Abstract
Butyrate produced by the intestinal microbiota is essential for proper functioning of the intestinal immune system. Total dependence on parenteral nutrition (PN) is associated with numerous adverse effects, including severe microbial dysbiosis and loss of important butyrate producers. We hypothesised that a lack of butyrate produced by the gut microbiota may be compensated by its supplementation in PN mixtures. We tested whether i.v. butyrate administration would (a) positively modulate intestinal defence mechanisms and (b) counteract PN-induced dysbiosis. Male Wistar rats were randomised to chow, PN, and PN supplemented with 9 mM butyrate (PN+But) for 12 days. Antimicrobial peptides, mucins, tight junction proteins, and cytokine expression were assessed by RT-qPCR. T-cell subpopulations in mesenteric lymph nodes (MLN) were analysed by flow cytometry. Microbiota composition was assessed in caecum content. Butyrate supplementation resulted in increased expression of tight junction proteins (ZO-1, claudin-7, E-cadherin), antimicrobial peptides (Defa 8, Rd5, RegIIIγ), and lysozyme in the ileal mucosa. Butyrate partially alleviated PN-induced intestinal barrier impairment and normalised IL-4, IL-10, and IgA mRNA expression. PN administration was associated with an increase in Tregs in MLN, which was normalised by butyrate. Butyrate increased the total number of CD4+ and decreased a relative amount of CD8+ memory T cells in MLN. Lack of enteral nutrition and PN administration led to a shift in caecal microbiota composition. Butyrate did not reverse the altered expression of most taxa but did influence the abundance of some potentially beneficial/pathogenic genera, which might contribute to its overall beneficial effect.
1. Introduction
Parenteral nutrition (PN) represents a life-saving treatment in patients with intestinal failure. However, PN and/or lack of enteral feeding are often associated with serious adverse effects, including impaired mucosal homeostasis, loss of immune reactivity [1], compromised intestinal barrier function, and generalised sepsis [2].
Proper gut barrier function depends on the integrity of physical barriers, i.e., tight junction proteins and adequate mucin production, sufficient production of antimicrobial compounds by Paneth cells and maintaining an optimal balance between immune tolerance to commensal microbiota and the defence against invading pathogens [3]. Lack of enteral feeding significantly affects all of these factors. Paneth cells, which are a specialised type of epithelial cell, release a spectrum of antimicrobial compounds when exposed to alloantigens [4]. The absence of enteral feeding decreases mRNA and protein expression of typical Paneth cell antimicrobials like lysozyme, cryptdin-4, and secretory phospholipase A2, thus compromising their function [5, 6]. The data concerning the effect of PN on the function of Paneth cells are inconclusive, as their antimicrobial functions have been shown to both increase [3] and decrease [7].
Goblet cells (GCs) continuously secrete glycoproteins (mucins) in order to repair and replace the intestinal mucus barrier [8]. Until recently, GCs were considered relatively passive players in promoting intestinal homeostasis and the host defence. However, recent reports indicate that GCs are able to sense and respond to danger signals (such as bacterial pathogens) as well as modulate the composition of the gut microbiome by modifying mucin secretion [9]. In a piglet model of enteral nutrition deprivation, GC expansion was established within a few days after the start of total or partial PN [10], which might reflect a higher degradation rate of the mucus layer, a lower rate of mucus secretion, or an altered rate of mucin turnover [11]. These data indicate that starvation alters mucus dynamics in the small intestine, which may in turn affect the intestinal defence capacity [11, 12].
The gut microbiota has an irreplaceable role in the maturation of mucosal and systemic immunity [13–15]. Depending on its composition, it may either promote a tolerogenic state in the intestinal mucosa [16–20] and instigate mechanisms preventing bacterial overgrowth or induce proinflammatory status associated with impaired gut barrier function [21]. PN itself, together with a lack of enteral feeding, generates a significant shift in microbiota composition. In rodent models, PN and starvation are associated with decreased gut microbiota diversity, the enrichment of potentially pathogenic and inflammation-promoting species, and the depletion of beneficial anaerobes [3, 7, 22]. Heneghan [7] hypothesises that the PN-associated shift in the gut microbiota may be part of a causal relationship with attenuated antimicrobial compound production.
Besides interacting directly with the host intestinal and immune cells, the gut microbiota may affect host intestinal homeostasis via fermentation products. Short-chain fatty acids (SCFA) have multiple beneficial effects on performance and intestinal health [23]. SCFA are produced by the fermentation of soluble fibre. To target intestinal SCFA production, an often-used treatment is to supplement the diet with prebiotics (dietary fibre), probiotics (mostly Lactobacillaceae or Bifidobacteriaceae), or a combination of both. Unfortunately, this approach is not applicable to all situations. Particularly PN-dependent patients with short bowel syndrome often exhibit an increased abundance of Lactobacillaceae as well as a lack of butyrate producers in the gut. Therefore, prebiotic/probiotic supplementation may result in D-lactate acidosis or Lactobacillus sepsis. The alternative to prebiotic/probiotic treatment is the direct administration of butyrate either per os or intravenously. To our knowledge, no study has been published on the effect of i.v. butyrate on the microbiota in a PN context. The purpose of this study was to determine whether the supplementation of a nutrition mixture with butyrate (9 mM) in the absence of enteral feeding would affect immune function and gut microbiota composition. In order to examine this hypothesis, we used a rat model of total parenteral nutrition and assessed the effect of i.v. butyrate on Paneth cell function, mucin production, intestine-associated immune cells, and the gut microbiome.
2. Materials and Methods
2.1. Animals and Experimental Design
Male Wistar rats (Charles River, initial weight 300-325 g) were kept in a temperature-controlled environment under a 12h light/dark cycle. For PN administration, the right jugular vein was cannulated with a Dow Corning Silastic drainage catheter (0.037 inch) as previously described [3]. Control animals underwent the same operation. The catheter was flushed daily with TauroLock HEP-100 (TauroPharm GmbH, Waldbüttelbrunn, Germany). After the operation, the rats were housed individually and connected to a perfusion apparatus (Instech, PA, USA), which allows free movement. For the next 48 hours, the rats were given free access to a standard chow diet (SD, SEMED) and provided Plasmalyte (BAXTER Czech, Prague, CZ) via the catheter at increasing rates (initial rate: 1 ml/hr; goal rate: 4 ml/hr) in order to adapt to the increasing fluid load. Two days after the operation, the rats were randomly divided into three groups. Rats in the experimental groups (PN; PN+But) were provided PN (205 kcal. kg−1. d−1; 10 hrs per day; rate 4 ml. hr−1; light period), the composition of which is given in Table S1. In the PN+But group, the PN mixture was supplemented with 9 mM butyrate. Stability of butyrate (monitored as butyric acid) in PN was tested using solid phase microextraction coupled to gas chromatography with mass spectrometric detector. Butyrate was stable at room temperature for at least 24 hours after its addition into PN.
PN alone, PN+But or Plasmalyte was administered for 12 days. All experiments were performed in accordance with the Animal Protection Law of the Czech Republic 311/1997 in compliance with the Principles of Laboratory Animal Care (NIH Guide for the Care and Use of Laboratory Animals, 8th edition, 2013) and approved by the Ethical Committee of the Ministry of Health, CR (approval no. 53/2014).
2.2. Histological Evaluation
Tissue samples (distal ileum, proximal colon) were fixed in 4% paraformaldehyde, embedded in paraffin blocks, and routinely processed. Sections cut at 4-6 μm were stained with haematoxylin/eosin and examined with an Olympus BX41 light microscope. Mucosal thickness was measured through specialised camera CANON EOS (Canon, Tokyo) and microscope imaging software system QuickPHOTO Camera 3.2 (Promicra, Prague) that provides advanced measuring, editing, annotating, and saving of acquired images and measurements. Before measuring, an appropriate calibration of objectives was performed. The measurement of mucosal thickness of each sample was repeated six times and the average thickness of mucosa was determined. The number of Paneth cells in a base of the Lieberkühn crypts was recorded per individual histological section of ileum and the average number per 10 crypts was calculated. The number of Goblet cells was recorded per 200 enterocytes in well preserved parts of thin sections of ileum and the average number was calculated.
