Summary
Background
Viral suppression among HIV-positive individuals is essential for protecting health and preventing HIV transmission. Financial incentives have shown promise in modifying various health behaviors in low-income countries but few studies have assessed whether they can improve HIV treatment outcomes. We aimed to determine the impact of time-limited financial incentives on viral suppression among HIV-positive adults in rural Uganda.
Methods
We conducted a randomized trial in four rural Ugandan parishes between June 27, 2016 and May 25, 2018. HIV-positive individuals aged ≥18 years were recruited from community health campaigns that included HIV testing services or at a local government health facility where HIV treatment is offered. Participants included those who were initiating antiretroviral therapy (ART) or already receiving ART. We measured participants’ viral load at baseline, 6, 12, 24, and 48 weeks and provided results along with viral load counseling. Participants randomized to the intervention group received financial incentives for viral suppression at 6, 12, and 24 weeks, with incentive amounts escalating from US$4 to US$12.5. The primary outcome was viral suppression (viral load<400 copies per mL) at 24 weeks. To assess longerterm effects of time-limited incentives, the secondary outcome was viral suppression at 48 weeks. This trial is registered with ClinicalTrials.gov (NCT02890459).
Findings
400 adults were enrolled in the study, 324 from community health campaigns and 76 from the government clinic. Of these, eight (2%) withdrew from the study and were not included in analyses. Over the 48-week follow-up period, 35 (9%) died or were lost-to-follow-up. Participants’ median daily income was US$0.79. At baseline, 300 participants (77%) were virally suppressed. In intention-to-treat analyses, 168 participants (84%) in the intervention group and 156 participants (82%) in the control group were virally suppressed at 24 weeks (odds ratio, OR, 1.14, 95% CI 0.68–1.93, p=0.62. In per-protocol analyses limited to participants with viral load measurements at 24 weeks, there was no difference between study groups in viral suppression (OR 0.91, 95% CI 0.45–1.83, p=0.78). Withdrawal of incentives at 24 weeks did not affect long-term viral suppression, with 176 (88%) and 154 (81%) participants in the intervention and control groups, respectively, being virally suppressed at 48 weeks (p=0.06).
Interpretation
Financial incentives had no effect on viral suppression among HIV-positive adults. High baseline viral suppression and provision of viral load results might have contributed to high viral suppression among participants. The results underscore the need for interventions that promote achievement of viral suppression among unsuppressed individuals.
Introduction
Antiretroviral therapy (ART) can significantly improve the health of HIV-positive persons and virtually eliminate the risk of HIV transmission to others.1 Realizing the benefits of ART requires treatment upon diagnosis of HIV with high and sustained adherence to a regimen that can achieve and maintain viral suppression. Despite substantial progress in scaling-up both HIV testing services and ART access in sub-Saharan Africa (SSA), roughly half of people living with HIV in the region are not virally suppressed.2 Moreover, in many settings there is a need for interventions that address not only the problem of achieving viral suppression among newly-diagnosed HIV-positive persons but also the challenge of maintaining viral suppression among those who have initiated ART since these individuals may have suboptimal levels of ART adherence and experience virologic rebound. For example, in estimates from eastern and southern Africa, 17% of HIV-positive persons accessing ART fail to achieve viral suppression.2
Insights from economics and psychology have identified several low-cost approaches for modifying behaviors that influence the effectiveness of biomedical HIV prevention and treatment. The use of monetary and non-monetary incentives, including lottery-based rewards, has been shown to promote several one-time behaviors such as uptake of HIV testing and medical male circumcision.3–5 However, evidence is mixed when it comes to repeated behaviors such as retention in clinical care and medication adherence.6 Several studies conducted in SSA recently have shown that financial incentives are effective in promoting retention and ART adherence, although these studies have not assessed viral suppression outcomes.7,8 In the US, on the other hand, one study showed that financial incentives lead to a modest increase in viral suppression rates while another showed no effects.9–11 With substantial variation in incentive designs and outcomes assessed, there is uncertainty about the optimal design of incentives and an accompanying need for operationally simple ways of implementing incentive interventions. Moreover, there is some evidence that time-limited incentives for healthy behaviors such as exercise or smoking cessation can lead to sustained behavior change.12,13 Few studies have addressed whether incentivizing adherence behaviors or viral suppression outcomes has similar long-term effects.
Given the importance of viral suppression for treatment effectiveness, we used behavioral economics and contingency management principles to study the effectiveness of financial incentives for both achieving and maintaining viral suppression in HIV-positive adults. We conducted a randomized trial of incentives in Uganda at a time when the country began to adopt World Health Organization guidelines that call for immediate offer of ART following diagnosis.14
Methods
Study design and participants
The study was a 2-group randomized clinical trial of financial incentives for viral suppression and was conducted between June 27, 2016 and May 25, 2018. It was a community-based study and included a 6-month intervention period followed by a 6-month follow-up period of no interventions. The study protocol is available in Supplement 1. The study occurred in four rural parishes in Mbarara District, Uganda, that are geographically adjacent to one another. The communities lie within the catchment area of the Bwizibwera Health Center IV, a government health facility that offers HIV treatment in concert with national guidelines along with other health services.
