Abstract
Maintenance of optimal conditions such as water parameters, diet, and feeding is essential to a healthy Xenopus laevis and Xenopus tropicalis colony and thus to the productivity of the lab. Our prior husbandry experience as well as the rapid growth of the National Xenopus Resource has given us a unique insight into identifying and implementing these optimal parameters into our husbandry operations. Here, we discuss our standard operating procedures which will be of use to both new and established Xenopus facilities.
Keywords: Xenopus laevis, Xenopus tropicalis, aquatic recirculating systems, husbandry
1. Introduction
A number of reasons make both Xenopus laevis and Xenopus tropicalis powerful systems for the study of developmental, cellular, and molecular biology as well as biomedical research modeling human disease [1, 2]. Many of these reasons are practical in nature and include a closed lifecycle with a relative ease of maintaining the animals in the lab, the ability to generate large quantities of equivalent research material all year round through the use of gonadotropic hormone injection to induce ovulation at any time of the year, and robust and relatively rapid development of early embryonic cultures [3]. These practical reasons in particular, are dependent on proper husbandry practices that are essential for maintaining a colony of healthy frogs capable of providing high quality eggs and embryos necessary for driving productivity of the lab forward.
One of the goals of the National Xenopus Resource (NXR) is to serve as a central repository and distribution center for a diverse number of Xenopus stocks of high interest to the research community [4]. Since the establishment of the NXR in 2010 we have grown our colony from zero frogs to now house over 3000 X. tropicalis and 5000 X. laevis. This rapid growth as well as the necessity to quickly replenish animal lines that we distribute, has motivated us to optimize Xenopus husbandry protocols for all life history stages, thus assuring rapid and robust growth from fertilization to sexual maturity.
Many researchers starting their own labs have limited understanding of the steps necessary for successfully establishing a new colony and frequently this task is delegated to institutional animal care facilities which may similarly lack the necessary expertise. Our goal in this chapter is to provide our protocols and experiences in establishing a new colony and maintaining it over the long term. We discuss a number of topics including recirculating Xenopus housing modules, optimal water parameters, diets and feeding schedules, and animal distribution.
2. Materials
2.1. Animals
Wild type outbred X. laevis and X. tropicalis can be purchased from a variety of commercial vendors, including Nasco, Xenopus One, and Xenopus Express. These companies are an excellent resource for obtaining large quantities of outbred Xenopus and they work closely with each researcher to provide animals at all stages. More specialized strains and lines of Xenopus, such as the inbred X. laevis J strain, are also available for purchase through regional stock centers, including the National Xenopus Resource (NXR, RRID:SCR_013731, http://www.mbl.edu/xenopus), the European Xenopus Resource Centre (EXRC, RRID:SCR_007164, https://xenopusresource.org/), and the Xenopus laevis Research Resource for Immunobiology (XLRRI, https://www.urmc.rochester.edu/microbiology-immunology/xenopus-laevis.aspx). In addition, these stock centers distribute transgenic and mutant lines for both X. laevis and X. tropicalis. Detailed information about the various suppliers, including contact information, can be found online at Xenbase (RRID:SCR_003280, http://www.xenbase.org/other/obtain.do).
2.2. Animal Housing
There are three main options for housing and maintaining Xenopus animals: recirculating, flow through, and static fill and dump systems. System choice will depend on the needs of the lab and available funds. Over the long term, we have found that recirculating aquatic systems are the best option for research laboratory sized operations. First, modern recirculating systems automatically measure and control a number of water parameters including temperature, pH, conductivity, and filter inflow and outflow pressures. This aids in maintaining the system water at peak quality for the housed animals, which is not possible with fill and dump systems. Second, although flow through systems also measure and regulate the same parameters, they are considerably less economical. In a flow through configuration a large volume of conditioned water is replaced daily, whereas in a recirculating system typically only 10% of its total volume is exchanged in a 24-hour period. Another choice that can be made during the system selection is the size of the tanks that will house the animals. This choice will depend on the species, age, number of animals in the colony, and the expected frequency of their use; whichever size is chosen, we recommend the use of transparent plastic tanks that permit for easy visual inspection of the animals inside. The following companies provide both stand-alone and multi-rack recirculating frog systems and have extensive experience in helping research labs selecting the most appropriate configurations for housing animals (see Note 1).
