Abstract
The impact that β-arrestin proteins have on G protein-coupled receptor trafficking, signaling and physiological behavior has gained much appreciation over the past decade. A number of studies have attributed the side effects associated with the use of naturally occurring and synthetic opioids, such as respiratory depression and constipation, to excessive recruitment of β-arrestin. These findings have led to the development of biased opioid small molecule agonists that do not recruit β-arrestin, activating only the canonical G protein pathway. Similar G protein-biased small molecule opioids have been found to occur in nature, particularly within kratom, and opioids within salvia have served as a template for the synthesis of other G protein-biased opioids. Here, we present the first report of naturally occurring peptides that selectively activate G protein signaling pathways at δ opioid receptors, but with minimal β-arrestin recruitment. Specifically, we find that rubiscolin peptides, which are produced as cleavage products of the plant protein rubisco, bind to and activate G protein signaling at δ opioid receptors. However, unlike the naturally occurring δ opioid peptides leuenkephalin and deltorphin II, the rubiscolin peptides only very weakly recruit β-arrestin 2 and have undetectable recruitment of β-arrestin 1 at the δ opioid receptor.
Keywords: delta opioid receptor, beta-arrestin, natural products, biased signaling, rubisco, G protein-coupled receptor
Introduction
Opioid receptors are G protein-coupled receptors (GPCRs) that are widely expressed throughout the body and regulate a diverse array of physiological functions, including pain sensation, respiration, mood and reward. Opioid receptors are activated by endogenous opioids, such as endorphins and enkephalins, by natural plant products such as Papaver somniferum (opium) and Salvia divinorum, and by synthetic opioids such as fentanyl and methadone. The current clinically-used opioids provide strong analgesic support in operative and post-operative settings, but their use is also associated with adverse effects including the development of tolerance, constipation, opioid use disorder and respiratory depression, which can ultimately prove fatal.
Studies utilizing β-arrestin 2 knockout mice revealed that certain opioid side effects may be associated with β-arrestin 2 recruitment upon μ-opioid receptor (μ-OR) activation (Raehal et al., 2005). These early studies ignited efforts to discover or develop opioids which exclusively activate G protein signaling without also eliciting β-arrestin recruitment. Such G protein-biased small molecules, like TRV130 (DeWire et al., 2013) and PZM21 (Manglik et al., 2016), have recently been synthesized and indeed appear to possess a reduced side effect profile. G protein-biased opioids also appear to occur naturally, as revealed by the pharmacological characterization of opioids found within Mitragynina speciosa (kratom) (Varadi et al., 2016). Additionally, naturally occurring opioids can serve as the basis of G protein-biased semi-synthetic opioids, as is the case for herkinorin, which is derived from naturally occurring salvinorin (Groer et al., 2007).
In 2001, two peptides YPLDL (rubiscolin-5) and YPLDLF (rubiscolin-6) were identified from spinach rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase), a 557 kDa enzyme highly common in photosynthetic organisms, that have micromolar affinity and potency at δ opioid receptors (δ-ORs) in vitro and ~500 fold selectivity in binding over the μ-OR and >30 fold in potency as measured by comparing rubiscolin-6 activity between mouse vas deferens (as estimate of δ-OR activity) and guinea pig ileum (as estimate of μ-OR activity) (Yang et al., 2003b; Yang et al., 2001). Interestingly a study investigating the effect of rubiscolin on skin inflammation also found that rubiscolin-6 prevented δ-OR internalization (Chajra et al., 2015). Strong internalization of δ-ORs by specific agonists has been linked to strong recruitment of β-arrestin, particularly β-arrestin 1 (Pradhan et al., 2016; Pradhan et al., 2010), and thus the aforementioned report of limited δ-OR internalization with rubiscolin-6 may suggest that rubiscolin peptides do not recruit β-arrestin 1, and potentially β-arrestin 2, to δ-ORs. This led us to hypothesize that these rubiscolin peptides possess G protein-bias when compared with the enkephalin and deltorphin-classes of peptides, which are known to recruit β-arrestin (Chiang et al., 2016; Molinari et al., 2010). Using cellular signaling assays we identified that the rubiscolin peptides indeed have a lower propensity to recruit β-arrestin than leu-enkephalin and deltorphin II, while acting as full agonists in a G protein-mediated cAMP assay.
