Abstract
Background:
Accountable care organizations’ (ACOs’) focus on formal clinical integration to improve outcomes overlooks actual patterns of provider interactions around shared patients.
Objective:
To determine whether such informal clinical integration relates to a health system’s performance in an ACO.
Research Design:
We analyzed national Medicare data (2008–2014), identifying beneficiaries who underwent coronary artery bypass grafting (CABG). After determining which physicians delivered care to them, we aggregated across episodes to construct physician networks for each health system. We used network analysis to measure each system’s level of informal clinical integration (defined by cross-specialty ties). We fit regression models to examine the association between a health system’s CABG mortality rate and ACO participation, conditional on informal clinical integration.
Subjects:
Beneficiaries age 66 and older undergoing CABG.
Measures:
90-day CABG mortality.
Results:
Over the study period, 3,385 beneficiaries were treated in 161 ACO-participating health systems. The remaining 49,854 were treated in 875 non-participating systems or one of the 161 ACO-participating systems prior to the ACO start date. ACO systems with higher levels of informal clinical integration had lower CABG mortality rates than non-participating ones (2.8% versus 5.5%; P<0.01); however, there was no difference based on ACO participation for health systems with lower to relatively moderate informal clinical integration. Regression results corroborate this finding (coefficient for interaction between ACO participation and informal clinical integration level is −0.25; P=0.01).
Conclusions:
Formal clinical integration through ACO participation may be insufficient to improve outcomes. Health systems with higher informal clinical integration may benefit more from ACO participation.
Keywords: accountable care organizations, physician networks, clinical integration, surgical outcomes
Introduction
Policymakers at the Centers for Medicare & Medicaid Services (CMS) are promoting the accountable care organization (ACO) model as a key step towards achieving the triple aim of improved population health, better patient experience, and lower healthcare costs.1 Medicare ACOs are groups of providers that voluntarily join together and contract with CMS to take responsibility for the cost and quality of care of a set of Medicare beneficiaries and share in the savings if total costs are reduced below benchmarked costs. Currently, nine million beneficiaries across all 50 states are covered by one of the nearly 500 ACOs in the Medicare Shared Savings Program.”2 CMS recently doubled down on the ACO model by incorporating it into new programs, including Next Generation ACOs, Accountable Health Communities, and the Comprehensive End Stage Renal Disease Care Model.
Despite CMS’s investments and growing provider participation, ACOs have enjoyed only modest success in shifting the cost-quality curve.3 One reason why may relate to ACOs’ focus on formal clinical integration—linking different types of providers through explicit contracts that establish relationships and governance—as the catalyst to reorganize care delivery. This focus on formal integration shifts attention away from the challenges of changing the actual patterns of healthcare delivery. Although these informal connections among providers may not map onto formal organizational structures or contracts4,5,6 they more accurately reflect the delivery of care. Thus, while ACOs formally integrate providers through contracts and common group affiliation, simultaneous efforts to increase informal clinical integration—understood here as the extent to which providers of different specialties collectively participate in patient care—might be a key success factor to improve healthcare quality and reduce costs. Insofar as the structure of patient-sharing networks among providers are associated with outcomes,7,8 ACO participation could provide less benefit to health systems with lower levels of informal clinical integration. Taken together, these considerations suggest an urgent need to better understand the role of informal clinical integration in ACOs.
In this context, we examined the relationship between ACO participation and health system performance at different levels of informal clinical integration. First, we used national Medicare data to identify older beneficiaries undergoing coronary artery bypass graft (CABG) surgery. The prevalence of CABG within older Americans, as well as the high costs associated with this procedure, make beneficiaries undergoing it an important target population where ACOs may focus. Next, we employed network analytical tools to measure informal clinical integration in health systems where these beneficiaries were treated. We then compared the statistical significance of changes in health systems’ CABG surgery outcomes before and after ACO participation, conditional on levels of informal clinical integration.
Methods
Data source and study population.
Our analyses were based on national Medicare claims from a 20% random sample of beneficiaries, including data from the Carrier, Denominator, Medicare Provider Analysis and Review, and Outpatient research identifiable files. Using the appropriate International Classification of Diseases, Ninth Revision, Procedure codes, we first identified 80,782 fee-for-service beneficiaries who underwent CABG between January 1, 2008, and December 31, 2014. We required that beneficiaries were 66 years or older and had continuous enrollment in Medicare Parts A and B for six months prior to surgery (to allow comorbidity assessment) and 90 days after discharge. To ensure sufficient variation in network-level measures after network construction—a projection of a bipartite network (see below)—we excluded cases from health systems that only performed one surgery in a given year. We also excluded cases for which we were missing control variable data. Following exclusions, our analytical sample comprised 53,239 beneficiaries across 1,036 health systems.
