Abstract
Objective
This in vitro and in vivo study was conducted to rationalize some of traditional medicinal uses of Ailanthus altissima in gastrointestinal, respiratory, and cardiovascular systems.
Materials
Crude extract of Ailanthus altissima (Aa.Cr) and its fractions were prepared and utilized in in vitro and in vivo studies. For in vitro studies, Aa.Cr was investigated on isolated rabbit jejunum, isolated tracheal strip, and isolated aorta of rat suspended in tissue organ bath. Platelet rich and platelet poor plasma were used to study platelet aggregation inhibitory activity. In vivo antidiarrheal effect of Aa.Cr was investigated on balb/c mice pretreated with castor oil to induce diarrhea and SD rats were used to study hypotensive activity.
Results
Concentration dependent spasmolytic effects of Aa.Cr and its DCM fraction (Aa.DCM) were observed on spontaneous and spasmogen induced contractions in jejunum isolated from rabbit, but effect against high potassium (high-K+) induced contractions was more potent. Moreover Aa.Cr showed parallel shifting of calcium response curve to the right side. While its aqueous fraction (Aa.aq) caused spasmogenesis of isolated rabbit jejunum, this effect was blocked partially with prior administration of atropine (1μM). Concentration dependent protection against castor oil induced diarrhea was also observed. Relaxant effect was observed by the application of Aa.Cr and Aa.DCM against high-K+ and carbachol (CCh) induced contractions in tracheal strips isolated from SD rats, while Aa.Aq caused partial relaxation of high-K+ induced contractions, but no effect was observed against CCh induced contractions. Relaxation of rat aorta by the application of Aa.Cr and its fractions was also observed. Inhibition of force of contraction in rabbit atrium was also observed. Inhibition of platelet aggregation was observed against epinephrine and ADP induced aggregation.
Conclusion
Keeping in view the observed results, it is concluded that smooth muscle relaxant, platelet aggregation inhibitory and hypotensive effect may be due to the blockage of calcium channels.
1. Introduction
Ailanthus altissima (Synonyms: Ailanthus cacodendron (Ehrh.) and Ailanthus glandulosa Desf.) belongs to Simaroubaceae family and is commonly called tree of heaven, smoke tree, God's tree, Chinese sumac, and sumac tree [1]. It is native to China, Indian occupied Kashmir, Vietnam, Taiwan, and Japan [2, 3]. This plant has peanut or cashew like fragrance [4, 5]. It has strong ornamental acceptance and due to this reason has become naturalized in many countries even in Pakistan [6].
A. altissima is widely used throughout Asia for the treatment of several disorders in traditional medicine. Its dried bark is listed as an official drug in modern Chinese materia medica as “chun bai pi”. This plant is used to treat gastrointestinal ailments like diarrhea, dysentery [7, 8], vermicide, and hemorrhage of intestine [9]. It is also used to cure leucorrhea, gonorrhea, cough, and gastric and intestinal complaints [10]. The bark is used to treat anemia, hemorrhage, diarrhea, and spermatorrhea and is considered as useful to treat epilepsy, diarrhea, asthma, and cardiac problems. In Chinese system of medicines, it is used to treat colds and gastric problems [11]. The leaves of A. altissima are used for the treatment of scabies and seborrhea [12]. Its bark is astringent, antispasmodic, anthelmintic, and parasiticidal [7, 13]. It possesses phosphodiesterase inhibitory [14], angiotensin converting enzyme inhibitory, analgesic, anti-inflammatory, antipyretic, antioxidant, and antiasthmatic activities [15]. Previous reported literature indicates that it possess pain reducing, ulcer healing, antipyretic [10], anticancer [16], COX inhibitory, and antioxidant potentials [17]. Also it has been reported that A. altissima possess antimicrobial and antifungal activities [18].
It has been reported that A. altissima contains hundreds of chemical constituents. A number of alkaloids with basic structure of β-carboline, canthine-6-one and canthine-6-one-3 N-oxide and indole have been reported [19, 20]. Presence of quercetin and isoquercetin is in leaves, ceryl alcohol in bark, 2,6-dimethoxy-p-benzoquinone, ailanthone, ailanthinone, chaparrinone, amarolide, and acetylamarolide in the wood of Ailanthus altissima [21, 22], while 1-acetyl-4-methoxy-β-carboline (I), canthin-6-one (VIIIa), 1-methoxycanthin-6-one (VIIIb), canthin-6-one-3N-oxide (IXa), 1-(2'-hydroxyethyl)-4-methoxy-β-carboline (IIa), 1-(1', 2'-dihydroxyethyl)-4-methoxy-β-carboline (IIIa), and 1-methoxycanthin-6-one-3N-oxide (IXb) have been isolated from its root [22, 23]. It has been reported that A. altissima possess (E,E)-2,4-decadienal, (E)-2-undecenal, (E)-2-decenal, hexanal, nonanal and furfural which have nematicidal activity [24]. Presence of flavonoids in this plant has also been reported [2].
2. Material and Experimental Protocols
2.1. Plant Collection and Extraction
Bark of A. altissima was collected from the botanical garden of Bahauddin Zakariya University, Mulan, Pakistan, authenticated and identified by a taxonomist (voucher no: R.R. Stewart 607). After shed drying, the bark material was grounded into coarse powder. One kilogram grounded bark was macerated in 2.5 liter of hydroalcoholic solution (30% water and 70% methanol). The macerate was filtrated by using muslin cloth and Whatman filter paper. The filtrate was dried on a rotary evaporator at controlled pressure and temperature (37°C) [25, 26], and a semisolid or honey like material was obtained. For the purpose of fractionation, 50g dried extract of Ailanthus altissima (Aa.Cr) was dissolved in 100 mL of distilled water and equivalent volume of dichloromethane was added to it. This solution was shifted to separating funnel and gently shaken and then kept on a stand. Two separate layers of solution were formed; both layers were separated and dried.
2.2. Drugs and Chemicals
High quality chemicals and drugs were used in this study and were purchased from reliable resources, i.e., Sigma Aldrich (merged with Merck Ag, Germany), Merck (Germany), and Alfa Acer (USA). Acetylcholine (Ach), carbachol, atropine sulphate, loperamide, verapamil hydrochloride, and potassium chloride were used as standard drugs for in vivo and ex vivo studies. Sodium chloride (NaCl), calcium chloride (CaCl2), sodium dihydrogen phosphate (NaH2PO4), potassium dihydrogen phosphate (KH2PO4), magnesium chloride (MgCl2), magnesium sulphate (MgSO4), potassium chloride (KCl), glucose (C6H12O6), and sodium citrate were used in the preparation of physiological salt solution. Before the execution of experiments, all solutions were freshly prepared in double deionized water.
2.3. Animals
Locally bred rabbits (1.2–1.8 kg), Sprague Dawley rats (150–300g), and BALB/c mice (20–35g) either males or females were used in this study. These animals were placed in the animal house of Pharmacology Department, Faculty of Pharmacy, Bahauddin Zakariya University, Multan. Temperature of the animal house was maintained at (23-25°C), humidity (50-55%) and 12 h light and dark cycle. Standard diet was provided to animals with full access to water. 16-20 hours before the start of experiments freedom for food was banned but ad libitum water. The rules set up by the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (1996) were followed in the execution of experiments. Care was exercised for the minimal number of animal usage defined by the principle of 3Rs, i.e., Replacement, Reduction, and Refinement. 3Rs principle was applied for the minimal number of animal usage. The protocols of this study were approved by the departmental ethical committee vide No.09/PEC/2015.
2.4. Preliminary Phytochemical Analysis
To analyze the presence of bioactive molecules like alkaloids, saponins, flavonoids, anthraquinones, tannins, and glycosides in plant extract, the following tests were performed [27–29].
2.4.1. Alkaloids
Plant extract was dissolved in distilled water. This solution was shifted into four different test tubes. Few drops of reagents, i.e., Mayer's reagent, Hager's reagent, Wagner's reagent, and Dragendorff's reagents, were added separately to these test tubes. Formation of precipitates indicated the presence of alkaloids.
2.4.2. Flavonoids
One gram of plant extract was dissolved in H2SO4 and boiled then filtered. 1% NH3 solution was added to the filtrate drop wise and observed for any change in color. Appearance of yellow color was considered as the presence of flavonoids.
2.4.3. Anthraquinones
1 gram of plant extract was boiled in H2SO4; this solution was treated with 10 mL of chloroform. By the addition of chloroform solution split into two layers, both layers were separated out and the upper layer was treated with dil. Ammonia solution. Observation of violet color was considered as indication for the presence of anthraquinone.
2.4.4. Saponins
1 gram of test material was dissolved in 10 mL of distilled water by vigorous shaking until the formation of froth. Then few drops of olive oil were added to it. Formation of emulsion or froth formation indicated the presence of saponins.