Methods
Drugs and chemicals:
[D-Ala2]-deltorphin II was purchased from Tocris, R&D systems (Minneapolis, MN, USA). Forskolin, leu-enkephalin, and piperidine were purchased from Sigma-Aldrich (St. Louis, MO, USA). [3H]DPDPE (lot 2399128, 49.8 Ci/mml) was purchased from Perkin Elmer (Waltham, MA). Fmoc-protected amino acids were purchased from NovaBiochem (Billerica, MA), Fmoc-L-Leu-Wang resin (100–200 mesh, 1% divinylbenzene, 0.745 meq/g) and Fmoc-L-Phe-Wang resin (200–400 mesh, 1% divinylbenzene, 0.443 meq/g) were purchased from Chem-Impex International (Wood Dale, IL). (1H-Benzotriazol-1-yloxy)(dimethylamino)-N,N-dimethylmethaniminium hexafluorophosphate (HBTU) was purchased from Oakwood Chemical (N. Estill, SC), N,Ndimethylformamide, dichloromethane, N,N-diisopropylethylamine and acetonitrile (HPLCgrade) were purchased from Fisher Scientific (Hampton, NH), trifluoroacetic acid was purchased from VWR Chemicals (Radnor, PA) and triisopropylsilane was purchased from Alfa Aesar (Ward Hill, MA). All reagents were used as received with no further purification. For the cellular assays, all peptides were dissolved in sterile water.
Synthesis and characterization of rubiscolin peptides.
Synthesis of both peptides was performed on a 0.022 mmol scale. Peptides were synthesized using standard Fmoc-based solid-phase synthesis with HBTU. Fmoc was deprotected with 20% piperidine in dimethylformamide. The resin was cleaved with 95% trifluoroacetic acid, 2.5% triisopropylsilane and 2.5% dichloromethane (1 mL) for one hour. After ether precipitation, peptides were purified using reverse-phase HPLC. Final pure peptides were identified (≥95% purity) using LC/MS (Agilent Technologies 6120 Quadrupole LC/MS).
Alignment analysis.
Maximum Common Substructure (MCS) was carried out for alignment. The atom-atom pairing was obtained from the 2D maximum common substructure while the unpaired atoms are typed using extended atom types to enable relevant atomic overly. MCS and subsequent alignment was carried out using ChemAxon similarity plugin (Englert and Kovacs, 2015; Kawabata, 2011; Raymond and Willett, 2002).
Membrane preparation.
CHO-OPRD cells stably expressing β-arrestin 2 and δOR (DiscoverX, Fremont, CA) were grown in T75 flasks in 10ml F12 media (Fisher Scientific) containing 10% FBS (Sigma, Lot 12M246), 300μg/ml hygromycin B (Fisher Scientific) and 800μg/ml geneticin (Fisher Scientific) until confluency. Cells were dislodged by treatment with 0.25% trypsin/EDTA (Fisher Scientific) for 5 minutes. The trypsin was then deactivated with antibiotic-free F12 and the resulting cell suspension centrifuged for 5 minutes at 400G at room temperature (Eppendorf 5804R). The supernatant was aspirated and the pellet resuspended in 50 mM Tris HCl pH 7.4 (prepared from powder stock, Sigma) and sonicated on ice for 30 seconds (Qsonica XL-2000, level 3). The membranes were then isolated by ultracentrifugation for 30 minutes at 20,000 rpm (46,500g) in a SW41Ti rotor (Beckman Coulter Life Sciences, Indianapolis, IN) in a precooled Optima L-100 XP centrifuge (Beckman Coulter) at 4°C. The pellet was resuspended in 50 mM Tris HCl buffer and sonicated once more to homogenize. The suspension was then pulled through a 28G needle, aliquoted and frozen at −80°C for future use.
Radioligand binding assay.
For the binding assay 50 μl of a dilution series of peptide was added to 50 μl of 3.3 nM [3H]DPDPE (Kd =3.87 nM) in a clear 96 well plate. Next, 100 μl of membrane suspension containing 7 μg protein was added to the agonists and incubated for 90 minutes at room temperature. The reaction mixture was then filtered over a GF-B filter plate (Perkin Elmer) followed by 4 quick washes with ice-cold 50 mM Tris HCl. The plate was dried overnight after which 50 μl scintillation fluid (Ultimagold uLLT) was added and radioactivity was counted on a Packard TopCount NXT scintillation counter. All working solutions were prepared in a radioligand assay buffer containing 50mM Tris HCl, 10mM MgCl2, and 1mM EDTA at pH 7.4.