Aquaneering, Inc., San Diego, CA, USA
Aquatic Enterprises, Inc., Seattle, WA, USA
Aqua Schwarz, GmBH, Göttingen, Germany
Iwaki Aquatic Systems and Services, Holliston, MA, USA
Tecniplast USA, West Chester, PA, USA
2.3. Food
sera Micron Growth Food (sera North America, Montgomeryville, PA, USA)
Brine Shrimp Flake (Brine Shrimp Direct, Ogden, UT, USA)
Nasco Frog Brittle (Powder) (Nasco, Fort Atkins, WI, USA)
Nasco Frog Brittle (Small Nuggets) for Post-Metamorphic Xenopus (Nasco, Fort Atkins, WI, USA)
Nasco Frog Brittle (Large Nuggets) for Adult Xenopus (Nasco, Fort Atkins, WI, USA)
Bio Vita Fry 1.2 mm Pellet (Bio-Oregon, Longview, WA, USA)
Bio Vita Fry 2.0 mm Pellet (Bio-Oregon, Longview, WA, USA)
BioTrout 4.0 mm Pellet (Bio-Oregon, Longview, WA, USA)
2.4. Water Quality Maintenance and Testing Kits
Sea salt used to control conductivity, we use Reef Salt from Seachem Laboratories (Madison, GA, USA)
Biocarbonate to buffer and regulate pH (ProLine brand, Pentair AES, Apopka, FL, USA)
Nitrifying bacteria (ProLine brand, Pentair AES, Apopka, FL, USA)
pH Aquarium Fresh Water Test Kit, pH range 6.0–7.6 (API, Chalfont, PA, USA)
Ammonia Fresh Water and Salt Water Test Kit (API, Chalfont, PA, USA)
Nitrite NO2 Fresh Water and Salt Water Test Kit (API, Chalfont, PA, USA)
Nitrate NO3 Fresh Water and Salt Water Test Kit (API, Chalfont, PA, USA)
2.5. Cleaning and Sterilization Reagents
Bleach (6.0% NaClO). Available from supermarket cleaning supplies isle. Dilute with pure water to make a 10% bleach solution (final concentration of NaClO is 0.6%), and use to sterilize tanks, tank accessories, frog nets, etc.
Ethanol. Source may depend on local regulations. Purchase 190 proof (95%) and dilute to 70% with pure water.
Dechlorinator (Na2S2O3). We use ProLine brand (Pentair AES, Apopka, FL, USA), which contains both the anhydrous (CAS# 7772-98-7) and pentahydrate salt (CAS# 10102-17-7) molecules. It can be acquired from other sources and whether the anhydrous or hydrate salt is used is not important. 1.6 to 2.6 ppm of Na2S2O3 per 1 ppm of chlorine is typically sufficient to dechlorinate water.
Virkon Aquatic (DuPont, Wilmington, DE, USA)
2.6. Anesthesia and Euthanasia Reagents
Ethyl 4-aminobenzoate, 98%; aka. Benzocaine (CAS# 94-09-7): make 10% stock in 95% Ethanol and store at room temperature.
Tricaine Methanesulfonate; aka. Tricaine-S, aka. MS 222 (CAS# 886-86-2)
3. Methods
3.1. Housing Animals
At the National Xenopus Resource, we house our adult, juvenile, and tadpole X. laevis and X. tropicalis in modular recirculating aquatic systems, and use both individual and multi-rack configurations (Figure 1a). To ensure high water quality, these recirculating systems incorporate several filtration steps, including biological, mechanical, activated carbon, and UV sterilization. Listed below we outline some guidelines that we have found help maintain the systems and keep animals healthy and productive.
Figure 1. Individual recirculating system configuration.
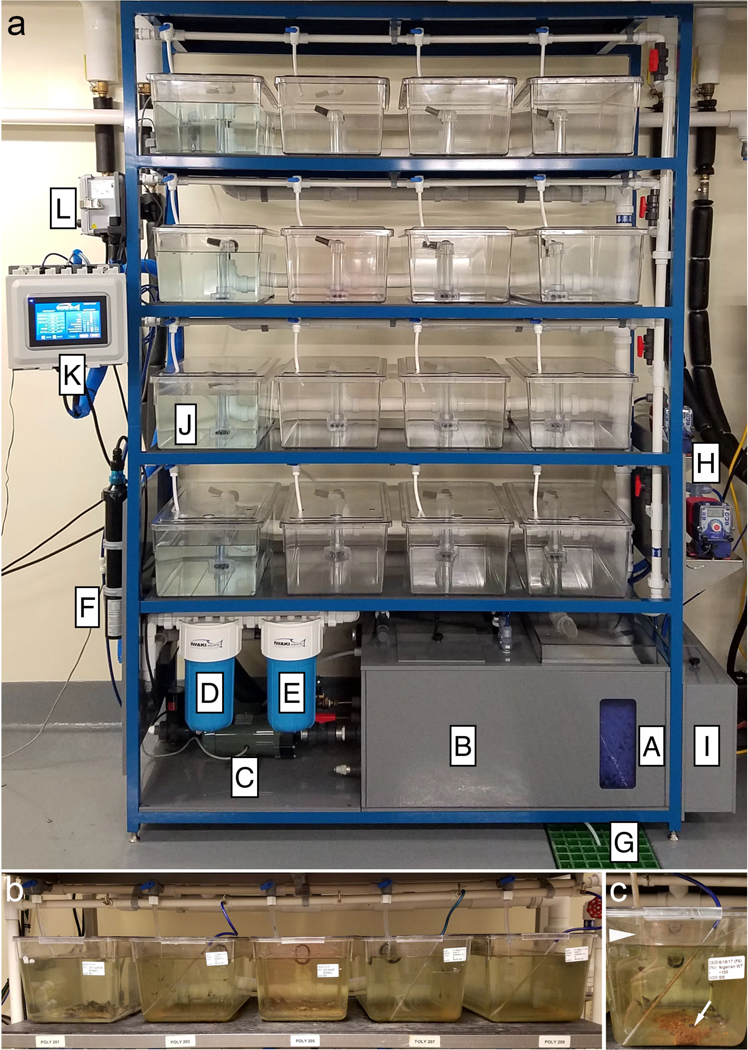
(a) Example of an individual recirculating aquatic system designed by Iwaki Aquatic, the ITS-X model. This system holds 16 × 23 L flood-and-flush tanks, and has the capacity to heat and chill the water. It has four filtration processes and digital user interface display of water quality levels. Specific aspects of the system are labeled. A-biofilter and aeration, B-water storage, submersible heater, and chilling coil, C-water pump, D-mechanical filter, E-carbon filter, F-UV lamp/filter, G-water effluent, H-pH/conductivity dosing pumps, I-pH/conductivity dosing reservoirs, J-23 L flood-and-flush tank, K-human machine interface, L-programmable logic controller with pH/Conductivity probes. (b) Nursey tank row. One row of 16 L nursery tanks shown as examples; the tanks have air lines added for aeration of water when growing tadpoles. (c) Single nursery tank. Arrowhead - incoming flow of water to the tank is much less than for adult frogs. Arrow - uneaten food and detritus settles to the bottom of the tank and provides tadpoles with additional food and places to hide.
