Abstract
Zebrafish is being increasingly used for metabolism and toxicity assessment. The drugs consumed in zebrafish metabolism studies are far less than those used in rat studies. In our study, zebrafish embryos were exposed to icariin, Baohuoside I (BI), Epimedin A (EA), Epimedin B (EB), Epimedin C (EC), Sagittatoside A (SA), Sagittatoside B (SB), and 2′′-O-rhamnosylicariside II (SC), respectively, to examine the toxicity and metabolic profiles of these flavonoids. The order of toxicity was SC, SB > EC, SA > BI, icariin, EA, EB. After 24 h exposure to SB and SC, the mortality of zebrafish larvae reached 100% and yolk sac swollen was obvious. Both SC and SB caused severe hepatocellular vacuolization and liver cells degeneration in adult zebrafish after 15 consecutive days' treatment. The metabolic profiles of these flavonoids with trace amount were also monitored in larvae. BI was the common metabolite shared by icariin, EA, EB, SA, and SB, via deglycosylation. Both BI and SC remained as the prototype in the medium, suggesting that it is hard for BI and SC to cleave the rhamnose residue. EC was metabolized into SC and BI in zebrafish, inferring that SC might be responsible for the toxicity observed in EC group. The metabolites of icariin, EA, EB, EC, and BI in zebrafish larvae coincided with results from rats and intestinal flora. These data support the use of this system as a surrogate in predicting metabolites and hepatotoxicity risk, especially for TCM compound with trace amount.
1. Introduction
Herba Epimedii known as YinYangHuo in Chinese is the dried leaf of the Epimedium and has been historically used in combination with other herbs to treat skeletal diseases in traditional Chinese medicine (TCM) [1]. Metabolic pathways of other Epimedium flavonoids, namely, Epimedin A (EA) [2], Epimedin B (EB) [3], Epimedin C (EC) [4], icariin [5], and Baohuoside I (BI) [6], were well described in both mammals and adult zebrafish (Danio rerio) [7]. However, no data have been reported on the metabolism of Epimedium flavonoids such as Sagittatoside A (SA), Sagittatoside B (SB), and 2′′-O-rhamnosylicariside II (SC), probably attributed to the difficult separation and purification process. Metabolic pathways observed in adult zebrafish are consistent with those obtained from rats, mainly involving deglycosylation, hydrogenation, hydroxylation, and glucuronidation. Drugs consumed in adult zebrafish metabolism studies equal approximately 2 mg, far less than those used in rat studies [7]. Therefore, zebrafish model is preferred for metabolic research of such trace compounds isolated from TCM.
The genomes of zebrafish share approximately 85% homology with humans [8] and their molecular and morphological basis of tissue and organ development is very similar to humans [9]. Easy maintenance, high proliferation (each adult female fish can lay about 300 eggs every week) [10], transparence up to 14 days, low cost, and convenient drug delivery make zebrafish an ideal model for exploring biological processes [11, 12]. Zebrafish model can predict the metabolism and excretion of chemicals from TCM, such as ibuprofen [13] and asperosaponin VI [14]. A large number of phase I and phase II metabolizing enzymes, along with intestinal flora in zebrafish larva, are sharing a strong analogy to humans', such as CYP2B6, 1A1, 3A4, 2E1, sulfotransferase, glucuronosyl-transferase, and glutathione-S-transferase [15]. When exposed to drug solutions, zebrafish can absorb compounds continuously from water via the cavity or the skin. The metabolites transformed by zebrafish will finally be excreted into the water.
Zebrafish developed functioning organs and tissues within 5 days after fertilization (dpf), including liver, heart, brain, bone, intestines, kidney, sensory organs, and nerve systems [16]. Equipped with the obvious advantages in identifying endpoints of toxicity [17], zebrafish larva is regarded as a highly predictive model for rapid evaluation of hepatotoxicity in vivo. Specifically, hepatocytes are already present from 2 dpf onwards. A transcriptomics-based hepatotoxicity comparison between the zebrafish embryo and traditional models (primary human hepatocytes, in vitro rat hepatocytes and in vivo rat liver) exhibited concordance in the metabolic pathway [18].
Epimedium is considered to be relatively safe and no major side effects have been discovered in acute or chronic toxicity studies [19]. Recently, a Chinese herbal prescription has been reported to cause acute liver failure [20]. Two elderly women suffering from osteoporosis developed significant elevation of alanine amino transferase (ALT) and aspartate amino transferase (AST) after taking XLGB capsules for several months [21]. And Epimedii occupies 70% of this prescription by content [22]. As reported by Chen et al. [23], vomit, nausea, and motion decrement were observed in mice after 3-day administration of Epimedium. Even worse, liver steatosis occurred after 15-day administration of Epimedium. The author speculated that the androgen-like effect of Epimedium is closely related to the hepatotoxicity. Moreover, Baohuoside I was a major metabolite of Epimedium extract. The cytotoxic effect of Baohuoside I has been proved in numerous research. For instance, Baohuoside I decreased the viability of human hepatoblastoma HepG2 cells in a concentration- and time-dependent manner [24]. Although there are no known warnings or contraindications for the use of Epimedium plants, a more rigorous safety evaluation is needed to determine any potentially harmful effects.