HIV-positive men and women were identified from multi-disease community health campaigns (CHCs) conducted in the study communities between June and July 2016 and at the Bwizibwera Health Center IV between October 3, 2016 and May 9, 2017. CHCs have been shown to achieve high levels of HIV testing coverage in a short period, and this model has been described elsewhere.15 All community members were invited to participate in the CHC. Adult men residing in the communities in particular were randomized to various financial incentives ranging in type (lottery or fixed incentive) and amount (US$1-$5) to attend the CHC and undergo HIV testing as part of a separate study of HIV testing incentives.3 Overall, 57% of community census-enumerated adult men and women attended the CHC and tested for HIV, with participation being higher among men likely due to the provision of incentives. To meet enrollment targets for this study, enrollment of additional HIV-positive adults who lived within the study communities occurred subsequently at the Bwizibwera Health Center IV.
Eligible participants were ≥18 years old, resided in one of the four study communities, and had an HIV-positive result by the Uganda Ministry of Health rapid HIV antibody test algorithm at the CHC or in health facility records. Key exclusion criteria included a self-reported intention to move away from the study area in the next six months. HIV-positive adults were eligible regardless of whether they were newly diagnosed. Given the importance of both achieving and maintaining viral suppression, eligibility to participate in the study and randomization to the study groups below did not depend on current ART or viral suppression status.
The Makerere University School of Medicine Research Ethics Committee, the Uganda National Council for Science and Technology, and the University of California San Francisco (UCSF) Committee on Human Research approved the study protocol. The latter institution served as the institutional review board of record for investigators from the University of Pennsylvania. Written informed consent was obtained from all individuals who met eligibility criteria and agreed to participate in the study.
Randomisation and masking
Participants were randomized to an intervention or control group in a 1:1 ratio in computer-generated blocks (block size 10) and pre-printed scratch cards were used to reveal study group assignment. Study group assignment was not masked from staff who enrolled participants and contacted them at follow-up visits. However, laboratory staff were not aware of participants’ intervention status.
Procedures
Study staff administered a brief baseline questionnaire to all participants. The baseline questionnaire obtained demographic and socioeconomic data on various participant characteristics including their daily wages and prior HIV care status. Participants’ time preferences (i.e. how much they value immediate vs. delayed rewards) were also assessed with an approach that has been used previously in other studies.16 Specifically, participants were asked to choose between a hypothetical monetary reward of $8 on the same day or a $9.50 reward in 14 days, with those who chose the immediate reward being classified as having a high discount rate.
Plasma HIV RNA (viral load) and CD4+ T lymphocyte cell count were measured for all participants at baseline. Participants recruited at the CHC were informed that the local health facility offered ART to all HIV-positive persons free-of-charge. Study staff also provided HIV viral load counseling to all participants, which included education on what an HIV “viral load” measurement is, how ART affects an HIV-positive person’s viral load, and how viral suppression relates to health and likelihood of transmitting HIV. All participants were given an unconditional cash transfer of 15,000 Uganda Shillings (~$4 in 2017 US$) to facilitate initial linkage to care or continued retention in care (if already in care). Participants identified at the CHC were also given a one-month supply of co-trimoxazole” (rINN), an antibiotic recommended for prevention of opportunistic infections in all HIV-positive persons, by Uganda Ministry of Health Guidelines17 and underwent symptom screening for active TB according to Ugandan Ministry of Health guidelines.
All participants were contacted for viral load testing at pre-defined time points of 6, 12, 24, and 48 weeks. Study activities were conducted by study staff who visited participants in communities rather than clinic staff who contacted patients at clinics. Testing typically occurred in participants’ homes or locations convenient to participants. At each interval, study staff provided viral load results (within two weeks of phlebotomy) and counseling to participants based on these results. Counseling procedures were adapted from those developed in the SEARCH Trial conducted in Kenya and Uganda.18 They were designed to ensure that all participants understood their viral load results. Social harms and adverse events were monitored passively over the duration of the study.
Participants in the intervention group were offered financial incentive payments for viral suppression at 6, 12, and 24 weeks. They could receive monetary payments at each period if their viral load was suppressed (HIV RNA <400 copies per mL). To simplify implementation and in recognition of the possibility that participants could receive care at various sites other than the Bwizibwera Health Center, incentives were not linked to medication adherence or clinic attendance measures.
The financial incentives also incorporated contingency management principles to motivate participants in the intervention group (regardless of their baseline viral suppression status) to be virally suppressed at 6, 12, and 24 weeks. Specifically, incentives escalated in value over time by 15,000 Ugandan Shillings (~US$4) per visit, from US$4 for being suppressed at 6 weeks, US$8 for being suppressed at 12 weeks, to US$12.50 for being suppressed at 24 weeks. The incentive amounts were chosen in consultation with a community advisory board prior to study enrollment, and aimed to offset the costs of retention in HIV care (including transport costs to reach clinic for monthly visits, and opportunity costs of time away from work), as well as reward viral suppression. Incentive payments were made in cash, and for those who were not virally suppressed at baseline the 6-week incentive condition was relaxed to allow payment if their HIV RNA had declined by ≥2 log10 since study enrollment. The incentives also featured a reset contingency, as participants in the intervention group who were not virally suppressed at a given study visit did not receive an incentive and subsequent incentives for viral suppression were reset to the initial value of US$4. Details about the financial incentive were explained to participants verbally and illustrated in a handout to participants (see Appendix).
