Abstract
Stress granules are macromolecular aggregates of mRNA and proteins assembling in response to stresses that promote the repression of protein synthesis. Most of the work characterizing stress granules has been done under acute stress conditions or during viral infection. Comparatively less work has been done to understand stress granule assembly during chronic stress, specifically regarding the composition and function of stress granules in this alternative context. Here, we describe key aspects of stress granule biology under acute stress, and how these stress granule hallmarks differ in the context of chronic stress conditions. We will provide perspective for future work aimed at further uncovering the form and function of both acute and chronic stress granules and discuss aspects of stress granule biology that have the potential to be exploited in human disease.
1. Introduction
RNA granules are a family of macromolecular aggregates that assemble in response to environmental cues. These granules are named based on their constituent parts and/or the process in which they function. The best-studied classes of RNA granules are germ granules, p-bodies, neuronal granules, and stress granules. Germ granules function in germ cells and regulate fertility, while p-bodies are associated with RNA decay and mRNA storage. Neuronal granules have been shown to regulate site-specific translation. Several excellent reviews have been written on these various RNA granule types [11,36,39,66,75]. Here we will focus on stress granules (SGs) because of their relationship with human disease and the gap in knowledge that exists in our understanding of SGs under acute and chronic stress. We will delineate differences between acute and chronic stress granules and highlight potential opportunities for therapies centered on SG disruption.
2. Acute stress granules
2.1. Components and conditions
Acute stress is generally defined as a stress that is applied transiently (usually for two hours or less) before analysis. Stress granules appear in the cytoplasm of cells under acute stress conditions such as oxidative, metabolic, hypoxic or thermal stress, as well as during cellular infection by many different viruses [2,28,31,58]. The type and duration of the stress can dictate incorporation of constituents within SGs, including or excluding many components of the translation machinery such as translation initiation factors EIF3, EIF4G and even PABP, 40S ribosomes, polyadenylated mRNAs, and SGs also recruit many signal transduction proteins and RNA binding proteins such as the SG nucleating factors G3BP1, G3BP2, TIA1 and TIAR [1]. In other words, SGs contain stalled 48S mRNP complexes which result from translation repression at the initiation step (Fig. 1). However, recent work has illustrated that stress granules can have a composition specific to the particular stress the cells are subjected to [5,56].
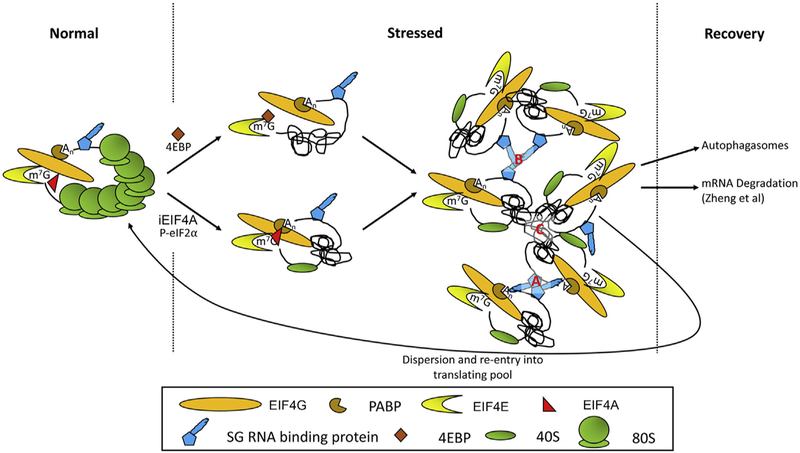
Fig. 1.
General features of acute stress granules. During unstressed conditions, mRNAs generally exist in a complex with ribosomes and translation initiation factors, as well as RNA binding proteins that stabilize the mRNA and promote its translation. During stress conditions, the cap-binding complex is disassembled or 40S ribosomes are stalled within the 5′UTR. Stress promotes disassembly of the cap-binding complex or inhibits 40S scanning via activation of 4EBPs, inhibition of EIF4A activity (iEIF4A), or inactivation of eIF2 by promoting eIF2α phosphorylation. Bound 80S ribosomes present within the open reading frame run off the mRNA under these conditions leaving exposed mRNA that collapses onto itself and is subject to RNA folding. At this point, RNA binding proteins also associate with the mRNA and the resulting complexes assemble into SG dependent on specific (A) and non-specific (B) protein:protein interactions requiring globular and disordered domains, respectively, as well as RNA:RNA interactions (C) resulting from free mRNA.
2.2. Pathways of induction
Stress granule assembly is a highly regulated, multi-step process[85], typically occurring in response to repression of protein translation. This process can be initiated in multiple ways. The most well-characterized stimuli uniformly induce translation repression by triggering eukaryotic initiation factor 2 α (eIF2α, EIF2S1) phosphorylation and subsequent accumulation of stalled translation initiation complexes [28]. mRNPs within SG rapidly exchange with mRNPs associated with the translation apparatus in the cytoplasm [6,29,32], and some SG components have half-residence times less than 20 s. However, restriction of translation initiation through mechanisms independent of eIF2α, such as inhibition of the RNA helicase EIF4A (with pateamine A, hippuristanol or silvesterol) or the nutrient sensor mTORC1 (with rapamycin or its analogs), has also been shown to promote SG formation in some contexts [10,12,17,76]. Thus, while several mechanisms promote formation of mRNPs that can be packaged into SGs by inhibition of function of the cap-binding complex eIF4f, the most well studied mechanism is through phosphorylation of eIF2α (Fig. 1).
2.3. Key interactions within SG
The critical interactions that have been shown to regulate SG assembly and persistence can be separated into three classes of interactions. The first are stable and specific protein:protein interactions occurring between globular domains that stably recognize interaction partners (Fig. 1, interaction A). Secondly, non-specific and less stable interactions, typically occurring between intrinsically disordered domains of proteins, have also been implicated in phase separation of mRNPs in stress granules (Fig. 1, interaction B). Finally, RNA:RNA interactions that result from exposed mRNA sequences appear upon the uncoating of mRNAs that occurs upon translation arrest have recently been shown to alter the propensity of mRNPs to phase separate (Fig. 1, interaction C). All of these are likely important for proper SG structure and function, but might differ in a stress specific manner.