For adult animals, we recommend the use of so called flood-and-flush tanks. These are self-cleaning tanks that are designed for automatic clearing of undissolved solids, such as leftover food and excretion products. Use of these tanks minimizes the time and effort required to net out the solids and in addition maintains cleaner water.
We do not recommend using the flood and flush tanks for growing tadpoles as this will result in flushing the animals out of the system. Tadpoles and froglets should be kept in tanks with only an overflow bulkhead (Figure 1b).
For growing tadpoles, we recommend that the tanks have air lines or bubblers installed to help oxygenate the water. Care should be taken however, as too fast a bubbling rate can actually have a negative impact on the tadpoles by increasing the total dissolved gas pressure. We generally keep the flow rate at no more that 10 × 3–5 mm bubbles per second in a 16 L tank (Figure 1b-c).
System readings should be checked and recorded daily to spot any potential developing problems; this is in addition to the automatic monitoring that is done by the systems.
Animal health and wellbeing should be confirmed via a daily visual inspection.
10% of the system water should be exchanged daily; most systems will change the water slowly over the course of 24 hours.
The animals should be kept in a 12 hours light/12 hours dark cycle, though this can be flexible, as others use 14 hours light/10 hours dark.
Animals can be provided with environmental enrichment; however, this should be kept to a minimum to limit the surface area available for algal growth. For X. laevis we recommend PVC piping cut lengthwise in half, of a length sufficient to allow the animals to hide under. Cut surfaces should be smoothened out to eliminate sharp edges that can injure the frogs. For X. tropicalis we recommend artificial aquarium foam lotus leaves that permit the animals to both hide underneath as well as rest on top.
The tank flow rate will vary with tank size and should be kept between 1.0–3.5 LPM for smaller to larger tanks respectively. Ideal flow should be high but without agitating the water.
To limit algal growth and detritus accumulation tanks should be spot cleaned as needed using aquarium scrubbing pads (see Note 2).
Tanks that are especially soiled should be replaced with clean tanks, and themselves disassembled and thoroughly cleaned. For cleaning, the tank and the lid should be scrubbed and rinsed with RO (Reverse Osmosis) water, sprayed with 10% bleach and allowed to stand for 30 minutes to 1 hour, rinsed with RO water again, sprayed with 70% ethanol and allowed to stand for 30 minutes to 1 hour, finally rinsed once more with RO water, allowed to dry, and put away.
Tank accessory parts, cleaning utensils, nets, and enrichment items should be cleaned following a similar process. Rinse in RO water and scrub if necessary, let soak for 1 hour in 10% bleach, let soak another hour in RO water with 10 g/L Na2S2O3, soak for 10 minutes in 70% ethanol, rinse with RO water, air dry and put away.
3.1.1. Establishing a New Recirculating Aquatic System
When setting up a new recirculating aquatic system, the biofilter must be established to ensure that the ammonia produced by frog excretion is converted to nontoxic byproducts. Nitrifying bacteria are used in recirculating systems to create the ammonia/nitrogen cycle [5]. Ammonia (NH3/NH4+) introduced from frog and food waste is oxidized by Nitrosomonas bacteria creating the byproduct nitrite (NO2−). High levels of nitrite are also harmful to frogs and it is further oxidized by Nitrobacter bacteria into non-toxic nitrate (NO3−). A startup container of bacteria can be purchased from aquatic system companies to seed the system.
Biomedia, on which the bacteria grow, should be rinsed before being placed into the biofilter.
On the first day, run one tank at the end of each row and place around 5–10 sentinel frogs total in the system. The UV lamp and water effluent exchange on the recirculating system should be OFF and no carbon included.
The next day, shut the system off except for the air pump to keep the biomedia aerated. Then, add the correct dosage of nitrifying bacteria to the biofilter, which depends on the amount of water in the system.