After 24 weeks, incentives were no longer offered and all participants were contacted at 48 weeks for viral load measurement.
Outcomes
The pre-specified primary outcome was viral suppression at 24 weeks, defined as having plasma HIV RNA<400 copies per mL. Pre-specified secondary outcomes included viral suppression at 48 weeks in order assess long-term effects of providing time-limited incentives, as well as viral suppression at 6 and 12 weeks in order to assess time to achieving viral suppression among participants who were not virally suppressed at enrollment and time to loss of viral suppression among those who were suppressed initially.
Statistical analysis
We estimated that a sample size of 400 participants would be needed to achieve >80% power to detect a difference of at least 15% in viral suppression with financial incentives (2-sided alpha-level of 0.05). P-values ≤0.05 were considered statistically significant. The power calculations assumed, on the basis of another study in the region, that 50% of HIV-positive adults would have HIV RNA <400 copies per mL at 24 weeks in the control group.19
We used chi-squared tests as well as unadjusted and adjusted logistic regression to estimate the effect of financial incentives on the primary outcome of viral suppression at 24 weeks. Adjusted logistic regression models controlled for participants’ gender and income due to significant differences between groups at enrollment. The primary analyses used an intention-to-treat approach in which participants who died or were lost-to-follow-up at a given interval were considered to be not virally suppressed. We also conducted per protocol analyses that were limited to participants with viral load measurements at 24 and 48 weeks.
We also performed subgroup analyses to estimate the effect of incentives on the primary outcome of 24-week viral suppression and the secondary outcomes of 48-week viral suppression as well as 6- and 12-week viral suppression (time to viral suppression). Specifically, in post hoc analyses, we examined the subgroup of participants who were virally suppressed at baseline to estimate effects of incentives on maintenance of viral suppression, and the subgroup of those not suppressed at baseline to estimate effects of incentives on achievement of viral suppression. Additionally, the effect of incentives on the primary outcome were examined among post hoc subgroups of participants based on gender and daily wage at baseline (≤median and >median) and pre-specified subgroups of participants with different time preferences (high vs. low discount rates). Participants with high discount rates were hypothesized to be more responsive to provision of incentives. Sensitivity analyses were performed with two alternative definitions of viral suppression at HIV RNA <40 copies per mL and HIV RNA <1,000 copies per mL. Analyses were conducted using Stata 14.0 (StataCorp).
Role of the funding source
The funder of the study had no role in study design, data collection, analysis, and interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
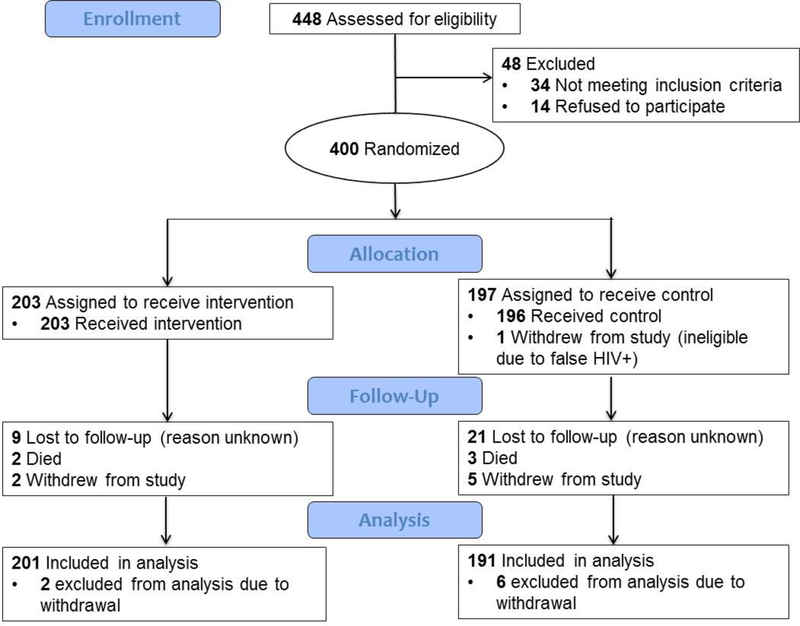
A total of 4,562 individuals aged ≥18 years came for HIV testing at the CHCs and 372 (8.2%) tested HIV-positive at the CHC. Of the 372 adults, 324 (87.1%) were enrolled in the study and an additional 76 were enrolled at the local health facility for a total sample of 400 participants. Following enrollment, 203 and 197 were randomized to the intervention and control groups respectively (Figure 1). Eight participants withdrew from the study and were not included in analyses. In the first 24 weeks, 33 participants died or were lost-to-follow-up, and study retention at 24 weeks was 92% and 87% in the intervention and control groups, respectively. Study retention at 48 weeks was also high given the low rates of loss-to-follow-up between 24–48 weeks, and there were also eight participants (five in the intervention group and three in the control group) who were followed-up at 48-weeks but not 24-weeks. The proportion retained at 48-weeks was significantly higher in the intervention group than the control group (94% vs. 85%, p<0.01).
Figure 1.
