaluation results were both at low and extremely low levels, thereby suggesting that we should be cautious about the results of this study.
4.1. Study Limitations
Some of the limitations of this study may affect the results, as follows. (1) The sample size of the included studies is small, most studies did not describe the sample size estimation, and most research methods are of low quality. (2) The beginning and end times of AT are unclear and differences in duration and frequency which may be the cause of clinical heterogeneity are significant. (3) Routine analgesia in the control group may also be the cause of clinical heterogeneity. All these factors limited the intensity of the research results. The number of included studies was <10. Hence, funnel plot was not used to analyse publication bias. Therefore, a considerable number of AT-related studies with unified and standardised operating standards and strict design are needed in the future to ensure high level of method quality.
4.2. Implications for Future Research and Practice
This review has some implications. First, the main advantages of AT are convenience, safety, and satisfactory postoperative analgesia [49, 50]. Therefore, healthcare workers should be encouraged to learn alternative therapy for postoperative pain. Standardised AT for postoperative pain management should be designed with evidence-based methods, such as the selection and identification of primary and auxiliary acupoints, manual compression guidance and treatment time. Second, patients with chronic pain after the hip operation have extremely high direct costs because of the utilisation of painkillers for years and lengthy rehabilitation programme to ensure the maintenance of patients' motility with sufficient quality of life. Therefore, further studies should pay attention to AT in both acute and chronic postoperative pain and further evaluate the effect of AT as an alternative therapy for pain control after THA. Third, further studies can also include objective evaluation indicators, such as pain effective rate. Other outcome indicators, such as Harris hip score, can be used to evaluate the effect of AT on rehabilitation after THA comprehensively. Most importantly, the methodological quality of future studies must be improved with the explicit descriptions of random sequence generation and allocation concealment which is a reasonable blinding design and an appropriate method for sample size calculation and describe the number and reason of exit in detail.
5. Conclusions
The present evidence for the effectiveness of perioperative AT on postoperative pain and intraoperative body mass-adjusted fentanyl amount for the patients after THA was affirmative, but prolongation of the time to first analgesic request and increase in the incidence of complications were not indicated. However, the results of this study still need to be verified by a multicentre, large sample, and high-quality research.
Acknowledgments
This study was supported by the Project of Science and Technology Program of Ningde (grant no. 20170021).
Disclosure
The funder (Project of Science and Technology of Ningde) has no role in the study design, data analysis, and manuscript preparation.
Conflicts of Interest
The authors declare no conflicts of interest in this study.
Supplementary Materials
This section contains a detailed description of search strategies including the databases of PubMed, Excerpta Medica Database (Embase), Cochrane Central Register of Controlled Trials, Web of Science, Science Direct, PsycINFO, Cumulative Index to Nursing and Allied Health Literature, and Allied and Complementary Medicine. All search strategies are based on PICOS principles.