Most work on specific interactions integral for SG formation (Fig. 1, interaction A) has predominantly focused on SG-nucleating proteins. Several SG-nucleating proteins have been described including Ras-GAP SH3 binding proteins 1 and 2 (G3BP1 and G3BP2), TIA1 and TIA1 cytotoxic granule associated protein like 1 (TIA1 and TIAR), FMRP (FMR1), FUS (TLS) and CAPRIN1 (RNG105). We do not consider all of these true nucleating proteins because some are only recruited to SGs under disease states (FUS; [26,65]) while others appear to function through other nucleating proteins (CAPRIN1 discussed below; [31]), or do not appear to alter SG assembly in their absence (FMR1; [20]). TIA1 and TIAR are bona fide nucleating proteins, but they lack discrete globular domains, and we thus focus our discussion on G3BP1. G3BP1 is the best studied nucleating protein, and can induce is SG assembly upon overexpression, and can induce eIF2α phosphorylation through PKR activity [31,61,62]. In some systems, inactivating G3BP1 can completely abrogate SG formation [73,81], while other systems require inactivation of both G3BP1 and its homolog G3BP2 [31,44,63]. Both G3BP1 and G3BP2 are RNA-binding proteins that have globular protein:protein interaction domains in the N-termini and protein:RNA interaction domains in the C-termini, in each case flanking a central intrinsically disordered region [30,59]. The domain layout of G3BP1 suggests a model where G3BP1 and G3BP2 can hold SG together by interacting with SG proteins on one end and RNA on the other. These features ultimately led to interest in the identification of additional proteins with which these two SG-nucleating proteins might be interacting. Subsequent work demonstrated that in addition to homo- and hetero-dimerization, G3BP1 and 2 interact with USP10 and Caprin1 [31,62,72,74]. USP10′s interaction with G3BP1 involves an FGDF motif within USP10 that binds a pocket within the N-terminal NTF2-like domain of G3BP1, and this binding inhibits SG condensation in response to some stresses [31,55]. Caprin1 competes with USP10 for binding to G3BP1, thus favoring SG condensation [31]. However, it is important to note that the interactions between G3BP1 and USP10 or Caprin1 do not fully dictate SG disassembly or assembly, as two different laboratories recently demonstrated that the unstressed G3BP1 interactome significantly overlaps the interactome of G3BP1 during stress [41,90]. Less than half of the G3BP1-interacting proteins interacted with G3BP1 specifically in stress conditions, and both USP10 and Caprin1 were shown to interact with G3BP1 in either context. Further work is required to determine the changes in the nature of these interactions that promote normal function within mRNPs in translation as compared to functions promoting SG condensation.
The second class of interactions involved in SG formation are weak and nonspecific interactions that do not require folded globular domains (Fig. 1, interaction B). Within SGs, these nonspecific interactions are mediated by intrinsically disordered and low complexity sequences intrinsic to many RNA binding proteins [30,38,57]. Despite the contribution of ordered protein-protein interactions, stress granules are phase-separated mRNPs that are largely dependent upon the aggregation of low complexity and intrinsically disordered domains within proteins (Fig. 1). SGs thus contain many RNA binding proteins containing such regions [26,38]. Indeed, recent work indicates that post-translational modification of the intrinsically disordered regions of G3BP1 and FUS by phosphorylation and arginine methylation [63,80] can alter SG formation.
Extending this concept, multiple post-translational modifications (PTMs) including deacetylation, arginine demethylation, poly(ADP) ribosylation, O-GlcNac modification, and phosphorylation have been shown to alter SG dynamics. Several of these modifications occur on specific SG nucleating proteins, and therefore might be expected to influence SG assembly. For example, G3BP1 is ADP-ribosylated [37], de-phosphorylated [63,78], and de-methylated to promote SG assembly[81]. TIA1 is also ADP-ribosylated under conditions in which SG form[37], and TIA1 oxidation has been shown to inhibit SG assembly [3]. FUS, which assembles into SG in the context of neurological disease, can be phosphorylated by DNA protein kinase thus rendering it less aggregation prone [47]. Finally, O-GlcNac modification of ribosomal proteins appears to regulate SG condensation [51]. On the other hand, while acetylation and ubiquitination have been shown to regulate SG dynamics [35,43], whether these effects are a consequence of modification of SG nucleating proteins or are indirect effects related to cellular stress have not been completely worked out. Importantly, the PTMs on specific proteins that regulate SG dynamics (i.e. G3BP1 and FUS as well as TIA1 to a lesser extent) concentrate in intrinsically disordered regions of the proteins suggesting they may act as a switch to trigger phase separation within a SG. How these PTMs and others in RNA binding proteins not yet discovered affect SG assembly and function remains underexplored.
Finally, consistent with a role for RNA in regulating SG formation, RNA can both self-assemble into RNA aggregates that resemble the SG transcriptome [83] and affect the ability of intrinsically disordered protein fragments to condense into phase separated mRNPs [38]. Thus RNA:RNA interactions are likely an important component of SG structure (Fig. 1, interaction C). G quartets, a well-studied RNA structure, form as a consequence of Hoogsteen base pairing between nearby guanines within RNA. Stacking of G-quartets then forms a right-handed helical structure known as a G-quadruplex [14]. These structures might contribute to SG condensation as guanosine has been shown in certain conditions to aggregate to form gel-like condensates similar to that formed by high concentrations of SG proteins [21,26]. Indeed, many SG-resident RNA binding proteins, such as FUS, YB1, FMRP and hnRNPA1, interact with G quadruplexes [8,40,87,91]. Recently, the interaction between YB1 and G-quadruplex structures within the 5′ cleavage product of a subset of mature tRNAs was speculated to be critical for SG formation, but researchers were unable to test this directly [40]. The involvement of G-quadruplexes in this process is ultimately dependent on conclusively showing that G-quadruplexes assemble in vivo. Because SGs assemble under conditions of global translation repression, ribosomes runoff and expose RNA sequences that may contain guanosine stretches involved in G-quadruplex formation, which could assemble into G-quadruplex structures. Assuming the appropriate cations are present within SGs to mediate G-quartet assembly, this process would be further promoted by high concentrations of RNA within SG resulting in an interaction of concentrated G-quadruplex structures that could in theory contribute to maintaining SG structure. Furthermore, hyperfolded, naked RNAs resulting from ribosome runoff could interact through RNA structural elements such as the G-quadruplex. One can surmise that RNA reorganization would also induce allosteric changes in RNA binding proteins containing intrinsically disordered domains to further promote assembly of SGs. Fig. 1 depicts the normally circular state of mRNAs within cells. When cells encounter a stress that abolishes translation initiation, ribosomes dissociate from RNAs causing the RNA to collapse into an aggregate, likely partly mediated by G-quadruplex structures, along with some translation initiation factors and RNA binding proteins. SG disassembly could then potentially occur by dissociation of intrinsically disordered protein interactions, routing of some components to autophagosomes, enhanced expression or activation of RNA chaperones, or alternatively, degradation of the SG constituents themselves. Future studies are needed to define the importance of G-quadruplexes and other RNA structures in the formation and disassembly of SG.