Allow bacteria to mix with the biomedia for 1–2 hours then turn the system back on, leaving the UV lamp and water effluent OFF and no carbon. Keep the filter pad and mechanical cartridge filter in the system.
Measure the NH3/NH4+, NO2−, and NO3− levels daily. In a couple of days to a few weeks the NH3/NH4+ levels will reach above 1–2 ppm. At this time, turn on the UV and water effluent, add carbon and start up 1–2 more tanks.
Continue to measure the water quality every 1–2 days. NO2− levels should then increase followed by NO3−. This process can take a couple weeks to 2 months.
Once both NH3/NH4+ and NO2− levels reach below 0.5 ppm, the biofilter has been established and is ready to receive more frogs.
3.1.2. System Sterilization
At times, it may be necessary to sterilize an entire frog housing system before introducing any animals into it. The following protocol can be used to recover a system following a disease outbreak, when repurposing it for use from one species to another, or prior to restarting a used or old system after an extended period of inactivity.
If the system is still in use shut it down completely turning off the water pump, the dosing pumps, any biomedia agitators and air bubblers, and unplugging pH and conductivity probes.
Remove all biomedia and either sterilize it by soaking in a 10% bleach solution for at least one day or discard it.
Clean the sump by netting out any detritus. Do this several times letting the detritus settle between each time, then scrub the inside of the sump and any sump parts.
Remove all filtration from the system including filter pads, filter cartridges, and carbon filters. Leave the UV bulb in place.
Disassemble the frog tanks. Scrub the tanks and the lids if necessary, then rinse with water, spray with 10% bleach and let stand for 1 hour, rinse with water, spray with 70% ethanol and let dry for 1 hour, rinse again with RO water, and finally allow to dry completely. Tank accessory parts should be scrubbed and rinsed, soaked 1 hour in 10% bleach, soaked 1 hour in a 10 g/L solution of Na2S2O3 in RO water, soaked 10 minutes in 70% ethanol, rinsed with RO water, and air dried.
Drain 75% of the sump volume.
Reassemble the system by putting back all the tanks as well as other inserts. For the carbon cartridge leave the carbon out.
Start the water pump letting the sump and all the tanks fill to normal capacity.
Add bleach to the sump. The final concentration of NaClO running through the system should be 0.06%.
Adjust the effluent rate to its lowest setting but no less than 1% volume exchange per day.
After 2–3 days increase the effluent rate to its maximum setting, preferably at least 50% volume exchange per day.
After 7 days begin testing the system pH daily. Once the system pH matches that of the influent water add Virkon Aquatic to the system. The amount of Virkon required is 3.2 g per 1 m2 of estimated internal surface area of the tanks and sump.
Return effluent to 1% exchange rate. Virkon will foam reaching and sterilizing surfaces in the system that water does not contact. At this point the system should be closely monitored as it is possible that the foam may overflow. To limit the risk of the overflow, water agitation in the tanks and sump should be reduced to a minimum and excessive foam should be sprayed with 70% ethanol to break up the bubbles.
After seven days change the effluent to 50% exchange rate to flush Virkon out of the system.
Observe the system daily. Virkon should be mostly gone once no foam can be seen. Let the system run for 2–3 more days to make sure Virkon is completely flushed out.
Replace all the rubber tubbing.
At this point the system is sterile and can be started up again using the procedure described in section 3.1.1.
3.2. Water Parameters and Animal Density
The optimal water parameters and the density at which animals are kept varies both by species and by the life history stage. In general, the animals can tolerate a range of values, with the average presumably being the optimal. In Table 1 we list the optimal values at which we keep our animals with the range of tolerance provided in parentheses where appropriate. All water is derived from RO Filtration. The systems are supplemented with Reef Salt and sodium bicarbonate to regulate conductivity and pH respectively. In mature systems water quality should be checked on a weekly basis. The readings for both X. laevis and X. tropicalis systems should be: NH3/NH4+ = 0 ppm (0–0.5 ppm), NO2− < 1 ppm, NO3− < 40 ppm, alkalinity > 40 ppm.
Table 1.
Water quality parameters for Xenopus
| Age | Water temperature (°C) | pH | Conductivity (µS) | Density | |
|---|---|---|---|---|---|
| X. laevis | Tadpole/Froglet | 24 (23–25) | 7.4 (7.2–7.6) | 1000 (900–1100) | 2–4 per 1L |
| Juvenile* | 22 (20–24) | 7.8 (7.0–8.5) | 1600 (1200–1800) | 1 per 1–2L | |
| Adult | 20 (18–20) | 7.8 (7.0–8.5) | 1600 (1200–1800) | 1 per 3L | |
| X. tropicalis | Tadpole/Froglet | 27 (26–27.5) | 7.0 (6.8–7.2) | 1000 (900–1100) | 2–4 per 1L |
| Juvenile* | 26 (25–27) | 7.0 (6.8–7.2) | 1200 (1000–1300) | 1 per 1L | |
| Adult | 25 (24–26) | 7.0 (6.8–7.2) | 1200 (1000–1300) | 1 per 1L |
8–12 month old X. laevis and 5–8 month old X. tropicalis.