Here, we assessed the metabolism and toxicity of Epimedium flavonoids by zebrafish model, aiming at (a) screening hepatotoxicant and illustrating the metabolic pathways of these flavonoids and unearthing the close relationship between metabolism and toxicity and (b) comparing the results with literatures and reinforcing the application of zebrafish in these fields.
2. Materials and Methods
2.1. Chemicals and Reagents
Icariin (purity > 98%) was obtained from the National Institute for Food and Drug Control (Beijing, China), while Baohuoside I (BI), Epimedin A (EA), Epimedin B (EB), Epimedin C (EC), Sagittatoside A (SA), Sagittatoside B (SB), and 2′′-O-rhamnosylicariside II (SC) were isolated and purified in our laboratory [2]. The purity (all > 98%) was determined by HPLC. Dimethyl sulphoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Test drugs were dissolved in DMSO or ddH2O and serial dilutions were made before experiments. HPLC-grade acetonitrile and methanol were purchased from Tedia Company (Fairfield, CT, USA). 0.5% DMSO or fish water was used as vehicle control. The concentration of DMSO keeps equal to or less than 0.5% because 0.5% DMSO had no effect on morphology and viability of zebrafish embryos (data not shown). Ultrapure water was produced using a Millipore Milli-Q Gradient system (Milford, MA, USA). All reagents were of analytical grade or better.
2.2. Zebrafish Husbandry
A wild-type Tubingen strain of zebrafish was supplied by Model Animal Research Center of Nanjing University (Nanjing, China). Adult zebrafish were maintained at 28°C, in a 12 h light and 12 h dark cycle conditions. The treatment was approved by the Animal Ethics Review Committee, Jiangsu Provincial Academy of Chinese Medicine. Embryos were collected immediately after natural spawning and cultured at 28°C for 24 h in egg water (5 mM NaCl, 0.17 mM KCl, 0.4 mM CaCl2, and 0.16 mM MgSO4) (Komoike and Matsuoka, 2013). Because the yolk sac can supply nutrition to zebrafish embryos, no feeding is requisite during the initial 9 to 10 dpf. This study and the experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Jiangsu Provincial Academy of Chinese Medicine.
2.3. The Toxicity Assays on Larval Zebrafish
In order to evaluate the potential toxicity of Epimedium flavonoids, zebrafish larvae (1 dpf, 24 h after fertilization) were exposed to DMSO vehicle, icariin, BI, EA, EB, EC, SA, SB, and SC (5 to 200 μM) in the egg culture water from 1 dpf to 6 dpf. Drug exposure was performed in 24-well plates, with 8 larvae in each well and two well replicates. The treatments were performed thrice independently. Major zebrafish organs and tissues were visually assessed. The fish were placed on glass slide for visual observation and image acquisition under a microscope. The morphology was under observation, and mortality of larval zebrafish was recorded every day. The observer was blinded from the separate exposure groups.
2.4. Metabolism of Epimedium Flavonoids on Zebrafish Larvae
To evaluate the metabolism of Epimedium flavonoids, zebrafish larvae were exposed to DMSO vehicle, icariin, BI, EA, EB, EC, SA, SB, and SC (25 μM) from 1 dpf to 6 dpf. Drug exposure was performed in 24-well plates, with 8 larvae in each well and three well replicates. The medium was collected to determine the metabolites at 1 dpf, 2 dpf, 3 dpf, 4 dpf, 5 dpf, and 6 dpf, respectively. The sample solution was evaporated to dryness with vacuum at room temperature. 80% methanol were added to dissolve the residue and further centrifuged at 12,000 rpm for 15 min. The supernatant (20 μL) was subjected to high performance liquid chromatography (HPLC) analysis with a Zorbax C-18 column (5 μm, 150 mm × 4.6 mm). The mobile phase consisting of 0.05% formic acid in water (A) and 0.05% formic acid in acetonitrile (B) was pumped at a flow rate of 1.0 mL/min. The column temperature was set at 30°C. The gradient elution program was set as follows: 0–5 min, 10% B; 5–15 min, 10–25% B; 15–40 min, 25–90% B; 40–45 min, 90% B.
2.5. Histology Examination for Liver Toxicity
To further examine the potential liver toxicity of flavone, adult zebrafish of mixed genders were used for histology examination on liver slices. The fish were treated with icariin, BI, EC (50 μM, respectively) or SC and SB (25 μM, respectively) for 15 days, with five fish in each group. Drug exposure was performed in small size brown fish tanks, with 50 mL incubation medium for per adult fish. To avoid the growth of microorganisms, solutions containing test drug were replaced every two days. At the end, the fish were sacrificed by embedding them in ice and subsequently fixed in 4% paraformaldehyde according to standard procedures. Sections (5 μm) were stained with hematoxylin and eosin (H&E) as previously reported [25] and analyzed for general structural changes in the liver with light microscopy.