2.4. Pro-survival functions
The formation of SGs appears to regulate several canonical signaling pathways (NF-κB, mTORC1, PKR, RIGI) within the stress response [30,27,53,59,76]. A decade ago, Takekawa and colleagues showed that acute cellular stresses induce pro-survival SG formation [2]. This seminal work demonstrated that SGs repress pro-apoptotic MAPK signaling through recruitment of RACK1 to SG. Expressing a mutant RACK1 protein, which is not recruited to SG, causes induction of apoptosis in response to some stressors in Cos7 cells. Subsequent studies have generally supported this function. Wei and coworkers showed that recruitment of ROCK1 to SGs inhibits apoptosis and pharmacological inhibition of SG assembly with cycloheximide or EHNA promoted cell death [79], thus affirming that acute SG function in a pro-survival capacity. While this finding is notable, it is unclear whether inhibition of SG themselves, inhibition of protein synthesis, or some other signaling pathway was affected that promoted cell death under their conditions. Finally, recent work indicates that TIA1 oxidation under conditions that promote SG assembly causes impaired SG formation and results in cell death [3]. Conversely, expression of a TIA1 mutant that cannot be oxidized rescues SG formation and impairs apoptosis. Collectively these studies strongly support a pro-survival function for SG formed in response to acute stress (Fig. 2).
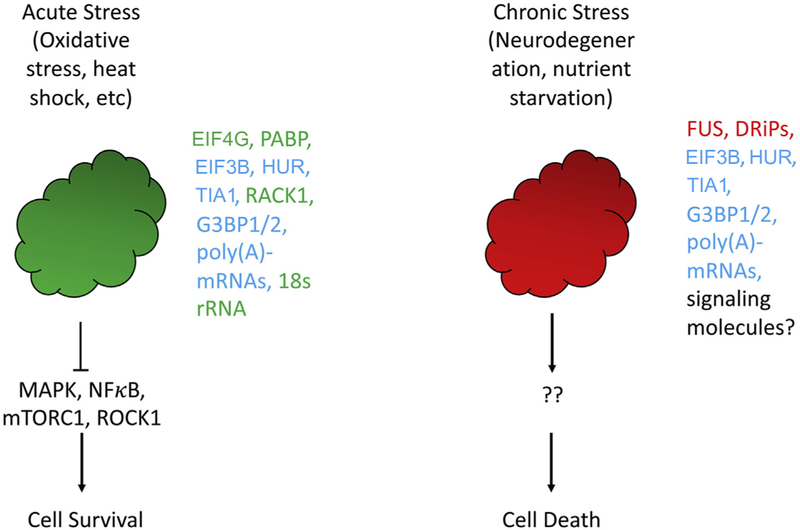
Fig. 2.
Acute and chronic SG differ in composition and effects on cell fate. Acute stress granules (left) contain many signaling components as well as 40S ribosomes, are very dynamic, and have a pro-survival function. In contrast, chronic SG (right) lack 40S ribosomes, are static and have a pro-death function. Components known to exist within each type of SG are listed (green, acute SG-specific; blue, present in both acute and chronic SG; red, chronic SG-specific).
Two mechanisms that might explain the pro-survival function of stress granules are the regulation of translation of a specific set of mRNAs or activation of pro-survival cellular signaling pathways independent of the former mechanism. These are not necessarily exclusive possibilities. Evidence for the former mechanism includes the observation that during acute stress, mRNAs for many proto-oncogenes that promote proliferation and survival accumulate in SGs and appear to be sorted based on RNA length and the presence of binding sites for SG resident RNA binding proteins [33,48]. Accumulation of these mRNAs in SGs suggests that they might be specifically regulated by SGs, although ribosome profiling analysis suggests these targets are translated at similar levels as mRNAs not enriched in SGs [48]. This mechanism and its role in promoting the cell survival function of SGs is not well understood; however, it is clear from multiple studies SG do not globally affect mRNA translation. Depletion of SG does not desensitize cells to stress-dependent translation repression.
The latter mechanism, regulation of cellular signaling pathways, is supported by the above-referenced study by Takekawa and colleagues[2]. This work documents repression of MAPK signaling contingent upon RACK1 association with SG. However, a function for acute SG in regulating cellular signaling has also been well-documented in the context of viral infection. SGs induced during viral infection recruit many innate immune proteins, and several studies have revealed that SGs serve as a platform for activation of Protein Kinase R (PKR) and the RIGI dsRNA helicase [53,62,89]. Some picornaviruses including polio-virus, coxsackie viruses, and EMCV cleave the SG nucleating protein G3BP1 to disassemble SG [18,49,86]. This cleavage enhances viral replication and quickly leads to cell death. Ectopic expression of a noncleavable mutant of G3BP1 in this context reduces viral replication and prolonged cell survival [49,86]. This pro-survival function of SGs has also been observed in other contexts, such as some types of cancer. In vitro, some sarcoma cells depend on SGs for improved survival [73], and colorectal cancer cells can upregulate SGs as a mechanism to survive chemotherapeutic agents [22]. Together, these studies highlight the need to better understand the specific signaling changes originating from SGs during stress that regulate cell fate. They similarly suggest that other yet-unknown factors may coordinate with SGs in determining cell fate in circumstances where SG regulation may be necessary but not sufficient for eliciting cell death.