3.3. Feeding
To ensure rapid growth and maturation of animals, as well as maintenance of healthy and productive adults it is essential to have a regular feeding schedule and use the appropriate food. The regimen described here has been developed in our facility and works well for animals kept in recirculating systems. The amount and frequency of feeding varies mainly by size and at early stages of development is identical for both X. laevis and X tropicalis.
3.3.1. Feeding Pre-metamorphic Tadpoles
Begin feeding when animals are older than stage 45. Feed them daily with sera Micron Growth Food while keeping them in a static container [6]. The amount used will depend on the size of the container in which the tadpoles are being grown and the number of tadpoles. A good rule of thumb is to add enough feed to turn the culture just slightly greenish. The following day the culture should not be green and if the tadpoles are feeding, strands of green digestion and excretion products should be present on the bottom of the container.
sera Micron can be sprinkled on top of the culture surface as most of it will eventually sink, however, some of it will invariably remain on the surface and over time settle on the sides of the container. To prevent settling on sides we recommend premixing the sera Micron with 0.1x MMR first, either in a weight boat by stirring it in with a Pasteur pipette or in a conical tube by vortexing, then adding this mixture to the tadpole culture.
When growing the tadpoles in the static tanks, large clutches of tadpoles will get dirty with time. Several steps can be taken to keep them relatively clean before the tadpoles reach a size large enough to be put in a recirculating system nursery tank. A pipette can be used to aspirate excretion products and settled food from the bottom of the container, more 0.1x MMR can be added to the culture, or a 50% volume exchange can be done with fresh 0.1x MMR. When exchanging 50% volume it is important that the MMR solution being added is at the same temperature as the one in which the tadpoles are growing.
At approximately two weeks of age tadpoles are transferred over to a nursery tank in a recirculating aquatic system. Once in the recirculating system the feeding schedule changes slightly, and tadpoles now should be fed twice daily, first in the early morning and second in the late afternoon. Just prior to feeding, the water flow in the tank should be decreased and kept at this lower flow rate for one to two hours; shutting off the water is not recommended during feeding as this affects the stability of water temperature. Food is measured into a 50 mL conical tube, resuspended with system water, and the whole mix is added to the tadpole tank. Initially only sera Micron is used, but as the animals grow their diet is supplemented with a 1:1 mix of brine shrimp flakes and Nasco frog brittle powder ground together to a powder like consistency (Table 2). Care should be taken to not overfeed. The feeding regimen described in Table 2 works well for 16 L nursery tank, but can be adjusted to work with tanks of different sizes.
As the tadpoles are typically not grown in the self-cleaning flood-and-flush systems, food detritus will accumulate on the bottom of the tank over time (Figure 1c). It is not essential to clean it out as this detritus is not detrimental to animal health and the tadpoles feed off the settled food on the bottom of the tank. Furthermore, cleaning the detritus poses a risk of accidentally disposing of animals when they are relatively small. Therefore, we typically do not clean the excess detritus until the tadpoles reach late metamorphosis/early froglet stage. At this point, floating detritus can be removed using a net.
Table 2.
Feeding regimen for Xenopus
| Age | Regimen | Food |
|---|---|---|
| Days 1–4 (In System) | 2x Daily | 0.1g Sera Micron |
| Days 5–6 | 2x Daily | 0.25g Sera Micron |
| Days 7–11 | 2x Daily | 0.4g Sera Micron |
| Days 12–20 | 2x Daily | 0.6g Sera Micron |
| Days 21–27 | 2x Daily | 0.4g Sera Micron + 0.2g Brine Shrimp Brittle Mix |
| Days 28–34 | 2x Daily | 0.4g Sera Micron + 0.4g Brine Shrimp Brittle Mix |
| Days 35+ | 2x Daily | 0.6g Sera Micron + 0.6g Brine Shrimp Brittle Mix |
| Post-Metamorhpic | 2x Daily | 1:1 ratio 1.2mm Bio Vita Fry Trout Pellets Nasco Post-Metamorphic Brittle/Powder |
| Juvenile froglets* | 5x Week 10–12 pellets/frog | 1:1 ratio 2mm Bio Vita Fry Trout Pellets Nasco Post-Metamorphic Brittle |
| Adult X. tropicalis | 3x Week 8–10 pellets/frog | 1:1 ratio 2mm Bio Vita Fry Trout Pellets Nasco Post-Metamorphic Brittle |
| Adult X. laevis** | 2x Weekly 6 pellets/frog | 1:1 ratio 4mm BioTrout Pellets Nasco Adult Brittle |
8–12 month old X. laevis and 5–8 month old X. tropicalis.
For J-strain adults feed 5x week if small, 3x week if large.
3.3.2. Feeding During and Post Metamorphosis.
As the animals undergo metamorphosis and mature into froglets their feeding schedule and food size is adjusted. Eventually, slightly different feeding regimens and diets are used for maintenance of adult X. laevis and X. tropicalis.