2.4.5. Tannins
For the identification of presence of tannins in plant extract, aqueous solution of plant extract was treated with drop wise addition of 1%FeCl3 solution. Appearance of bluish-black or brownish-green color indicated tannins presence.
2.4.6. Reducing Sugar Test
Mixture of aqueous solution of plant extract and Fehling's solution was heated. Formation of brick red color precipitates indicates reducing sugars.
2.4.7. Deoxy-Sugar in Glycosides
For the detection of deoxy sugars in glycosides, Keller-Killiani test was adopted. In this test plant extract was dissolved in chloroform. This solution was treated with H2SO4 carefully along the wall of test tube. If deoxy sugars are present in the sample, then brown color will appear at the interface.
2.5. Ex Vivo Experiments on Isolated Tissues
2.5.1. Experiments for Spasmolytic Activity on Jejunum Preparation
Rabbit jejunum was used to study the possible spasmolytic activity of Aa.Cr and its fractions. After scarifying the rabbit, jejunum was removed and small pieces about 2–3cm in length were made. The jejunum pieces were suspended in 15 mL tissue bath filled with normal Tyrode's solution. The temperature of the bath was kept constant at 37°C with continuous supply of carbogen and a tension of 1g was applied to the tissue. The tissue was kept free to equilibrate for a period of 30–45 minutes and during this period no drug was applied, but it was repeatedly washed with fresh Tyrode's solution. Then the tissue was stabilized by repeated exposure to acetylcholine (0.3μM) after each 3-minutes interval. Spasmolytic action of plant material was investigated by cumulative administration of test drug. This effect was measured as percentage of jejunum spontaneous contractions obtained immediately before the application of 1st dose of test materials. Calcium channel blocker activity was studied by the application of Aa.Cr to the jejunal strip preexposed to 80 mmol/L K+ [26, 30, 31]. To confirm that the spasmolytic activity of the A. altissima is due to the blockage of calcium channel or any other mechanism involved, concentration response curves of calcium (CRCs) were constructed. For this purpose, the tissue was stabilized in calcium free and K+ rich Tyrode's solution. After the stabilization and incubation, the tissue was treated with Aa.Cr and left free for a period of 45 minutes. Then calcium was administered in a cumulative manner and the contractile response was observed [32].
2.5.2. Study of the Relaxant Effect on Isolated Rat Tracheal Tissue
To study the relaxant effect of the test drug rat tracheal tissues were used. 24-hour fasted rat was killed by a sharp blow on its head and the trachea was carefully separated. After removing the adhesive tissues, the trachea was cut open ventrally. Small tracheal strips with a width of 3-4 mm having minimum 2-3 cartilages were formed. These strips were individually hung in a tissue organ bath containing Krebs's solution at 37°C with constant carbogen perfusion. 1g tension was applied to tissue till the end of experiment. After hanging, the tissue was allowed to equilibrate up to 45 minutes prior to the application of any drug. After equilibration, the tissue was exposed to 80 mmol of potassium (high-K+) or carbachol (1μM) to induce contractions in it. Then relaxant effect of plant extracts was observed on these precontracted tracheal tissues via isometric transducers (MLT 0201, Panlab, Spain) connected to PowerLab data acquisition system [25, 30].
2.5.3. Vasodilation Study on Isolated Rat Aorta
Vasodilation potential of plant extract and its fractions was evaluated by using isolated rat aorta with adaptation of the reported procedure [18–20, 22]. SD rat was sacrificed by a sharp blow on its head and descending thoracic aorta was dissected out and pieces of 2–3mm were formed. Each isolated rat aortic tissue segment was suspended in tissue organ bath filled with Kreb's solution. Temperature of tissue organ bath was fixed at 37°C with continuous supply of carbogen. Then, 2g preload tension was applied to the tissue and left it to equilibrate for a period of 1h. Tissue was repeatedly exposed to phenylephrine (1μM) or high potassium (K+-80 mM) for the purpose of stabilization. The vasodilator effect of plant material was studied by cumulative addition of plant extract to precontracted aortic rings. Changes in the tension of precontracted aortic rings were measured via isometric transducer MLT 0201 (Panlab, Spain) which was in connection with PowerLab data acquisition system (ADInstruments, Australia) coupled with LabChart software. Vasodilation effect of the test material was calculated as %age relaxant effect by considering the base line response of the tissue as 100% relaxant effect and PE and high-K+ contractions as 100% contractile effect.
2.5.4. Study of Ionotropic and Chronotropic Effect on Isolated Rabbit Atria
Right atrium was isolated carefully from heart of rabbit and hung in tissue organ bath having Kreb's solution with the continuous supply of carbogen. Ionotropic and chronotropic effect of atria were recorded under 1.0 g pressure by isometric transducer associated with PowerLab. Atrial tissues were permitted to equilibrate for a time of 30 min with persistent buffer change at regular intervals preceding administration of any drug [21]. After stabilization of the tissue, it was exposed to isoprenaline (1μM) and acetylcholine (1μM) to check the responsiveness of the tissue. Then test materials were administered cumulatively and the responses were noted on rate and force of contractions. Negative inotropic and chronotropic effects of the test drugs were calculated as %age of the baseline response of isolated atrium.
2.5.5. Study of Platelet Aggregation Inhibitory Activity
Platelet aggregation inhibitory effect of the plant extract was determined by using the method as described by Imran et al. (2015). Blood samples were collected from the healthy human volunteers with age range of 24±4 who have not taken any medicine at least one week prior to experimentation. Sodium citrate (3.8% w/v) solution as anticoagulant was mixed with blood in a ratio of 9:1 (blood: sodium citrate solution). Blood sample was subjected to centrifugation at a speed of 1500 rpm for 15 minutes. Supernatant was separated out as platelet rich plasma (PRP). Remaining blood sample was further centrifuged at a speed of 4,000 rpm for a period of 20 minutes to get platelet poor plasma (PPP). Antiplatelet activity of the test drugs was determined by using chronolog 490-2D aggregometer (Chrono-log Corporation, Haverton, PA 19083, USA) which works on the principal of light transmission aggregometry (LTA). After maintaining the temperature of the machine, 0.23 mL of PRP was taken in a siliconized microcuvette with spacer at the bottom and placed in PRP chamber. Final volume of the cuvette was made up to 0.25 ml by the addition 10 μL of standard aggregating agent ADP (5μM) or phenylephrine (40 μM) and 10μL of extract dissolved in normal saline. Similarly, 450 μL of PPP was taken in a cuvette and placed in PPP chamber as a reference. PRP was first incubated with 10μL of test material for a period of five minutes then 10μL of ADP or PE was added to it to make up the volume 0.25 mL, placed in PRP chamber, and response was observed. Results were calculated as percentage inhibition with respect to time.
2.6. In Vivo Experiments
2.6.1. In Vivo Antidiarrheal Activity
BALB/c mice were segregated into six groups randomly having six animals in each. These mice were placed in individual cages which were lined with blotting paper. On the day of experiment, all animals were orally exposed to castor oil (10 mL/kg) for the induction of diarrhea. One hour after castor oil treatment, Group-I animals (negative control) were treated with 0.9% sodium chloride solution (10 mL/kg) while group-II (positive control) was orally given loperamide (15 mg/kg). While group-III was orally treated with Aa.Cr (100 mg/kg), group-IV with Aa.Cr (200 mg/kg), group-V with Aa.Cr (300 mg/kg), group-VI was treated with Aa.Cr (500 mg/kg). After treatment all animals were observed up to 6 h and total number of feces was counted individually for each animal [33].
2.6.2. Invasive Hypotensive Activity
Hypotensive activity of plant extracts was studied on SD rats by the previously described method [21, 24]. Ketamine (50–80 mg/kg) and diazepam (5mg/kg) were used intraperitoneally to anesthetize the rats. The anesthetized rat was placed in a supine position on dissecting board. Temperature of the rat was kept constant by putting it on isothermic warming cushion at 37°C. Trachea, jugular vein, and carotid artery were uncovered by making a small incision and cannulated. Spontaneous breathing was maintained by cannulation of trachea with 18 gauge polyethylene tube (PE-20). The jugular vein was cannulated to administer drug/s with PE-50. Cannulation was performed in carotid artery with similar tubing filled with heparin solution (60 IU/mL) and attached with disposable BP transducer (MLT0699, ADInstruments, Sydney, Australia), further connected to PowerLab data acquisition system coupled with LabChart software for the measurement of blood pressure. The transducer was calibrated prior to experiment with the help of mercury sphygmomanometer. Surface exposed for cannulation was kept covered with filter paper moistened with normal saline. The rat was administered 0.1 mL of heparin to prevent clotting of blood. Standard medications were administered in 0.1mL followed by 0.1mL of 0.9% normal saline flush through the jugular vein. To check the animal behavior towards hypertensive and hypotensive drugs acetylcholine (1μg/kg) and epinephrine (1μg/kg) were administered intravenously to every animal before the administration of any drug. After attaining the equilibrium, 0.1 mL of the extract or drug was administered intravenously followed by normal saline (0.1 mL) flush [24]. Changes in systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) were calculated and compared with the response of verapamil used as standard hypotensive drug.