3. Chronic stress granules
3.1. Components and conditions
Chronic stress is characterized by prolonged exposure to a given stress condition. Given that the majority of acute SG studies have historically been performed in vitro with cells grown on plastic matrices, chronic stress conditions are broadly defined as stresses lasting from four to six hours (or longer) in duration. Studies of chronic stress may intuitively be more relevant to human diseases, given that stresses attributable to pathological circumstances may last days to months dependent on the disease and organ affected. Based on the variation of SG residents under acute stress conditions [5], and data suggesting that chronic SG are fundamentally different from acute SG, there is a current need to better understand the role of SGs during chronic stress conditions and their relevance to disease.
The composition of SGs that assemble under various chronic stress conditions has not been established in the level of detail that has been accomplished for acute stress conditions. However, some recent examples have begun to provide information on the former topic. While many cell culture models of virus infection are acute stresses in which cells are rapidly overcome by the virus and rarely survive, some chronic models also exist. One study utilized a Chikungunya virus replicon to impose chronic stress in the context of a persistent infection. These conditions induced formation of stable NSP3:G3BP1 aggregates lasting days [64]. While these authors did not expect that these RNA granules contained many canonical SG components, this possibility has not yet been rigorously investigated, nor has whether SG formation coincides with global translational repression in this context. During picornavirus infection, normal SGs are disassembled early in infection via cleavage of the SG nucleating protein G3BP1 [18,49,86]. However, EIF4G cleavage by the picornavirus 2A protease during infection may promote formation of persistent, atypical SGs that lack canonical SG components but contain cellular mRNAs. This has led to speculation these persistent SGs would harbor repressed cellular mRNAs, while disassembly of normal SG through G3BP1 cleavage would release viral genomic RNA to promote its translation [88].
Another example where chronic SGs assemble in response to chronic stress is in neurodegenerative disease. Some neurodegenerative diseases are associated with mutations in HNRNPA family members. In this context, these mutant proteins promote the maturation of SGs into irreversible fibrils that enhance the pathology of the disease [23,46]. Previous work indicates that autophagy is partly responsible for SG clearance [7], suggesting that beneficial effects of autophagy-inducing drugs might be partly owed to enhanced clearance of SG clearance during treatment. Indeed, drugs that promote autophagy have been effective in treatment of neurological disorders [45], and individuals with deleterious mutations in proteins of the autophagic pathway have increased incidence of neurodegenerative disease. These results from the autophagy field suggest that pro-death SG may be induced in the diseased state and might contribute to neuron cell death. Even beyond viral pathogens and deleterious genetic variants, chronic nutrient starvation has been shown to promote SG formation. Similar to acute SGs, chronic SGs arising from a dearth of nutrients contain translation initiation factors, RNA binding proteins, and poly(A) mRNAs. However, these chronic SGs lack 18S rRNA as well as two 40S-associated proteins, RPS6 and RACK1, that are commonly found in SGs under acute stress conditions. SGs forming in response to chronic nutrient starvation do not exchange with cytoplasmic mRNP pools, suggesting their cellular role might vary from the classical paradigm of acute SG function. However, unlike irreversible fibrils in neurodegeneration, the static SGs that form under chronic nutrient starvation are still capable of being cleared once nutrients are restored [60]. This indicates that while nutrient starvation SGs are not dynamic, they do not mature to the state of the irreversible fibrils observed in neurological disease, and therefore may represent a middle ground between dynamic and reversible acute SGs and static and irreversible chronic SGs. This is an emerging area of SG research that will require additional studies to determine the key differences in chronic SG, their relationship with acute SGs, and the contribution of low complexity sequences in each.
3.2. Pathways of induction
While many acute stresses promote SG condensation via eIF2α phosphorylation, chronic stresses have been shown to overcome eIF2α phosphorylation to ultimately reduce SG formation [68]. Alternatively, chronic stress in some contexts can promote increases in eIF2α phosphorylation, such as during the epithelial to mesenchymal transition [15,34] and in neurodegenerative diseases such as frontotemporal dementia and ALS [71]. These conditions are expected to promote SG assembly, although it remains to be formally tested what effect these SGs may have on cell fate.
We recently showed that chronic nutrient starvation conditions in which cells are deprived of glucose, glutamine, and serum induces eIF2α phosphorylation at serine 51. When MEFs homozygous for the S51A mutant of eIF2α were exposed to these conditions, SG did not form and these MEFs were capable of surviving significantly longer than their wild-type counterparts, in which SG readily formed. These data indicate that similar to its role in the context of acute stresses, eIF2α phosphorylation is important for induction of SG formation under chronic nutrient stress conditions (Fig. 1). This likely occurs via activation of PKR-like endoplasmic reticulum kinase (PERK). It is unclear whether impairment of the cap-binding complex important for protein synthesis initiation is also important for SG formation under these conditions; however, it is notable that mTORC1 is rapidly shut down under these conditions despite the presence of amino acids within the culture medium.
While both acute and chronic stress granules require eIF2α phosphorylation, at least under most conditions described to date, there are significant differences in the systems used to study SG induction. Under acute conditions, the dose of the stress is typically much higher than would be expected to be encountered under physiological conditions. Furthermore, the time frame before analysis is shorter thereby preventing analysis of any adaptive changes the cell may use to counter the effects of the stress. Therefore, while much work has been done to investigate cell survival in response to acute stress conditions, this work may describe only initial sensitivity of a given cell type to a given stress. Thus, future work should delineate how acute and chronic stress yield different responses despite similar translation repression. The differing composition and function of acute and chronic SG is one mechanism that might help to explain these different cellular responses.