X. laevis animals younger than 8 months should continue to be fed twice a day. At 8 months of age, feeding is changed to once a day, five days/week. After 12 months, outbred wild type X. laevis are fed only twice a week. Inbred J strain X. laevis animals should continue to be fed five days/week; depending on their size feeding can eventually be reduced to 3 days/week.
X. tropicalis animals younger than 5 months should be fed twice a day. Once they reach 5 months of age, feeding should be changed to once a day. After 8 months, adult X. tropicalis should be fed three days/week.
Observe the animals few minutes after feeding. Add more food if all the food in the tank is gone and they are still actively feeding.
Typically, animals will ignore food that has been sitting in the tank water for extended amount of time. At one to three hours post feeding visually inspect the tanks and if necessary net out any excess food settled on the bottom. Make sure to use a different, clean net for each tank.
Overfeeding can result in poor water quality, accumulation of waste, and additional system maintenance.
3.4. Transportation and Quarantine Procedures
Shipping animals is relatively straightforward but requires some planning and coordination, by both the shipper and the receiver, to limit the stress on the animals associated with the transport as well as prevent any potential problems. Any paperwork and health-tests that may be required by law or by the receiving institution should be done ahead of time. If possible, the animals should be shipped over night and it is recommended that they are also shipped early in the week in case some problem occurs that causes their delivery to be delayed to a later date. Animals should be unpacked as soon as they arrive and any packing material such as shredded sponge or peat moss should be rinsed off before they are put in the system. Adult animals should be allowed to rest one to three weeks before being used for breeding. Finally, shipping cryopreserved sperm, testes, and tadpoles is also a possibility if the receiving facility cannot accommodate intake of adult animals.
3.4.1. Shipping Tadpoles
Prior to shipping tadpoles, it is important to check the temperature at the origin and the destination to assure that the tadpoles will not experience temperature conditions that may be detrimental to their survival. If necessary, ice or heating pack can be included in the shipping container. “Handle with care” and “Live tadpoles” stickers should be used to label the container and assure it is handled gently.
Use a 50 mL conical tube for shipping tadpoles younger than stage 48. Fill the tube with 35 mL to 40 mL of 0.1x MMR plus 10 μg/mL of gentamicin. Put no more than 50 tadpoles per tube. Parafilm the whole tube to enhance its structural integrity and pack into a Ziploc bag as a secondary container. Ship in either a Styrofoam box with bubble wrap or an insulated mailer envelope.
Tadpoles older than stage 48 can be shipped in 1 L to 4 L volume plastic containers. We recommend the use of Cubitainers. Fill the Cubitainer 80% with frog system water or 0.1x MMR and add the tadpoles. Keep the density of tadpoles to 10–20 per 1 L; do not overcrowd. Parafilm the cap and ship secured in a Styrofoam box.
3.4.2. Shipping Adult Animals
As with tadpoles, prior to shipping adults check the temperature at the origin and destination to assure the conditions are viable for animal survival. X. laevis can tolerate temperatures as low as 5 °C and X. tropicalis can be shipped if the temperature is at least 10 °C; the animals can tolerate up to 30 °C. If the temperatures are outside the animal tolerance range, but it is absolutely necessary to ship the animals, then arrangements should be made with companies that specialize in shipping under climate controlled conditions. Label the shipping container with “Handle with care” and “Live frogs” stickers to assure they are handled gently.
To ship X. laevis use a 43 cm × 43 cm × 23 cm (17” × 17” × 9”) cardboard box with a compatible Styrofoam box and lid. With the Styrofoam box inside the cardboard box, and the cardboard box closed, drill 12 air holes through the top of both boxes with a 5/16” drill bit. Mark the lid of the Styrofoam box and the inside of the cardboard box to aid in orientation. Remove the Styrofoam box, fill half way with dried peat moss or shredded sponge, and let the moss/sponge soak in RO water for at least one hour. Drain most of the water out leaving the moss/sponge damp but not soaking wet.
Density is critical to ensure the frogs arrive healthy to their destination. Place up to 10 adult X. laevis female wild type frogs (~130 g each) in the Styrofoam box and tape its edge. Up to 12 female J strain animals or 15 male frogs can be shipped per box since they are much smaller in size. Put the taped Styrofoam box back in the cardboard box, using the markings on the inside of the cardboard and the lid of the Styrofoam to align the air holes. Add any necessary shipping documents, tape the top of the cardboard box, label, and ship.
To ship X. tropicalis we use a 12 cup (3 L) plastic food container with a snap top. Drill air holes in the lid with a ¼” drill bit. Fill the container half way with dried peat moss or shredded sponge and let soak in RO water for at least one hour. Drain the water so that the moss/sponge is damp but not soaking wet.
Place up to 5 adult frogs in the plastic container and seal it. Up to four containers can fit in a 43 cm × 43 cm × 23 cm (17” × 17” × 9”) Styrofoam box. When shipping X. tropicalis always use a heat pack/hand warmer. Let the heat pack warm up, wrap it in damp paper towels, place it in a plastic bag with holes in it for ventilation, and tape it to the inside lid of the Styrofoam box. Make sure to also drill air holes in the lid of the Styrofoam box and the accompanying cardboard box.