2.7. Acute Toxicity Assay
For acute toxicity assay, three groups of mice, five mice in each group, were selected. 1st group was given Aa.Cr 1 g/kg orally while the 2nd and 3rd groups received Aa.Cr 2 g/kg and 3 g/kg in a volume of 10 mL/kg, respectively. The animals were observed for 24 h for behavioral changes and mortality but with full access to water and food [25, 31].
2.8. Statistical Analysis of Data
For isolated tissues, the data were represented as mean ± SEM (n = number of individual experiments) and median effective concentrations (EC50) with 95% confidence intervals. The concentration response curves were analyzed by nonlinear regression. While Two-Way ANOVA followed by Tukey's multiple comparison test was used to determine the significant difference in various concentration, Student t-test was utilized to analyze hypotensive effect. One-Way ANOVA followed by Dunnett's multiple comparison test with defined control group as a reference was used for the analysis of antidiarrheal and antiaggregatory activities. In all cases, P values <0.05 were considered to be statistically significant. All statistical tests were performed by using GrphPad prism software (V 6.01).
3. Results
3.1. Preliminary Phytochemical Reports
Phytochemical evaluation of Aa.Cr showed that alkaloids, saponins, tannins, glycosides, flavonoids, and triterpenoids were present in it.
3.2. In Vitro Activities
3.2.1. Spasmolytic Effect on Isolated Jejunum of Rabbit
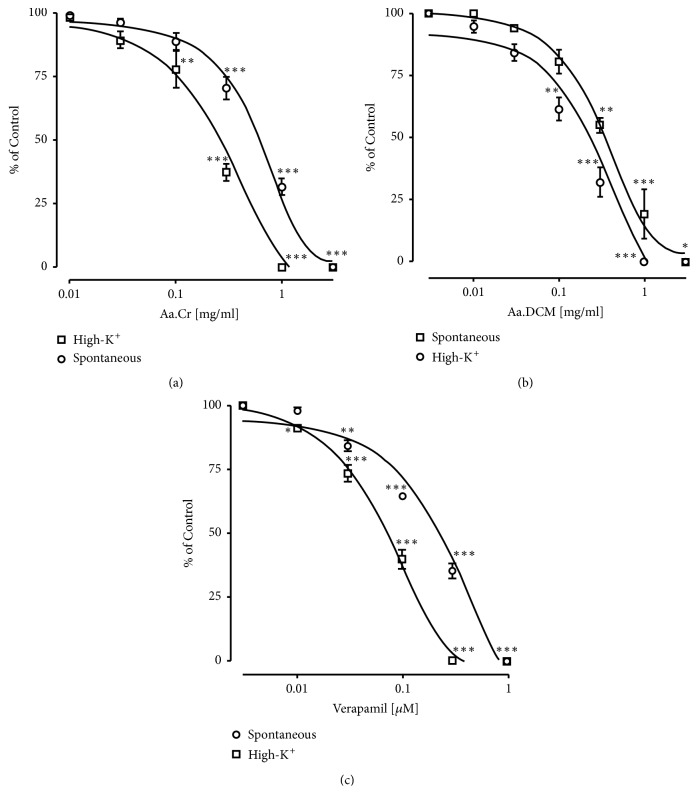
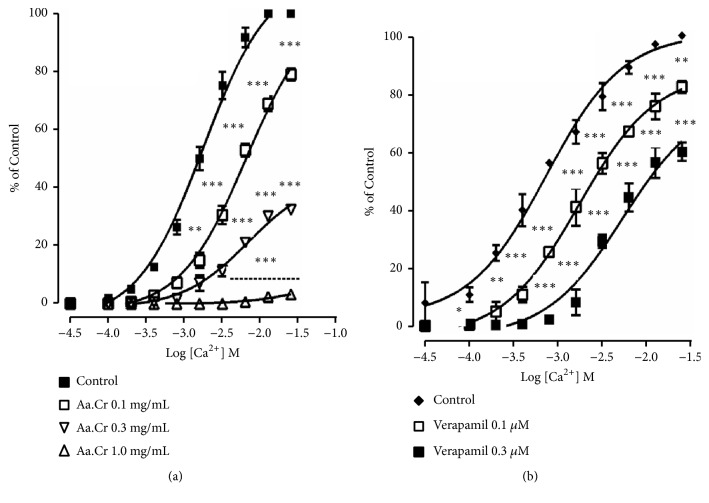
Concentration dependent inhibition of spontaneous contractions and high-K+ induced contractions in isolated piece of rabbit jejunum was observed by the application of verapamil-a standard calcium channel antagonist. Similarly, inhibition of spontaneous contractions and high-K+ induced contractions in isolated rabbit jejunum was observed after the application of Aa.Cr. Observed respective EC50 values of Aa.Cr were 0.98 ± 0.60 mg/mL and 0.40 ± 0.12 mg/ mL. Similar results were observed by Aa.DCM but at lower concentration having respective EC50 values 0.45 ± 0.09 mg/mL and 0.22 ± 0.07 mg/mL against spontaneous contractions and high-K+ induced contractions in isolated jejunum of rabbit (Figure 1). While Aa.Aq caused spasmogenesis against spontaneously contracting isolated rabbit jejunum, this spasmogenic effect was partially blocked with pretreatment of atropine (1μM) (data not shown), a cholinergic antagonist. The behavior of the jejunum observed after the application of Aa.Cr and Aa.DCM showed similarity to the behavior of calcium channel blockers, i.e., verapamil. To confirm the presence of calcium antagonistic activity in Aa.Cr, concentration response curves (CRCs) of calcium were constructed in the presence and absence of Aa.Cr (0.1-1.0 mg/ mL, n= 4) or verapamil (0.01-0.03 μM, n = 4) in a Ca++ free but K+-rich Tyrode's solution. The CRCs were shifted towards right with inhibition of maximum contractile effect (Figure 2).
Figure 1.
Concentration response curves showing concentration dependent spasmolytic effect of (a) Aa.Cr, (b) Aa.DCM, and (c). Verapamil on spontaneous and high-K+ induced contractions in isolated rabbit jejunum. Data is represented as mean ± SEM of 6-8 individual experiments and statistically analyzed with the implication of Two-Way ANOVA followed by Tukey's multiple comparison test. ∗ P<0.05, ∗∗ P<0.01, ∗∗∗ P<0.001 shows a comparison of concentration dependent effects on the basis of individual doses (specified effect compared with the effect of preceding dose).
Figure 2.
Graph showing concentration response curves (CRCs) of calcium (a) in the absence and presence of Aa.Cr (b) in the absence and presence of verapamil in isolated rabbit jejunum preparation. Curves were constructed in Ca++-free but K+-rich Tyrode's solution. Data values are presented as mean ± SEM of 6-8 individual experiments and statistically analyzed with the Two-Way ANOVA followed by Dunnett's multiple comparison test. ∗ P<0.05, ∗∗ P<0.01, ∗∗∗ P<0.001 show a comparison of doses with respect to control.
3.2.2. Relaxant Effect on Isolated Rat Trachea
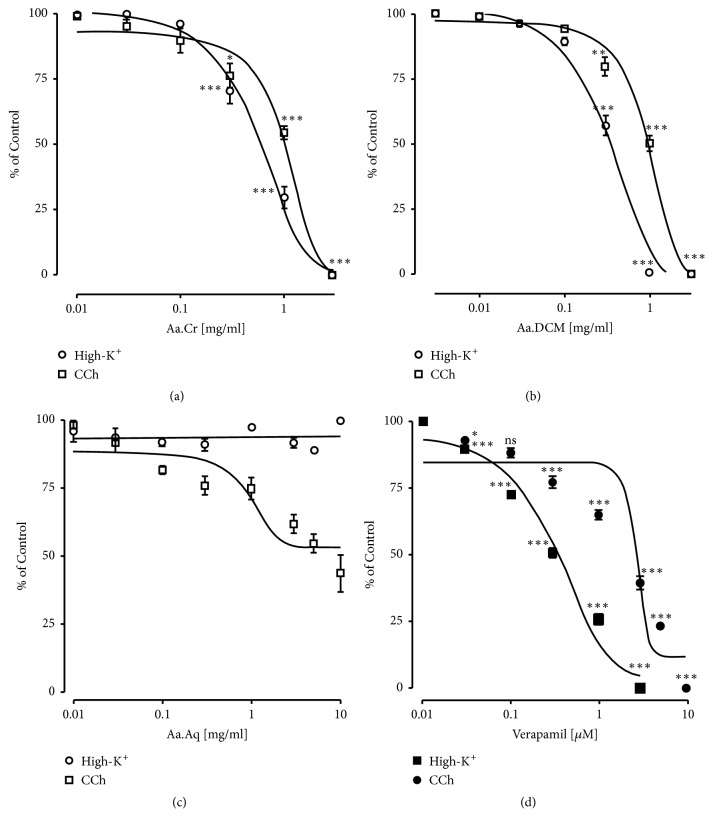
Tracheal relaxant effect was observed by the application of Aa.Cr to precontracted isolated trachea (Figure 3). EC50 value of Aa.Cr against the contractions induced by the application of high-K+ was 0.90±0.06 mg/mL and 3.96±0.16 mg/mL against CCh induced contractions. Aa.DCM also showed relaxant effect on high-K+ and CCh induced tracheal constriction but at low concentration with EC50 values of 0.37±0.08 mg/mL and 1.58±0.06 mg/mL. respectively. Similar response of the tissue was observed by the application of verapamil. While Aa.Aq caused relaxation of high-K+ induced contractions in isolated rat trachea, no effect was observed on CCh induced contractions (Figure 3).