References
- 1.Fan Z., Ma J., Ma X., et al. The efficacy of dexamethasone on pain and recovery after total hip arthroplasty. Medicine. 2018;97(13):p. e0100. doi: 10.1097/MD.0000000000010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam Y., Chan P., Fu H., Yan C., Chiu K. A review of the clinical approach to persistent pain following total hip replacement. Hong Kong Medical Journal. 2016;22(6):600–607. doi: 10.12809/hkmj164969. [DOI] [PubMed] [Google Scholar]
- 3.Lovald S. T., Ong K. L., Lau E. C., Schmier J. K., Bozic K. J., Kurtz S. M. Mortality, cost, and downstream disease of total hip arthroplasty patients in the medicare population. The Journal of Arthroplasty. 2014;29(1):242–246. doi: 10.1016/j.arth.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Bergen N. U. O. B. The Norwegian Arthroplasty Register. 2015. [Google Scholar]
- 5.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. The Journal of Bone & Joint Surgery. 2007;89(4):780–785. doi: 10.2106/jbjs.f.00222. [DOI] [PubMed] [Google Scholar]
- 6.Goebel S., Steinert A. F., Schillinger J., et al. Reduced postoperative pain in total hip arthroplasty after minimal-invasive anterior approach. International Orthopaedics. 2012;36(3):491–498. doi: 10.1007/s00264-011-1280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerr D. R., Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery—a case study of 325 patients. Acta Orthopaedica. 2008;79(2):174–183. doi: 10.1080/17453670710014950. [DOI] [PubMed] [Google Scholar]
- 8.Chung F., Un V., Su J. Postoperative symptoms 24 hours after ambulatory anaesthesia. Canadian Journal of Anesthesia. 1996;43(11):1121–1127. doi: 10.1007/BF03011838. [DOI] [PubMed] [Google Scholar]
- 9.Kehlet H., Jensen T. S., Woolf C. J. Persistent postsurgical pain: risk factors and prevention. The Lancet. 2006;367(9522):1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 10.Liu S. S., Buvanendran A., Rathmell J. P., et al. Predictors for moderate to severe acute postoperative pain after total hip and knee replacement. International Orthopaedics. 2012;36(11):2261–2267. doi: 10.1007/s00264-012-1623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kehlet H., Andersen L. Ø. Local infiltration analgesia in joint replacement: the evidence and recommendations for clinical practice. Acta Anaesthesiologica Scandinavica. 2011;55(7):778–784. doi: 10.1111/j.1399-6576.2011.02429.x. [DOI] [PubMed] [Google Scholar]
- 12.Baratta J. L., Gandhi K., Viscusi E. R. Perioperative pain management for total knee arthroplasty. Journal of Surgical Orthopaedic Advances. 2014;23(1):22–36. doi: 10.3113/JSOA.2014.0022. [DOI] [PubMed] [Google Scholar]
- 13.Young A. C., Buvanendran A. Pain management for total hip arthroplasty. Journal of Surgical Orthopaedic Advances. 2014;23(1):13–21. doi: 10.3113/JSOA.2014.0013. [DOI] [PubMed] [Google Scholar]
- 14.Husted H., Hansen H. C., Holm G., et al. What determines length of stay after total hip and knee arthroplasty? A nationwide study in Denmark. Archives of Orthopaedic and Trauma Surgery. 2010;130(2):263–268. doi: 10.1007/s00402-009-0940-7. [DOI] [PubMed] [Google Scholar]
- 15.Lee H., Ernst E. Acupuncture analgesia during surgery: a systematic review. PAIN. 2005;114(3):511–517. doi: 10.1016/j.pain.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 16. Traditional Medicine Growing Needs and Potential—WHO Policy Perspectives on Medicines. 2002.
- 17.Oleson T. Auriculotherapy Manual: Chinese and Western Systems of Ear Acupuncture. 4th. Edinburgh, UK: Elsevier; 2013. [Google Scholar]
- 18.W. H. Organization. Lyon, France: 1990. Report of the working group on auricular acupuncture nomenclature. [Google Scholar]