3.4.3. Shipping Sperm and Testes
Shipping cryopreserved sperm on dry ice is a simple way of distributing distinct animal lines. Sperm can be used immediately or stored for extended period of time either at −80 °C or in liquid nitrogen. In general, fertilization from cryopreserved sperm is efficient and animals generated develop normally, however, it has also been demonstrated that cryopreserving sperm does cause some plasma membrane damage and DNA fragmentation in the spermatozoa [7, 8].
As an alternative to shipping cryopreserved sperm, fresh Xenopus laevis testes can be shipped. Fill a 50-mL conical tube with 35 mL to 40 mL of ice cold 1x MMR with 10 μg/mL of gentamicin. Add freshly isolated testes. Parafilm the entire tube to help maintain integrity. Put the tube in a Ziploc bag as a secondary container and wrap in bubble wrap. As a shipping container use either a Styrofoam box or an insulated mailer envelope. Put a freezer pack on the bottom of the container and cover with either paper towels or some bubble wrap to prevent direct contact between the freezer pack and the conical tube. Make sure the conical tube is secured and will not toss around during shipping by either taping it to the side of the shipping container or filling the container with bubble wrap.
3.5. Health
Daily visual checks are necessary for maintenance of a healthy animal colony. Attention should be paid to both physical and behavioral indicators of potential health problems looking for discoloration or redness of the skin, apparent wounds, abnormal swimming, changes in body shape and size, bloating, and lethargic response to external stimulation. Any frogs showing these signs should be isolated from their tank. Whereas, frogs with visible wounds, bloating, or skin discoloration might recover after being placed for several days in a bath of 1x MMR with 10 μg/mL gentamicin, other animals should be euthanized and necropsied. Close attention should be taken with animals that go through sudden stressors such as temperature spikes, as it can lead to susceptibility to sickness or mortality. In bloated animals, pressure can sometimes be released by creating a small laterally located puncture in the posterior third of the ventral abdomen with a 16 gauge needle to release the accumulated fluid or gas. This should only be attempted by a trained individual or a veterinarian as care must be taken that the puncture goes through both skin and the abdominal muscle but is shallow enough to not damage any of the viscera. Animals that do not show improvement should be euthanized and necropsied.
Suboptimal quarantine protocols, in particular when bringing new animals into the colony, may lead to introduction of disease causing agents. Many frog pathogens are dormant with the disease outbreaks caused by stress, such as that associated with shipping or less than ideal husbandry conditions. Several known pathogens of Xenopus have been previously characterized in the literature and include Ranavirus, bacteria (Mycobacterium spp., Chlamydophila spp.), Chytrid fungus, and Cryptosporidium and even in colonies not showing any signs of sickness, regular tests (once or twice per year using qPCR) are recommended [9–13]. Frogs showing signs of disease as well as other frogs from the same tank should be immediately isolated from the system. If a disease is spread throughout the colony immediate aggressive treatment or euthanasia are recommended. If a colony is euthanized, the aquatic housing system should be sterilized before next use.
3.5.1. Euthanasia
When necessary, anesthetic overdose can be used as an efficient and humane way of euthanasia in Xenopus. Prepare a bath of 0.15% benzocaine in frog water, or 0.5% MS222 in water buffered to pH 7.0. After 30 minutes, check that the frog is unresponsive to foot pinching and lacks a swallowing reflex when its throat is rubbed. Put the euthanized animal in a bag and freeze at −20 °C over-night. Discard the carcass following local and institutional regulations.
3.6. Record keeping
Good record keeping is essential for maintenance of a healthy frog colony. Records can be kept on paper or in a digital format, but it is essential that water parameters are recorded daily. In addition, a mortality log should be kept to document the tank from which the dead animal came, any particular abnormalities of the carcass, and whether it was a recently mated female. These records will allow establishment of an operational baseline which can be used to identify whether water parameters or frequency of animal death look abnormal and thus catching potential developing problems early. Figure 2 shows an example of such a log.
Figure 2. Record keeping.
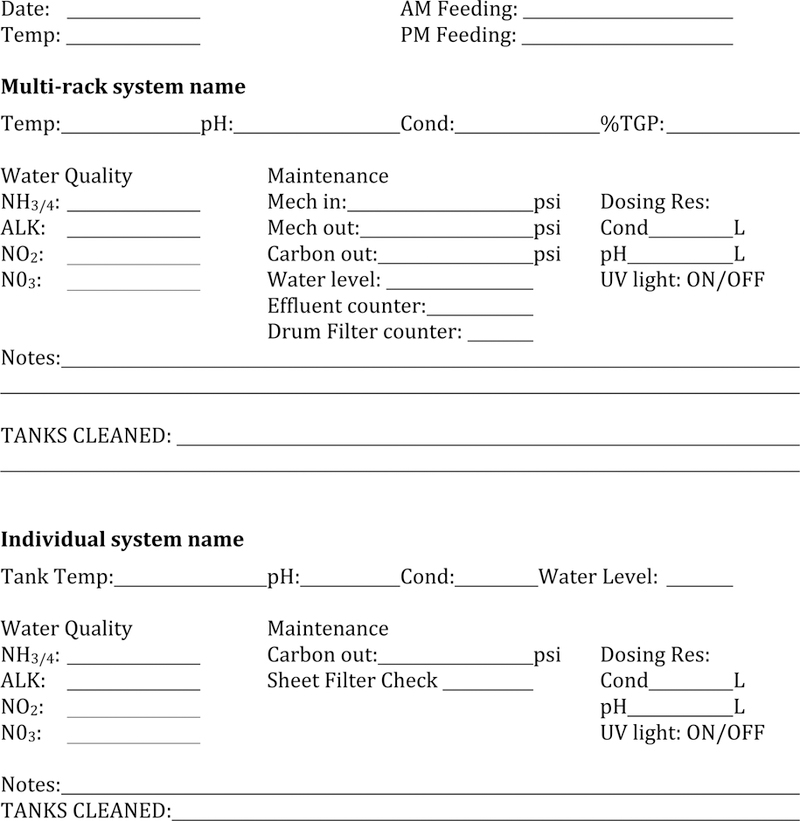
Two examples of format for record keeping of either multi-rack system or individual system. Various values/parameters are recorded daily.