Creating the health system networks
We defined the health system that cared for a beneficiary as encompassing the hospital where her CABG procedure was performed and all physicians who billed services during her surgical episode. We constructed a claims window from 90-days prior to the beneficiary’s hospitalization for surgery until 90 days following discharge. The length of this window was informed by current Medicare value-based purchasing initiatives wherein health systems are accountable for beneficiaries up to 90 days post-discharge. We then aggregated across all episodes within a year to construct the physician network for each health system. Following established methodology, we recorded a relationship (network tie) between two physicians if they shared care of (i.e., billed services for) a beneficiary during the same episode.8,9,10 Previous research using patient-sharing data demonstrated a strong correlation between network ties and actual professional interactions.11 Additional studies used patient-sharing data to assess the relationship between network characteristics and clinical or health-economic outcomes.7,8,9,12 Thus, the network data are an appropriate proxy for physician-to-physician interactions and well-suited for our objective—to understand the relationship between formal clinical integration through ACO participation and patterns of physician interaction in CABG care.
Lastly, we identified physician specialties using Medicare specialty codes. We grouped specialties into three categories: primary care, surgical specialty care, and medical specialty care. In keeping with existing the literature on physician networks, we excluded specialties that are generally not involved in direct patient care (e.g., radiology, pathology).8
Measuring informal clinical integration.
We defined informal clinical integration as the extent to which physicians from different specialties were involved in each beneficiary’s surgical episode. This definition is closely related to concepts of integration in social systems.13 For instance, sociologists have characterized racial integration in schools as the extent to which network ties connect students of different races.14 Such mixing is also considered to increase “opportunities for contact” among members of different groups.11,12 In ACOs, social identification through formal integration may similarly increase opportunities for physicians from different specialties to interact—an outcome that may support better patient care.15 However, we note that we are not attempting to measure care coordination; rather, informal clinical integration may represent an important antecedent.
We measure informal clinical integration using a network property called “disassortativity,” which can vary from −1 to 1.16 Disassortativity, which has been used in previous research using network data, measures the propensity within a network for ties to form between dissimilar nodes (i.e., nodes of different types).8 Thus, for each health system, disassortativity increases as the care of CABG patients involves a more diverse set of physicians. Further information on this measure is included in the Appendix. Consistent with prior literature,8 we stratified health system-years into terciles based on their level of informal clinical integration: Tercile 1, lower integration (−0.236 to 0.029, N=2,321), Tercile 2, relatively moderate integration (0.029 to 0.059, N=2,320), and Tercile 3, higher integration (0.059 to 0.515, N=2,320). These ranges, while similar to those published in previous work, are context-specific. That is, in another context, values defining Tercile 3 (higher integration) may be considered relatively low.
Capturing Medicare ACO participation (formal integration)
Formal integration refers to the establishment of a shared goal or identity through contractual or economic mechanisms.11 In our study, we operationalized formal integration as entry into a Medicare ACO contract.
We defined a beneficiary as being “exposed” to formal integration if her CABG procedure was performed in a health system’s acute care hospital whose ACO contract start date preceded the surgery date. Contract information was based on the Leavitt Partners ACO Database,17 which uses press releases, news articles, government announcements, conferences, personal and industry interviews, and other public records to track Medicare, Medicaid, and commercial ACOs. The first Medicare ACOs began in 2012, so only beneficiaries who underwent surgery between 2012 and 2014 had the potential for exposure. Out of 53,239 cases in the final sample, 3,385 were performed in one of 161 ACO participating health systems. The remaining 49,854 were treated in one of 875 non-participating health systems or one of the 161 ACO health systems prior to the ACO start date.
Measuring surgical outcomes.
To evaluate the association between informal clinical integration and post-surgical outcomes in ACO-participating health systems, we measured 90-day operative mortality, defined as death from any cause during a beneficiary’s hospitalization through the first 90 days following discharge. Given that early, in-hospital mortality may be influenced more by surgeon skill than informal clinical integration of providers responsible for perioperative care, in supplementary analyses we also excluded patients who died in the first 3 days following surgery.
Control variables
We included control variables at the beneficiary, health system, and community levels. At the beneficiary level, we accounted for differences in the level of comorbidity using a modification of the Charlson index.18 We also included various demographic factors, such as sex, race, age, and socioeconomic status indicators. At the health system level, we compared surgical volume, the number and types of physicians involved in surgical episodes, the proportion of treated beneficiaries that live outside of the hospital’s core-based statistical area, the type of hospital that performed the surgery, whether the hospital used electronic health records, and whether the system belonged to a Pioneer ACO. At the community-level, we include population and medical capacity measures based on the health service area for the treating hospital.19,20 Detailed information about each control variable and the data source is provided in the Online Appendix.
Statistical analyses
Our longitudinal data includes beneficiary-level observations for patients who underwent a CABG procedure at some point during the 2008–2014 period, nested within health systems. As CABG procedures are observed at irregular intervals both within and across health systems, there is no time-specific ordering of observations with the exception that all “treated” observations occur later than “untreated” observations within a given health system. This data structure enables us to assess both within and between-group variation, which is important for our regression tests.