- 19.Barlow T., Downham C., Barlow D. The effect of complementary therapies on post-operative pain control in ambulatory knee surgery: a systematic review. Complementary Therapies in Medicine. 2013;21(5):529–534. doi: 10.1016/j.ctim.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Crespin D. J., Griffin K. H., Johnson J. R., et al. Acupuncture provides short-term pain relief for patients in a total joint replacement program. Pain Medicine. 2015;16(6):1195–1203. doi: 10.1111/pme.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu M.-S., Chen K.-H., Chen I.-F., et al. The efficacy of acupuncture in post-operative pain management: a systematic review and meta-analysis. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0150367.e0150367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usichenko T. I., Lehmann C., Ernst E. Auricular acupuncture for postoperative pain control: a systematic review of randomised clinical trials. Anaesthesia. 2008;63(12):1343–1348. doi: 10.1111/j.1365-2044.2008.05632.x. [DOI] [PubMed] [Google Scholar]
- 23.Wetzel B., Pavlovic D., Kuse R., et al. The effect of auricular acupuncture on fentanyl requirement during hip arthroplasty: a randomized controlled trial. The Clinical Journal of Pain. 2011;27(3):262–267. doi: 10.1097/AJP.0b013e3181fd516c. [DOI] [PubMed] [Google Scholar]
- 24.Usichenko T. I., Dinse M., Hermsen M., Witstruck T., Pavlovic D., Lehmann C. Auricular acupuncture for pain relief after total hip arthroplasty—a randomized controlled study. PAIN. 2005;114(3):320–327. doi: 10.1016/j.pain.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Usichenko T. I., Lysenjuk V. P., Dinse M., Pavlovic D., Lehmann C. Auricular acupuncture reduces intraoperative fentanyl requirement during total hip arthroplasty - a randomized double-blinded study. Acupuncture & Electro-Therapeutics Research. 2007;32(1-2):142–143. doi: 10.3727/036012906815844265. [DOI] [PubMed] [Google Scholar]
- 26.Lv M. L. Observation of 49 cases of pain relief after hip joint replacement by auricular point buried-bean. Zhejiang Journal of Traditional Chinese Medicine. 2017;52(7):502–504. [Google Scholar]
- 27.Kong F. F., Pei J. B. Analgesic efficacy of auricular pressure therapy on patients after the total hip resurfacing. Zhejiang Journal of Traditional Chinese Medicine. 2010;(4):281–282. [Google Scholar]
- 28.Cui Y., Ni L. Y., Shen H. F. Effect of auricular point buried-bean on pain after total hip arthroplasty. Public Medical Forum Magazine. 2016;20(16):2255–2256. [Google Scholar]
- 29.Xu B. B. Application of auricular therapy plus celecoxib preemptive analgesia in total hip arthroplasty. Journal of Emergency in Traditional Chinese Medicine. 2014;23(4):766–767. [Google Scholar]
- 30.Shen F. Y., Zhou M. Effect of Auricular Pressure on analgesia and patient-controlled analgesia after total hip arthroplasty in elderly patients. Jiangsu Journal of Traditional Chinese Medicine. 2017;(9):61–62. [Google Scholar]
- 31.Tian J. J., Ding P., Zhai K. K. The therapeutic effect observation of auricular acupoint pressing in relieveing pain after total hip replacement. Clinical Journal of Traditional Chinese Medicine. 2016;(1) [Google Scholar]
- 32.Higgins G. S., Cochrane J. P. Handbook for Systematic Reviews of Interventions | Cochrane Training. 2018. [Google Scholar]
- 33.Gan T. J., Meyer T., Apfel C. C., et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesthesia & Analgesia. 2003;97(1):62–71. doi: 10.1213/01.ane.0000068580.00245.95. [DOI] [PubMed] [Google Scholar]
- 34.Guay J. Postoperative pain significantly influences postoperative blood loss in patients undergoing total knee replacement. Pain Medicine. 2006;7(6):476–482. doi: 10.1111/j.1526-4637.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- 35.Horn C. C., Wallisch W. J., Homanics G. E., Williams J. P. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. European Journal of Pharmacology. 2014;722(1):55–66. doi: 10.1016/j.ejphar.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salerno A., Hermann R. Efficacy and safety of steroid use for postoperative pain relief: update and review of the medical literature. The Journal of Bone & Joint Surgery—American Volume. 2006;88(6):1361–1372. doi: 10.2106/jbjs.d.03018. [DOI] [PubMed] [Google Scholar]