2.6. Statistical Analysis
Data were analyzed using Graphpad Prism 5.01 (GraphPad Software Inc., San Diego, CA, USA). Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by posttests. Quantitative data are presented as the mean ± SD. Differences were considered significant at P < 0.05.
3. Results
3.1. Toxicity of Epimedium Flavonoids on Zebrafish Larvae
Embryos (1 dpf) were exposed to graded concentrations (5-200 μM) of BI, icariin, EA, EB, EC, SA, SB, and SC for 1-5 days (2-6 dpf). Morphologies of the zebrafish embryos in each group were visually examined under a microscope, such as pericardial edema, crooked body, tail malformation, yolk sac swollen, and spinal deformity. No death or morphological abnormalities were observed in icariin, BI, EA, or EB-treated fish after 1-5 days of exposure, at concentrations up to 200 μM. Likewise, EC at low concentration (50 μM) did not reveal any negative effect on larvae mortality from 2 to 6 dpf. By contrast, the survival rates of larvae (5 dpf) in 100 and 200 μM EC groups dropped to 6% and 0%, respectively. For SA at 100 μM, the mortality rate at 4 dpf was 16.7%. However, the mortality at 2 dpf increased abruptly from 0% to 100% between the concentrations of 50 to 100 μM (SB and SC) after 24 h exposure (Figure 1). Moreover, SB and SC incubation resulted in the whitening and clotting of the larvae. The body length of embryo at 3 dpf was shorter in both SB and SC (50 μM) groups than the control group, suggesting that SB and SC might have developmental toxicity.
Figure 1.
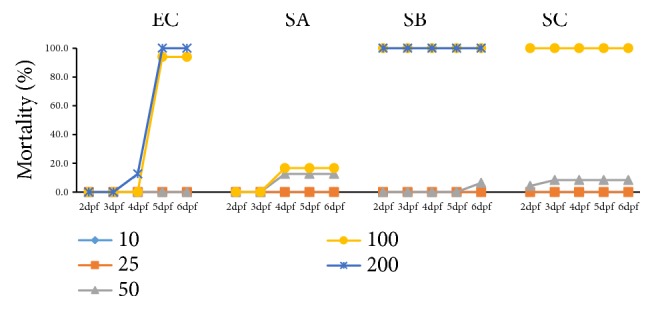
Mortality curves of zebrafish larvae. From 2 dpf to 6 dpf, after exposure to EC, SA, SB, and SC (10, 25, 50, 100, and 200 μM).
In the phenotype evaluation (3 dpf), both SB and SC (50 μM) notably delayed the absorption of yolk sac (Figure 2), indicating potential hepatotoxicity of these two compounds. These results suggested that icariin, BI, EA, and EB have negligible toxicity for zebrafish embryos, while EC and SA have slight toxicity for the larvae. SB and SC are much more toxic to zebrafish embryos than icariin, BI, EA, EB, EC, and SA, as demonstrated by 100% mortality rate and yolk sac swollen. The order of toxicity was SC, SB > EC, SA > BI, icariin, EA, EB. The maximum nonlethal concentration (MNLC) of these flavonoids in larvae was close to 50 μM, so concentration of 0-50 μM was used for subsequent metabolism and target organ toxicity experiments.
Figure 2.

Visual phenotype of yolk sac in zebrafish at 3 dpf after exposure to drugs from 1 dpf to 3 dpf. SB and SC notably delayed the absorption of yolk sac. Normal phenotype of yolk sac was found in icariin, BI, EA, and EB groups, whereas slight yolk sac swollen was found in EC and SA groups. Scale bars = 1,000 μm.