For our initial analytic step, we assessed the differences in various beneficiary-, health system-, and community-level characteristics between ACO participating and non-participating health systems, as well as among health systems at different levels of informal clinical integration, using parametric and non-parametric tests where appropriate. To evaluate the relationship between 90-day mortality and health system ACO participation, we compared unadjusted mortality rates based on level of informal clinical integration and ACO participation.
We also fitted a linear probability model with mortality as the outcome, regressed on variables for ACO participation, informal clinical integration, and control variables for patient, health system, and community-level characteristics. To understand whether informal clinical integration moderates ACO effects, we included an interaction term between the ACO participation indicator and the level of informal clinical integration. To account for correlation of outcomes within health systems, we included health system random effects. The random effects estimator has the benefit of capturing variation both within and between groups. We also performed sensitivity analyses to assess the robustness of our findings, such as changing the inclusion criteria for number of CABG procedures performed, removing control variables, controlling for emergent surgery, excluding patients that died within 3-days of surgery, and using health system fixed effects. All regression results are described in the Online Appendix, Table 1.
All analyses were performed using Stata IC version 14.2, and all statistical tests were two-tailed, with probability of Type 1 error at 0.05. The study was determined to be exempt from institutional review board oversight.
Results
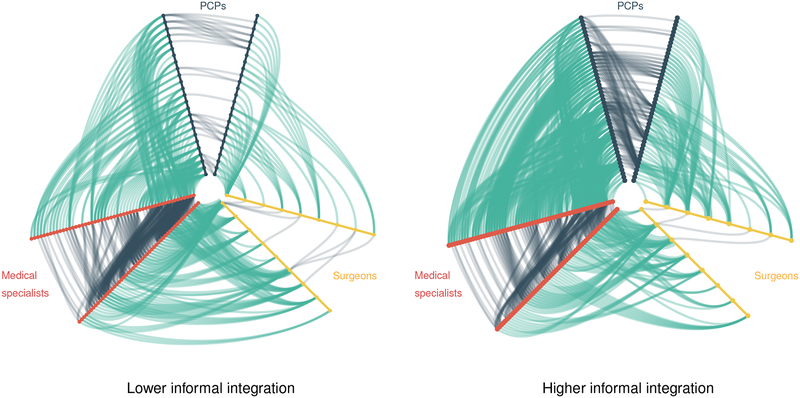
Across the 1,036 health systems, informal clinical integration varied considerably, from −0.236 (lower integration) to 0.515 (higher integration). In Figure 1 we use hive plots to illustrate differences in the relative frequency of within-specialty versus cross-specialty ties among primary care physicians, medical specialists, and surgeons in two ACO-participating health systems with lower (left) and higher (right) informal clinical integration. Each “V” axis of the hive plots represents physicians of a particular specialty such that lines within a V are within-specialty connections and lines between two Vs are between-specialty connections. In the system with higher informal clinical integration (right-hand plot), the cross-specialty ties among physicians comprise a greater proportion of total ties within the physician network (62% of ties in the higher integration network are cross-specialty versus 49% in the lower integration network). Consequently, the care of beneficiaries involves a more balanced representation of physicians from each of the three specialty groups.
Figure 1: Lower and higher informal clinical integration in CABG treatment within two ACO-participating systems.
The colored “V-shaped” axes of each hive plot represent groups of physicians from a specific specialty, with each node representing an individual physician. Lines within each “V” represent within-specialty ties among physicians. Lines between each “V” represent cross-specialty ties among physicians. The network on the left comprises 86 physicians and 13 CABG beneficiaries; 391 of 793 (49%) total ties are cross-specialty. The network on the right comprises 117 physicians and 19 CABG beneficiaries; 641 of 1039 (62%) total ties are cross-specialty.
Comparisons of beneficiary, health system, and community characteristics by ACO participation and level of informal clinical integration are shown in Table 1; further comparisons are provided in Appendix Table 2. ACO participation differed across integration tercile groups (p<0.01), as did the average Charlson score (p<0.01). There were also statistically significant differences in the number of CABG procedures per year (p<0.01) and propensity for the health system to be centered around an academic hospital (p<0.01). Accordingly, ACO-participating health systems treated beneficiaries with higher levels of comorbid illness (p<0.01), performed slightly more CABG procedures (p<0.01), and were more likely to have an academic affiliation (p<0.01).
Table 1.