2.3. Intestinal Permeability
Intestinal permeability of the isolated segments of ileum was measured as previously described [3]. Briefly, cca 8 cm segment of terminal ileum was resected, inverted, filled with 1 ml Tris buffer (125 mmol/l NaCl, 10 mmol/l fructose, 30 mmol/l Tris, pH=7.5), and closed at both ends. The segments were put into container with heated (37°C) Tris buffer containing HRP enzyme (0.04μg/l) and incubated for 45 min. After incubation, the inner content of the segment was precisely collected and HRP concentration was determined using spectrophotometry (450 nm, substrate: tetramethylbenzidine).
2.4. Immunohistochemistry
Paraffin sections (4 μm) were deparaffinised in xylene and rehydrated in graded ethanol. Endogenous peroxidase was blocked, with proteinase K digestion (Dako, Glostrup, Denmark) used for antigen retrieval. The primary antilysozyme antibody (rabbit polyclonal, Dako, Glostrup, Denmark) was detected using Histofine Simple Stain Rat MAX PO (Nichirei, Japan). Lysozyme staining intensity was assessed by two independent blinded observers (scale 0 to 3), with average scores presented for each group.
2.5. Flow Cytometry
Single cell suspensions from mesenteric lymph nodes (MLN) were obtained by gently fragmenting and filtering the tissues through 100 μm cell strainers (Sigma Aldrich), with lymphocytes isolated by centrifugation on Ficoll (ρ = 1.077 g/ml, GE Healthcare). Isolated cells were frozen and stored at -80°C until analysis. Prior to staining, the lymphocytes were thawed and incubated for two hours in RPMI 1640 + 10% FCS, 2mM L-glutamine, 1% Pen/Strep. Panels for both effector and regulatory T cells were stained simultaneously. First, cells were surface-stained using the following anti-rat antibodies: anti-CD45-FITC (OX-1, Thermo Fisher Scientific), anti-CD4-BV-786 (OX-35, BD Biosciences), and anti-CD8α-PerCP-e710 (OX-8, Thermo Fisher Scientific), for the effector T-cell panel, and anti-CD45-FITC, anti-CD4-BV-786, and anti-CD25-PE (OX-39, Thermo Fisher Scientific) for the regulatory T-cell panel. Second, the cells in both panels were fixed and permeabilised using an intracellular staining kit (Anti-Mouse/Rat Foxp3 Staining Set APC, Thermo Fisher Scientific) either with Foxp3 antibody (FJK-16s, regulatory T cells) or PBS (effector T cells) in conjunction with 15-min blocking using 2% normal rat serum (regulatory T cells only, Thermo Fisher Scientific). Immediately after staining with anti-Foxp3-APC, the lymphocytes were analysed using the BD LSR II flow cytometer (BD Biosciences). The stability of the antigens of interest after one freezing/thawing cycle was tested in an independent experiment (Figure S1).
2.6. RT-qPCR
Pieces of the distal ileum (5-8 cm from the ileocaecal valve) were rapidly dissected, flushed first with cold saline and then with RNA later, and opened along the mesenteric border, and the mucosa was then scraped using a glass slide and immediately frozen in liquid nitrogen. To determine cytokine expression, Peyer's patches were dissected from the rest of the ileum. Total RNA was extracted using the RNeasy PowerMicrobiome Kit (Qiagen, Hilden, Germany). A DNAase step was included to avoid possible DNA contamination. A standard amount of total RNA (1600 ng) was used to synthesise first-strand cDNA with the High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA, USA). The RT-PCR amplification mixture (25ul) contained 1 ul template cDNA, SYBR Green Master Mix buffer (QuantiTect, Qiagen, Hilden, Germany) and 400nM (10 pmol/reaction) of sense and antisense primers. Primers were designed based on known rat sequences taken from the GeneBank Graphics database: https://www.ncbi.nlm.nih.gov. Primer design was performed with Primer3 software: http://bioinfo.ut.ee/primer3-0.4.0/primer3/ (Table 1). The reaction was run on the ViiA 7 Real-Time PCR System (Thermo Fisher Scientific, USA). Results were analysed using SDS software, ver. 2.3 (Applied Biosystems, Foster City, CA, USA). The expression of genes of interest was normalised to the housekeeper gene Rplp2 and calculated using the ΔΔCt method.
Table 1.
Primer sequences (5′ - 3′).
| sense (forward) | antisense (reverse) | ||
|---|---|---|---|
| Defa 8 | NM_001033077.1 | GGTCCAGGCTGATCACATTC | TTATGTCCTCCCTGGTGTCC |
| lysozyme | NM_012771.3 | AAGGCATTCGAGCATGGGTG | TGAGAAAGAGACAGTGTGAGCTG |
| RegIII | NM_173097.1 | GAGCCTCAGGATTTCTGAG | TCAAATGAGAGGAAGGAAGG |
| Muc3 | XM_017598596.1 | CAACGAAGAACAAGAAAACG | TGGGCTCTTCTGAATCTGG |
| Muc2 | NM_173097.1 | CCAATATCACCTGCCCTGAC | AGCAAGAACACCCATGATCC |
| Fcgbp | NM_001164657.2 | TCTCCCCATGTCCCAACTG | GTTTGAATTCAGGGGCTCAG |
| IFNγ | NM_138880.2 | CCAAGTTCGAGGTGAACAAC | CCAGAATTCTTCTTATTGGCACAC |
| IL-10 | NM_012854.2 | CTGCAGGACTTTAAGGGTTACTTG | TTCTCACAGGGGAGAAATCG |
| TNFα | NM_012675.3 | ACGTCGTAGCAAACCACCAAG | TGTGGGTGAGGAGCACATAG |
| IFNγ | NM_138880.2 | CCAAGTTCGAGGTGAACAAC | CCAGAATTCTTCTTATTGGCACAC |
| ZO-1 | NM_001106266.1 | TGTTCCTGTGAGTCCTTCAG | AAGGTGGGAGGATGCTATTG |
| Cldn7 | NM_031702.1 | CATCGTGGCAGGTCTTGCTG | GTGCACGGTATGCAGCTTTG |
| Igha | NC_005105.4 | ATCCCACCATCTACCCACTGA | ATTGTTCCAGCGCTCGGCA |
| IL-4 | NM_201270.1 | CCACGGAGAACGAGCTCATC | GAGAACCCCAGACTTGTTCTTCA |
| Cdh1 | NM_031334.1 | GAAGACCAGGACTTTGATTTG | TCAGAACCACTCCCCTCATAG |
| Rplp2 | NM_001030021.1 | TCGCTCAGGGTGTTGGCAAG | AGGCCAAATCCCATGTCGTC |
2.7. Determination of Microbiota Composition
Microbiota composition was determined in caecum content. All samples were frozen at -20°C until required. DNA was isolated using the QIAamp PowerFecal DNA Kit (Qiagen). Extracted DNA was used as a template in amplicon PCR to target the hypervariable V4 region of the bacterial 16S rRNA gene. A 16S metagenomics library was prepared according to the Illumina 16S Metagenomic Sequencing Library Preparation protocol, with some modifications described below. Each PCR was performed in triplicate, with the primer pair consisting of Illumina overhang nucleotide sequences, an inner tag and gene-specific sequences (forward: TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-InnerTag-GTGYCAGCMGCCGCGGTAA; reverse: GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGC-InnerTag-GGACTACNVGGGTWTCTAAT) [24, 25]. The Illumina overhang served to ligate the Illumina index and adapter. Each inner tag—a unique sequence of 7–9 bp—was designed to differentiate samples into groups. After PCR amplification, triplicates were pooled and the amplified PCR products were determined by gel electrophoresis. PCR clean-up was performed with Agencourt AMPure XP beads (Beckman Coulter Genomics). Samples with different inner tags were equimolarly pooled based on fluorometrically measured concentrations using the Qubit® dsDNA HS Assay Kit (Invitrogen™, USA) and microplate reader (Synergy Mx, BioTek, USA). Pools were used as a template for the second PCR with Nextera XT indexes (Illumina, USA). Differently indexed samples were quantified using the KAPA Library Quantification Complete Kit (Kapa Biosystems, USA) and equimolarly pooled according to the measured concentration. The prepared library was checked with the 2100 Bioanalyzer Instrument (Agilent Technologies, USA), with concentrations measured by qPCR shortly prior to sequencing. The library was diluted to a final concentration of 8 pM with the addition of 20 % PhiX DNA (Illumina, USA). Sequencing was performed using the Miseq Reagent Kit v2 according to the manufacturer's instructions (Illumina, USA).