CONSORT Flow Diagram
Participants had a mean age of 37 years and 56% (n=220) were female (Table 1). Participants’ daily wages had a mean of US$1.36 and median of US$0.79. Most participant characteristics were similar between study groups, although the intervention group had significantly higher representation of women and lower wages. Clinical characteristics of participants at enrollment were also similar between groups, with 76% reporting they were previously in care and CD4 count having a median (IQR) of 529 (360–706) cells/μL. Seventy-seven percent (n=300) participants were virally suppressed at baseline.
Table 1.
Participant characteristics
| Intervention group | Control group | Total | |
|---|---|---|---|
| Number enrolled | 203 | 197 | 400 |
| Demographics | |||
| Median age (IQR) (n=392) | 36 (31–45) | 37 (30–45) | 37 (31–45) |
| Gender (n=392) | |||
| Male | 77 (38%) | 95 (50%) | 172 (44%) |
| Female | 124 (62%) | 96 (50%) | 220 (56%) |
| Marital status (n=391) | |||
| Married/cohabitating relationship | 118 (59%) | 117 (62%) | 235 (60%) |
| Never married | 16 (8%) | 18 (9%) | 34 (9%) |
| Divorced/widowed/separated | 67 (33%) | 55 (29%) | 122 (31%) |
| Highest school attended (n=383) | |||
| No school | 33 (17%) | 32 (17%) | 65 (17%) |
| Primary to Secondary | 157 (81%) | 149 (79%) | 306 (80%) |
| > Secondary | 5 (3%) | 7 (4%) | 12 (3%) |
| Home has electricity (n=377) | 11 (6%) | 9 (5%) | 20 (5%) |
| Daily wage, US$, median (IQR) (n=377) | $0.79 (0.53–1.32) | $0.79 (0.26–1.32) | $0.79 (0.39–1.32) |
| ≤ median wage | 125 (65%) | 99 (53%) | 224 (59%) |
| > median wage | 66 (35%) | 87 (47%) | 153 (41%) |
| Daily wage, US$, mean (SD) (n=377) | $1.63 (4.37) | 1.09 (1.57) | 1.36 (3.27) |
| High discount rate (n=374)** | 87 (46%) | 105 (56%) | 192 (51%) |
| Previously in HIV care (n=373) | 143 (76%) | 141 (76%) | 285 (76%) |
| Baseline clinical measures | |||
| Median CD4 count, cells per μL (IQR) (n=392) | 532 (393–724) | 517 (349–676) | 529 (360–706) |
| Virally suppressed, <400 copies per mL (n=392) | 153 (75%) | 147 (77%) | 300 (77%) |
| Virally unsuppressed, ≧400 copies per mL | 50 (25%) | 46 (23%) | 96 (24%) |
| VL unsuppressed, previously in care* | 17 (40%) | 18 (40%) | 35 (40%) |
| VL unsuppressed, newly diagnosed* | 24 (60%) | 27 (60%) | 53 (60%) |
Data are n (%) unless specified otherwise. Data are excluded for 8 participants who withdrew from the study. In addition, some participants are missing socio-economic characteristics because baseline data obtained on laptop computers were lost when several computers malfunctioned during data collection activities.
Self-reported.
Determined based on responses to questions administered at baseline about participants’ time preferences for immediate vs. delayed monetary rewards.
In primary intention-to-treat analyses, viral suppression was high in both study groups at 24 weeks, with 84% and 82% in the intervention and control groups having HIV RNA <400 copies per mL, respectively (Table 2). There was no significant difference between groups in the proportion of participants who were virally suppressed at 24 weeks (odds ratio, OR, 1.14, 95% CI 0.68–1.93). Results were similar with adjustment for participant gender and income. In per protocol analyses there were again no significant differences in viral suppression, with at least 90% of participants being virally suppressed in both study groups (OR 0.91, 95% CI 0.45–1.83). Sensitivity analyses showed that effects of the financial incentives were similar for alternative definitions of 24-week viral suppression as HIV RNA <40 copies per mL and <1,000 copies per mL (Appendix Table 1).
Table 2:
Effect of financial incentives on viral suppression at 24 weeks
| Proportion virally suppressed |
Unadjusted odds ratio (95% CI) | Unadjusted p value | Adjusted odds ratio (95% CI) | Adjusted p value | ||
|---|---|---|---|---|---|---|
| Intervention group | Control group | |||||
| Intention-to-treat analyses | 168/201 (84%) | 156/191 (82%) | 1/14 (0.68–1.93) | 0.62 | 1.14 (0.66–1.97) | 0.63 |
| Per-protocol analyses | 168/187 (90%) | 156/172 (91%) | 0.91 (0.45–1.83) | 0.78 | 0.80 (0.39–1.65) | 0.55 |
| Subgroup analyses | ||||||
| Baseline suppressed | 141/153 (92%) | 132/147 (90%) | 1.34 (0.60–2.96) | 0.48 | 1.56 (0.67–3.62) | 0.30 |
| Baseline unsuppressed | 27/48 (56%) | 24/44 (55%) | 1.07 (0.47–2.44) | 0.87 | 1.14 (0.47–2.73) | 0.78 |
| Men | 62/77(81%) | 73/95 (77%) | 1.25 (0.60–2.61) | 0.56 | 1.32 (0.61–2.85) | 0.48 |
| Women | 106/124 (85%) | 83/96 (86%) | 0.92 (0.43–1.99) | 0.84 | 0.96 (0.44–2.11) | 0.93 |
| Median daily wage or less | 67/87 (77%) | 50/61 (82%) | 0.77 (0.39–1.54) | 0.46 | 0.76 (0.38–1.52) | 0.44 |
| Above median daily wage | 93/104 (89%) | 102/125 (82%) | 2.61 (0.97–7.00) | 0.06 | 2.39 (0.88–6.48) | 0.09 |
| High discount rate* | 74 (85%) | 82 (80%) | 1.39 (0.65–2.99) | 0.40 | 1.41 (0.64–3.10) | 0. 39 |
| Low discount rate* | 82 (82%) | 64 (82%) | 0.99 (0.46–2.15) | 0.99 | 0.97 (0.44–2.10) | 0.93 |
Data are n/N (%) unless otherwise specified. Viral suppression was defined as HIV RNA of less than 400 copies per mL. Odds ratio was adjusted for sex and median daily wage.