Patient, health system, and community characteristics by ACO participation, level of informal clinical integration
| Lower integration |
Moderate integration |
Higher integration |
Overall p-value |
Non-ACO | ACO Pre-Joining | ACO Post-Joining | Overall p-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Beneficiary characteristics | ||||||||||||||
| Patient mortality (90-day) | 0.08 | 0.27 | 0.07 | 0.25 | 0.05 | 0.22 | <0.01 | 0.07 | 0.25 | 0.07 | 0.25 | 0.07 | 0.25 | 0.94 |
| Charlson score | 2.05 | 1.97 | 1.96 | 1.92 | 1.71 | 1.75 | <0.01 | 1.91 | 1.89 | 1.99 | 1.93 | 2.16 | 2.04 | <0.01 |
| Emergent surgery | 0.19 | 0.39 | 0.21 | 0.41 | 0.23 | 0.42 | <0.01 | 0.22 | 0.41 | 0.19 | 0.4 | 0.19 | 0.39 | <0.01 |
| Died 3 days post-CABG (0 = No, 1 = Yes) | 0.01 | 0.12 | 0.01 | 0.11 | 0.01 | 0.12 | 0.63 | 0.01 | 0.12 | 0.01 | 0.12 | 0.01 | 0.11 | 0.99 |
| Sex (0 = Male, 1 = Female) | 0.30 | 0.46 | 0.30 | 0.46 | 0.29 | 0.45 | 0.07 | 0.3 | 0.46 | 0.3 | 0.46 | 0.28 | 0.45 | 0.31 |
| White (0 = No, 1 = Yes) | 0.93 | 0.25 | 0.94 | 0.24 | 0.95 | 0.22 | <0.01 | 0.94 | 0.24 | 0.94 | 0.24 | 0.94 | 0.24 | 1.00 |
| Black (0 = No, 1 = Yes) | 0.04 | 0.20 | 0.04 | 0.19 | 0.03 | 0.16 | <0.01 | 0.04 | 0.19 | 0.04 | 0.19 | 0.04 | 0.19 | 0.94 |
| Age | 75.00 | 5.92 | 74.84 | 5.83 | 74.62 | 5.69 | <0.01 | 74.78 | 5.81 | 75.03 | 5.88 | 75.01 | 5.96 | <0.01 |
| Lives in a rural area | 0.01 | 0.08 | 0.01 | 0.08 | 0.01 | 0.08 | 1.00 | 0.01 | 0.09 | 0.01 | 0.08 | 0.00 | 0.05 | 0.86 |
| % living below federal poverty line (mean) | 14.19 | 3.92 | 14.87 | 3.76 | 15.70 | 4.23 | <0.01 | 15.18 | 3.91 | 13.88 | 3.92 | 13.88 | 4.17 | <0.01 |
| % with a bachelor’s degree (mean) | 27.55 | 7.33 | 26.02 | 6.99 | 24.36 | 6.86 | <0.01 | 25.16 | 6.85 | 27.99 | 7.23 | 31.59 | 7.31 | <0.01 |
| Health system characteristics | ||||||||||||||
| Total CABG beneficiaries (mean) | 25.20 | 18.83 | 19.35 | 13.75 | 13.89 | 9.17 | <0.01 | 19.22 | 14.7 | 24.19 | 18.55 | 21.68 | 16.23 | <0.01 |
| Total physicians caring for CABG (mean) | 175.16 | 145.17 | 111.63 | 73.21 | 64.10 | 37.72 | <0.01 | 113.34 | 93.84 | 162.36 | 151.83 | 152.04 | 111.71 | <0.01 |
| Proportion of cross-specialty ties | 0.53 | 0.06 | 0.56 | 0.06 | 0.61 | 0.06 | <0.01 | 0.57 | 0.07 | 0.55 | 0.06 | 0.54 | 0.06 | <0.01 |
| Proportion of beneficiaries from outside the CBSA | 0.59 | 0.24 | 0.61 | 0.24 | 0.58 | 0.26 | <0.01 | 0.59 | 0.24 | 0.6 | 0.25 | 0.62 | 0.25 | <0.01 |
| Academic hospital (0 = No, 1 = Yes) | 0.73 | 0.44 | 0.64 | 0.48 | 0.56 | 0.50 | <0.01 | 0.6 | 0.49 | 0.78 | 0.42 | 0.77 | 0.42 | <0.01 |
| Government hospital (0 = No, 1 = Yes) | 0.08 | 0.27 | 0.08 | 0.26 | 0.08 | 0.27 | 0.11 | 0.1 | 0.31 | 0.03 | 0.18 | 0.01 | 0.11 | <0.01 |
| For-profit hospital (0 = No, 1 = Yes) | 0.09 | 0.29 | 0.11 | 0.32 | 0.13 | 0.34 | <0.01 | 0.15 | 0.35 | 0.03 | 0.18 | 0.03 | 0.16 | <0.01 |