2.8. Data Processing and Statistical Analysis
Sequencing data, i.e., raw sequences, were processed using standard bioinformatic procedures within QIIME 1.9.1 package [26]. In short, these include quality filtering, chimera removal, open reference clustering, and taxonomic identification based on the SILVA 123 database and UCLUST algorithm [27]. Raw sequences were filtered according to default quality requirements in QIIME 1.9.1 (-r: 3; -p: 0.75; -n:0; -q:3). Chimeras were detected and filtered using the UCHIME algorithm with the Gold database. Data were afterwards clustered at the 97% similarity threshold against SILVA database version 123. Representative sequences were aligned, and a phylogenetic tree was constructed and taxonomic identity determined by the USEARCH algorithm. The data were treated as compositional (proportions of total read count in each sample, nonrarefied) and prior to all statistical analyses were transformed using centered log-ratio transformation [28]. Sequencing data are available from ENA database under the accession number PRJEB28521. All analyses were performed in R, version 3.4.2. [29].
Gene expression data and flow cytometry data are presented as mean ± SD. Statistical analysis was performed using the Kruskal-Wallis test with multiple comparisons. Differences were considered statistically significant at the level of p<0.05. For testing group pairwise differences in microbial composition, we applied ANOVA test with Tukey's honest significance. The statistical analyses were performed on each of the six taxonomy levels (Phylum, Class, Order, Family, Genus, and OTUs) separately. The resulting p-values were adjusted for multiple hypothesis testing using the Benjamini-Hochberg procedure. Results were considered significant at FDR<=10%. Hierarchical clustering with Euclidean distance and the average-linkage algorithm was used to cluster microbial profiles in the heatmap and the radar chart.
3. Results
3.1. Ileal and Colonic Architecture
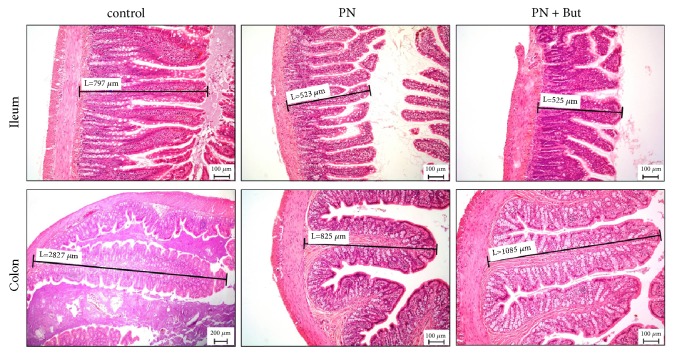
Compared with controls, we observed significantly reduced mucosal thickness in the ileum (550±40 versus 746±28 μm, p<0.05) and colon (886±90 versus 2750±110 μm, p<0.01) (Figure 1) in rats totally dependent on PN. Butyrate supplementation had no effect on these parameters (ileum: 535±32; colon: 1020±103 μm).
Figure 1.
Histology of the intestinal mucosa. Mucosal thickness was assessed in the small intestine (ileum) and the large intestine (colon). Sections of intestinal tissues were stained with H&E (magnification x100).
3.2. Butyrate Stimulates Paneth Cell Function
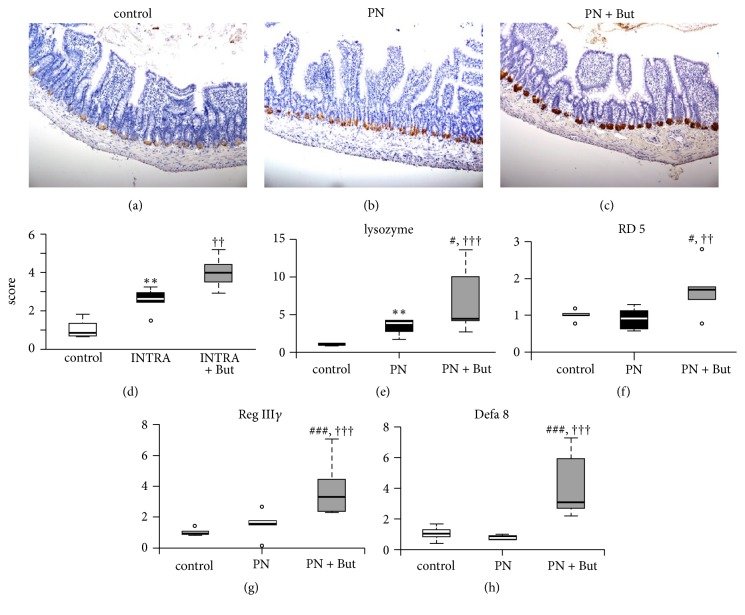
To examine the potential Paneth cell alterations associated with butyrate administration, we determined the expression of Paneth cell-produced compounds. First, we examined the expression of lysozyme. Immunohistochemical staining confirmed its presence in Paneth cell granules in the ileum in all groups (Figures 2(a)–2(c)). Based on staining intensity, PN administration substantially increased lysozyme expression compared with controls. Supplementation of the PN mixture with butyrate resulted in the further elevation of lysozyme-specific staining intensity (Figure 2(d)). Corresponding results were obtained at the mRNA level (Figure 2(e)). Next, we determined the expression of other antimicrobial peptides, i.e., α-defensins (Rd5, Defa8) and RegIIIγ (Figures 2(f)–2(h)). Whereas PN alone had no effect, we found significantly increased expression of all three compounds in the PN+But group. The number of Paneth cells per crypt was similar in all three groups (control: 4.7±0.8; PN: 5.3±0.9; PN+But: 4.8±0.8). In conclusion, our data show that supplementation of the PN mixture with butyrate is associated with increased Paneth cell function, as measured by the expression of antimicrobial peptides.
Figure 2.
Host defence peptide proteins and mRNA expression in the ileum. (a)–(c) Lysozyme staining, magnification x 200; (d) lysozyme staining quantification; (e) lysozyme mRNA expression; (f) RD5 mRNA expression; (g) Defa8 mRNA expression; (h) RegIIIγ mRNA expression. mRNA expression is given as a fold change over the control group. Results are presented using Tukey box-and-whisker plots as quartiles (25%, median, and 75%). ∗∗p <0.01 PN versus control; ††p <0.01; †††p<0.001 PN+But versus control; #p<0.05, ###p<0.001 PN+But versus PN.