Figure 3.
Concentration response curves showing the relaxant effect of (a) Aa.Cr, (b) Aa.DCM, (c) Aa.Aq, and (d) Verapamil against high-K+ and carbachol (CCh) induced contractions in isolated rat tracheal tissue. Data values are represented as mean ± SEM of 5-8 individual experiments. Two-Way ANOVA followed by Tukey's post-test was utilized to determine the significant difference among various doses of extract on carbachol and high-K+ induced contraction. ∗ P<0.05, ∗∗ P<0.01, ∗∗∗ P<0.001 indicate the specified effect compared with the effect of preceding dose.
3.2.3. Vasodilation Effect
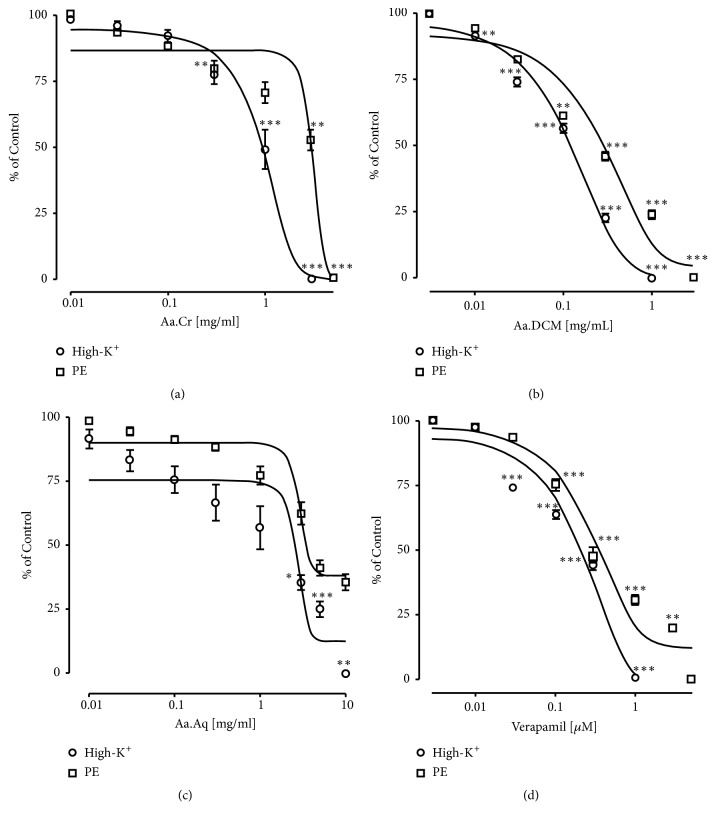
To study the vasodilation potential of extract, contractions were induced in isolated rat aortic tissues by the application of high-K+ and PE. When Aa.Cr was applied to these precontracted aortic tissues, concentration dependent vasodilation was observed. EC50 value of Aa.Cr against high-K+ vasoconstrictions was 1.09±0.07 mg/mL and against phenylephrine (PE) induced vasoconstrictions was 4.76±0.13 mg/mL. But Aa.DCM showed similar results but at lower concentrations with EC50 values of 0.13±0.06 mg/mL against high-K+ induced vasoconstrictions and 0.29±0.09 mg/mL against PE induced vasoconstrictions. Aa.Aq caused 100% relaxation of high-K+ induced vasoconstrictions at the concentration of 10.00 mg/mL with EC50 value of 2.42±0.10 mg/mL, but 64.41±2.97% relaxation with EC50 value of 3.58 ± 0.11 mg/mL was observed against PE induced constrictions (Figure 4).
Figure 4.
Concentration response curves showing the vasodilation effect of (a) Aa.Cr, (b) Aa.DCM, (c) Aa.Aq, and (d) Verapamil against high-K+ and phenylephrine (PE) induced vasoconstriction in isolated rat aorta. Data values are represented as mean ± SEM of 5-8 individual experiments. Two-Way ANOVA followed by Tukey's multiple comparison post-test was utilized to determine the level of significance ( ∗ P<0.05, ∗∗ P<0.01 ∗∗∗ P<0.001) among various concentrations of extract, when challenged with phenylephrine (PE) and high-K+ induced contraction.
3.2.4. Effect on Isolated Atria of Rabbit
Rabbit atrium was used to study the effect of test drugs on cardiac rate and force of contractions. When Aa.Cr was applied cumulatively to carefully isolated rabbit atrium, concentration dependent decrease in force of contraction was observed with EC50 value of 4.09 mg/mL (3.71-4.40), but effect on rate of contraction was not prominent (Figure 5).
Figure 5.
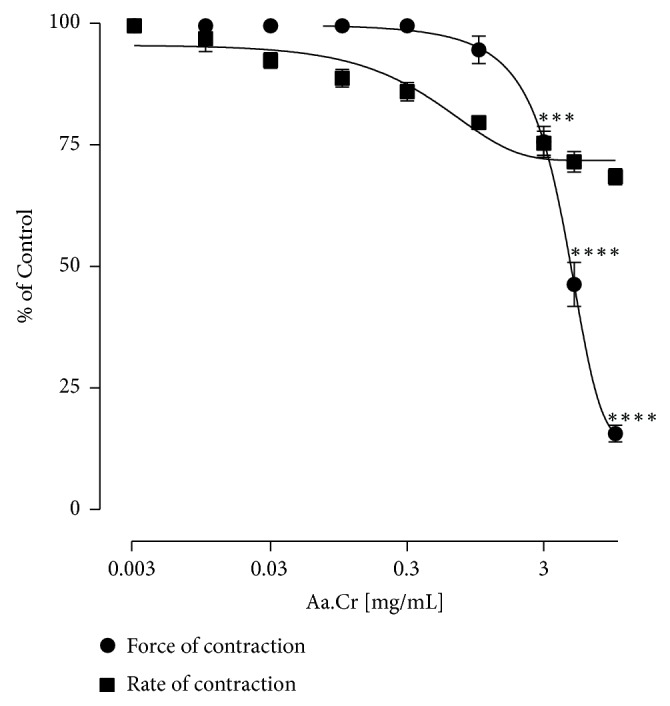
Graph showing the concentration dependent effect of Aa.Cr on isolated rabbit atrium (Data sets are represented as mean ±SEM of 5-6 individual experiments). Two-Way ANOVA followed by Tukey's post-test was utilized to determine the significant difference among various concentrations (∗∗∗ P<0.001).
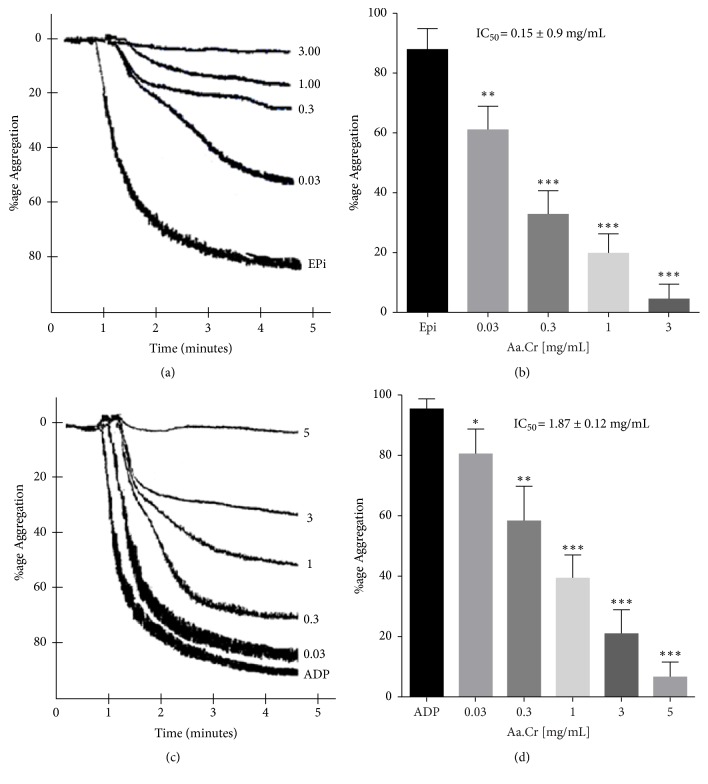
3.2.5. Platelet Aggregation Inhibitory Response
Concentration dependent inhibition of platelet aggregation was observed against epinephrine (Epi) and ADP induced aggregations (Figure 6). Respective IC50 values of Aa.Cr against EPi and ADP induced aggregation were 0.15 ± 0.9 mg/mL (mean ± SEM, n = 4) and 1.87 ± 0.12 mg/mL (mean ± SEM, n = 5-7).