| Electronic health records (0 = No, 1 = Yes) | 0.97 | 0.16 | 0.97 | 0.17 | 0.97 | 0.18 | 0.06 | 0.96 | 0.19 | 0.98 | 0.13 | 1.00 | 0.05 | <0.01 |
| Health system integration | 0.01 | 0.02 | 0.04 | 0.01 | 0.09 | 0.03 | <0.01 | 0.04 | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 | <0.01 |
| Pioneer ACO (0 = No, 1 = Yes) | 0.02 | 0.13 | 0.01 | 0.09 | 0.01 | 0.07 | <0.01 | 0.00 | 0.00 | 0.03 | 0.16 | 0.06 | 0.25 | <0.01 |
| Joined ACO (0 = No, 1 = Yes) | 0.08 | 0.26 | 0.06 | 0.23 | 0.05 | 0.21 | <0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | <0.01 |
| Community characteristics | ||||||||||||||
| Total resident population (log) | 6.13 | 1.02 | 5.90 | 0.95 | 5.55 | 0.84 | <0.01 | 5.9 | 1.01 | 5.97 | 0.89 | 5.96 | 1 | <0.01 |
| Total black population (log) | 3.90 | 1.78 | 3.48 | 1.77 | 2.71 | 1.73 | <0.01 | 3.48 | 1.88 | 3.57 | 1.71 | 3.4 | 1.72 | <0.01 |
| Total Hispanic population (log) | 3.72 | 1.59 | 3.30 | 1.54 | 2.86 | 1.45 | <0.01 | 3.33 | 1.62 | 3.49 | 1.44 | 3.53 | 1.65 | <0.01 |
| Acute care hospital beds per 1,000 residents | 1.99 | 0.48 | 1.97 | 0.48 | 2.00 | 0.52 | 0.01 | 2.02 | 0.51 | 1.92 | 0.44 | 1.86 | 0.38 | <0.01 |
| PCPs per 100,000 residents | 72.58 | 18.16 | 69.09 | 15.38 | 65.93 | 14.10 | <0.01 | 68.01 | 16.26 | 73.39 | 16.34 | 75.39 | 17.67 | <0.01 |
| Medical specialists per 100,000 residents | 55.99 | 18.10 | 50.38 | 14.26 | 44.87 | 11.32 | <0.01 | 49.71 | 15.26 | 54.98 | 16.61 | 57.52 | 18.99 | <0.01 |
| Surgeons per 100,000 residents | 37.01 | 7.97 | 36.19 | 7.78 | 34.87 | 7.62 | <0.01 | 36.09 | 7.97 | 36.54 | 7.71 | 36.71 | 7.38 | <0.01 |
ACO: accountable care organization; CBSA: core-based statistical area; PCP: primary care physician
p-values from Kruskal-Wallis test except for categorical variables (χ2 test)
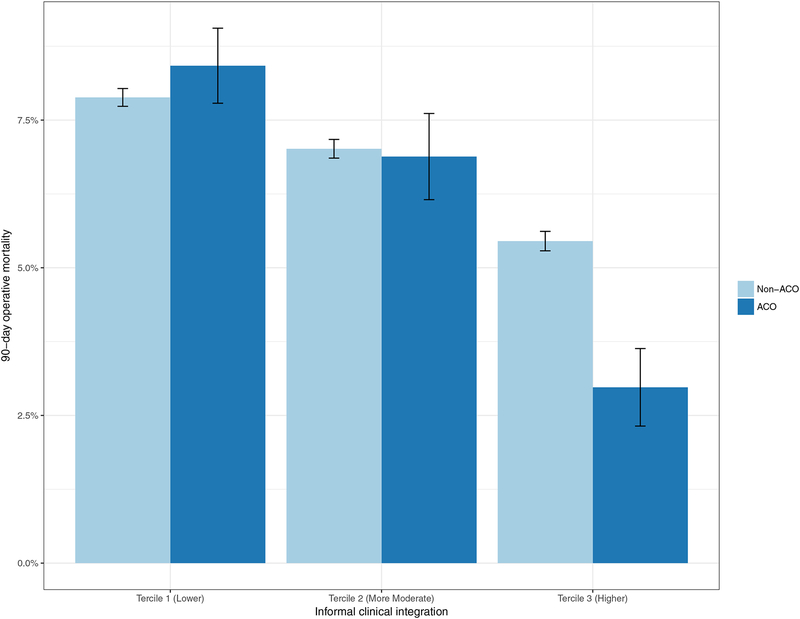
There was a statistically significant and negative relationship between mortality and the level of informal clinical integration (p<0.01 for all comparisons between tercile groups). Lower integration systems had the highest mortality rate (8%), and mortality rates decreased with increasing integration (7% at more moderate and 5% at higher integration systems). While there was no statistically significant difference in patient mortality between the ACO and non-ACO participating health systems, a closer examination suggests that the association between ACO participation and bypass surgery outcomes may be conditional on the level of informal clinical integration. Figure 2 shows unadjusted mortality rates by level of informal clinical integration and ACO participation, with error bars denoting ±1 standard error for each mean. Importantly, although health systems with higher levels of informal clinical integration that participated in ACOs had better mortality outcomes (2.8% versus 5.5% for ACO and non-ACO at higher integration; P<0.01), lower and more moderate integration systems did not show any mortality differences based on ACO participation.