3.3. Butyrate Promotes Mucin Production
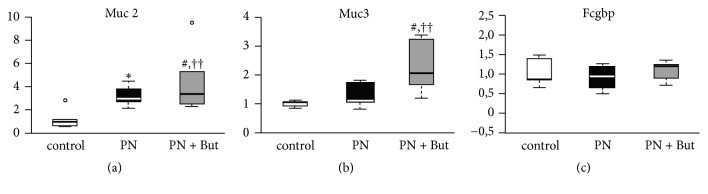
GCs specialise in producing and secreting mucin glycoproteins and other factors to form a protective mucus layer in the intestine. We assessed their function according to the number of GCs (normalised as the GC number per 200 enterocytes) and by mRNA expression of three GC products in the ileum. GC numbers tended to be higher in the PN group compared with controls, but the difference was not statistically significant (53.2±5 versus 44±9.4, p=0.08). The addition of butyrate resulted in a further increase in abundance (63.6±8.5, p<0.01 versus controls, p<0.05 versus PN). Expression of Muc2, the main secretory mucin, increased in the PN group compared with controls and was further potentiated by butyrate. Muc3, the dominant transmembrane mucin, was elevated only in the PN+But group. Fcgbp expression was not affected in any group (Figure 3). These data indicate that in response to the absence of enteral feeding GCs increase activity and that butyrate supplementation significantly stimulates this process.
Figure 3.
mRNA expression of mucosa-forming genes in the ileum. (a) Muc2; (b) Muc3; (c) Fcgbp. mRNA expression is given as a fold change over the control group. Results are presented using Tukey box-and-whisker plots as quartiles (25%, median, and 75%). ∗p<0.05 PN versus control; ††p<0.01 PN+But versus control; #p<0.05; ##p<0.01 PN+But versus PN.
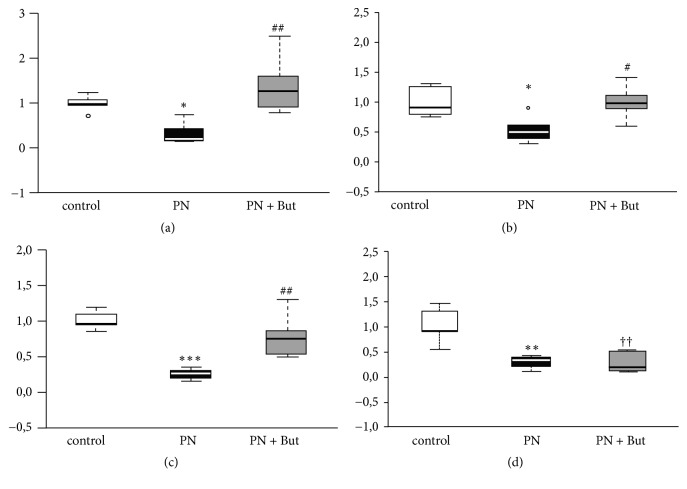
3.4. Butyrate Alleviates PN-Induced Small Intestinal Permeability
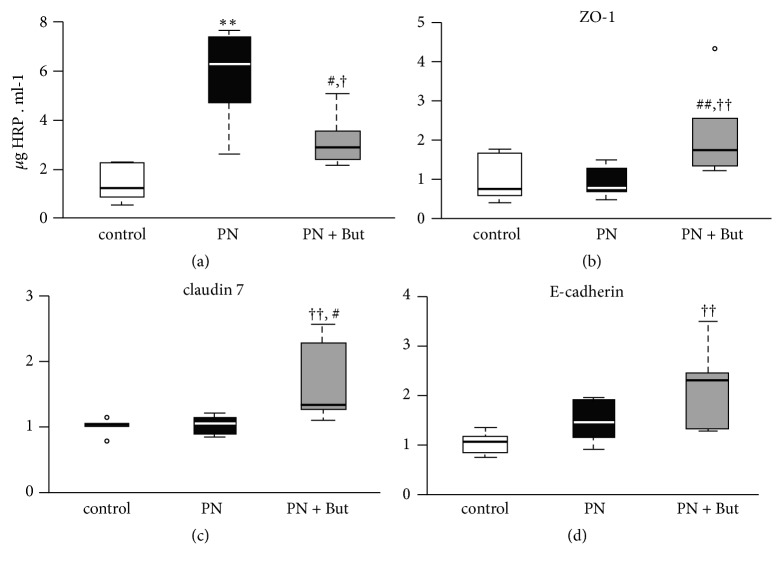
The effect of butyrate on small intestinal integrity was assessed by in vitro permeability for HRP and by the expression of tight junction proteins. Ileal segments of both the PN and PN+But groups were more permeable for HRP compared with controls (Figure 4(a)). Butyrate supplementation decreased intestinal permeability compared with the PN group, although it did not match the control level. The expression of tight junction proteins (ZO-1, claudin-7, E-cadherin) was similar in the control and PN groups and significantly increased in the PN+But group (Figures 4(b)–4(d)). In summary, these findings support the hypothesis that butyrate alleviates the detrimental effect of PN on intestinal permeability via the stimulation of tight junction protein expression.
Figure 4.
The effect of butyrate on tight junction proteins mRNA expression and intestinal permeability. (a) ZO-1 mRNA; (b) E-cadherin mRNA; (c) claudin-7 mRNA; (d) HRP leakage in vitro. mRNA expression is given as a fold change over the control group. Results are presented using Tukey box-and-whisker plots as quartiles (25%, median, and 75%). ∗p<0.05 PN versus control; ††p<0.01 PN+But versus control; #p<0.05; ##p<0.01 PN+But versus PN.
3.5. Effect of Butyrate on Lymphocyte Phenotypes and Cytokine Expression
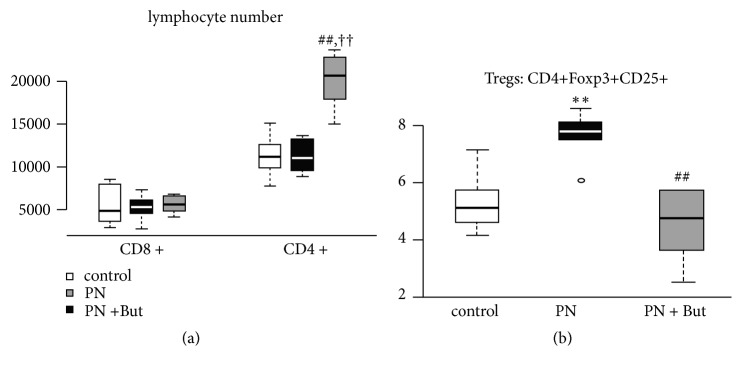
In order to determine the effect of butyrate on gut-associated T-cell subpopulations, we isolated lymphocytes from MLN and analysed them by flow cytometry (Figure 5). In MLN, PN alone did not affect the total number of CD4+ or CD8+ lymphocytes, CD4+/CD8+ ratio (2.3±0.5 versus 2.4±0.4) or percentage of different CD8+ subpopulations, but it did increase the percentage of CD4+Foxp3+CD25+ (Treg). Butyrate supplementation led to a significant rise in CD4+ lymphocytes but did not change the total number of CD8+ lymphocytes, resulting in an increased CD4+/CD8+ ratio (3.5±0.2).
Figure 5.
The effect of butyrate on the distribution of T-cell subpopulations in mesenteric lymph nodes. (a) Total CD4+ and CD8+ lymphocyte numbers; (b) Tregs subpopulation. Results are presented using Tukey box-and-whisker plots as quartiles (25%, median, and 75%). ∗∗p<0.01 PN versus control; ††p<0.05 PN+But versus control; ##p<0.01 PN+But versus PN.
In the PN group, we found significant attenuation of IL-10 (Figure 6(a)) and IL-4 mRNA (Figure 6(b)) expression in Peyer's patches as well as IgA mRNA expression (Figure 6(c)) in the intestinal mucosa. In the PN+But group, the expression of both cytokines increased to the levels observed in controls and IgA expression was nearly normalised. IFNγ expression was decreased in both PN-dependent group compared with controls (Figure 6(d)). Taken together, butyrate added to a PN mixture is associated with an increase in the total CD4+ lymphocyte population, normalisation of the Tregs subpopulation in MLN, and an increase in gut mucosal immunity.