Figure 6.
Original tracings (a and c) and Bar graph (b and d) showing the concentration dependent platelet aggregation inhibitory effect of Aa.Cr against epinephrine (EPi) and ADP induced platelet aggregation (data represented as mean ± SEM, n = 5-7 individual experiments). One-Way ANOVA followed by Dunnett's multiple comparison test was used to compare the response of various concentrations with respect to control aggregation by epinephrine (20 μM) and ADP (5 μM). ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001 as compared to control values.
3.3. In Vivo Activities
3.3.1. Antidiarrheal Effect
Aa.Cr when given orally to the mice which were already treated with castor oil (10 mg/kg) protected them from diarrhea and this protection was dose dependent. Nonsignificant protection was observed in the group treated with Aa.Cr 50 mg/kg while the protection observed at the doses of 100 mg/kg (P ≤ 0.01), 300 mg/kg (P ≤ 0.001), and 500 mg/kg (P ≤ 0.001) was significant like verapamil 10mg/kg (P ≤ 0.001) as shown in Figure 7.
Figure 7.
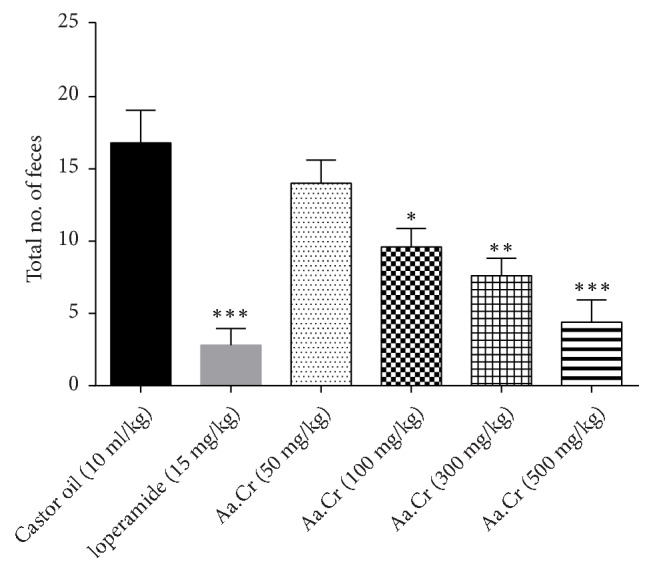
Bar graph indicating antidiarrheal effect of Aa.Cr. Diarrhea was induced by oral administration of castor oil 10 ml/kg. Data sets are presented as mean ± SEM and One-Way ANOVA followed by Dunnett's multiple comparison test was applied for data analysis, ∗P < 0.01, ∗∗P < 0.001, ∗∗∗P < 0.0001 in comparison with control.
3.3.2. Invasive Hypotensive Activity
Normotensive anesthetized rats have been used usually for the study of hypotensive effect of test drugs. When Aa.Cr was given intravenously to preanesthetized rats, dose dependent hypotensive response was evident (Table 1). Significant reduction in blood pressure parameters was observed as compared to normotensive control. Aa.Cr at the dose of 3 mg/kg decreased systolic blood pressure (SBP) to 139.81±3.41 mmHg versus normal SBP 149.00±3.05 mmHg, diastolic blood pressure (DBP) to 101.80±4.18 mmHg versus normal DBP 110.00±5.29mmHg, and mean arterial pressure (MAP) to 114.48±3.45mmHg versus normal MAP 123.45±4.53 mmHg, while Aa.Cr at the dose of 10 mg/kg decreased SBP to 120.25±4.11 mmHg versus normal SBP, DBP to 80.32±5.04 mmHg versus normal DBP, and MAP to 93.63±3.69 mmHg versus normal MAP. Maximum reduction of blood pressure was observed at the dose of 30 mg/kg. Reduction of SBP by the administration of Aa.Cr 30 mg/kg was up to 75.65±3.04 versus normal SBP, DBP was reduced to 48.86±2.45 mmHg versus normal DBP, and reduction of MAP was up to 57.79±2.61 mmHg versus normal MAP.
Table 1.
Hypotensive effect of crude extract of Ailanthus altissima (Aa.Cr) in mmHg on various blood pressure parameters in normotensive anesthetized rats.
| Group | SBP | DBP | MAP |
|---|---|---|---|
| Control | 149.0 ± 3.05 | 110.0 ± 5.29 | 123.45 ± 4.53 |
| Verapamil (1 mg/kg) | 84.33 ± 3.48 | 63.67 ± 2.96 | 70.56 ± 3.12 |
| Aa.Cr (3 mg/kg) | 139.81±3.41 | 101.80±4.18 | 114.48±3.45 |
| Aa.Cr (10 mg/kg) | 120.25±4.11∗∗ | 80.32±5.04∗ | 93.63±3.69∗∗ |
| Aa.Cr (30 mg/kg) | 75.65±3.04∗∗∗ | 48.86±2.45∗∗∗ | 57.79±2.61∗∗∗ |
Data are expressed as mean ±SEM (n = 5 individual experiments). Student t-test was utilized to analyze data, ∗ P<0.05, ∗∗ P<0.01, ∗∗∗ P<0.001.
4. Discussions
Ailanthus altissima is a member of Simaroubaceae family and is wildly used in traditional medicines and homeopathy for the treatment of various disorders including gastrointestinal, respiratory, cardiovascular, neurological, and peripheral disorders. This study was framed to give the pharmacological proofs for its antidiarrheal, antiasthmatic, hypotensive and platelet aggregation inhibitory effects by using both in vitro and in vivo techniques. Its primary phytochemical investigation showed the presence of alkaloids, saponins, triterpenoids, tannins, glycosides, and flavonoids. Previously reported literature indicates that flavonoids, alkaloids, and tannins have a potential for the relaxation of smooth muscles [34–37].
Free cytosolic calcium has a vital role in regulation of smooth muscle contraction and the entry of this calcium into the cell is through voltage operated calcium channels (L-Type) [38]. Free calcium into the cytoplasm activates ryanodine receptors on sarcoplasmic reticulum and results in the opening of calcium channels present on the membrane of sarcoplasmic reticulum. Calcium rushes into the cytoplasm from these sarcoplasmic stores. This calcium binds with calmodulin and calcium calmodulin complex results. This complex activates an enzyme myosin light chain kinase (MLCK). MLCK activates myosin heads and results in the formation of cross bridges and contraction of the smooth muscles occurs. Contraction of vascular smooth muscles increases peripheral vascular resistance which leads to increase in blood pressure whereas contraction of bronchial smooth muscles results in bronchoconstriction and asthma. Similarly contraction of gastrointestinal smooth muscles leads to diarrhea [25, 39]. Blockage of calcium channels inhibits the influx of calcium into the cytoplasm which results in the relaxation of smooth muscles and this blockage of voltage operated calcium channels is beneficial for the treatment of smooth muscle disorders like asthma, diarrhea, and hypertension [40, 41]. Application of high concentration of potassium (high-K+) to smooth muscle tissues results in the opening of voltage operated calcium channels which leads to smooth muscle contraction [42]. Calcium channel blockers have inhibitory role against both spontaneously contracting smooth muscles and smooth muscles preexposed to high-K+ but prominent effect against high potassium induced contractions as compared to inhibition of spontaneous contractions [43, 44]. Similarly when Aa.Cr was tested against spontaneous contractions and the contractions induced by the application of high-K+ in isolated rabbit jejunum, inhibition of both contractions was observed but inhibitory response was prominent against the contractions induced by high-K+ as observed by the verapamil. Similar response was observed by the application of Aa.DCM to the isolated jejunum of rabbit but at lower concentration than Aa.Cr. Blockage of calcium channels was further confirmed when preincubated jejunum was treated with calcium; inhibition of calcium contractile effect was a result with the shifting of concentration response curves of calcium towards right. These in vitro results were confirmed by protection against castor oil induced diarrhea. Calcium channel blockers routinely used for the treatment of gastrointestinal disorders like diarrhea and dysentery and the presence of calcium channel blocking activity in A. altissima provide a mechanistic proof for its use in the treatment of diarrhea. Aa.Aq showed spasmogenic effect when applied to isolated jejunum which was blocked with pretreatment of tissue with atropine. These findings showed that spasmolytic and spasmogenic potential is present in A. altissima but spasmolytic effect is dominant. This type of behavior (having both agonistic and antagonistic effects) is present in most herbal drugs which is beneficial to overcome the adverse effect of the herbal drugs [45].