Figure 2.
90-day operative mortality following CABG procedure by level of informal clinical integration and ACO participation.
We further corroborated this interaction relationship using regression analysis (Table 2). As previously stated, our data structure, with multiple observations on each group, enabled us to assess both between and within group variation, before and after ACO participation. The results of the linear probability model for mortality with health system random effects support that ACO participation is not significantly associated with mortality rate. However, both the level of informal clinical integration (p<0.01) and the interaction of informal clinical integration and ACO participation (p=0.01) are significantly associated with lower mortality. In context, at the mean level of integration for Tercile 1 (“lower,” 0.01), our model predicts that ACO-participating systems will be associated with a higher mortality rate than non-participants; 8.0% versus 7.5%, respectively. However, at the mean level of integration for Tercile 3 (“higher, 0.09), our model predicts that ACO-participating health systems will have a mortality rate of 4.4% compared to 5.9% for non-participants.
Table 2.
Regression results for 90-d operative mortality for CABG beneficiaries (2008–2014)
| Variable | Main model |
|---|---|
| Beneficiary characteristics | |
| Charlson score | 0.01*** (0.00) |
| Sex (0 = Male, 1 = Female) | 0.02*** (0.00) |
| White (0 = No, 1 = Yes) | 0.00 (0.01) |
| Black (0 = No, 1 = Yes) | 0.00 (0.01) |
| Age | 0.00*** (0.00) |
| Lives in a rural area | 0.00 (0.01) |
| % living below federal poverty line (mean) | 0.00† (0.00) |
| % with a bachelor’s degree (mean) | −0.00*** (0.00) |
| Health system characteristics | |
| Total CABG beneficiaries | −0.00*** (0.00) |
| Total physicians caring for CABG (mean) | 0.00*** (0.00) |
| Proportion of cross-specialty ties | −0.07*** (0.02) |
| Proportion of beneficiaries from outside the CBSA | 0.00 (0.01) |
| Academic hospital (0 = No, 1 = Yes) | 0.00 (0.00) |
| Government hospital (0 = No, 1 = Yes) | 0.00 (0.00) |
| For-profit hospital (0 = No, 1 = Yes) | 0.01* (0.00) |
| Electronic health records (0 = No, 1 = Yes) | −0.01 (0.01) |
| Pioneer ACO (0 = No, 1 = Yes) | 0.00 (0.01) |
| Community characteristics | |
| Total resident population (log) | 0.01 (0.00) |
| Total black population (log) | −0.00* (0.00) |
| Total Hispanic population (log) | −0.00 (0.00) |
| Acute care hospital beds per 1,000 residents | 0.01* (0.00) |
| PCPs per 100,000 residents | −0.00 (0.00) |
| Medical specialists per 100,000 residents | −0.00 (0.00) |
| Surgeons per 100,000 residents | −0.00 (0.00) |
| ACO membership and informal clinical integration | |
| Joined ACO (0 = No, 1 = Yes) | 0.01 (0.01) |
| Health system integration | −0.20*** (0.04) |
| Joined ACO * integration | −0.25* (0.10) |
| Constant | −0.24*** (0.03) |
| Year fixed effects | Yes |
| Observations | 53,239 |
| R2 | 0.03 |
Standard errors (clustered by health system) in parentheses. ACO: accountable care organization; CBSA: core-based statistical area; PCP: primary care physician
< 0.1
p < .05
p < .01
p < .001
To test the robustness of this finding, we conducted a series of sensitivity analyses (reported in Appendix Table 1). Notably, we found that adjusting for emergent CABG procedures and cases in which patients died within three days of surgery increased the robustness of the associations. Additionally, the results were consistent in tests accounting for loss of observations due to incomplete data for control variables, using a threshold of 10 bypass procedures per year for health systems, and using health system fixed effects.
Discussion
Medicare ACOs are widely viewed as an important organizational innovation that will reduce fragmentation in the delivery of healthcare. Yet, despite rapid expansion since 2012, many ACOs are still struggling to achieve their cost and quality targets. Our principal finding indicates that ACO participation among health systems with lower levels of informal clinical integration (the extent to which physicians from different specialties were involved a beneficiary’s care) is associated with higher 90-day operative mortality following CABG surgery. In contrast, ACO participating systems with higher levels of informal clinical integration appear to be associated with considerably lower mortality than their non-participant counterparts. The results highlight the potential importance of informal clinical integration for healthcare reform and underscore a need to focus on both formal and informal clinical integration as ACOs become increasingly prevalent.