Figure 6.
The effect of butyrate on cytokine and IgA mRNA expression. (a) IL-10 expression in Peyer's patches; (b) IL-4 expression in Peyer's patches; (c) IgA expression in the intestinal mucosa; (d) IFNγ expression in Peyers patches. mRNA expression is expressed as a fold change over the control group. Results are presented using Tukey box-and-whisker plots as quartiles (25%, median, and 75%). ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001 PN versus control; ††p<0.01 PN+But versus control; #p<0.05; ##p<0.01 PN+But versus PN.
3.6. Effect of Butyrate Supplementation on the Microbiota
Microbiota composition was assessed via sequencing of the 16S rRNA gene in caecum content sampled at the time of sacrifice. Alpha diversity was assessed in terms of species richness (OTU numbers, Chao1 index) or evenness (Shannon index, Simpson index) (Table 2). Caecal microbiota in PN+But group tend to be less diverse compared with control or PN groups, but this tendency reached the statistical significance only when OTUs number is concerned.
Table 2.
Alpha diversity.
| OTUs | Chao1 | Shannon | Simpson | |
|---|---|---|---|---|
| control | 477 (180) | 814 (340) | 6.23 (1.14) | 0.96 (0.03) |
| PN | 429 (109) | 726 (289) | 6.04 (1.43) | 0.96 (0.1) |
| PN+But | 328 (157)† | 583 (288) | 4.53 (3.44) | 0.85 (0.34) |
Data are given as median and IQR. † p <0.05 PN+But vs. control.
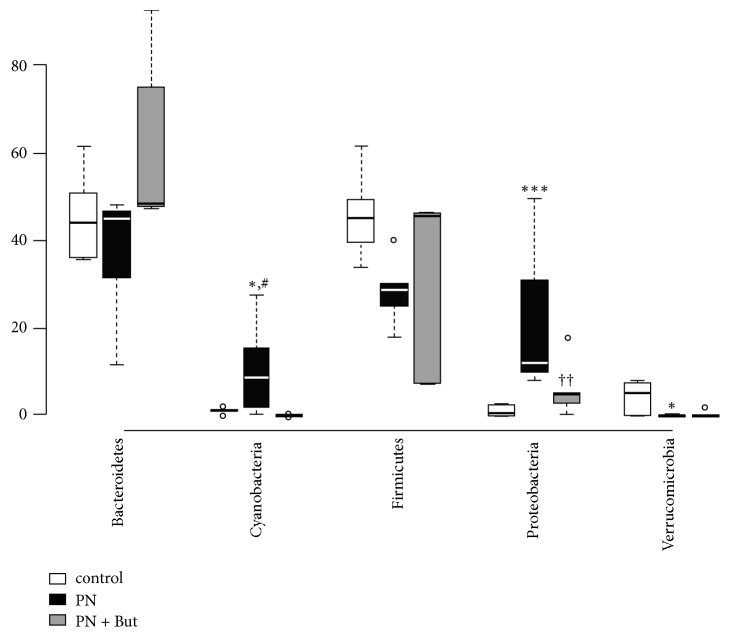
The absence of enteral feeding in combination with PN administration had a significant effect on gut microbiota composition. At the phylum level, Proteobacteria significantly increased in both PN-dependent groups. Butyrate administration was associated with a decrease in Proteobacteria abundance, but this trend did not reach statistical significance. Butyrate supplementation counteracted the deregulation of Cyanobacteria observed in the PN group (Figure 7).
Figure 7.
Microbiota composition in the caecum: phylum level. Results are presented using Tukey box-and-whisker plots as quartiles (25%, median, and 75%) and outliers (open circles). ∗p<0.05, ∗∗∗p<0.001 PN versus control; ††p<0.05 PN+But versus control; #p<0.05 PN+But versus PN.
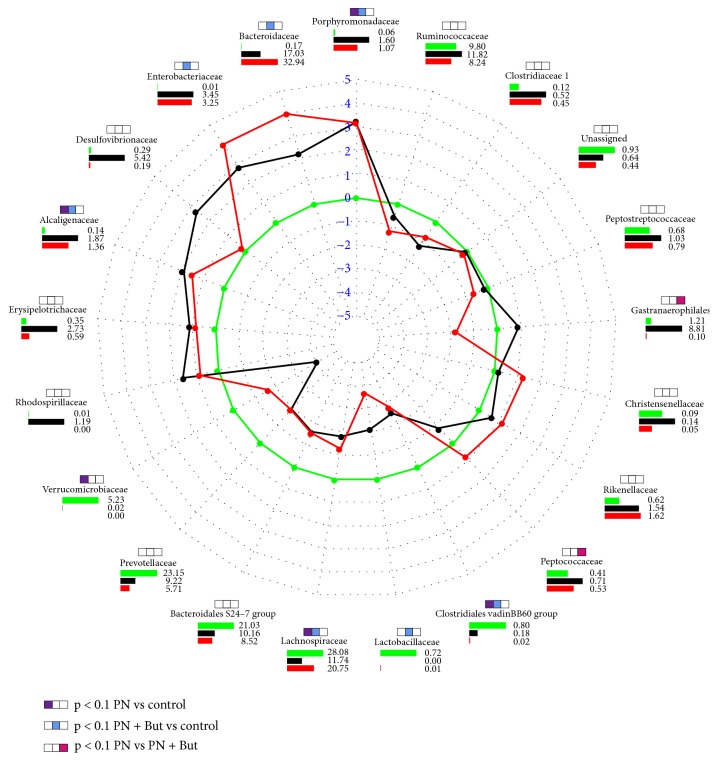
The distribution pattern of abundant (<1%) bacterial families is shown in Figure 8. Porphyromonadaceae and Alcaligenaceae were significantly elevated while the Clostridiales vadinBB60 was reduced in both PN-dependent groups compared with controls. The abundance of Bacteroidaceae, Enterobacteriaceae, Lachnospiraceae, and Lactobacillaceae was significantly altered only in one of the PN-dependent groups compared with controls, but the trend was similar, i.e., of the same orientation, in both of them. Butyrate supplementation had significant effect on the abundance of Peptococcaceae and one unidentified taxon belonging to Gastranaerophilales.
Figure 8.
Distribution pattern of all abundant (<1%) bacterial families in the caecum. Lines show the fold change versus controls; green line represents the null change (controls versus controls). Bar charts demonstrate the relative abundance (%) of each family. Colour key: green = controls; black = PN; red = PN+But. The significance (p<0.05) is shown using coloured boxes above the family names.