A. altissima has been used to treat respiratory tract like cold, cough, and asthma [46]. For the validation of its traditional uses its crude extract (Aa.Cr) was applied to tracheal strip isolated from SD rat and showed relaxant effect against high-K+ induced and carbachol (CCh) induced tracheal contractions. Potent effect against high-K+ induced contractions as compared to the effect against CCh induced contractions was seen like verapamil. High-K+ is famous to induce contractions in smooth muscles by opening voltage gated calcium ion channels [47]. Carbachol (CCh) has a reputation to induce contractions in isolated trachea by binding with and stimulating M3 receptors. M3 receptors are muscarinic receptors which when stimulated result in activation of an enzyme phospholipase C [48]. Phospholipase C converts phosphoinositol 4,5-bisphosphate to inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). IP3 results in opening of voltage gated calcium channels in cell membrane wile DAG results in the activation of protein kinase C (PKC) [49, 50]. Ultimate result of IP3 and DAG formation is the contraction of bronchoconstriction [51]. Aa.Cr caused bronchodilation of both high-K+ and CCh induced bronchoconstriction but prominent against high-K+ induced bronchoconstriction. Similar behavior was observed by the application of Aa.DCM to isolated tracheal tissues of rat. But Aa.Aq caused bronchodilation of high-K+ induced bronchoconstriction, but no effect was observed against CCh induced bronconstriction. These results indicate that bronchodilation is due to the blockage of calcium channels (voltage gated) as observed in the case of rabbit jejunum, thus giving a proof that use of A. altissima for the treatment of asthma is due to the blockage of calcium channels. These results also showed smooth muscle relaxant effect of crude effect of A. altissima is concentrated in its organic fraction.
A. altissima has a reputation to treat cardiovascular problems [13, 52, 53]. Therefore, it was evaluated first on isolated aorta of rat then on rabbit atrium to study its effect on blood vessel tone and cardiac rate and force of contraction. Aa.Cr when applied on phenylephrine and high-K+ induced vasoconstrictions showed a concentration dependent vasorelaxant effect. The relaxant effect was more dominant against high-K+ induced vasoconstriction indicating that calcium channel blocker effect is dominant mechanism as observed in the case of jejunum and trachea. Similarly, dichloromethane fraction of crude extract (Aa.DCM) also showed relaxation of precontracted aortic tissues. Aa.Aq showed complete relaxation of high-K+ induced vasoconstrictions and incomplete relaxation of PE induced vasoconstrictions. Vasorelaxant effect of Aa.DCM was dominant over Aa.Cr and Aa.Aq showing that smooth muscle relaxant effect is concentrated in dichloromethane fraction as compared to aqueous fraction. Relaxation of blood vessels results in decrease in peripheral vascular resistance. When applied to isolated atrium of rabbit, Aa.Cr decreased rate and force of contractions and the results were dependent on concentration. Decrease in cardiac rate and force of contraction may lead to reduction of cardiac output. Blood pressure is directly proportional to peripheral vascular resistance (PVR) and cardiac output (CO). Any drug which increases PVR or CO results in increased BP, but a drug which decreases PVR or CO results in decreased BP [54–58]. As observed above, Aa.Cr possesses vasodilation potential which may cause decrease in PVR and BP. The above cardiovascular results were confirmed in vivo. Intravenous application of Aa.Cr to preanesthetized rats showed concentration dependent fall in blood pressure. These results justify the traditional use of A. altissima in cardiovascular disorders like hypertension. In the light of these results, we can say that spasmolytic, bronchodilator, vasorelaxant, and hypotensive activities of A. altissima may be due to the blockage of voltage operated calcium channels, but the involvement of other ion channels and receptors cannot be ruled out.
Platelets play an important role in hemostasis maintenance [59] by the aggregation and formation of platelet plug. Uncontrolled aggregation of platelets may lead to progression of various cardiovascular disorders like stroke, atherosclerosis, myocardial infarction, and hypertension [60, 61]. ADP and EPi play an important role in the aggregation of platelets. ADP binds with G-protein coupled receptors called P2Y1 and P2Y12. ADP has agonistic effect on these receptors. When ADP binds with its receptors, stimulation of receptors takes place. Stimulation of P2Y1 results in the release of Ca++ via diacylglycerol (DAG) and inositol triphosphate (IP3) pathway, but activation of P2Y12 receptors results in inactivation of adenylyl cyclase enzyme which leads to inhibition of release of cAMP. Released calcium activates platelets and events of aggregation start up [62–64]. Epinephrine causes aggregation of platelets by stimulation of α 2-receptors [65–67]. Concentration dependent inhibition of aggregation was seen when ADP and EPi were applied to PRP incubated with Aa.Cr, but the effect against EPi was more potent. Therefore, it may be said that Aa.Cr caused inhibition of platelet aggregation by antagonizing P2Y1, P2Y12, and α 2-receptors. As it has been observed in our isolated tissue experiments, Aa.Cr possessed calcium channel blocking activity, so the involvement of calcium channel potential cannot be ignored.
It has been reported that phytochemical constituents like alkaloids, flavonoids, and tannins present in herbal drugs possess smooth muscle relaxant effects [36, 68, 69]. We have also observed that Aa.Cr contains alkaloids, tannins, and flavonoids. So it can be said that the observed smooth muscle relaxant effects may be due to the presence of these phytochemicals.
5. Conclusion
In short, it can be concluded that Ailanthus altissima may possess calcium antagonistic compounds which could mediate the relaxation of jejunum, tracheal and aortic tissue in ex vivo experiments. These compounds may belong to battery of alkaloids, tannins, and saponins, but the role of other phytochemical constituents and involvement of other ion channels and receptors cannot be ruled out. Inhibition of platelet aggregation may be due to antagonistic effect on P2Y1, P2Y12, and α 2-receptors and therefore downstream inhibition of calcium channel pathways would be a potential target of Aa.Cr. However, this is the preliminary part of experiments and further investigation deems necessary to explore the mechanistic pathways linked with the identification and isolation of active compound (s) from Ailanthus altissima.
List of Abbreviations
- Aa.Aq:
Aqueous fraction of Aa.Cr
- Aa.Cr:
Crude extract of Ailanthus altissima
- Aa.DCM:
Dichloromethane fraction of Aa.Cr
- ADP:
Adenosine diphosphate
- CCh:
Carbachol
- CRC:
Concentration response curves
- DAG:
Diacylglycerol
- DBP:
Diastolic blood pressure
- EPi:
Epinephrine
- High-K+:
High concentration of potassium (K-80 mM)
- IP3:
Inositol triphosphate
- MAP:
Mean arterial pressure
- PE:
Phenylephrine
- PPP:
Platelet poor plasma
- PRP:
Platelet rich plasma
- SBP:
Systolic blood pressure.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
We, the authors of this article, declare that there are no conflicts of interest among us regarding this work.