Cell culture and biased signaling assays:
The cAMP inhibition assay was performed as previously described (Chiang et al., 2016). For β-arrestin recruitment assays, U2OS-humanδ-OR PathHunter β-arrestin-1 cells (DiscoverX) and CHO-humanδ-OR PathHunter β-arrestin-2 cells were plated (2500 cells/well, 10 μl) prior to stimulation with 2.5 μl δ-OR agonists for 90 minutes at 37°C/5%CO2, after which cells were incubated with 6 μl cell PathHunter assay buffer for 60 minutes at room temperature as per the manufacturer’s protocol. Luminescence for each of these assays was measured using a FlexStation3 plate reader (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis:
Each data point for binding and arrestin recruitment was run in duplicate, and for the cAMP assay in triplicate. For figure 2, the averages of each run were combined to provide a composite figure in favor of a representative figure. All data are presented as means ± standard error of the mean and were performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA). In order to calculate Log (τ/KA), we followed the operational model equation in Prism 7 as previously described (van der Westhuizen et al, 2014). Subsequently bias factors were calculated using leu-enkephalin as the reference compound.
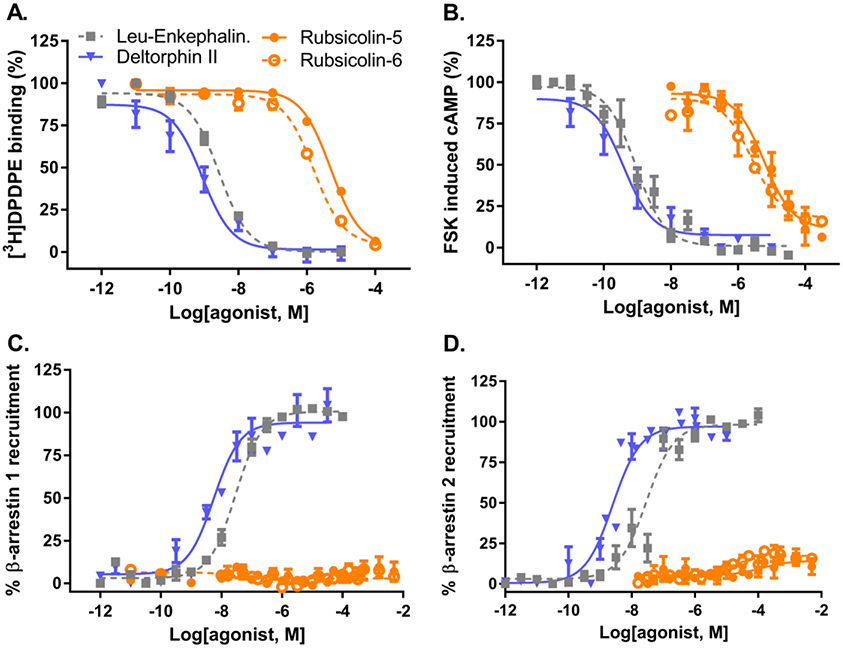
Figure 2. Pharmacological characterization of rubiscolin peptides at δ-ORs.
A) Displacement of [3H]DPDPE from δ-ORs (inset shows saturation binding for [3H]DPDPE on δ-ORs). B) Inhibition of forskolin-induced cAMP production in HEK293-δ-OR cells. C) β-arrestin 1 recruitment in U2OS δ-OR PathHunter cells. D) β-arrestin 2 recruitment in CHO δ-OR PathHunter cells. Composite figures are shown (see Table 1 for details).
Results
Synthesis of rubiscolin peptides.