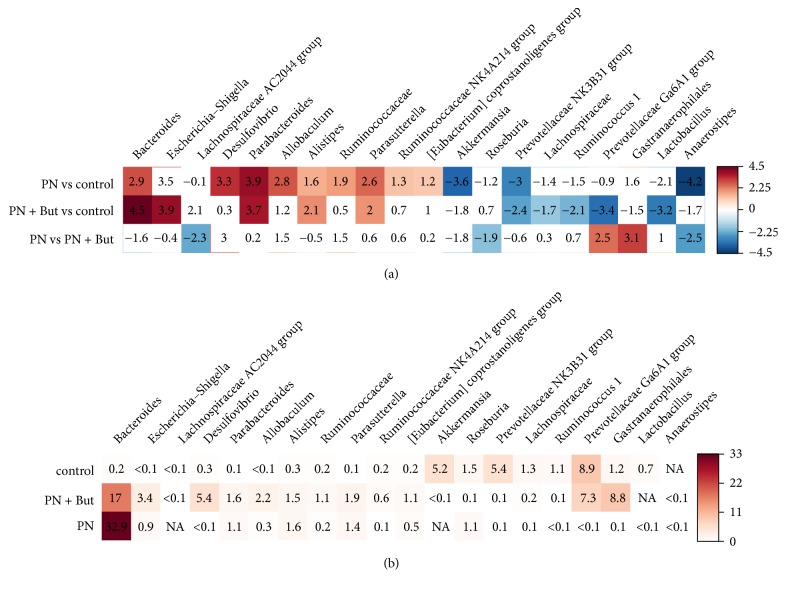
We identified 20 genera that were significantly differently (p < 0.05) represented in at least one of the PN-dependent groups compared with controls (Figure 9). Five genera were deregulated in both the PN and PN+But groups, i.e., Bacteroides, Parabacteroides, Alistipes, Parasutterella (increased), and Prevotellaceae NK4A214 group (decreased). Compared with the PN group, butyrate supplementation resulted in the increased abundance of Anaerostipes, Lachnospiraceae AC2044 group, and Roseburia but decreased the representation of the Prevotellaceae Ga6A1 group and unidentified bacteria from the Gastranaerophilales order. Similar trend (PN+But < PN) was observed in case of Desulfovibrio sp. (p=0.055). All relevant statistical data are shown in Table S2. Our data confirm the profound effect of the lack of enteral feeding on microbiota composition. Butyrate supplementation counteracted only some of the alterations.
Figure 9.
Heatmap showing the fold change (a) and the abundance (%) (b) of genera that were differently (p<0.05) represented in at least two groups. Positive values correspond with an increase and negative values with a decrease in the first group compared with the second group. Shades of blue represent fold change decrease while shades of brown represent fold change increase. Uncoloured fields are not significant at p<0.05.
4. Discussion
4.1. Butyrate and Nonimmune Defence Systems
In the intestine, the basic line of defence (independent of immune cells) consists of a tight attachment of epithelial cells mediated by tight junction proteins, a mucin layer secreted by GCs, host defence peptides produced by Paneth cells, and enterocyte products like RegIIIγ and Muc3. All of these factors prevent bacteria from coming into contact with the subepithelial layer and thus inducing the inflammatory response. PN administration disturbs these systems [6, 7, 10, 30–33], resulting in the increased exposure of antigens to the immune system, increased intestinal permeability, and the establishment of proinflammatory status in the intestine.
Although there is abundant evidence (obtained both in vitro and in vivo) that butyrate affects all components of this defence system, the mechanism is not yet fully understood and controversies remain. Muc 2, secreted by GCs, is the major structural component of the intestinal mucus. Muc 3 is a transmembrane mucin produced by enterocytes and the major component of glycocalyx, which plays an active role in the intestinal mucosal defence [34]. Studies published thus far have only focused on the effect of butyrate on mucin production when administered per rectum or in cell lines in vitro; furthermore, these results are rather inconsistent [35, 36]. Gaudier [37] reported that, in vitro, butyrate grossly stimulated Muc2 expression but only in a glucose-deprived medium, while the effect of butyrate was dose-dependent and inhibitory at higher concentrations. These findings indicate that the effect of butyrate on mucus formation is context-dependent. The stimulatory effect of butyrate on host defence peptides and tight junction protein expression has been proved both in vitro and after dietary supplementation in vivo [38–42]. Nevertheless, to our knowledge, no study has evaluated the effect of butyrate administered parenterally. Our data show that supplementation of a PN mixture with butyrate at a concentration within physiological limits (9 mM) upregulates the expression of all components of the nonimmune defence—including mucins, host defence peptides, and tight junction proteins in the ileum—while also improving intestinal permeability. We conclude that enforcement of the intestinal barrier may represent one of the beneficial effects of i.v. butyrate in the context of total dependence on PN and the absence of enteral nutrition and/or butyrate producers.
4.2. Butyrate and Immune Functions
Total dependence on PN in critically ill patients is accompanied by decreased immune responsiveness, reduced gut-associated lymphoid tissue (GALT) mass, diminished IgA secretion, and increased risk of generalised sepsis [43]. Nevertheless, it seems that the main factor responsible for immune dysfunction in PN-dependent patients is not PN administration itself, but the lack of enteral feeding [1]. One consequence of the absence of enterally provided nutrients is low SCFA content in the gut. SCFA and, in particular, butyrate have been shown to influence immune cells towards anti-inflammatory and tolerogenic phenotypes [44] and to induce the differentiation of Foxp3+ Treg lymphocytes [45–47]. In mice, an SCFA mixture administered per os increased the numbers of IgA-secreting lamina propria B cells, IgA expression or levels of secreted IgA in various compartments of the intestine, and IgA and IgG levels in the blood circulation [48]. On the other hand, Kespohl et al. [49] showed that the effect of butyrate depends on its concentration. In vitro, low butyrate concentration (0.25 mM) facilitated differentiation of Tregs while higher dose (1 mM) induced proliferation of IFNγ producing T cells. Furthermore, oral treatment with 100 mM butyrate potentiated the inflammatory status during acute colitis in germ-free mice.
To our knowledge, only one study has focused on the effect of butyrate when added to PN on GALT. In mice, 47 mM butyrate administered in i.v. in nutrition mixture partially restored a PN-induced drop in lymphocyte numbers in Peyer's patches and intestinal IgA levels [50]. In our study, butyrate administration was associated with the increased expression of anti-inflammatory cytokines IL-4 and IL-10. Compared with controls, IFNγ expression was reduced in both PN and PN+But groups suggesting that 9 mM butyrate concentration in PN mixture does not induce proinflammatory response. Butyrate supplementation was associated with an increase in CD4+ lymphocyte numbers and an increase in the CD4+/CD8+ ratio in MLN. Rather surprisingly, we observed an increase of Tregs in MLN of rats administered a PN mixture without butyrate, while the addition of butyrate resulted in a decrease in Tregs percentage to the control level. Treg cells expressing transcription factor Foxp3 are believed to play a key role in limiting inflammatory responses in the intestine [51], as they inhibit bystander T-cell activation either by a contact-dependent mechanism or through soluble factors [52]. Paradoxically, Foxp3+ Tregs are more common in the inflamed intestinal mucosa of IBD patients, leading to a reciprocal drop in circulating Treg frequency in the peripheral blood; this likely reflects sequestration of these cells to the site of inflammation [53, 54]. In a rat sepsis model, the prosurvival treatment was associated with a decrease in spleen Tregs [55] and in septic patients the persistence of elevated Treg indicated poor outcomes [56]. We hypothesise that in our experimental setting decreased Treg frequency in MLN in the PN+But group reflects the lower inflammatory status of the intestinal epithelium, thus reducing the need to produce an anti-inflammatory response.
IgA production by plasmatic B cells in the submucosal layer is regulated by Th1 and Th2 cytokines produced by different T-helper subpopulations. While Th1 cytokines (IFNγ) downregulate IgA production, Th2 cytokines (IL-4, IL-5, IL-6, and IL-10) stimulate it [57]. Hanna [58] reported that PN depressed both IL-4 and IL-10 levels in small intestine homogenates but that IFNγ levels remained unchanged, resulting in an imbalance between pro-/anti-IgA-regulating cytokines and a subsequent reduction in IgA production. Our data confirm this observation concerning the effect of nonsupplemented PN. Butyrate supplementation resulted in increases in IL-4 and IL-10 expression to control levels and the near normalisation of IgA expression. In contrast to Hanna et al. we found decreased IFNγ expression in both PN-dependent groups. These data suggest that intraepithelial Th2 helpers are one of the targets of butyrate and that butyrate supplementation may restore the PN-induced cytokine imbalance. The interpretation of our data may be limited by the fact that we determined only mRNA expression of particular genes and mRNA and protein expressions do not necessarily correlate. Nevertheless, we observed corresponding changes at functional level (intestinal permeability, mucosa thickness) supporting the relevance of mRNA data.