3.3. Key interactions within SG
The protein:protein and RNA:RNA interactions that serve to hold chronic SG together are likely to be similar to those that have been described for acute SGs. While the interactions that serve to hold chronic SGs together have not yet been well-defined, yet some basic principles have been observed. As in the context of acute stresses, depletion of G3BP1 and G3BP2 completely impairs SG condensation under chronic stresses. This suggests that specific protein:protein interactions associated with these proteins reprise some role in SGs arising from chronic nutrient starvation. However, a role for the G3BP1 and G3BP2 interactions with CAPRIN1 and/or USP10 under chronic nutrient starvation has not yet been investigated. Yet, structural differences between chronic and acute SGs may mediate differential recruitment or exclusion of distinct factors that ultimately engender an altered impact on cell fate. Indeed, RACK1 is absent from chronic nutrient starvation SGs. Therefore, while the interactions between the SG nucleating factors G3BP1 and G3BP2 are likely similar between acute and chronic SG, one can reasonably conclude that these interactions are unlikely to affect cell fate by themselves.
These initial findings in chronic nutrient starvation models parallel results from studies in neurodegenerative diseases suggesting that prolonged aggregation of SG proteins promote assembly of fibrils that eventually recruit pathological aggregates that induce neuronal cell death [4,84]. In fact, expression of RNA binding protein mutants associated with neurodegenerative disease (e.g. FUS, HNRNPA1, C9ORF72, and ATAXIN2) all promote SG aggregation. This is in large part associated with intrinsically disordered domains, and these interactions have been suggested to promote the fibril assembly associated with disease [23,46,47].
Finally, as with acute SGs, it is reasonable to speculate that RNA:RNA interactions are involved in chronic SG formation. Nucleotide repeats within the C9ORF72 RNA, again associated with neurological disease, have been shown to assemble into G-quadruplexes that promote assembly of stress granule-like structures [13]. However, further studies are required to investigate the relative contribution of RNA and protein in phase separation of SGs. While the SG that form in neurological disease differ from those forming under acute stresses, they also differ from those that form under chronic nutrient starvation. Unlike in neurodegenerative diseases, chronic nutrient starvation induces SG that are reversible despite not being dynamic as observed under conditions of acute stress. To explore the possibility of targeting chronic SGs for specific pharmacological interventions, much work remains.
3.4. Pro-death function
While the individual signals and mechanisms remain largely unclear, it is clear that cellular context, stress type, and stress duration may have largely different effects on SG composition and function. SGs are important for cancer cell viability and stress resistance [22,73] when cells are exposed to acute stresses in vitro. However, in an in vivo tumor setting cells are often exposed to chronic stress, leading to the question of whether chronic nutrient starvation induced pro-survival SG similar to acute stress conditions. One of the most well-accepted strategies for inactivating SG is to deplete the SG nucleating proteins G3BP1 and G3BP2. Using this model in the context of chronic nutrient starvation, we found that SG depletion resulted in improved survival. These results suggest that at least some chronic stresses induce pro-death SG in contrast to acute stress conditions (Fig. 2).
The pro-death function we observed during chronic nutrient starvation is reminiscent of studies of acute stress with either H2O2 or selenite. These stresses induce non-canonical SG formation, and selenite-induced SG have been suggested to function in a pro-death capacity [12,17]. However, the SGs described in these previous studies differ in composition and are induced by different pathways of translation repression as compared to chronic nutrient starvation. Given that these studies also predated a robust strategy for SG depletion, it remains unclear whether SGs themselves or regulation of translation of subsets of mRNAs is primarily responsible for the pro-death effects observed within this context.
4. Targeting SG or SG subcomplexes in disease
4.1. Rationale for targeting SGs in disease
Many therapies target single proteins that are overexpressed or activated in a given disease state. However, despite the protein concentration or activity being elevated in diseased cells, the therapeutic index of these approaches may be suboptimal since the protein may also be expressed (at lower levels) in unaffected tissues. Because SGs appear only during stress conditions, they have the potential to be a comparatively tractable target for drug discovery (under the rationale that only stressed cells would be expected to be vulnerable to drugs targeting SG-dependent functions). Specific interactions within SGs that depend on interaction of globular domains which are critical for SG assembly could be targeted using conventional drug design platforms. Nonspecific, weak interactions contributed by intrinsically disordered domains could also potentially be exploited to interfere with SG assembly or function. While this strategy would be more difficult because of the importance of these domains in cellular signaling and other processes, recent work has suggested it would be both possible and beneficial to disrupt nonspecific and weak interactions within SGs in the context of neurological disease [23]. Finally, regulation of subsets of mRNAs by SG resident complexes is another mechanism that could be exploited to influence cell fate of diseased cells. Together, these strategies reveal a vast array of strategies that can be exploited to target SG.
4.2. Molecular targets to exploit
In order to effectively target SG within disease, it will be important to understand which type and function of SG might need to be targeted in a given context. For example, while it would be useful to inhibit pro-death chronic SGs to preserve functioning neurons in the context of neurodegenerative disease, inhibition of SGs functioning as pro-survival factors during chemotherapeutic stress would render cancer cells more sensitive to existing interventions.
Both acute and chronic SGs could be concomitantly targeted by the inhibition of protein:protein interactions common to both entities. This would be useful in cases where SG can be locally targeted with nano-particles or similar approaches, or in cases in which only one of the acute or chronic SGs are forming. For example, G3BP1 and G3BP2 are present in both acute and chronic SG and regulate their assembly. G3BP1 is bound by the small molecules resveratrol and the polyphenol epigallocatechin gallate (EGCG) [52,69]. While a role for these small molecules in regulating SG assembly has not been directly shown, Oi et al did demonstrate that resveratrol interacts with the same N-terminal pocket in G3BP1 mediating the interaction of this protein with CAPRIN1 to promote SG assembly [31,52,55]. Therefore, inhibition of SG via G3BP1 (and likely also G3BP2) might be achieved by employing derivatives of these compounds, which have already been proven to be safe (Table 1). Interestingly, this same N-terminal pocket bound by resveratrol has also been shown to interact with the FGDF domains in USP10 and the Alphavirus protein NSP3 [55], which inhibit SG assembly by competing with CAPRIN1 binding. Alphaviruses subvert G3BP1 function by recruiting this protein from SGs and into replication factories via NSP3, promoting viral genome replication [16,54]. Peptides derived from NSP3 or other FGDF-containing proteins have the potential to specifically target cells under stress without affecting adjacent unstressed cells. Because recent research has shed light onto the structural determinants of SG formation, it is now feasible to develop drugs that target key interactions within the SG similar to those described above (Table 1). Of course, this possibility will become more attractive as additional studies are published in this area, and additional protein:protein interactions critical to SG formation that might confer vulnerability to this process may remain to be discovered.