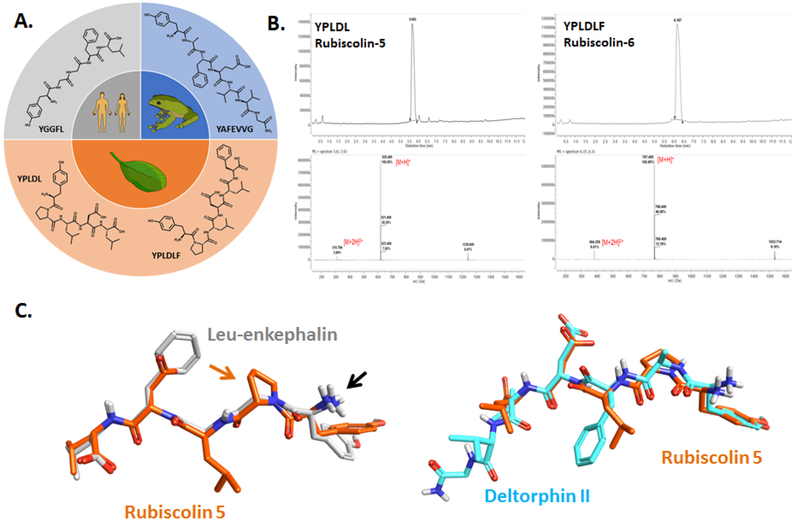
The rubiscolin, deltorphin II and leu-enkephalin peptides can all be found in nature (Figure 1A), however to ensure purity of the rubiscolin peptides, we elected to synthesize rather than extract these peptides [see methods for details]. HPLCMS analysis of the produced rubiscolin 5 and 6 peptides revealed single, sharp elution peaks with >99% purity (RT = 5.68 and 6.19 minutes, respectively) displaying mass spectra with parent peaks characteristic of the [M+H]+ ions of our desired products (m/z = 620.4 and 767.4 for rubiscolins 5 and 6, respectively), enabling their use in cellular assays to characterize their pharmacology (Figure 1B).
Figure 1. Origin and structural differences of naturally-occurring δ-OR peptides.
A) Chemical structures of the naturally-occurring peptides leu-enkephalin (human), [D-Ala2]-deltorphin II (frog), rubiscolin-5/YPLDL and rubiscolin-6/YPLDLF (spinach). B) HPLC-MS spectra for rubiscolin-5 and rubiscolin-6. C) Maximum common structure alignment for rubiscolin-5 (orange) with leu-enkephalin (gray) or deltorphin II (cyan). Black arrow points to the conserved amino-group. Orange arrow points to the proline residue unique to the rubiscolins.
Maximal common substructure alignment of naturally occurring δ-OR peptides.
The peptide ligands rubiscolin-5, and deltorphin II were subject to alignment using a maximal common substructure (MCS) method with leu-enkephalin. The conformation of leu-enkephalin used was derived from its crystal structure in complex with DPP3 (RCSB PDB ID: 5E3A). The MCS alignment between rubiscolin-5 and leu-enkephalin or deltorphin II provided similarity scores of 0.72 and 0.6 respectively, suggesting substantial overlap. Notable differences in the leu-enkephalin aligned rubiscolin-5 structure are the presence of an acidic aspartate in place of the hydrophobic phenylalanine of leu-enkephalin, and the presence of a bulky, hydrophobic leucine in place of leu-enkephalin’s glycine (Figure 1C). Interestingly, deltorphin II shows similar features to the rubiscolin compounds in this area with an acidic glutamate and hydrophobic phenylalanine (Figure 1C).
Rubiscolin peptides are G protein-biased δ-OR agonists.
In order to determine the signaling properties of rubiscolin peptides at the δ-OR, we first confirmed that rubiscolin peptides indeed bind to the δ-OR with radioligand binding using membranes prepared from the PathHunter cells. We compared the affinity of the rubiscolin peptides to the highly potent and selective δ-OR peptides deltorphin II and leu-enkephalin. Deltorphin II displayed the strongest affinity for the δ-OR followed by leu-enkephalin, rubiscolin-6 and rubiscolin-5 (Figure 2A, Table 1).
Table 1: Pharmacological characterization of naturally occurring δ-OR peptides.
Affinity of peptide agonists for the δ-OR is depicted as the pKi, which is a negative log value of the Ki, which is the concentration at which 50% of the δ-OR are occupied by the peptide. Potency and efficacy (α, normalized to leu-enkephalin) for the δ-OR peptide agonists to inhibit cAMP production is depicted as concentration of 50% inhibition (pIC50) and the SEM. Potency (pEC50) and efficacy (α, normalized to leu-enkephalin) of δ-OR agonists to recruit β-arrestin 1 and 2 are depicted with SEM. The number of repetitions for each drug is indicated in parentheses.