3.2. Metabolism of EA, EB, Icariin, SA, SB, and BI on Zebrafish Larvae
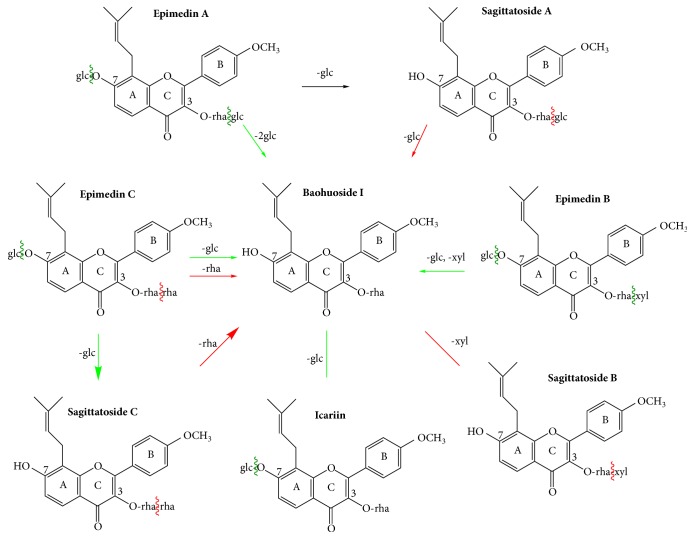
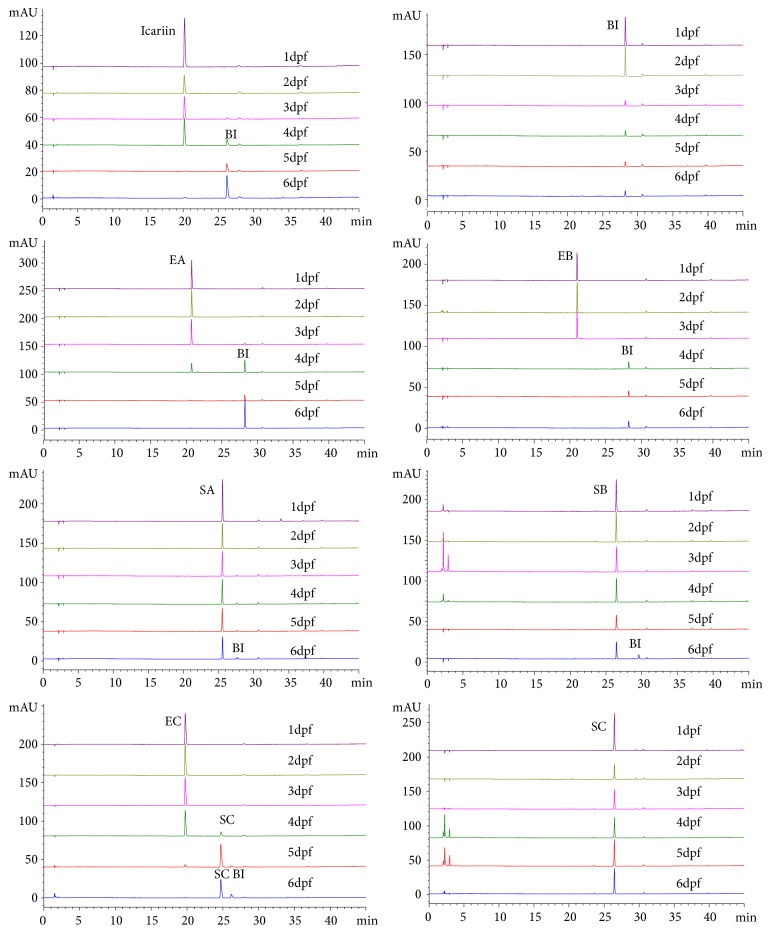
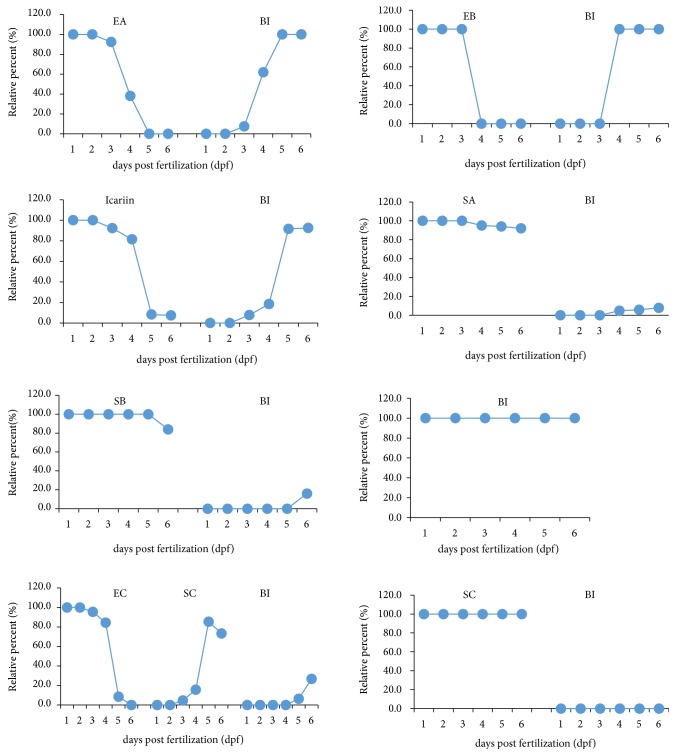
After 24 h to 120 h exposure, metabolites of flavonoids from Herba Epimedii were detected by HPLC analysis with a Zorbax C-18 column. Icariin was transformed into BI via one step of hydrolysis of glucosides. EA was directly transformed into BI via cleavage of both 7-OH and 3-OH glucose residues. Likewise, EB was transformed into BI rather than SB, through cleavage of 7-OH glucose residue and 3-OH xylose residue (Figure 3). Both EA and EB underwent two steps of hydrolysis, and only the final metabolite BI can be observed in the chromatogram (Figures 4 and 5). These results were in accordance with previous reports from in vivo rat model and in vitro intestinal bacteria metabolism model [26, 27]. At 4 dpf, 67% EA, 100% EB, and 10% icariin were metabolized into BI. At 5 dpf, both EA and icariin were entirely transformed into BI. In the medium of BI-treated group, only the prototype of BI was detected, even after 5 days of treatment (6 dpf). No other metabolites were yielded, suggesting that rhamnose residue at the 3-OH of ring C is hardly eliminated from BI.
Figure 3.