Determined based on responses to questions administered at baseline about participants’ time preferences for immediate versus delayed monetary rewards.
In intention-to-treat analyses, viral suppression at 24 weeks was more likely among those who were virally suppressed initially as compared to those who were not (91% vs. 55%). In subgroup analyses, however, financial incentives had no effect on the likelihood of maintaining viral suppression (OR 1.34, 95% CI 0.60–2.96) or achieving viral suppression (OR 1.07, 95% CI 0.47–2.44). The effect of the incentives was also not significant among men or among women. It was only among participants with above-median daily wages at enrollment that the intervention had a positive and marginally significant effect on viral suppression at 24 weeks (OR 2.61, 95% CI 0.97–7.00, p=0.057). In addition, while there was some evidence that incentives were more effective among participants with high discount rates than low discount rates, the effects were not statistically significant in either of two subgroups.
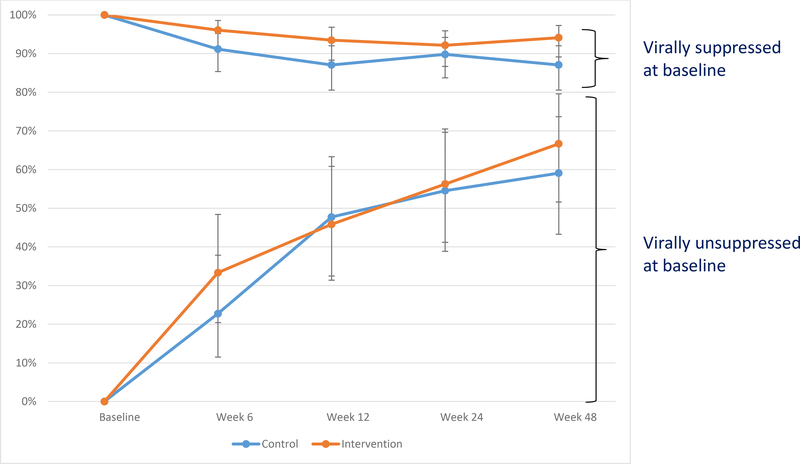
Temporal patterns in achieving and maintaining viral suppression in subgroups that were unsuppressed and suppressed at baseline are shown in Figure 2 and Table 3. During the 48-week study period, there was an increase in achievement of viral suppression for participants who were not virally suppressed initially, irrespective of study group. There were no significant differences between study groups at 6 weeks and 12 weeks, however, indicating that incentives had no effect on time to viral suppression. For participants who were virally suppressed initially, about 90% maintained viral suppression in both groups throughout the study period but incentives did result in a small and marginally significant increase in the likelihood of maintaining suppression at 6 weeks (adjusted odds ratio, AOR, 3.62 95% CI 1.14–11.47) and at 12 weeks (AOR 2.30, 95% CI 0.99–5.30).
Figure 2.
Percent of participants achieving viral suppression over time, stratified by baseline viral suppression
Table 3.
Effect of financial incentives on viral suppression at 6, 12, and 48 weeks
| Proportion virally suppressed, n/N(%) | Unadjusted Odds Ratio (95% CI) | Adjusted* Odds Ratio (95% CI) | Adjusted P-value | ||
|---|---|---|---|---|---|
| Intervention group | Control group | ||||
| Baseline suppressed | |||||
| 6 weeks | 147/153 (96%) | 134/147 (91%) | 2.38 (0.88–6.43) | 3.62 (1.14–11.47) | 0.03 |
| 12 weeks | 143/153 (93%) | 128/147 (87%) | 2.12 (0.95–4.73) | 2.30 (0.99–5.30) | 0.05 |
| 48 weeks | 144/153 (94%) | 128/147 (87%) | 2.38 (1.04–5.44) | 3.00 (1.21–7.44) | 0.02 |
| Baseline unsuppressed | |||||
| 6 weeks | 16/48 (33%) | 10/44 (23%) | 1.7 (0.67–4.29) | 2.01 (0.73–5.55) | 0.18 |
| 12 weeks | 22/48 (46%) | 21/44 (48%) | 0.93 (0.41–2.10) | 0.98 (0.40–2.38) | 0.96 |
| 48 weeks | 32/48 (67%) | 26/44 (59%) | 1.38 (0.59–3.24) | 1.40 (0.57–3.46) | 0.46 |
Notes: results based on intention-to-treat analyses, viral suppression defined as HIV RNA <400 copies per mL
Adjusted for gender and median daily wage
In the 6-month, post-intervention, follow-up period, there was no evidence that the withdrawal of financial incentives led to worse clinical outcomes at 48 weeks. In intention-to-treat analyses, 176 participants (88%) in the intervention group were virally suppressed at 48 weeks compared to 154 (81%) in the control group (p=0.06). Among those who were virally suppressed at baseline, the intervention group had significantly higher viral suppression at 48 weeks than the control group (94% vs. 87%, AOR 3.00, 95% CI 1.21–7.44), indicating that the 6- and 12-week differences in viral suppression were sustained in the long-term. Among those not suppressed initially, there was no significant difference in viral suppression between groups (67% vs. 59%, p=0.45).