| Affinity | cAMP | β-arrestin1 | β-arrestin2 | ||||
|---|---|---|---|---|---|---|---|
| Compounds | pKi | pIC50 | α | pEC50 | α | pEC50 | α |
| Leu-enkephalin | 8.8±0.1 (4) | 9.0±0.1 (9) | 100 | 7.5±0.1 (11) | 100 | 7.5±0.1 (10) | 100 |
| Deltorphin II | 9.5±0.3 (5) | 9.4±0.1 (4) | 95±2 | 8.3±0.1 (5) | 94±6 | 8.7±0.2 (6) | 103±4 |
| Rubiscolin-5 | 5.5±0.1 (4) | 5.2±0.1 (6) | 85±9 | ND (9) | ND | 4.1±0.3 (7) | 15±4 |
| Rubsicolin-6 | 6.0±0.1 (3) | 5.8±0.2 (4) | 77±8 | ND (7) | ND | 5.1±0.3 (6) | 20±3 |
Next we confirmed that all four peptides were indeed able to inhibit forskolin-induced cAMP production by activation of the inhibitory Gαi-protein pathway. All four peptides displayed full agonism and followed the same rank order in potency as observed for their affinity (Figure 2B, Table 1). Next, we assessed the ability of these peptides to recruit β-arrestin, finding that deltorphin II and leu-enkephalin recruit both β-arrestin 1 (Figure 2C) and βarrestin 2 (Figure 2D), with deltorphin II showing a higher potency (Table 1). Interestingly, in both assays β-arrestin recruitment induced by the rubiscolins ranged from low to undetectable even at concentrations of 316 μM (Figure 2C and 2D). Importantly, because the PathHunter cell line was used for our binding studies, this limited β-arrestin recruitment cannot be attributed to a lack of binding to the δ-OR.
Discussion
Delta opioid receptor peptides like leu-enkephalin, DPDPE and DADLE are all strongly recruit β-arrestin; still, G protein-biased synthetic δ-OR peptides have been produced over 10 years ago as exemplified by UFP-512 (Chiang et al., 2016; Molinari et al., 2010). Nevertheless, in our assays, the potency for leu-enkephalin for cAMP inhibition (G protein) is 1.5 log steps stronger than for β-arrestin recruitment, suggesting leu-enkephalin is G protein-biased. Relative to leu-enkephalin, we find that deltorphin II is less biased (Table 2, bias factor < 1). Distinguishingly, here we present the first report of rubiscolin-5 as naturally-occurring G protein-biased δ-OR peptide, with a bias factor 2 when comparing cAMP versus β-arrestin 2 (Table 2) and undetectable rubiscolin-5 induced β-arrestin 1 recruitment to the δOR at concentrations as high as 2mM (Figure 2C and D). Rubiscolin-6 similarly did not recruit β-arrestin 1 to an extent that would allow us to calculate a bias factor. The cAMP- βarrestin 2 bias factor for rubiscolin-6 was 0.52 making it less G protein-biased than leuenkephalin, but more biased than deltorphin II.
Table 2: Bias factors for naturally occurring δ-OR peptides.
Transduction coefficient (t), (KA) and bias factor determination. Log (τ/KA) was determined using the operational model equation (van der Westhuizen et al, 2014). ΔLog (τ/KA) was calculated using Leu-enkephalin as the reference compound. Bias Factor = 10(ΔLog (τ/KA)[cAMP] - ΔLog (τ/KA)[βARR]). [ND = not determinable].
| cAMP | β-arrestinl | β-arrestin2 | Bias Factor | |||||
|---|---|---|---|---|---|---|---|---|
| Compounds | Log (τ/KA) |
ΔLog (τ/KA) |
Log (τ/KA) |
ΔLog (τ/KA) |
Log (τ/KA) |
ΔLog (τ/KA) |
cAMP-βarr 1 | cAMP-βarr 2 |
| Leu-enkephalin | 9.05 | 0 | 7.56 | 0 | 7.53 | 0 | 1 | 1 |
| Deltorphin II | 9.43 | 0.39 | 8.20 | 0.64 | 8.56 | 1.07 | 0.55 | 0.21 |
| Rubiscolin-5 | 5.13 | −3.92 | ND | ND | 3.26 | −4.23 | ND | 2.04 |
| Rubiscolin-6 | 5.41 | −3.63 | ND | ND | 4.14 | −3.35 | ND | 0.52 |
In terms of opioid receptor pharmacology, studies thus far have highlighted the benefits of biased signaling that favors G protein over β-arrestin. For μ-ORs, G protein-biased signaling may reduce tolerance, constipation and respiratory depression (DeWire et al., 2013; Manglik et al., 2016), although recent studies have placed some doubts about the strength of this hypothesis (Altarifi et al., 2017; Hill et al., 2018). For the κ-ORs, G protein-biased ligands may avoid aversive effects commonly associated with (unbiased) κ-OR (Bruchas and Chavkin, 2010). For the δ-ORs β-arrestin recruitment has been associated with increased alcohol use, whereas G protein-biased δ-OR agonists reduce alcohol intake in mice (Chiang et al., 2016; Robins et al., 2018).