Major metabolic pathway of flavonoids from Herba Epimedii on larval zebrafish. BI was the common metabolite shared by icariin, EA, EB, SA, and SB, via one or two steps of deglycosylation. EC was transformed into SC. But it is hard for BI and SC to cleave the rhamnose residue. Green arrow and wavy line mean easy deglycosylation, and red arrow and wavy line mean relatively hard deglycosylation process.
Figure 4.
HPLC chromatogram of the metabolism on larval zebrafish. The incubation medium from icariin, BI, EA, EB, EC, SA, SB, and SC groups was analyzed by HPLC at 1 dpf to 6 dpf.
Figure 5.
The variation of the contents of the metabolites and the parent compound on zebrafish larvae. In EA, EB, SA, SB, and icariin groups, the content of the metabolite BI was rising. Both BI and SC remained as the prototype in the medium from 1dpf to 6 dpf. In EC group, two metabolites SC and BI were detected.
Glucose or xylose residue on the 3-OH of ring C was cleaved from SA and SB, generating the same metabolite BI. But the deglycosylation rate of SA and SB at 3-OH is much slower than the hydrolysis speed for EA, EB, and icariin. In detail, only a small amount of BI was found in the medium of SA group until 72 h incubation (4 dpf), and the content of BI merely reached 7.9% at 6 dpf. For SB group, BI was not detected until 6 dpf. In summary, BI was the common metabolite shared by icariin, EA, EB, SA, and SB, via fast or slow hydrolysis steps.
3.3. Metabolism of EC and SC on Zebrafish Larvae
Within the initial 48 h treatment (from 1 dpf to 3 dpf), EC were mainly excreted as the prototype (Figures 4 and 5). The metabolite SC could not be found in the medium of the EC zebrafish larvae group until 72 h incubation (4 dpf). By comparing the retention time of the reference standard compound, the peak was identified as SC, forming via the cleavage of glucose residue at the 7-OH. At 4 dpf, 20% of EC was transformed into SC and the transformation rate reached 92% at 5 dpf. A small amount of BI can be detected in the solution of EC group at 5 dpf, probably via the elimination of rhamnose residue at the 3-OH from SC. The relative content for SC and BI was 85.3% and 6.2% at 5 dpf, respectively. At 6 dpf, EC prototype could not be detected anymore in the solution, and the relative content for SC and BI changed into 73.3% and 26.7%, respectively. It can be inferred that a portion of the metabolite SC has been further metabolized into BI.
When it comes to the metabolic pathway of SC, unexpectedly, only SC prototype was detected in the medium from 1 dpf to 6 dpf, without generating the metabolite BI. But the transformation of SC into BI at 5 dpf and 6 dpf was observed in EC-treated group. We speculated that incubation with EC from 1 dpf to 5 dpf has an impact on the development of the intestine and CYP450 enzyme system in larvae, thus promoting the transformation of SC into BI. This might explain the different phenomenon in EC and SC groups.
3.4. Hepatotoxicity of SB and SC on Zebrafish Adults
Liver histopathology evaluations on adult zebrafish were conducted to evaluate the hepatotoxicity of SA, SB, SC, EC, BI, and icariin. Given that zebrafish embryos are well tolerated to EA and EB treatment and both of them are mainly metabolized into BI, evaluations on BI hepatotoxicity can also elucidate whether EA and EB are safe to the larvae. The MNLC dose (50 μM) was chosen for histology study on zebrafish adults. However, in both SB and SC groups at 50 μM, zebrafish adults died within 30 min incubation. Herein, a lower concentration of 25 μM for SB and SC was chosen, in order to examine the liver toxicity after long-term treatment. All the fish survived till the end of the 15-day treatment.
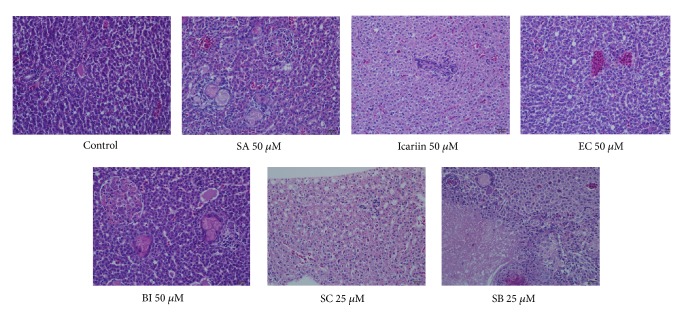
As demonstrated in H&E staining (Figure 6), zebrafish liver in the untreated and vehicle (0.5% DMSO) group showed the structural integrity of the hepatic cell. Liver section of zebrafish exposed to SA, BI, and icariin (50 μM) for 15 days appeared similar to the vehicle group. No apparent changes were observed in hepatocytes in EC group (50 μM), except slight edema and negligible vacuolization. In SC-treated group (25 μM), the liver sections showed severe hepatocellular vacuolization, mild degeneration of liver cells, edema, and slight infiltration of inflammatory and, while SB (25 μM) caused a certain degree of cells necrosis, moderate edema with mild vacuolar changes, loose cell-to-cell contact, lymphocyte and mononuclear infiltration, and Kupffer cell proliferation in mesenchyme, suggesting that SB and SC are hepatotoxic even at lower concentrations.