4.3. Butyrate and the Microbiota
The gut microbiome in animal models of PN is characterised by a significant shift in microbiota composition, particularly a loss of Firmicutes and an enrichment of Bacteroidetes and Proteobacteria [3, 7]. In our study, we observed a shift towards an unfavourable microbiota composition, particularly an enrichment of Proteobacteria and the reduction of bacteria involved in butyrate production (Lactobacillaceae or Lachnospiraceae) in both PN-dependent groups. While the abundance of butyrate producers was not affected by butyrate supplementation we observed a trend, albeit not statistically significant, towards Proteobacteria reduction in butyrate-administered animals. Interestingly, butyrate supplementation (but not enteral deprivation/PN administration alone) was associated with a tendency to the loss of diversity.
Although the effects of dietary fibre on the gut microbiota have been described elsewhere [59], information concerning the direct effect of butyrate on the gut microbiota is scarce. Dietary butyrate was reported to reduce coliform bacteria [60] and to increase the abundance of Lactobacillus [42, 61] and butyrate producers Blautia and Anaerostipes [42]. We observed no radical effect of i.v. butyrate, as it did not attenuate deregulation of the main contributors to PN-induced dysbiosis. Nevertheless, butyrate supplementation has been associated with an increased abundance of several potentially beneficial genera (Anaerostipes, Roseburia, and Lachnospiraceae AC2044 group), a decreased abundance in the opportunistic human pathogen Desulfovibrio [62], and a trend towards attenuation in Proteobacteria dominance. We suggest that this subtle shift in microbiota composition may contribute, along with other mechanisms, to the overall beneficial effect of butyrate.
4.4. Conclusion
We report that supplementation of a PN mixture with butyrate resulted in a significant enhancement of gut defence systems, i.e., increased expression of mucins, tight junction proteins and host defence peptides, and improvement of PN-induced aggravation of intestinal permeability. Lack of enteral nutrition and/or PN administration led to a shift in caecal microbiota composition. Although butyrate did not reverse the altered expression of most taxa, it did influence the abundance of several potentially beneficial or pathogenic genera which might contribute to its overall advantageous effect. We conclude that supplementation of a PN mixture with butyrate may represent a prospective therapeutic approach for mitigating the adverse effects of parenteral nutrition.
Acknowledgments
This study was supported by the Ministry of Health of the Czech Republic, grant no. 15-28745A AZV CR and by MH CR-DRO (“Institute for Clinical and Experimental Medicine–IKEM, IN 00023001”).
Data Availability
The FASTQ data used to support the findings of this study have been deposited in the EBI repository (PRJEB29257), link: https://www.ebi.ac.uk/ena/data/view/PRJEB29257.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Supplementary Materials
Table S1: composition of parenteral nutrition mixture. Table S2: statistical analysis of sequencing data. Figure S1: stability of the antigens of interest after one freezing/thawing cycle.
References
- 1.Wildhaber B. E., Yang H., Spencer A. U., Drongowski R. A., Teitelbaum D. H. Lack of enteral nutrition - effects on the intestinal immune system. Journal of Surgical Research. 2005;123(1):8–16. doi: 10.1016/j.jss.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Alverdy J. C., Aoys E., Moss G. S. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988;104(2):185–190. [PubMed] [Google Scholar]
- 3.Hodin C. M., Visschers R. G. J., Rensen S. S., et al. Total parenteral nutrition induces a shift in the firmicutes to bacteroidetes ratio in association with paneth cell activation in rats. Journal of Nutrition. 2012;142(12):2141–2147. doi: 10.3945/jn.112.162388. [DOI] [PubMed] [Google Scholar]
- 4.Iida T., Onodera K., Nakase H. Role of autophagy in the pathogenesis of inflammatory bowel disease. World Journal of Gastroenterology. 2017;23(11):1944–1953. doi: 10.3748/wjg.v23.i11.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodin C. M., Lenaerts K., Grootjans J., et al. Starvation compromises Paneth cells. The American Journal of Pathology. 2011;179(6):2885–2893. doi: 10.1016/j.ajpath.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierre J. F., Heneghan A. F., Tsao F. H. C., et al. Route and type of nutrition and surgical stress influence secretory phospholipase A2 secretion of the murine small intestine. Journal of Parenteral and Enteral Nutrition. 2011;35(6):748–756. doi: 10.1177/0148607111414025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heneghan A. F., Pierre J. F., Tandee K., et al. Parenteral nutrition decreases paneth cell function and intestinal bactericidal activity while increasing susceptibility to bacterial enteroinvasion. Journal of Parenteral and Enteral Nutrition. 2014;38(7):817–824. doi: 10.1177/0148607113497514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler K. B., Tuvim M. J., Dickey B. F. Regulated mucin secretion from airway epithelial cells. Frontiers in Endocrinology. 2013;4, article 129 doi: 10.3389/fendo.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allaire J. M., Morampudi V., Crowley S. M., et al. Frontline defenders: goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2018;314(3):G360–G377. doi: 10.1152/ajpgi.00181.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conour J. E., Ganessunker D., Tappenden K. A., Donovan S. M., Gaskins H. R. Acidomucin goblet cell expansion induced by parenteral nutrition in the small intestine of piglets. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2002;283(5):G1185–G1196. doi: 10.1152/ajpgi.00097.2002. [DOI] [PubMed] [Google Scholar]
- 11.Smirnov A., Sklan D., Uni Z. Mucin dynamics in the chick small intestine are altered by starvation. Journal of Nutrition. 2004;134(4):736–742. doi: 10.1093/jn/134.4.736. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q., Cadrin M., Herrmann H., et al. Keratin 20 serine 13 phosphorylation is a stress and intestinal goblet cell marker. The Journal of Biological Chemistry. 2006;281(24):16453–16461. doi: 10.1074/jbc.M512284200. [DOI] [PubMed] [Google Scholar]
- 13.Mazmanian S. K., Cui H. L., Tzianabos A. O., Kasper D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Christensen H. R., Frøkiær H., Pestka J. J. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. The Journal of Immunology. 2002;168(1):171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov I. I., Frutos R. D. L., Manel N., et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host & Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwasaki A., Kelsall B. L. Freshly isolated peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. The Journal of Experimental Medicine. 1999;190(2):229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelsall B. L., Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunological Reviews. 2005;206:132–148. doi: 10.1111/j.0105-2896.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 18.Kelly D., Campbell J. I., King T. P., et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nature Immunology. 2004;5(1):104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 19.Lee J., Mo J., Katakura K., et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nature Cell Biology. 2006;8(12):1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 20.Beutler B., Rietschel E. T. Innate immune sensing and its roots: the story of endotoxin. Nature Reviews Immunology. 2003;3(2):169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 21.Demehri F. R., Barrett M., Teitelbaum D. H. Changes to the intestinal microbiome with parenteral nutrition: review of a murine model and potential clinical implications. Nutrition in Clinical Practice. 2015;30(6):798–806. doi: 10.1177/0884533615609904. [DOI] [PubMed] [Google Scholar]
- 22.Miyasaka E. A., Feng Y., Poroyko V., et al. Total parenteral nutrition-associated lamina propria inflammation in mice is mediated by a MyD88-dependent mechanism. The Journal of Immunology. 2013;190(12):6607–6615. doi: 10.4049/jimmunol.1201746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutrition Research Reviews. 2010;23(2):366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 24.Apprill A., Mcnally S., Parsons R., Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquatic Microbial Ecology. 2015;75(2):129–137. doi: 10.3354/ame01753. [DOI] [Google Scholar]