Currently only antagonist-bound δ-OR structures have been resolved (Fenalti et al., 2015; Granier et al., 2012), in contrast to κ-ORs and μ-ORs for which agonist-bound structures are available. Still, although less optimal, homology modeling can be performed to use the agonist-bound OR structures to create a computational model of an agonist bound conformation of the δ-OR. Recent studies using computational modeling and X-ray crystallography have suggested that drug conformation can have a significant impact on specific receptor conformations inducing receptor states that are more or less susceptible to recruit β-arrestin (McCorvy et al., 2018; Wacker et al., 2017). While common substructure analysis did not show major differences in conformation between rubiscolin-5 and leuenkephalin or deltorphin II, it is noteworthy that the rubiscolin peptides contain a proline residue. Proline residues, due to their cyclic structure are known to induce kinks and instill a degree of inflexibility to the peptide. It is possible that this proline residue in rubiscolins induces a certain conformation that the more flexible enkephalin and deltorphin II circumvent. With our finding that rubiscolin peptides are G protein-selective δ-OR agonists, it may be of interest to dock rubiscolin-6, or YPLDLV a more potent and δ-OR-selective synthetic analog (Yang et al., 2003b) to potentially obtain insight into which regions of the δ-OR are being engaged or spared to confer G protein selectivity onto rubiscolin when compared with deltorphin II or leu-enkephalin.
Rubiscolin-6 is more potent and has stronger affinity for the δ-OR than rubiscolin-5, but both peptides are rather weak in comparison to leu-enkephalin and deltorphin II, which may limit their therapeutic potential. One advantage however of the rubisocolin peptides is their oral bioavailability; rubiscolins are produced by digestive pepsin cleavage of rubisco, and rubiscolin-6 at a dose of 100 mg/kg, p.o. produced analgesia in a tail-pinch assay (Yang et al., 2001), enhanced memory consolidation, (Yang et al., 2003a) and reduced anxiety-like behavior (Hirata et al., 2007) in a manner that was shown to be reversible by the δ-OR antagonist naltrindole, suggesting that the behavior was δ-OR mediated.
Overall, we provide evidence for the first known naturally-occurring, orally bioavailable G protein-selective δ-OR peptide agonist. The attributes of rubiscolin-6 may make it an interesting drug in treating alcohol use disorder (Chiang et al., 2016; Robins et al., 2018), or as an analgesic with fewer side effects.
Highlights.
Rubiscolin-5 and −6 are naturally occurring peptides
Rubiscolin-5 and −6 bind to δ opioid receptors
Rubiscolin-5 and −6 activate G-protein signaling at δ opioid receptors
Rubiscolin-5 and −6 do not recruit β-arrestin 1
Rubiscolin-5 and −6 only weakly recruits β-arrestin 2
Acknowledgments
Role of the funding source
This project was funded by grants from the National Institutes for Health to RMvR (R03DA045897, R01AA025368, R21AA026949) and the Purdue University Department of Medicinal Chemistry and Molecular Pharmacology. DJT was supported through a start-up package from Purdue University School of Pharmacy and from the Purdue University Center for Cancer Research NIH grant P30 CA023168.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS, 2017. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol 31, 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C, 2010. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 210, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chajra H, Amstutz B, Schweikert K, Auriol D, Redziniak G, Lefevre F, 2015. Opioid receptor delta as a global modulator of skin differentiation and barrier function repair. Int J Cosmet Sci 37, 386–394. [DOI] [PubMed] [Google Scholar]
- Chiang T, Sansuk K, van Rijn RM, 2016. Beta-arrestin 2 dependence of delta opioid receptor agonists is correlated with alcohol intake. Br J Pharmacol 173, 323–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD, 2013. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344, 708–717. [DOI] [PubMed] [Google Scholar]
- Englert P, Kovacs P, 2015. Efficient heuristics for maximum common substructure search. Journal of chemical information and modeling 55, 941–955. [DOI] [PubMed] [Google Scholar]