Figure 6.
H&E staining of liver sections from adult zebrafish. The representative figures indicate normal liver in vehicle group as well as icariin, EC, and BI (50 μM) groups after 15-day treatment. Liver degeneration, hepatocellular vacuolization, and infiltration of inflammatory were found in zebrafish treated with SB and SC (25 μM).
4. Discussions
The zebrafish, as a model organism, is being increasingly used for pharmaceutical metabolism. In the present study, zebrafish larva was applied to explore the metabolism of EA, EB, EC, icariin, SA, SB, SC, and BI, after 24 h to 120 h incubation. Before we carried out the metabolism and toxicity study, stability test of these compounds in the medium has been performed. As shown in supplementary Figure (S1), these compounds keep stable till the fifth day. BI was the common metabolite shared by icariin, EA, EB, SA, and SB, via one or two steps of deglycosylation. EC was transformed into SC. The metabolism of SA, SB, and SC was reported for the first time. SA and SB were deprived with glucose or xylose, mainly converting into BI. But it is hard for BI and SC to cleave the rhamnose residue. Both BI and SC remained as the prototype in the medium from 1 dpf to 6 dpf. In general, the metabolic pathways of icariin, EA, EB, EC, and BI in zebrafish larvae coincided with results obtained from rodent model, in vitro intestinal flora, and zebrafish adult model [3–7, 27]. In addition, the metabolic pathway of SA, SB, and SC is similar to that of the homologous flavonoids EA and BI, consolidating the application of zebrafish embryo in drug metabolism predicting.
Based on the characteristic of the metabolic pathway, Epimedii flavonoids can be divided into two categories. One category includes EA, EB, icariin, SA, SA, and BI, all of which share the same metabolite BI. The deglycosylation speeds for glycoside, xyloside, or rhamnoside from the parent compounds were different among these flavonoids. Glucoside at 3-OH or 7-OH and xyloside at 3-OH were easily and quickly cleaved from icariin, EA, and EB, respectively. But the hydrolysis speed of glucose at 3-OH from SA or xylose at 3-OH from SB slowed down considerably. In contrast, rhamnose residue at 3-OH was hardly eliminated from BI or SA. The other category includes EC and SC, both of which share the same metabolite SC. EC can easily cleave the glucose, transforming into SC. But the elimination of rhamnoside from the metabolite SC in EC-treated group indeed took some time. The transformation of SC into BI was not detected until 5 dpf in EC-treated group.
The applicability of the zebrafish larva in metabolism study further supports the use of this system in toxicity screening. The zebrafish has been widely used for estimating the hepatoxicity of chemicals [28]. The liver is known as an important target organ for xenobiotic chemicals. High concentrations of xenobiotics may end up in the liver after intestinal absorption. Primary hepatocytes, liver slices, and human hepatocyte HepaRG line have been established to measure liver injury [29]. However, the reductionistic nature and the loss of functionality severely limited the wide use of these in vitro methods. In next-generation RNA sequencing, strong similarities were found between whole zebrafish embryo and mouse liver in pathways associated with hepatotoxicity. At 3 dpf, the liver of zebrafish is fully functioning, including the CYP450 enzymes, which are essential for metabolizing xenobiotics [30]. In addition, embryogenesis of zebrafish is finished within 5 dpf. Thus, hepatic responses can be expected after exposure to hepatotoxicants in zebrafish embryo.
In this paper, zebrafish larva was used for hepatotoxicity testing of Epimedium flavonoids. Among them, SB and SC exerted remarkable hepatotoxicity. When zebrafish larvae were treated with EC (200 μM) for 3 days (4 dpf), no significant toxicity was observed, as revealed by whole fish survival and normal yolk sac morphology. But when the incubation time reached 4 days (5 dpf), the mortality of EC group (both 100 and 200 μM) surged to almost 100%. According to the metabolic results, the generation of SC in the solution of EC group was negligible at 4 dpf. But afterwards, more and more EC was transformed into SC. 92% of EC has been transformed into SC at 5 dpf and EC could not be detected anymore in the medium at 6 dpf. Therefore, we speculated that the metabolite SC might be responsible for the lethal effect of EC after long-time transformation.
When it comes to the hepatotoxicity assessment in adult zebrafish, degeneration of liver cells was found after 15 days of SC treatment (25 μM). However, no obvious liver injury was found after 15 days of EC treatment. According to our previous research, EC was mainly metabolized into SC in adult zebrafish after 24 h exposure, but only a small quality of SC was generated [7], far less than 25 μM. More importantly, in order to avoid the growth of germs in adult fish experiments, the incubation medium containing the test drug was replaced every other day, whereas the incubation solution was not changed throughout the zebrafish larvae experiment. Therefore, it is reasonable that no apparent hepatotoxicity was spotted in EC-treated adult zebrafish group (50 μM) after 15 days of treatment, but 100% mortality of larvae was found in EC group at 5 dpf. In summary, the hepatotoxicity of SB and SC are most distinguished, and the toxicity induced by EC in larva is probably attributed to the metabolite SC.