- 25.Caporaso J. G., Lauber C. L., Walters W. A., et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Acadamy of Sciences of the United States of America. 2011;108(supplement 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caporaso J. G., Kuczynski J., Stombaugh J., et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edgar R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 28.Aitchison J. The Statistical Analysis of Compositional Data. London, UK: Chapman and Hall; 1986. (Monographs on Statistics and Applied Probability). [DOI] [Google Scholar]
- 29.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 30.Busch R. A., Heneghan A. F., Pierre J. F., et al. Bombesin preserves goblet cell resistin-like molecule β during parenteral nutrition but not other goblet cell products. Journal of Parenteral and Enteral Nutrition. 2016;40(7):1042–1049. doi: 10.1177/0148607115585353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J., Tian F., Zheng H., et al. N-3 polyunsaturated fatty acid-enriched lipid emulsion improves Paneth cell function via the IL-22/Stat3 pathway in a mouse model of total parenteral nutrition. Biochemical and Biophysical Research Communications. 2017;490(2):253–259. doi: 10.1016/j.bbrc.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 32.Omata J., Pierre J. F., Heneghan A. F., et al. Parenteral nutrition suppresses the bactericidal response of the small intestine. Surgery. 2013;153(1):17–24. doi: 10.1016/j.surg.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X., Yang H., Nose K., et al. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;294(1):G139–G147. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- 34.Shekels L. L., Ho S. B. Characterization of the mouse Muc3 membrane bound intestinal mucin 5′ coding and promoter regions: regulation by inflammatory cytokines. Biochimica et Biophysica Acta—Gene Structure and Expression. 2003;1627(2-3):90–100. doi: 10.1016/S0167-4781(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 35.Hamer H. M., Jonkers D. M., Renes I. B., et al. Butyrate enemas do not affect human colonic MUC2 and TFF3 expression. European Journal of Gastroenterology & Hepatology. 2010;22(9):1134–1140. doi: 10.1097/MEG.0b013e32833a6ca0. [DOI] [PubMed] [Google Scholar]
- 36.Jiminez J. A., Uwiera T. C., Wade Abbott D., Uwiera R. R. E., Inglis G. D. Butyrate supplementation at high concentrations alters enteric bacterial communities and reduces intestinal inflammation in mice infected with Citrobacter rodentium. mSphere. 2017;2(4) doi: 10.1128/mSphere.00243-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaudier E., Jarry A., Blottière H. M., et al. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2004;287(6):G1168–G1174. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- 38.Cresci G. A., Glueck B., McMullen M. R., Xin W., Allende D., Nagy L. E. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. Journal of Gastroenterology and Hepatology. 2017;32(9):1587–1597. doi: 10.1111/jgh.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dou X., Han J., Song W., et al. Sodium butyrate improves porcine host defense peptide expression and relieves the inflammatory response upon toll-like receptor 2 activation and histone deacetylase inhibition in porcine kidney cells. Oncotarget . 2017;8(16):26532–26551. doi: 10.18632/oncotarget.15714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunkara L. T., Achanta M., Schreiber N. B., et al. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS ONE. 2011;6, article e27225(11) doi: 10.1371/journal.pone.0027225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong H., Guo B., Gan Z., et al. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Scientific Reports. 2016;6, article 27070 doi: 10.1038/srep27070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou D., Pan Q., Xin F.-Z., et al. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World Journal of Gastroenterology. 2017;23(1):60–75. doi: 10.3748/wjg.v23.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierre J. F. Gastrointestinal immune and microbiome changes during parenteral nutrition. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2017;312(3):G246–G256. doi: 10.1152/ajpgi.00321.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rooks M. G., Garrett W. S. Gut microbiota, metabolites and host immunity. Nature Reviews Immunology. 2016;16(6):341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arpaia N., Campbell C., Fan X., et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bollrath J., Powrie F. Immunology. Feed your Tregs more fiber. Science. 2013;341(6145):463–464. doi: 10.1126/science.1242674. [DOI] [PubMed] [Google Scholar]
- 47.Smith P. M., Howitt M. R., Panikov N., et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim M., Qie Y., Park J., Kim C. H. Gut microbial metabolites fuel host antibody responses. Cell Host & Microbe. 2016;20(2):202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kespohl M., Vachharajani N., Luu M., et al. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4(+) T cells. Frontiers in Immunology. 2017;8, article 1036 doi: 10.3389/fimmu.2017.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murakoshi S., Fukatsu K., Omata J., et al. Effects of adding butyric acid to PN on gut-associated lymphoid tissue and mucosal immunoglobulin a levels. Journal of Parenteral and Enteral Nutrition. 2011;35(4):465–472. doi: 10.1177/0148607110387610. [DOI] [PubMed] [Google Scholar]
- 51.Josefowicz S. Z., Niec R. E., Kim H. Y., et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482(7385):395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lord J. D. Promises and paradoxes of regulatory T cells in inflammatory bowel disease. World Journal of Gastroenterology. 2015;21(40):11236–11245. doi: 10.3748/wjg.v21.i40.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maul J., Loddenkemper C., Mundt P., et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128(7):1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 54.Lord J. D., Valliant-Saunders K., Hahn H., Thirlby R. C., Ziegler S. F. Paradoxically increased FOXP3+ T cells in IBD do not preferentially express the isoform of FOXP3 lacking exon 2. Digestive Diseases and Sciences. 2012;57(11):2846–2855. doi: 10.1007/s10620-012-2292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao S., Ren J., Sun L., Gu G., Yuan Y., Li J. Fish oil-supplemented parenteral nutrition prolongs survival while beneficially altering phospholipids' fatty acid composition and modulating immune function in rat sepsis. Shock. 2011;36(2):184–190. doi: 10.1097/SHK.0b013e31821e4f8b. [DOI] [PubMed] [Google Scholar]
- 56.Venet F., Chung C.-S., Monneret G., et al. Regulatory T cell populations in sepsis and trauma. Journal of Leukocyte Biology. 2008;83(3):523–535. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- 57.Kramer D. R., Sutherland R. M., Bao S., Husband A. J. Cytokine mediated effects in mucosal immunity. Immunology & Cell Biology. 1995;73(5):389–396. doi: 10.1111/j.1440-1711.1995.tb03887.x. [DOI] [PubMed] [Google Scholar]
- 58.Hanna M. K., Kudsk K. A. Nutritional and pharmacological enhancement of gut-associated lymphoid tissue. Canadian Journal of Gastroenterology and Hepatology. 2000;14(supplement D):145D–151D. doi: 10.1155/2000/308787. [DOI] [PubMed] [Google Scholar]
- 59.Whelan K. Mechanisms and effectiveness of prebiotics in modifying the gastrointestinal microbiota for the management of digestive disorders. Proceedings of the Nutrition Society. 2013;72(3):288–298. doi: 10.1017/S0029665113001262. [DOI] [PubMed] [Google Scholar]
- 60.Galfi P., Bokori J. Feeding trial in pigs with a diet containing sodium n-butyrate. Acta Veterinaria Hungarica. 1990;38(1-2):3–17. [PubMed] [Google Scholar]
- 61.Jellbauer S., Raffatellu M. An intestinal arsonist: pathobiont ignites IBD and flees the scene. Gut. 2014;63(7):1034–1035. doi: 10.1136/gutjnl-2013-305589. [DOI] [PubMed] [Google Scholar]
- 62.Verstreken I., Laleman W., Wauters G., Verhaegen J. Desulfovibrio desulfuricans bacteremia in an immunocompromised host with a liver graft and ulcerative colitis. Journal of Clinical Microbiology. 2012;50(1):199–201. doi: 10.1128/JCM.00987-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: composition of parenteral nutrition mixture. Table S2: statistical analysis of sequencing data. Figure S1: stability of the antigens of interest after one freezing/thawing cycle.
Data Availability Statement
The FASTQ data used to support the findings of this study have been deposited in the EBI repository (PRJEB29257), link: https://www.ebi.ac.uk/ena/data/view/PRJEB29257.