References
- 1.Hu S. Y. Ailanthus. Arnoldia; 1979. [Google Scholar]
- 2.Albouchi F., Hassen I., Casabianca H., Hosni K. Phytochemicals, antioxidant, antimicrobial and phytotoxic activities of Ailanthus altissima (Mill.) Swingle leaves. South African Journal of Botany. 2013;87:164–174. doi: 10.1016/j.sajb.2013.04.003. [DOI] [Google Scholar]
- 3.Albright T. P., Chen H., Chen L., Guo Q. The ecological niche and reciprocal prediction of the disjunct distribution of an invasive species: the example of Ailanthus altissima. Biological Invasions. 2010;12(8):2413–2427. doi: 10.1007/s10530-009-9652-8. [DOI] [Google Scholar]
- 4.Evans C., Moorhead D., Bargeron C., Douce G. Invasive Plant Responses to Silvicultural Practices in the South. Tifton, GA, USA: The University of Georgia Bugwood Network; 2012. [Google Scholar]
- 5.Mansouritorghabeh H., Jabbari-Azad F., Sankian M., Varasteh A., Farid-Hosseini R. The most common allergenic tree pollen grains in the middle east: a narrative review. Iranian Journal of Medical Sciences. 2017 [PMC free article] [PubMed] [Google Scholar]
- 6.Marwat K. B., Hashim S., Ali H. Weed management: a case study from north-west Pakistan. Pakistan Journal of Botany. 2010;42:341–353. [Google Scholar]
- 7.Rashid S., Ahmad M., Zafar M., et al. Ethnobotanical survey of medicinally important shrubs and trees of Himalayan region of Azad Jammu and Kashmir, Pakistan. Journal of Ethnopharmacology. 2015;166:340–351. doi: 10.1016/j.jep.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 8.Murad W., Ahmad A., Gilani S. A., Khan M. A. Indigenous knowledge and folk use of medicinal plants by the tribal communities of Hazar Nao Forest, Malakand District, North Pakistan. Journal of Medicinal Plants Research. 2011;5(7):1072–1086. [Google Scholar]
- 9.Yasari E., Vahedi A. Study of iranian biospherical reservation areas for medicinal plants diversity. World Academy of Science, Engineering and Technology, International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering. 2011;5:53–56. [Google Scholar]
- 10.Rashed K., Slowing K., Said A., Cueto M. Analgesic, antipyretic and antiulcer activities of Ailanthus altissima (Mill.) Swingle. Phytopharmacology. 2012;3:341–350. [Google Scholar]
- 11.Luís Â., Gil N., Amaral M. E., Domingues F., Duarte A. P. C. Ailanthus altissima (Miller) Swingle: a source of bioactive compounds with antioxidant activity. Bioresources. 2012;7(2):2105–2120. [Google Scholar]
- 12.Gu X., Fang C., Yang G., et al. Acaricidal properties of an Ailanthus altissima bark extract against Psoroptes cuniculi and Sarcoptes scabiei var. Experimental and Applied Acarology. 2014;62(2):225–232. doi: 10.1007/s10493-013-9736-0. [DOI] [PubMed] [Google Scholar]
- 13.Laskar S. A brief resume on the genus Ailanthus: chemical and pharmacological aspects. Phytochemistry Reviews. 2010;9:379–412. [Google Scholar]
- 14.Rahimi R., Ghiasi S., Azimi H., Fakhari S., Abdollahi M. A review of the herbal phosphodiesterase inhibitors; future perspective of new drugs. Cytokine. 2010;49(2):123–129. doi: 10.1016/j.cyto.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Jin M., Yang J. H., Lee E., et al. Antiasthmatic activity of luteolin-7-O-glucoside from Ailanthus altissima through the downregulation of T helper 2 cytokine expression and inhibition of prostaglandin E2 production in an ovalbumin-induced asthma model. Biological & Pharmaceutical Bulletin. 2009;32(9):1500–1503. doi: 10.1248/bpb.32.1500. [DOI] [PubMed] [Google Scholar]
- 16.Wang R., Lu Y., Li H., et al. Antitumor activity of the Ailanthus altissima bark phytochemical ailanthone against breast cancer MCF7 cells. Oncology Letters. 2018;15(4):6022–6028. doi: 10.3892/ol.2018.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo Y. M., Lee B. H., Rashed K., Said A. Antioxidant and cyclooxygenase (COX) inhibitory activities of Ailanthus altissima (Mill.) Swingle leaves. IUFS Journal of Biology. 2012;71(2):77–84. doi: 10.1055/s-0031-1282758. [DOI] [Google Scholar]
- 18.Khan A. S. Trees with Antimicrobial Activities, Medicinally Important Trees. Springer International Publishing; 2017. [DOI] [Google Scholar]
- 19.Souleles C., Waigh R. Indole alkaloids of ailanthus altissima. Journal of Natural Products. 1984;47(4):741–741. doi: 10.1021/np50034a040. [DOI] [Google Scholar]
- 20.Anderson L. A., Harris A., Phillipson J. D. Production of cytotoxic canthin-6-one alkaloids by Ailanthus altissima plant cell cultures. Journal of Natural Products. 1983;46(3):374–378. doi: 10.1021/np50027a014. [DOI] [PubMed] [Google Scholar]
- 21.De Feo V., De Martino L., Quaranta E., Pizza C. Isolation of phytotoxic compounds from tree-of-heaven (Ailanthus altissima Swingle) Journal of Agricultural and Food Chemistry. 2003;51(5):1177–1180. doi: 10.1021/jf020686+. [DOI] [PubMed] [Google Scholar]
- 22.Ohmoto T., Koike K. Studies on the constituents of Ailanthus altissima Swingle. III. The alkaloidal constituents. Chemical & Pharmaceutical Bulletin. 1984;32(1):170–173. doi: 10.1248/cpb.32.170. [DOI] [PubMed] [Google Scholar]
- 23.Ohmoto T., Koike K., Sakamoto Y. Studies on the constituents of Ailanthus altissima Swingle. II. Alkaloidal constituents. Chemical & Pharmaceutical Bulletin. 1981;29(2):390–395. doi: 10.1248/cpb.29.390. [DOI] [Google Scholar]
- 24.Caboni P., Ntalli N. G., Aissani N., Cavoski I., Angioni A. Nematicidal activity of (E, E)-2, 4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. Journal of Agricultural and Food Chemistry. 2012;60(4):1146–1151. doi: 10.1021/jf2044586. [DOI] [PubMed] [Google Scholar]
- 25.Rahman H. M. A., Ahmed K., Rasool M. F., Imran I. Pharmacological evaluation of smooth muscle relaxant and cardiac-modulation potential of Phyla nodiflora in ex-vivo and in-vivo experiments. Asian Pacific Journal of Tropical Medicine. 2017;10(12):1146–1153. doi: 10.1016/j.apjtm.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Janbaz K. H., Akhtar T., Saqib F., et al. Pharmacological justification of use of Solena heterophylla Lour. in gastrointestinal, respiratory and vascular disorders. Journal of Translational Medicine. 2015;13, article 134 doi: 10.1186/s12967-015-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S., Sharma U. K., Sharma A. K., Pandey A. K. Protective efficacy of Solanum xanthocarpum root extracts against free radical damage: phytochemical analysis and antioxidant effect. Cellular and Molecular Biology. 2012;58(1):171–178. doi: 10.1170/T938. [DOI] [PubMed] [Google Scholar]
- 28.Mishra A. K., Kehri H. K., Sharma B., Pandey A. K. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Annals of Clinical Microbiology and Antimicrobials. 2009;8, article 9 doi: 10.1186/1476-0711-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onwukaeme D., Ikuegbvweha T., Asonye C. Evaluation of phytochemical constituents, antibacterial activities and effect of exudate of Pycanthus Angolensis weld warb (Myristicaceae) on corneal ulcers in rabbits. Tropical Journal of Pharmaceutical Research. 2007;6(2):725–730. doi: 10.4314/tjpr.v6i2.14652. [DOI] [Google Scholar]
- 30.Imran I., Hussain L., Zia-Ul-Haq M., Janbaz K. H., Gilani A. H., De Feo V. Gastrointestial and respiratory activities of Acacia leucophloea. Journal of Ethnopharmacology. 2011;138(3):676–682. doi: 10.1016/j.jep.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Rahman H. M. A., Bashir S., Mandukhail S. R., Huda S., Gilani A. H. Pharmacological evaluation of gut modulatory and bronchodilator activities of achyranthes aspera Linn. Phytotherapy Research. 2017;31(11):1776–1785. doi: 10.1002/ptr.5907. [DOI] [PubMed] [Google Scholar]
- 32.Yasin M., Janbaz K. H., Imran I., Gilani A.-U., Bashir S. Pharmacological studies on the antispasmodic, bronchodilator and anti-platelet activities of abies webbiana. Phytotherapy Research. 2014;28(8):1182–1187. doi: 10.1002/ptr.5112. [DOI] [PubMed] [Google Scholar]
- 33.Abdur Rahman H. M., Bashir S., Gilani A. H. Calcium channel blocking activity in Desmostachya bipinnata (L.) explains its use in gut and airways disorders. Phytotherapy Research. 2013;27(5):678–684. doi: 10.1002/ptr.4761. [DOI] [PubMed] [Google Scholar]
- 34.Ajay M., Gilani A. H., Mustafa M. R. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sciences. 2003;74(5):603–612. doi: 10.1016/j.lfs.2003.06.039. [DOI] [PubMed] [Google Scholar]