The metabolic pathways of icariin, EA, EB, EC, and BI in zebrafish larvae coincided with results from rats and intestinal flora. Not only metabolism and excretion, but the consequent changes in toxicity can also be simultaneously elucidated in zebrafish model. This convenient, time-saving, and predictive animal model can serve as an intermediate step between cell-based evaluation and mammalian animal testing.
Acknowledgments
The authors received grants from National Natural Science Foundation of China (Grant Nos. 81573833, 81303275, 81503253, 81603500, 30973978, and 81873055); 2015 Annual Traditional Chinese Medicine for Public Interest Research from Ministry of Finance of China (201507004-10); National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2017ZX09301-056, 2017ZX09301-051); Natural Science Foundation of Jiangsu Province of China (BK20141507); “333 Project” of Jiangsu Province (BRA2014348); and Foundation for High-Level Talent in Six Areas of Jiangsu Province (2013-YY006).
Contributor Information
Jie Song, Email: momo198420@hotmail.com.
Yingjie Wei, Email: wyj970@163.com.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
All animal experiments were reviewed and approved by the Animal Care and Use Committee of the Animal Laboratory Center of Jiangsu Research Institute of TCM and adhered to the guidelines of the Guide for the Care and Use of Laboratory Animals.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Jie Song, Yang Zhan, E. Sun, Ziqi Shi, and Liang Feng performed the research. Yingjie Wei and Xiaobin Jia designed the research study. Jie Song, Ying Chen, and Yingjie Wei analyzed the data. Jie Song and Ying Chen wrote the paper. All authors approved the final version of the manuscript. Rongling Zhong and Ying Chen contributed equally to this work.
Supplementary Materials
Figure S1. A: HPLC of total flavonoids of Epimedium koreanum in the days 0 and 5, respectively. B: HPLC of mixed reference substance, including 1 EA, 2 EB, 3 EC, 4 icariin, 5 SA, 6 SB, 7 SC, and 8 BI.
References
- 1.Wang L., Li Y., Guo Y., et al. Herba epimedii: an ancient Chinese herbal medicine in the prevention and treatment of osteoporosis. Current Pharmaceutical Design. 2016;22(3):328–349. doi: 10.2174/1381612822666151112145907. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J., Ma Y. H., Zhou Z., et al. Intestinal absorption and metabolism of epimedium flavonoids in osteoporosis rats. Drug Metabolism and Disposition. 2015;43(10):1590–1600. doi: 10.1124/dmd.115.064386. [DOI] [PubMed] [Google Scholar]
- 3.Cui L., Sun E., Zhang Z., et al. Metabolite profiles of epimedin B in rats by ultraperformance liquid chromatography/quadrupole-time-of-flight mass spectrometry. Journal of Agricultural and Food Chemistry. 2013;61(15):3589–3599. doi: 10.1021/jf304625x. [DOI] [PubMed] [Google Scholar]
- 4.Cui L., Sun E., Zhang Z., Tan X., Xu F., Jia X. Metabolite profiles of epimedin C in rat plasma and bile by ultra-performance liquid chromatography coupled with quadrupole-TOF-MS. Biomedical Chromatography. 2014;28(10):1306–1312. doi: 10.1002/bmc.3174. [DOI] [PubMed] [Google Scholar]
- 5.Qian Q., Li S.-L., Sun E., et al. Metabolite profiles of icariin in rat plasma by ultra-fast liquid chromatography coupled to triple-quadrupole/time-of-flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2012;66:392–398. doi: 10.1016/j.jpba.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 6.Sun E., Xu F., Qian Q., Cui L., Tan X., Jia X. Ultra-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry analysis of icariside II metabolites in rats. Natural Product Research (Formerly Natural Product Letters) 2014;28(19):1525–1529. doi: 10.1080/14786419.2014.921684. [DOI] [PubMed] [Google Scholar]
- 7.Wei Y., Li P., Fan H., et al. Metabolite profiling of four major flavonoids of Herba Epimedii in zebrafish. Molecules. 2012;17(1):420–432. doi: 10.3390/molecules17010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milan D. J., Jones I. L., Ellinor P. T., MacRae C. A. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. American Journal of Physiology-Heart and Circulatory Physiology. 2006;291(1):H269–H273. doi: 10.1152/ajpheart.00960.2005. [DOI] [PubMed] [Google Scholar]
- 9.Chen J.-N., Fishman M. C. Zebrafish tinman homolog demarcates the heart field and initiates myocardial differentiation. Development. 1996;122(12):3809–3816. doi: 10.1242/dev.122.12.3809. [DOI] [PubMed] [Google Scholar]