The critical assumption of ACOs is that formal integration will improve integration and promote care coordination. From an economic perspective, shared financial incentives among ACO members should reduce the transaction costs associated with coordinating work across the boundaries of existing organizations.21 From a sociological standpoint, common group identity may engender social identification among ACO members, diminishing “silos” and enabling providers—in particular, physicians22—to collaborate more effectively across traditional boundaries of organization or specialty.23 The present study predominantly focused on the latter, engaging with what is viewed as a central component of health system integration: “the theme of overcoming disciplinary, sectoral, and institutional ‘silos.’”24 Put differently, our focus was to evaluate an element of ACO implementation25—is ACO participation associated with increased cross-specialty participation among physicians involved in CABG care?
Research across disciplines suggests that formal integration does not imply informal clinical integration. For instance, sociologists theorized that important structural features in society, such as socioeconomic or racial disparities, emerged from patterns of informal social interaction that are constrained, but not dictated, by the existence of formal organizations.13 Similarly, research in healthcare has highlighted that integration must be achieved in spite of, not due to, group or organizational affiliations.18 In fact, early studies of ACOs found that many ACO participants did not view the ACO as unifying entity.26 Thus, it is no surprise that our results suggest that ACO participation alone is not associated with better CABG outcomes. Instead, it appears that structure of informal networks may characterize different types of contexts within which ACOs may be more, or less, successful. More specifically, in the early stages, the best ACOs may be those in which patient care is delivered through physician networks that already display a higher level of informal clinical integration. Indeed, it may be challenging for clinical leaders and administrators to quickly alter the informal clinical integration of a health system due to complex factors such as referral relationships, capacity, or patient needs. As previous studies of ACOs found,17,19,22 one of the most challenging aspects of implementing an ACO may be generating enough physician buy-in to alter the existing routines and structure of care delivery.
Though our study only focused on quality, increasing informal clinical integration may also benefit the cost containment side of ACOs. Foremost, reducing patient mortality is likely to be associated with lower overall costs for an ACO. Second, achieving higher informal clinical integration does not mean simply promoting more referrals between ACO physicians, which may actually increase costs.27 Instead, higher informal clinical integration may depend more on pruning network ties, such as reducing the within-specialty ties that come from a lack of continuity between patient and physician, or increasing the between-specialty ties that come when necessary work is referred out to best place for the patient.
Our findings should be viewed in the context of several limitations. First, we focused specifically on older beneficiaries who underwent CABG surgery. As such, our sample is not necessarily representative of the entire Medicare population, though it does represent an important subpopulation in terms of morbidity and expenditures. Additionally, our sample only includes data through 2014 and therefore only captures the effect of ACO membership for a short duration after the programs began in 2012. It is possible that prolonged “exposure” to ACO participation will increase informal clinical integration and enable lower integration systems to improve outcomes. Further, it is also unclear whether the observed relationships are unique to our context of CABG procedures within Medicare ACOs, or if they might be generalizable to other types of procedures or ACOs. Similarly, although clinical outcomes are a critical component of ACO performance, we do not assess cost outcomes. Finally, our measure of informal clinical integration is somewhat limited in that it cannot directly capture the level of interaction among physicians. Put differently, although we directly measure the level of patient sharing among physicians—and know that this is a strong predictor of interaction11—the informal clinical integration index is a proxy for actual interaction. Moreover, our measure captures the presence of cross-specialty participation within CABG care delivery, an essential component of integration that becomes increasingly important with case complexity.19
Despite some limitations, our study’s findings have important policy implications. CMS continues to highlight the overall success of its various ACO models in terms of cost-savings, quality, and increasing interest from healthcare providers. However, conspicuously absent are guidelines or best practices with regards to how ACO participants should seek to reorganize their clinical practices, leaving those decisions to the individual groups. Consequently, performance varies widely among ACOs. More concerning, CMS has yet to quantify the administrative costs associated with ACOs that do not meet cost and quality benchmarks. Considering that recent research has also called into question whether ACOs are a net positive for CMS after the shared savings incentive payments are factored in,3 it seems prudent to question whether ACO participation should be a more selective process. Perhaps evidence-based screening that accounts for factors that provide some indicator of a group of providers’ collective capability to implement changes in clinical practice, such as informal clinical integration, may increase the likelihood of success. Formal clinical integration through ACO participation and its shared financial incentive may not be enough to alter physician behavior, but it may unlock additional benefits for health systems and groups of providers that are already well integrated informally.
Supplementary Material
Funding:
This study was supported by 1R01HS024525 01A1 and 1R01HS024728 01 The views expressed in this article do not reflect the views of the federal government.
Footnotes
Disclosures: The authors have no conflict of interest to declare regarding the content of this manuscript.