- 35.Yang Z., Pan A., Zuo W., Guo J., Zhou W. Relaxant effect of flavonoid naringenin on contractile activity of rat colonic smooth muscle. Journal of Ethnopharmacology. 2014;155(2):1177–1183. doi: 10.1016/j.jep.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 36.Moloudizargari M., Mikaili P., Aghajanshakeri S., Asghari M. H., Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacognosy Reviews. 2013;7(14):199–212. doi: 10.4103/0973-7847.120524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto T., Kobayashi T., Ishida K., et al. Vasodilator effect of cassiarin a, a novel antiplasmodial alkaloid from cassia siamea, in rat isolated mesenteric artery. Biological & Pharmaceutical Bulletin. 2010;33(5):844–848. doi: 10.1248/bpb.33.844. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura E., Suzuki H. Dual concentration-dependent effects of phorbol 12, 13-dibutyrate on spontaneous and acetylcholine-induced electrical responses recorded from isolated circular smooth muscle of the guinea-pig stomach antrum. Journal of Smooth Muscle Research. 2004;40(6):259–270. doi: 10.1540/jsmr.40.259. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M., Leung F.-P., Huang Y., Bian Z.-X. Increased colonic motility in a rat model of irritable bowel syndrome is associated with up‐regulation of L‐type calcium channels in colonic smooth muscle cells. Neurogastroenterology & Motility. 2010;22(5):e162–e170. doi: 10.1111/j.1365-2982.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- 40.Ohama T., Hori M., Ozaki H. Mechanism of abnormal intestinal motility in inflammatory bowel disease: how smooth muscle contraction is reduced? Journal of Smooth Muscle Research. 2007;43(2):43–54. doi: 10.1540/jsmr.43.43. [DOI] [PubMed] [Google Scholar]
- 41.Wright D. B., Tripathi S., Sikarwar A., et al. Regulation of GPCR-mediated smooth muscle contraction: implications for asthma and pulmonary hypertension. Pulmonary Pharmacology and Therapeutics. 2013;26(1):121–131. doi: 10.1016/j.pupt.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Gharib Naseri M. K., Mazlomi H., Goshaiesh M., Vakilzadeh G., Heidari A. Antispasmodic effect of Zataria multiflora Boiss. Leaf extract on the rat uterus. Iranian Journal of Pharmaceutical Research. 2010:131–136. [Google Scholar]
- 43.Shah A. J., Gowani S. A., Zuberi A. J., Ghayur M. N., Gilani A. H. Antidiarrhoeal and spasmolytic activities of the methanolic crude extract of Alstonia scholaris L. are mediated through calcium channel blockade. Phytotherapy Research. 2010;24(1):28–32. doi: 10.1002/ptr.2859. [DOI] [PubMed] [Google Scholar]
- 44.Gilani A. H., Mandukhail S.-U., Iqbal J., et al. Antispasmodic and vasodilator activities of Morinda citrifolia root extract are mediated through blockade of voltage dependent calcium channels. BMC Complementary and Alternative Medicine. 2010;10, article 2 doi: 10.1186/1472-6882-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilani A. H., Mehmood M. H., Janbaz K. H., Khan A.-U., Saeed S. A. Ethnopharmacological studies on antispasmodic and antiplatelet activities of Ficus carica. Journal of Ethnopharmacology. 2008;119(1):1–5. doi: 10.1016/j.jep.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 46.Mani M., Sachan N., Tandon A., Wahi A. K. Anti-diarrhoeal activity of methanolic extract of root bark of Ailanthus altissima Swingle (Family: Simaroubaceae) on experimental animals. International Journal of Pharmaceutical Sciences and Research. 2010;1:197–202. [Google Scholar]
- 47.Hoe S.-Z., Lee C.-N., Mok S.-L., Kamaruddin M. Y., Lam S.-K. Gynura procumbens Merr. decreases blood pressure in rats by vasodilatation via inhibition of calcium channels. Clinics. 2011;66(1):143–150. doi: 10.1590/s1807-59322011000100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Z., Balenga N., Cooper P. R., et al. Regulator of G-protein signaling–5 inhibits bronchial smooth muscle contraction in severe asthma. American Journal of Respiratory Cell and Molecular Biology. 2012;46(6):823–832. doi: 10.1165/rcmb.2011-0110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katzung B. G., Chatterjee K. Vasodilators and the treatment of angina pectoris. Basic and Clinical Pharmacology. 2012;7:20–25. doi: 10.1007/BF03312503. [DOI] [Google Scholar]
- 50.Widmaier E. P., Raff H., Strang K. T. Vander’s human physiology. The Mechanisms of Body Function. 2011 [Google Scholar]
- 51.Townsend E. A., Siviski M. E., Zhang Y., Xu C., Hoonjan B., Emala C. W. Effects of ginger and its constituents on airway smooth muscle relaxation and calcium regulation. American Journal of Respiratory Cell and Molecular Biology. 2013;48(2):157–163. doi: 10.1165/rcmb.2012-0231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duke J. A. Handbook of Medicinal Herbs. CRC Press; 2002. [DOI] [Google Scholar]
- 53.Hwang Y. J., Woo H., Kim I., et al. Effect of apoptosis induction of Ailanthus altissima on human lung carcinoma cells. Journal of Agriculture & Life Sciences. 2011;45:91–96. doi: 10.1126/science.1201396. [DOI] [Google Scholar]
- 54.Siddiqui A. Effects of vasodilation and arterial resistance on cardiac output. Journal of Clinical & Experimental Cardiology. 2011;2, article 170 doi: 10.4172/2155-9880.1000170. [DOI] [Google Scholar]
- 55.Katzung B. G., Trevor A. J. Basic & Clinical Pharmacology. New York, NY, USA: McGraw-Hill; 2015. [Google Scholar]
- 56.Brunton L. L. Goodman and Gilman’s Manual of Pharmacology and Therapeutics. New York, NY, USA: McGraw-Hill; 2014. [Google Scholar]
- 57.Rang H. P., Ritter J. M., Flower R. J., Henderson G. Rang & Dale’s Pharmacology E-Book: with Student Consult Online Access. Elsevier Health Sciences; 2014. [Google Scholar]
- 58.Mitchell A., Bührmann S., Saez A. O., et al. Clonidine lowers blood pressure by reducing vascular resistance and cardiac output in young, healthy males. Cardiovascular Drugs and Therapy. 2005;19(1):49–55. doi: 10.1007/s10557-005-6890-6. [DOI] [PubMed] [Google Scholar]
- 59.Łukasik Z. M., Makowski M., Makowska J. S. From blood coagulation to innate and adaptive immunity: the role of platelets in the physiology and pathology of autoimmune disorders. Rheumatology International. 2018;38(6):959–974. doi: 10.1007/s00296-018-4001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puurunen M. K., Hwang S.-J., Larson M. G., et al. ADP platelet hyperreactivity predicts cardiovascular disease in the FHS (Framingham Heart Study) Journal of the American Heart Association. 2018;7(5) doi: 10.1161/JAHA.118.008522.e008522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mekhfi H., Haouari M. E., Legssyer A., et al. Platelet anti-aggregant property of some Moroccan medicinal plants. Journal of Ethnopharmacology. 2004;94(2-3):317–322. doi: 10.1016/j.jep.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 62.Gachet C. P2 receptors, platelet function and pharmacological implications. Thrombosis & Haemostasis. 2008;99(3):466–472. doi: 10.1160/th07-11-0673. [DOI] [PubMed] [Google Scholar]
- 63.Hardy A. R., Conley P. B., Luo J., Benovic J. L., Poole A. W., Mundell S. J. P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood. 2005;105(9):3552–3560. doi: 10.1182/blood-2004-07-2893. [DOI] [PubMed] [Google Scholar]
- 64.Mosa R. A., Oyedeji A. O., Shode F. O., Singh M., Opoku A. R. Triterpenes from the stem bark of Protorhus longifolia exhibit anti-platelet aggregation. African Journal of Pharmacy and Pharmacology. 2011;5(24):2698–2714. [Google Scholar]
- 65.Yun-Choi H. S., Park K. M., Pyo M. K. Epinephrine induced platelet aggregation in rat platelet-rich plasma. Thrombosis Research. 2000;100(6):511–518. doi: 10.1016/S0049-3848(00)00353-4. [DOI] [PubMed] [Google Scholar]
- 66.Woodman R., Brown C., Lockette W. Chlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesis. Hypertension. 2010;56(3):463–470. doi: 10.1161/HYPERTENSIONAHA.110.154476. [DOI] [PubMed] [Google Scholar]
- 67.Bismuth-Evenzal Y., Gonopolsky Y., Gurwitz D., Iancu I., Weizman A., Rehavi M. Decreased serotonin content and reduced agonist-induced aggregation in platelets of patients chronically medicated with SSRI drugs. Journal of Affective Disorders. 2012;136(1-2):99–103. doi: 10.1016/j.jad.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 68.Budriesi R., Ioan P., Micucci M., Micucci E., Limongelli V., Chiarini A. Stop Fitan: antispasmodic effect of natural extract of chestnut wood in guinea pig ileum and proximal colon smooth muscle. Journal of Medicinal Food. 2010;13(5):1104–1110. doi: 10.1089/jmf.2009.0210. [DOI] [PubMed] [Google Scholar]
- 69.Moradi M.-T., Rafieian-Koupaei M., Imani-Rastabi R., et al. Antispasmodic effects of yarrow (Achillea millefolium L.) extract in the isolated ileum of rat. African Journal of Traditional, Complementary and Alternative Medicines. 2013;10(6):499–503. doi: 10.4314/ajtcam.v10i6.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.