Adverse events were rare in both study groups. Six participants (3%) in the control group and four (2%) in the intervention group experienced adverse events. All adverse events were unrelated to study participation. Six of the events were serious adverse events, including two deaths in the intervention group and three deaths in control group. Four of the deaths were attributed to acute illnesses with hospitalization and one was due to acute alcohol intoxication. One participant experienced a serious injury (tibia fracture) following an auto accident. The remaining four adverse events included one participant reporting a stillborn birth, and three transient acute illnesses due to malaria, acute gastroenteritis and pneumonia, and typhoid.
Discussion
In this randomized trial of incentives for viral suppression, escalating financial incentives delivered three times over a 6-month period did not increase the likelihood of viral suppression at 24 weeks among HIV-positive adults. Viral suppression at 24 weeks was high among all participants and subgroup analyses of participants who were virally unsuppressed and suppressed at baseline showed that incentives did not affect the likelihood of either achieving or maintaining viral suppression at 24 weeks, respectively. Incentives did appear to increase the likelihood of maintaining viral suppression initially, but effects were small in magnitude. Encouragingly, there was some evidence that incentives led to higher longterm maintenance of viral suppression 24 weeks after discontinuation of incentives and there was no evidence that discontinuation of incentives led to a deterioration in outcomes for those not suppressed at baseline.
The main finding that incentives were not effective has several potential explanations. First, a much higher-than-anticipated share of participants (77%) were virally suppressed at baseline. Since our primary recruitment strategy was a community-wide HIV testing campaign, we expected to identify many HIV-positive adults who were previously unaware of their status or were not receiving HIV care. Yet a majority of adults identified through the campaign were already virally suppressed, perhaps due to prior HIV prevention and treatment efforts in the region. Nonetheless, it seems unlikely this alone would alter the overall interpretation of our findings since the proportion achieving viral suppression at 24-weeks was almost identical in the intervention and control groups. A more striking finding is that the viral suppression at 24- and 48-weeks among those not suppressed at baseline were about 60%. While it is encouraging that such a high percentage of individuals who were tested in community-based settings became virally suppressed, there is much room for improvement. Our study did not determine reasons for failure to achieve viral suppression, but possibilities include failure to access ART, non-adherence, as well as both acquired (from prior ART) and transmitted drug-resistant virus. Our findings suggest that achieving viral suppression requires more intensive interventions beyond financial incentives alone.
Second, a lower-than-anticipated share of participants who were virally suppressed at baseline had virologic rebound over the 48-week follow-up period. This finding is in contrast to evidence that retention in care and durable viral suppression are challenges facing many HIV care programs.20 While virologic rebound may have been more likely with a longer follow-up period, this study suggests for HIV-positive persons already on ART and virally suppressed, interventions should only be targeted to those at risk of virologic rebound. An alternative explanation is that the home-based viral load measurement and counseling for all participants at regular intervals contributed to higher motivation to both stay in care and adherent to medications. Ultimately, regular biofeedback on adherence and viral load could have resulted in a more “active” control group and reduced the overall effectiveness of incentives. If the control group had received clinic-based viral load measurement that was conducted less frequently, there is a possibility their viral suppression rates would have been lower.
Finally, a third explanation is that the provision of incentives may have been too infrequent and not linked to daily behaviors like medication adherence. Providing frequent incentives offers immediate rewards to individuals and may thereby motivate adherence and promote viral suppression. This would be consistent with behavioral economics insights that the frequency and immediacy of costs and benefits can have strong influences on behavior.21 Such approaches may ultimately be necessary, especially for those who not virally suppressed at baseline, but they requires use of adherence monitoring that provides real-time data and therefore are more resource-intensive. A related explanation is that the incentive amounts used in this study may have been too small to motivate adherence behaviors over a long time period. However, this seems less plausible in light of the finding that participants with above-median wages were more responsive to the intervention, perhaps because their opportunity costs of time were higher and created a more prominent barrier to care-seeking.