Table 1.
Proteins and treatments that can interfere with SG assembly.
| SG Protein | Treatment/Effectors | Description | Reference |
|---|---|---|---|
| TDP43/FUS | VEGF | VEGF treatment of cells in CSF from ALS patients | Shantanu et al. [67]. J. Chem. Neuro. |
| C9ORF72* | Antisense Therapy | Development of antisense oligonucleotides against C9ORF72 | Andrew Scott. (2017). Nature. Ionis Pharmaceuticals |
| FUS | Rapamycin, Torkinib, Paroxetine, promethazine | Autophagy Inducers | Marrone et al. [42]. Stem Cell Reports. |
| TDP43 | Auranofin, chlerythrine, riluzole | Thioredoxin reductase inhibitor, TTX-sensitive sodium channels | Oberstadt et al. [50]. Scientific Reports. |
| G3BP1* | Epigallocatechin gallate | Direct G3BP1 binding | Sim et al. [69]. Cane. Prev. Res. |
| G3BP1* | Resveratrol | NTF2-like domain of G3BP1 | Oi et al. [52]. Oncogene. |
| G3BP1 | NSP3 and USP10 FGDF tetrapeptides | Interacts with the NTF2-like domain of G3BP1 | Panas et al. [55], Plos Pathog. |
| TDP43, FUS, α-synuclein* | Hsp104p variants | Disaggregation of toxic proteins | Jackrel and Shorter [25]. Prion. |
| FUS, TAF15, EWSR1, hnRNPA1, hnRNPA2 | Karyopherinβ2 and Importinα-karyopherinβ1 | Disaggregation and relocalization of RNA binding proteins to the nucleus | Guoet al. [23]. Cell. |
| Defective ribosomal products (DRiPs) | HSPB8-BAG3-HSP70 and HSP70 | Disrupts toxic protein aggregation | Ganassi et al. [19]. Mol. Cell, [43]. EMBO J. |
| G3BP1 | PRMT1 and 5 | Antagonizes SG condensation | Tsai et al. [74,80]. J. Biol. Chem. |
| G3BP1 | JMJD6 | Depletion enhances SG assembly | Tsai et al. [82]. J. Biol. Chem. |
| Unknown | Staufen1 | Binds dsRNA to inhibit SG condensation | Thomas et al. [77]. J. Cell Sci. |
These studies did not specifically look at SGs, but it can be inferred that these might be useful in their inhibition.
One strategy to specifically perturb pro-survival SGs would be to target the PTMs needed for SG assembly and signaling. For example, HDAC6 and the arginine demethylase JMJD6 have been shown to promote SG formation [35,81]. In contrast, the arginine methyltransferases PRMT1, 5 and 8 have been shown to antagonize SG assembly. Therefore, inhibition of HDAC6 or JMJD6 and/or activation or PRMT 1, 5 or 8 may be a feasible approach to SG inhibition. It is important to note, however, that strategies targeting these molecules may suffer from drawbacks associated with indirect and epigenetic effects, and it is too early to tell whether the same PTMs are important in facilitating assembly of chronic SGs.
In contrast, specific inhibition of pro-death chronic SGs may be achieved via multiple approaches. Shorter and colleagues show that pathological aggregates in neurodegenerative disease can be reversed by transient expression of the nuclear import receptor Karyopherin-β2, which appears to interfere with intermolecular interactions responsible for fibril formation [23]. While the understanding of this mechanism is in its infancy, it is possible that small peptides that phenocopy this effect could be developed to improve survival of neurons harboring toxic fibrils (Table 1). Another strategy to target chronic SGs in neuro-degenerative disease would be to pharmacologically promote autophagy, which is known to contribute to SG clearance, would eliminate pro-death granules and resulting toxic fibrils, and has received attention for beneficial responses in mouse models of various neurological diseases (reviewed in [45]). Additionally, variants of the yeast disaggregase Hsp104 have been shown to disassemble FUS and TDP-43 aggregates that have been associated with ALS and FTD [70], and the human nuclear transport Karyopherin-β2 has been shown to reverse FUS, TAF15, EWSR1, HNRNPA1, and HNRNPA2 aggregates associated with disease [23]. Modifications of disaggregation by these molecules may prove useful in the clinic in the near future.
In metastatic cells, one would most likely want to promote assembly of pro-death SGs in an attempt to cull migrating cells responsible for disease progression. While pro-survival SGs are thought to form in response to chemotherapeutic agents [22], it might be possible to convert these SGs to pro-death SGs as more studies are done to characterize and mechanistically define chronic pro-death SGs. If treatment exploited the different characteristics of pro-death and pro-survival SGs to inhibit the pro-survival ones, drug resistant cell populations could likely be curbed resulting in treatment with higher efficacy.
5. Future directions
The significant recent advances in the understanding of SG function have tremendous implications for the importance of these cellular compartments in cellular function and disease pathology; however, these advances also highlight how much there still is to learn. Chronic stress is highly relevant to disease states including ischemia, neurological disease, cancer and even prolonged viral infection. Many signaling molecules relevant to disease states have been shown to concentrate in acute SGs, but only a few have been shown to be regulated by SGs (e.g. PKR, mTORC1, RIG-I and TRAF2), and none have been characterized within the context of chronic stress. Chronic stress involves adaptive mechanisms to promote cellular fate and function that rely on changes in gene expression both at the transcriptional and post-transcriptional levels. Recent studies in U2OS cells show that acute SGs do not regulate global translation [31,48,63], although regulation of subsets of mRNAs within these contexts remain to be extensively characterized. Do chronic SGs regulate translation globally, or that of context-specific functional subsets of mRNAs? The latter possibility has the potential to be crucial to adaptive mechanisms impacting the response to the stress, cell fate decisions, and ultimately to the progression of disease. Acute SGs concentrate many mRNAs for protooncogenes, and enhanced translation of subsets of mRNAs in cancer models has previously been shown to promote cancer progression [9,24], suggesting the SGs arising from chronic stress conditions may function via analogous mechanisms. However, the mechanisms responsible for selecting mRNAs for SG incorporation are not known. Finally, robust studies showing that SG are important in whole animals in the context of disease are sparse. It is imperative moving forward to explore these topics as well as to better understand the regulatory mechanisms and context of chronic SG function in disease to effectively target SGs and promote a desired cellular response. Studies of acute SG will provide insight into the mechanisms for study under chronic stress conditions and have the potential to be used as a roadmap in these newer studies. Completion of these studies are necessary before extensive resources should be committed to development of drugs targeting SG in disease.