- Fenalti G, Zatsepin NA, Betti C, Giguere P, Han GW, Ishchenko A, Liu W, Guillemyn K, Zhang H, James D, Wang D, Weierstall U, Spence JC, Boutet S, Messerschmidt M, Williams GJ, Gati C, Yefanov OM, White TA, Oberthuer D, Metz M, Yoon CH, Barty A, Chapman HN, Basu S, Coe J, Conrad CE, Fromme R, Fromme P, Tourwe D, Schiller PW, Roth BL, Ballet S, Katritch V, Stevens RC, Cherezov V, 2015. Structural basis for bifunctional peptide recognition at human deltaopioid receptor. Nature structural & molecular biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK, 2012. Structure of the delta-opioid receptor bound to naltrindole. Nature 485, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano TE, Bohn LM, 2007. An opioid agonist that does not induce mu-opioid receptor--arrestin interactions or receptor internalization. Mol Pharmacol 71, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, Bailey C, Kelly E, Henderson G, 2018. The novel mu-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol 175, 2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Sonoda S, Agui S, Yoshida M, Ohinata K, Yoshikawa M, 2007. Rubiscolin6, a delta opioid peptide derived from spinach Rubisco, has anxiolytic effect via activating sigma1 and dopamine D1 receptors. Peptides 28, 1998–2003. [DOI] [PubMed] [Google Scholar]
- Kawabata T, 2011. Build-up algorithm for atomic correspondence between chemical structures. Journal of chemical information and modeling 51, 1775–1787. [DOI] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang XP, Sassano MF, Giguere PM, Lober S, Da D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK, 2016. Structure-based discovery of opioid analgesics with reduced side effects. Nature 537, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorvy JD, Butler KV, Kelly B, Rechsteiner K, Karpiak J, Betz RM, Kormos BL, Shoichet BK, Dror RO, Jin J, Roth BL, 2018. Structure-inspired design of betaarrestin-biased ligands for aminergic GPCRs. Nat Chem Biol 14, 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari P, Vezzi V, Sbraccia M, Gro C, Riitano D, Ambrosio C, Casella I, Costa T, 2010. Morphine-like opiates selectively antagonize receptor-arrestin interactions. J Biol Chem 285, 12522–12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Perroy J, Walwyn WM, Smith ML, Vicente-Sanchez A, Segura L, Bana A, Kieffer BL, Evans CJ, 2016. Agonist-Specific Recruitment of Arrestin Isoforms Differentially Modify Delta Opioid Receptor Function. J Neurosci 36, 3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, Evans C, Kieffer BL, 2010. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci 30, 16459–16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Walker JK, Bohn LM, 2005. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314, 1195–1201. [DOI] [PubMed] [Google Scholar]
- Raymond JW, Willett P, 2002. Maximum common subgraph isomorphism algorithms for the matching of chemical structures. Journal of computer-aided molecular design 16, 521–533. [DOI] [PubMed] [Google Scholar]
- Robins MT, Chiang T, Mores KL, Alongkronrusmee D, van Rijn RM, 2018. Critical Role for Gi/o-Protein Activity in the Dorsal Striatum in the Reduction of Voluntary Alcohol Intake in C57Bl/6 Mice. Frontiers in psychiatry 9, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi A, Marrone GF, Palmer TC, Narayan A, Szabo MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, Hunkele A, Pagirsky J, Eans SO, Medina JM, Xu J, Pan YX, Borics A, Pasternak GW, McLaughlin JP, Majumdar S, 2016. Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit beta-Arrestin-2. J Med Chem 59, 8381–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, Lansu K, Schools ZL, Che T, Nichols DE, Shoichet BK, Dror RO, Roth BL, 2017. Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 168, 377–389 e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Kawamura Y, Yoshikawa M, 2003a. Effect of rubiscolin, a delta opioid peptide derived from Rubisco, on memory consolidation. Peptides 24, 325–328. [DOI] [PubMed] [Google Scholar]
- Yang S, Sonoda S, Chen L, Yoshikawa M, 2003b. Structure-activity relationship of rubiscolins as delta opioid peptides. Peptides 24, 503–508. [DOI] [PubMed] [Google Scholar]
- Yang S, Yunden J, Sonoda S, Doyama N, Lipkowski AW, Kawamura Y, Yoshikawa M, 2001. Rubiscolin, a delta selective opioid peptide derived from plant Rubisco. FEBS Lett 509, 213–217. [DOI] [PubMed] [Google Scholar]