Our findings are largely consistent with those from two prominent randomized trials of financial incentives for viral suppression in the US, despite the very different study setting and considerably larger incentive amounts used in the US. One study found that financial incentives along with patient navigation had no long-term effect on viral suppression among patients with elevated viral loads and substance use, while another showed that incentives led to modest increases in viral suppression.9,10 It is noteworthy, however, that recent studies in both the US and in east Africa that incentivized outcomes such as medication adherence periodically (although not daily) and clinic attendance have shown improvements in those outcomes.7,8,22 A study in Tanzania used incentive amounts that were larger than those in our study whereas another in Uganda used incentives that were offered in the form of lower-cost, lottery-based rewards. While these studies did not assess viral suppression as an outcome, they suggest there may be value in incentivizing behaviors that affect viral suppression for some individuals. Approaches that combine incentives for process-oriented behaviors as well as outcomes such as viral suppression may have greater overall effectiveness and warrant further development and testing.
This study also provides suggestive evidence on the important question of whether providing timelimited incentives affects long-term motivation and clinic outcomes of patients. Despite the null finding at 24 weeks, it is notable that outcomes in the intervention group did not deteriorate in the 24 weeks after incentives were withdrawn. Viral suppression at 48 weeks was actually significantly higher in the intervention group than the control group among participants who were virally suppressed at baseline, an encouraging finding for future studies of incentives for promoting adherence and viral suppression.
This study has several limitations. As noted above, a considerably higher-than-anticipated proportion of participants were virally suppressed at baseline, thereby limiting the number of participants who may have been most responsive to incentives for viral suppression. Secondly, the control group in the study received periodic viral load monitoring and counseling. Measurement of viral suppression at intermediate intervals (6 and 12 weeks) necessitated having an active control group, but this may also have reduced the potential to detect an additional effect of incentives for reasons noted above. The study lacked measurement of key HIV care engagement variables such as clinic attendance and medication adherence as well as drug resistance testing, all of which would provide further insight into why some participants were not virally suppressed. The decision to incentivize viral suppression alone was made in recognition of the fact that patients in the study setting can obtain HIV treatment at several different facilities and in order to simplify implementation of incentive-based interventions. Linking incentives to medication adherence, for example, would require objective measurement of adherence and potential increase the cost, and reduce the scalability, of the intervention. Lastly, since all participants were contacted at their homes by study staff and offered viral load testing and counseling based on the results, the control group may have stayed more engaged in care over time than is typically the case. It is possible that offering incentives in a clinic-based setting may have a larger effect on viral suppression.
In summary, financial incentives offered over a 6-month period had no effect on viral suppression among HIV-positive adults. Provision of viral load results and high baseline viral suppression may have contributed to high viral suppression in both study groups. Our findings underscore the need for more interventions that promote achievement of viral suppression among unsuppressed individuals.
Supplementary Material
Research in Context.
Evidence before this study
Despite substantial progress in scaling-up HIV services, including treatment with antiretroviral therapy (ART), roughly half the people living with HIV in sub-Saharan Africa are not virally suppressed. A burgeoning literature has shown that financial incentives can promote healthy behaviors. Several randomized trials have demonstrated that incentives increase uptake of services such as HIV testing and medical male circumcision, as well retention of HIV+ pregnant women in care during pregnancy. We conducted a search of PubMed on October 30, 2018 to review the literature on financial incentives to promote viral suppression among HIV-positive individuals. Using the search terms incentive* AND HIV AND viral AND suppression without language or date restrictions, we retrieved 21 articles. Three of the articles reported results from randomized trials of incentives in which viral suppression was a primary outcome. Two large trials conducted in the United States showed small to modest effects of financial incentives on viral suppression rates, while another pilot trial in the United States showed that commitment contracts linked to clinic attendance and ART adherence led to higher viral suppression. No such studies have been conducted in sub-Saharan Africa. With substantial variation in incentive designs and outcomes assessed, there is considerable uncertainty about the optimal design of incentives and an accompanying need for operationally simple ways of implementing incentive interventions. Few studies have addressed a related and important question of whether discontinuation of incentives leads to long-term improvement or deterioration in outcomes.
Added value of this study
To our knowledge, this is the first study to test the effect of periodic financial incentives for viral suppression in sub-Saharan Africa. Using community- and clinic-based recruitment of HIV-positive adults, a randomized trial design, and measurement of viral suppression as a primary outcome, the study found that financial incentives did not increase the likelihood of viral suppression at 24 weeks. Higher-than-expected baseline viral suppression levels along with provision of viral load results and counseling to all participants may provide some explanation for why incentives were not effective in increasing viral suppression rates. However, subgroup analyses showing that participants who were unsuppressed at baseline did not respond to financial incentives suggest that alternative approaches are necessary. Encouragingly, there was no evidence that discontinuation of incentives after 24 weeks led to a deterioration in outcomes at 48 weeks, a novel finding that broadens understanding about the long-term effects of incentives.
Implications of all the available evidence
There is a vital need for innovative interventions to promote achievement of viral suppression among HIV-positive individuals who are unsuppressed. While the provision of financial incentives linked to viral suppression is unlikely to be successful, future research is needed to test alternative incentive designs that reward process-oriented behaviors such as linkage and adherence in addition to outcomes such as viral suppression alone. Future studies should also test additional, more intensive interventions designed to increase viral suppression rates.