- 10.Yang X., Zhao W., Wang Z., et al. Toxicity of hemimethyl-substituted cucurbit[7]uril. Food and Chemical Toxicology. 2017;108:510–518. doi: 10.1016/j.fct.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Vliegenthart A. D. B., Tucker C. S., Del Pozo J., Dear J. W. Zebrafish as model organisms for studying drug-induced liver injury. British Journal of Clinical Pharmacology. 2014;78(6):1217–1227. doi: 10.1111/bcp.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howe K., Clark M. D., Torroja C. F., et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones H. S., Trollope H. T., Hutchinson T. H., Panter G. H., Chipman J. K. Metabolism of ibuprofen in zebrafish larvae. Xenobiotica. 2012;42(11):1069–1075. doi: 10.3109/00498254.2012.684410. [DOI] [PubMed] [Google Scholar]
- 14.Wei Y. J., Xue X. L., Liu W., Wang C. M., Zhan Y., Jia X. B. Metabolism study of asperosaponin VI by using zebrafish. Yao Xue Xue Bao. 2013;48(2):281–285. [PubMed] [Google Scholar]
- 15.Podechard N., Chevanne M., Fernier M., et al. Zebrafish larva as a reliable model for in vivo assessment of membrane remodeling involvement in the hepatotoxicity of chemical agents. Journal of Applied Toxicology. 2017;37(6):732–746. doi: 10.1002/jat.3421. [DOI] [PubMed] [Google Scholar]
- 16.McGrath P., Li C.-Q. Zebrafish: a predictive model for assessing drug-induced toxicity. Drug Discovery and Therapy. 2008;13(9-10):394–401. doi: 10.1016/j.drudis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Hill A. J., Teraoka H., Heideman W., Peterson R. E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicological Sciences. 2005;86(1):6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 18.Driessen M., Vitins A. P., Pennings J. L. A., Kienhuis A. S., Water B. V. D., van der Ven L. T. M. A transcriptomics-based hepatotoxicity comparison between the zebrafish embryo and established human and rodent in vitro and in vivo models using cyclosporine A, amiodarone and acetaminophen. Toxicology Letters. 2015;232(2):403–412. doi: 10.1016/j.toxlet.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Ma H., He X., Yang Y., Li M., Hao D., Jia Z. The genus Epimedium: an ethnopharmacological and phytochemical review. Journal of Ethnopharmacology. 2011;134(3):519–541. doi: 10.1016/j.jep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Zheng M., Zhou Y., Lv X. A case of liver failure caused by combination of Xianling Gubao Capsule and diclofenac sodium sustained - release capsules. Chinese Journal of Pharmacovigilance. 2014;11:507–508. [Google Scholar]
- 21.Pei D., Cao Y. Acute drug-induced liver injury caused by Xianlinggubao in one patient. Chinese Hepatology. 2009;14:457–458. [Google Scholar]
- 22.Cheng Y., Liu Y., Wang H., et al. A 26-week repeated dose toxicity study of xian-ling-gu-bao in sprague-dawley rats. Journal of Ethnopharmacology. 2013;145(1):85–93. doi: 10.1016/j.jep.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 23.Chen J., Cai H. Adverse reactions of Zuangguguanjie Wan and cause analysis. Adverse Drug Reactions Journal. 2000;1:15–19. [Google Scholar]
- 24.Geng Y.-D., Zhang C., Shi Y.-M., et al. Icariside II-induced mitochondrion and lysosome mediated apoptosis is counterbalanced by an autophagic salvage response in hepatoblastoma. Cancer Letters. 2015;366(1):19–31. doi: 10.1016/j.canlet.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Gopal V., Agrawal N., Mandal S. C. Biological parameters for evaluating the toxic potency of petroleum ether extract of wattakaka volubilis in wistar female rats. Journal of Pharmacopuncture. 2014;17(3):7–15. doi: 10.3831/KPI.2014.17.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu F., Chen Y. J., Kano Y., Yao X. S. Metabolism of orally administered icariin in rats. Yao Xue Xue Bao = Acta Pharmaceutica Sinica. 1999;24:222–226. [Google Scholar]
- 27.Xu W., Zhang X. M., Zhang Y. P., Shen Z. Y., Zhang W. D. Metabolism of icariin and icariside II by rat intestinal bacteria in vitro. World Science and Technology-Modernization of Traditional Chinese Medicine and Materia Medica. 2006;8:98–106. [Google Scholar]
- 28.Nam H. S., Hwang K. S., Jeong Y. M., et al. Expression of miRNA-122 induced by liver toxicants in zebrafish. BioMed Research International. 2016;2016:7. doi: 10.1155/2016/1473578.1473578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guillouzo A., Corlu A., Aninat C., Glaise D., Morel F., Guguen-Guillouzo C. The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chemico-Biological Interactions. 2007;168(1):66–73. doi: 10.1016/j.cbi.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Alderton W., Berghmans S., Butler P., et al. Accumulation and metabolism of drugs and CYP probe substrates in zebrafish larvae. Xenobiotica. 2010;40(8):547–557. doi: 10.3109/00498254.2010.493960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A: HPLC of total flavonoids of Epimedium koreanum in the days 0 and 5, respectively. B: HPLC of mixed reference substance, including 1 EA, 2 EB, 3 EC, 4 icariin, 5 SA, 6 SB, 7 SC, and 8 BI.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.