Acknowledgments
The National Xenopus Resource is supported by a grant from the National Institutes of Health (P40 OD010997).
Footnotes
Notes
Some labs use home-made recirculating systems for housing their animals. In particular, in areas where municipal water is not highly chlorinated, tap water purified through a carbon filter can be used instead of reconstituted water. In such a configuration general water hardness provided by dissolved calcium and magnesium ions needs to be monitored as well, since moderate to very hard water is required to produce high quality eggs and normal embryonic development [14].
Detergents should never be used to clean any equipment used for housing and handling adult frogs and embryos.
References
- 1.Gurdon JB, Hopwood N (2000) The introduction of Xenopus laevis into developmental biology: of empire, pregnancy testing and ribosomal genes. Int J Dev Biol 44:43–50. [PubMed] [Google Scholar]
- 2.Tandon P, Conlon F, Furlow JD, Horb ME (2017) Expanding the genetic toolkit in Xenopus: Approaches and opportunities for human disease modeling. Dev Biol 426:325–335. 10.1016/j.ydbio.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wlizla M, Falco R, Peshkin L, et al. (2017) Luteinizing Hormone is an effective replacement for hCG to induce ovulation in Xenopus. Dev Biol 426:442–448. 10.1016/j.ydbio.2016.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearl EJ, Grainger RM, Guille M, Horb ME (2012) Development of Xenopus resource centers: the National Xenopus Resource and the European Xenopus Resource Center. Genesis 50:155–163. 10.1002/dvg.22013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hem LJ, Rusten B, Ødegaard H (1994) Nitrification in a moving bed biofilm reactor. Water Research 28:1425–1433. 10.1016/0043-1354(94)90310-7 [DOI] [Google Scholar]
- 6.Showell C, Conlon FL (2009) Natural mating and tadpole husbandry in the western clawed frog Xenopus tropicalis. Cold Spring Harb Protoc 2009:pdb.prot5292–pdb.prot5292. 10.1101/pdb.prot5292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearl E, Morrow S, Noble A, et al. (2017) An optimized method for cryogenic storage of Xenopus sperm to maximise the effectiveness of research using genetically altered frogs. Theriogenology 92:149–155. 10.1016/j.theriogenology.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrow S, Gosálvez J, López-Fernández C, et al. (2016) Effects of freezing and activation on membrane quality and DNA damage in Xenopus tropicalis and Xenopus laevis spermatozoa. Reprod Fertil Dev 29:1556–1566. 10.1071/RD16190 [DOI] [PubMed] [Google Scholar]
- 9.De Jesús Andino F, Chen G, Li Z, et al. (2012) Susceptibility of Xenopus laevis tadpoles to infection by the ranavirus Frog-Virus 3 correlates with a reduced and delayed innate immune response in comparison with adult frogs. Virology 432:435–443. 10.1016/j.virol.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfrey D, Williamson H, Silverman J, Small PLC (2007) Newly identified Mycobacterium species in a Xenopus laevis colony. Comp Med 57:97–104. [PubMed] [Google Scholar]
- 11.Reed KD, Ruth GR, Meyer JA, Shukla SK (2000) Chlamydia pneumoniae infection in a breeding colony of African clawed frogs (Xenopus tropicalis). Emerging Infect Dis 6:196–199. 10.3201/eid0602.000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tinsley RC, Coxhead PG, Stott LC, et al. (2015) Chytrid fungus infections in laboratory and introduced Xenopus laevis populations: assessing the risks for U.K. native amphibians. Biol Conserv 184:380–388. 10.1016/j.biocon.2015.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green SL, Bouley DM, Josling CA, Fayer R (2003) Cryptosporidiosis associated with emaciation and proliferative gastritis in a laboratory-reared South African clawed frog (Xenopus laevis). Comp Med 53:81–84. [PubMed] [Google Scholar]
- 14.Godfrey EW, Sanders GE (2004) Effect of water hardness on oocyte quality and embryo development in the African clawed frog (Xenopus laevis). Comp Med 54:170–175. [PubMed] [Google Scholar]