References
- 1.Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: The extended hospital medical staff. Health Affairs. 2007;26(1):w44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Medicare and Medicaid Services. Medicare Shared Savings Program Fast Facts. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/SSP-2018-Fast-Facts.pdf
- 3.McWilliams JM. Cost containment and the tale of care coordination. New England Journal of Medicine. 2016;375(23):2218–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pentland A Social physics: How good ideas spread—The lessons from a new science. New York: Penguin Press; 2014. [Google Scholar]
- 5.Rosenkopf L, Schleicher T. Below the tip of the iceberg: the co-evolution of formal and informal interorganizational relations in the wireless telecommunications industry. Managerial and Decision Economics. 2008;29(5):425–41. [Google Scholar]
- 6.McEvily B, Soda G, Tortoriello M. More formally: Rediscovering the missing link between formal organization and informal social structure. The Academy of Management Annals. 2014;8(1):299–345. [Google Scholar]
- 7.Hollingsworth JM, Funk RJ, Garrison SA, Owen-Smith J, Kaufman SA, Pagani FD, Nallamothu BK. Association between physician teamwork and health system outcomes after coronary artery bypass grafting. Circulation: Cardiovascular Quality and Outcomes. 2016; CIRCOUTCOMES. 116.002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funk RJ, Owen-Smith J, Kaufman SA, Nallamothu BK, Hollingsworth JM. Association of Informal Clinical Integration of Physicians with Cardiac Surgery Payments. JAMA surgery. 2017: E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everson J, Funk RJ, Kaufman SR, Owen-Smith J, Nallamothu BK, Pagani FD, Hollingsworth JM. Repeated, close physician coronary artery bypass grafting teams associated with greater teamwork. Health services research. 2018. April;53(2):1025–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landon BE, Keating NL, Barnett ML, Onnela JP, Paul S, O’Malley AJ, Keegan T, Christakis NA. Variation in patient-sharing networks of physicians across the United States. JAMA. 2012. July 18;308(3):265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnett ML, Landon BE, O’Malley AJ, Keating NL, Christakis NA. Mapping physician networks with self-reported and administrative data. Health Services Research. 2012; 31(11): 2368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghomrawi HM, Funk RJ, Parks ML, Owen-Smith J, Hollingsworth JM. Physician referral patterns and racial disparities in total hip replacement: A network analysis approach. PloS one. 2018. February 20;13(2):e0193014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blau PM. A macrosociological theory of social structure. American Journal of Sociology. 1977; 83(1): 26–54. [Google Scholar]
- 14.Race Moody J., school integration, and friendship segregation in America. American Journal of Sociology. 2001; 107(3):679–716. [Google Scholar]
- 15.Burns LR, Muller RW. Hospital-Physician Collaboration: Landscape of Economic Integration and Impact on Clinical Integration. The Milbank Quarterly. 2008; 86(3): 375–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman Mark EJ. Mixing patterns in networks. Physical Review E 67, no. 2 (2003): 026126. [DOI] [PubMed] [Google Scholar]
- 17.Colla CH, Lewis VA, Tierney E, Muhlestein DB. Hospitals participating in ACOs tend to be large and urban, allowing access to capital and data. Health Affairs. 2016. March 1;35(3):431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charslon ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 19.The Darmouth Atlas of Health Care. Available at: http://www.dartmouthatlas.org. Accessed April 24, 2018.
- 20.American Community Survey. Available at: https://www.census.gov/programs-surveys/acs/. Accessed April 24, 2018.
- 21.Williamson OE. The economics of organization: The transaction cost approach. American Journal of Sociology. 1981; 87(3): 548–77. [Google Scholar]
- 22.Kreindler SA, Larson BK, Wu FM, Gbemudu JN, Carluzzo KL, Struthers A, Van Citters AD, Shortell SM, Nelson EC, Fisher ES. The rules of engagement: physician engagement strategies in intergroup contexts. Journal of Health Organization and Management. 2014; 28(1): 41–61. [DOI] [PubMed] [Google Scholar]
- 23.Kogut B, Zander U. What firms do? Coordination, identity, and learning. Organization science. 1996; 7(5):502–18. [Google Scholar]
- 24.Kreindler SA, Dowd DA, Star ND, Gottschlk T. Siloes and social identity: the social identity approach as a framework for understanding and overcoming divisions in health care. The Milbank Quarterly. 2012; 90(2): 347–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher EC, Shortell SM, Kreindler SA, Van Citters AD, Larson BK. A framework for evaluating the formation, implementation, and performance of accountable care organizations. Health Affairs. 2012; 31(11): 2368–2378. [DOI] [PubMed] [Google Scholar]
- 26.Kreindler SA, Larson BK, Wu FM, Carluzzo KL, Gbemudu JN, Struthers A, Van Citters AD, Shortell SM, Nelson EC, Fisher ES. Interpretations of integration in early accountable care organizations. The Milbank Quarterly. 2012; 90(3): 457–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Z, Sequist TD, and Barnett ML. Patient referrals: a linchpin for increasing the value of care. JAMA. 2014; 312(6): 597–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.