- 37.Myles P. S., Williams D. L., Hendrata M., Anderson H., Weeks A. M. Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. British Journal of Anaesthesia. 2000;84(1):6–10. doi: 10.1093/oxfordjournals.bja.a013383. [DOI] [PubMed] [Google Scholar]
- 38.Cui Z., Liu X., Teng Y., Jiang J., Wang J., Xia Y. The efficacy of steroid injection in total knee or hip arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy. 2015;23(8):2306–2314. doi: 10.1007/s00167-014-3049-7. [DOI] [PubMed] [Google Scholar]
- 39.Oleson T. D. Auriculotherapy stimulation for neuro-rehabilitation. NeuroRehabilitation. 2002;17(1):49–62. [PubMed] [Google Scholar]
- 40.Noling L. B., Clelland J. A., Jackson J. R., Knowles C. J. Effect of transcutaneous electrical nerve stimulation at auricular points on experimental cutaneous pain threshold. Physical Therapy in Sport. 1988;68(3):328–332. doi: 10.1093/ptj/68.3.328. [DOI] [PubMed] [Google Scholar]
- 41.Woodward Krause A., Clelland J. A., Knowles C. J., Jackson J. R. Effects of unilateral and bilateral auricular transcutaneous electrical nerve stimulation on cutaneous pain threshold. Physical Therapy in Sport. 1987;67(4):507–511. doi: 10.1093/ptj/67.4.507. [DOI] [PubMed] [Google Scholar]
- 42.Chang L. H., Hsu C. H., Jong G. P., et al. Auricular acupressure for managing postoperative pain and knee motion in patients with total knee replacement: a randomized sham control study. Evidence-Based Complementary and Alternative Medicine. 2012;2012:7. doi: 10.1155/2012/528452.528452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han J. Acupuncture analgesia: areas of consensus and controversy. PAIN. 2011;152(3):S41–S48. doi: 10.1016/j.pain.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Moffet H. H. Sham acupuncture may be as efficacious as true acupuncture: a systematic review of clinical trials. The Journal of Alternative and Complementary Medicine. 2009;15(3):213–216. doi: 10.1089/acm.2008.0356. [DOI] [PubMed] [Google Scholar]
- 45.Lewith G. T., Machin D. On the evaluation of the clinical effects of acupuncture. PAIN. 1983;16(2):111–127. doi: 10.1016/0304-3959(83)90202-6. [DOI] [PubMed] [Google Scholar]
- 46.Alimi D., Rubino C., Pichard-Léandri E., Fermand-Brulé S., Dubreuil-Lemaire M., Hill C. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. Journal of Clinical Oncology. 2003;21(22):4120–4126. doi: 10.1200/JCO.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Sator-Katzenschlager S. M., Michalek-Sauberer A. P-Stim™ auricular electroacupuncture stimulation device for pain relief. Expert Review of Medical Devices. 2014;4(1):23–32. doi: 10.1586/17434440.4.1.23. [DOI] [PubMed] [Google Scholar]
- 48.Memtsoudis S. G., Poeran J., Zubizarreta N., et al. Association of multimodal pain management strategies with perioperative outcomes and resource utilization. Anesthesiology. 2018;128(5):891–902. doi: 10.1097/ALN.0000000000002132. [DOI] [PubMed] [Google Scholar]
- 49.Tan J. Y., Molassiotis A., Wang T., Suen L. K. Adverse events of auricular therapy: a systematic review. Evidence-Based Complementary and Alternative Medicine. 2014;2014:20. doi: 10.1155/2014/506758.506758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeh M.-L., Tsou M.-Y., Lee B.-Y., Chen H.-H., Chung Y.-C. Effects of auricular acupressure on pain reduction in patient-controlled analgesia after lumbar spine surgery. Acta Anaesthesiologica Taiwanica. 2010;48(2):80–86. doi: 10.1016/s1875-4597(10)60018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This section contains a detailed description of search strategies including the databases of PubMed, Excerpta Medica Database (Embase), Cochrane Central Register of Controlled Trials, Web of Science, Science Direct, PsycINFO, Cumulative Index to Nursing and Allied Health Literature, and Allied and Complementary Medicine. All search strategies are based on PICOS principles.