Acknowledgements
We gratefully acknowledge our research staff, community advisory board members, and especially the communities and participants involved in this study. This study was supported by a grant from the National Institute of Mental Health (NIMH) at the National Institutes of Health (R01MH105254). The content is solely the responsibility of the authors and does not represent views of NIMH.
FUNDING National Institute of Mental Health (NIMH) at the National Institutes of Health
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing statement
Deidentified participant data that were collected for this study can be obtained by contacting the corresponding author. Data will be made available after approval of a short proposal summarizing the analyses to be performed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Harsha Thirumurthy, Division of Health Policy, Perelman School of Medicine and Center for Health Incentives and Behavioral Economics, University of Pennsylvania, Philadelphia, PA, USA.
Alex Ndyabakira, Infectious Diseases Research Collaboration, Kampala, Uganda.
Kara Marson, Division of HIV, Infectious Diseases and Global Medicine, University of California, San Francisco, CA, USA.
Devy Emperador, Division of HIV, Infectious Diseases and Global Medicine, University of California, San Francisco, CA, USA.
Prof Moses Kamya, Makerere University, Kampala, Uganda.
Prof Diane Havlir, Division of HIV, Infectious Diseases and Global Medicine, University of California, San Francisco, CA, USA.
Dalsone Kwarisiima, Infectious Diseases Research Collaboration, Kampala, Uganda.
Gabriel Chamie, Division of HIV, Infectious Diseases and Global Medicine, University of California, San Francisco, CA, USA.
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. Ending AIDS: Progress towards the 90–90-90 targets. 2017.
- 3.Chamie G, Schaffer EM, Ndyabakira A, et al. Comparative effectiveness of novel non-monetary incentives to promote HIV testing: a randomized trial. AIDS. 2018;32:1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thirumurthy H, Masters SH, Rao S, et al. Effect of Providing Conditional Economic Compensation on Uptake of Voluntary Medical Male Circumcision in Kenya. JAMA : the journal of the American Medical Association. 2014;312(7):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornton R The Demand for, and Impact of, Learning HIV Status. American Economic Review. 2008;98(5):1829–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maughan-Brown B, Smith P, Kuo C, et al. A Conditional Economic Incentive Fails to Improve Linkage to Care and Antiretroviral Therapy Initiation Among HIV-Positive Adults in Cape Town, South Africa. AIDS patient care and STDs. 2018;32(2):70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linnemayr S, Stecher C, Mukasa B. Behavioral economic incentives to improve adherence to antiretroviral medication. AIDS. 2017;31(5):719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCoy SI, Njau PF, Fahey C, et al. Cash vs. food assistance to improve adherence to antiretroviral therapy among HIV-infected adults in Tanzania. AIDS. 2017;31(6):815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Sadr WM, Donnell D, Beauchamp G, et al. Financial Incentives for Linkage to Care and Viral Suppression Among HIV-Positive Patients: A Randomized Clinical Trial (HPTN 065). JAMA Intern Med. 2017;177(8):1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metsch LR, Feaster DJ, Gooden L, et al. Effect of Patient Navigation With or Without Financial Incentives on Viral Suppression Among Hospitalized Patients With HIV Infection and Substance Use: A Randomized Clinical Trial. Jama. 2016;316(2):156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shelus V, Taylor J, Greene E, et al. It’s all in the timing: Acceptability of a financial incentive intervention for linkage to HIV care in the HPTN 065 (TLC-Plus) study. PLoS One. 2018;13(2):e0191638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360(7):699–709. [DOI] [PubMed] [Google Scholar]
- 13.Charness G, Gneezy U. Incentives to exercise. Econometrica. 2009;77(3):909–931. [Google Scholar]
- 14.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection Recommendations for a public health approach - Second edition Geneva: World Health Organization;2016. [PubMed] [Google Scholar]
- 15.Chamie G, Kwarisiima D, Clark TD, et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS One. 2012;7(8):e43400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thirumurthy H, Hayashi K, Linnemayr S, et al. Time Preferences Predict Mortality among HIV-Infected Adults Receiving Antiretroviral Therapy in Kenya. PLoS One. 2015;10(12):e0145245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uganda Ministry of Health. Consolidated Guidelines for prevention and treatment of HIV in Uganda. 2016.
- 18.Kwarisiima D, Kamya MR, Owaraganise A, et al. High rates of viral suppression in adults and children with high CD4+ counts using a streamlined ART delivery model in the SEARCH trial in rural Uganda and Kenya. Journal of the International AIDS Society. 2017;20(Suppl 4):21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen M, Balzer L, Kwarsiima D, et al. Association of Implementation of a Universal Testing and Treatment Intervention With HIV Diagnosis, Receipt of Antiretroviral Therapy, and Viral Suppression in East Africa. Jama. 2017;317(21):2196–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox MP, Rosen S. Retention of Adult Patients on Antiretroviral Therapy in Low- and Middle-Income Countries: Systematic Review and Meta-analysis 2008–2013. Journal of acquired immune deficiency syndromes. 2015;69(1):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donoghue T, Rabin M. Doing it now or later. American Economic Review. 1999;89(1):103–124. [Google Scholar]
- 22.Alsan M, Beshears J, Armstrong WS, et al. A commitment contract to achieve virologic suppression in poorly adherent patients with HIV/AIDS. AIDS. 2017;31(12):1765–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.