Acknowledgements
The authors would like to thank Dr. Richard Lloyd for critical reading of this manuscript. This work was supported by an American Cancer Society - Athena Water Breast Cancer Research Scholar Grant (RSG-15-088-01RMC) and National Institutes of Health Public Health Service grants (NCI CA185769, and NCI CA190467) to J.R.N. The authors declare no conflicts of interest in writing this manuscript.
References
- [1].Anderson P, Kedersha N, Ivanov P, Stress granules, P-bodies and cancer, Biochim. Et Biophys. Acta (2014), 10.1016/j.bbagrm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M, Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways, Nat. Cell Biol. 10 (11) (2008) 1324–1332, 10.1038/ncb1791. [DOI] [PubMed] [Google Scholar]
- [3].Arimoto-Matsuzaki K, Saito H, Takekawa M, TIA1 oxidation inhibits stress granule assembly and sensitizes cells to stress-induced apoptosis, Nat. Commun 7 (2016) 10252, 10.1038/ncomms10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ash PEA, Vanderweyde TE, Youmans KL, Apicco DJ, Wolozin B, Pathological stress granules in Alzheimer’s disease, Brain Res. 1584 (2014) 52–58, 10.1016/j.brainres.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aulas A, Fay MM, Lyons SM, Achorn CA, Kedersha N, Anderson P, Ivanov P, Stress-specific differences in assembly and composition of stress granules and related foci, J. Cell Sci. 130 (5) (2017) 927–937, 10.1242/jcs.199240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bley N, Lederer M, Pfalz B, Reinke C, Fuchs T, Glaß M, et al. , Stress granules are dispensable for mRNA stabilization during cellular stress, e26–e26, Nucl. Acids Res. 43 (4) (2015), 10.1093/nar/gku1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buchan JR, Kolaitis R-M, Taylor JP, Parker R, Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function, Cell 153 (7) (2013) 1461–1474, 10.1016/j.cell.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cammas A, Lacroix-Triki M, Pierredon S, Le Bras M, Iacovoni JS, Teulade-Fichou M-P, et al. , hnRNP A1-mediated translational regulation of the G quadruplex-containing RON receptor tyrosine kinase mRNA linked to tumor progression, Oncotarget 7 (13) (2016) 16793–16805 10.18632/oncotarget.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chaudhury A, Cheema S, Fachini JM, Kongchan N, Lu G, Simon LM, et al. , CELF1 is a central node in post-transcriptional regulatory programmes underlying EMT, Nat. Commun 7 (2016) 13362, 10.1038/ncomms13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dang Y, Kedersha N, Low W-K, Romo D, Gorospe M, Kaufman R, et al. , Eukaryotic initiation factor 2alpha-independent pathway of stress granule induction by the natural product pateamine A, J. Biol. Chem 281 (43) (2006) 32870–32878, 10.1074/jbc.M606149200. [DOI] [PubMed] [Google Scholar]
- [11].De Graeve F, Besse F, Neuronal RNP granules: from physiological to pathological assemblies, Biol. Chem 399 (7) (2018) 623–635, 10.1515/hsz-2018-0141. [DOI] [PubMed] [Google Scholar]
- [12].Emara MM, Fujimura K, Sciaranghella D, Ivanova V, Ivanov P, Anderson P, Hydrogen peroxide induces stress granule formation independent of eIF2α phosphorylation, Biochem. Biophys. Res. Commun 423 (4) (2012) 763–769, 10.1016/j.bbrc.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fay MM, Anderson PJ, Ivanov P, ALS/FTD-associated C9ORF72 repeat RNA promotes phase transitions in vitro and in cells, Cell Reports 21 (12) (2017) 3573–3584, 10.1016/j.celrep.2017.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fay MM, Lyons SM, Ivanov P, RNA G-quadruplexes in biology: principles and molecular mechanisms, J. Mol. Biol 429 (14) (2017) 2127–2147, 10.1016/j.jmb.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Feng Y-X, Sokol ES, Del Vecchio CA, Sanduja S, Claessen JHL, Proia TA, et al. , Epithelial-to-mesenchymal transition activates PERK-eIF2α and sensitizes cells to endoplasmic reticulum stress, Cancer Discovery 4 (6) (2014) 702–715, 10.1158/2159-8290.CD-13-0945. [DOI] [PubMed] [Google Scholar]
- [16].Fros JJ, Domeradzka NE, Baggen J, Geertsema C, Flipse J, Vlak JM,Pijlman GP, Chikungunya Virus nsP3 blocks stress granule assembly by recruitment of G3BP into cytoplasmic foci, J. Virol 86 (19) (2012) 10873–10879, 10.1128/JVI.01506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fujimura K, Sasaki AT, Anderson P, Selenite targets eIF4E-binding protein-1 to inhibit translation initiation and induce the assembly of non-canonical stress granules, Nucl. Acids Res. 40 (16) (2012) 8099–8110, 10.1093/nar/gks566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fung G, Ng CS, Zhang J, Shi J, Wong J, Piesik P, et al. , Production of a dominant-negative fragment due to G3BP1 cleavage contributes to the disruption of mitochondria-associated protective stress granules during CVB3 infection, PLoS One 8 (11) (2013) e79546, 10.1371/journal.pone.0079546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ganassi M, Mateju D, Bigi I, Mediani L, Poser I, Lee HO, et al. , A surveillance function of the HSPB8-BAG3-HSP70 chaperone complex ensures stress granule integrity dynamism, Mol. Cell 63 (5) (2016) 796–810, 10.1016/j.molcel.2016.07.021. [DOI] [PubMed] [Google Scholar]
- [20].Gareau C, Houssin E, Martel D, Coudert L, Mellaoui S, Huot M-E, et al. , Characterization of fragile X mental retardation protein recruitment and dynamics in Drosophila stress granules, PLoS One 8 (2) (2013) e55342, 10.1371/journal.pone.0055342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gellert M, Lipsett MN, Davies DR, Helix formation by guanylic acid, PNAS 48(12) (1962) 2013–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grabocka E, Bar-Sagi D, Mutant KRAS enhances tumor cell fitness by upregulating stress granules, Cell 167 (7) (2016) 1803–1813.e12, 10.1016/j.cell.2016.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guo L, Kim HJ, Wang H, Monaghan J, Freyermuth F, Sung JC, et al. , Nuclear-import receptors reverse aberrant phase transitions of RNA-binding proteins with prion-like domains, Cell 173 (3) (2018) 677–692.e20, 10.1016/j.cell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hussey GS, Chaudhury A, Dawson AE, Lindner DJ, Knudsen CR, Wilce MCJ, et al. , Identification of an mRNP complex regulating tumorigenesis at the translational elongation step, Mol. Cell 41 (4) (2011) 419–431, 10.1016/j.molcel.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jackrel ME, Shorter J, Engineering enhanced protein disaggregases for neurode-generative disease, Prion 9 (2015) 90–109, 10.1080/19336896.2015.1020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, et al. , Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels, Cell 149 (4) (2012) 753–767, 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kedersha N, Anderson P, Mammalian stress granules and processing bodies, Methods Enzymol. 431 (2007) 61–81, 10.1016/S0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- [28].Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, Anderson P, Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules, Mol. Biol. Cell 13 (1) (2002) 195–210, 10.1091/mbc.01-05-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, et al. , Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules, J. Cell Biol. 151 (6) (2000) 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kedersha N, Ivanov P, Anderson P, Stress granules and cell signaling: more than just a passing phase? Trends Biochem. Sci 38 (10) (2013) 494–506, 10.1016/j.tibs.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kedersha N, Panas MD, Achorn CA, Lyons S, Tisdale S, Hickman T, et al. , G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits, J. Cell Biol. 212 (7) (2016) 845–860, 10.1083/jcb.201508028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, et al. , Stress granules and processing bodies are dynamically linked sites of mRNP remodeling, The J. Cell Biol. 871–884 (2005), 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Khong A, Matheny T, Jain S, Mitchell SF, Wheeler JR, Parker R, The stress granule transcriptome reveals principles of mRNA accumulation in stress granules, Mol. Cell 68 (4) (2017) 808–820.e5, 10.1016/j.molcel.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koromilas AE, Roles of the translation initiation factor eIF2α serine 51 phosphorylation in cancer formation and treatment, BBA 1849 (7) (2015) 871–880, 10.1016/j.bbagrm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- [35].Kwon S, Zhang Y, Matthias P, The deacetylase HDAC6 is a novel critical component of stress granules involved in the stress response, Genes Dev. 21 (24) (2007) 3381–3394, 10.1101/gad.461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lehtiniemi T, Kotaja N, Germ granule-mediated RNA regulation in male germ cells, Reprod. (Cambridge, England) 155 (2) (2018) R77–R91, 10.1530/REP-17-0356. [DOI] [PubMed] [Google Scholar]
- [37].Leung AKL, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P, Poly(ADPRibose) regulates stress responses and MicroRNA activity in the cytoplasm, Mol. Cell 42 (4) (2011) 489–499, 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lin Y, Protter DSW, Rosen MK, Parker R, Formation and maturation of phase-separated liquid droplets by RNA-binding proteins, Mol. Cell 60 (2) (2015) 208–219, 10.1016/j.molcel.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu-Yesucevitz L, Bassell GJ, Gitler AD, Hart AC, Klann E, Richter JD, et al. , Local RNA translation at the synapse and in disease, J. Neurosci.: Off. J. Soc. Neurosci 31 (45) (2011) 16086–16093, 10.1523/JNEUROSCI.4105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lyons SM, Gudanis D, Coyne SM, Gdaniec Z, Ivanov P, Identification of functional tetramolecular RNA G-quadruplexes derived from transfer RNAs, Nat. Commun 8 (1) (2017) 1127, 10.1038/s41467-017-01278-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Markmiller S, Soltanieh S, Server KL, Mak R, Jin W, Fang MY, et al. , Context-dependent and disease-specific diversity in protein interactions within stress granules, Cell 172 (3) (2018) 590–604.e13, 10.1016/j.cell.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Marrone L, Poser I, Casci I, Japtok J, Reinhardt P, Janosch A, et al. , Isogenic FUS-eGFP iPSC reporter lines enable quantification of FUS stress granule pathology that is rescued by drugs inducing autophagy, Stem Cell Rep. 10 (2) (2018) 375–389, 10.1016/j.stemcr.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mateju D, Franzmann TM, Patel A, Kopach A, Boczek EE, Maharana S, et al. , An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function, EMBO J. 36 (12) (2017) 1669–1687, 10.15252/embj.201695957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Matsuki H, Takahashi M, Higuchi M, Makokha GN, Oie M, Fujii M, Both G3BP1 and G3BP2 contribute to stress granule formation, Genes Cells 18 (2) (2012) 135–146, 10.1111/gtc.12023. [DOI] [PubMed] [Google Scholar]
- [45].Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, et al. , Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities, Neuron 93 (5) (2017) 1015–1034, 10.1016/j.neuron.2017.01.022. [DOI] [PubMed] [Google Scholar]
- [46].Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, et al. , Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization, Cell 163 (1) (2015) 123–133, 10.1016/j.cell.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Monahan Z, Ryan VH, Janke AM, Burke KA, Rhoads SN, Zerze GH, et al. , Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity, EMBO J. 36 (20) (2017) 2951–2967, 10.15252/embj.201696394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Namkoong S, Ho A, Woo YM, Kwak H, Lee JH, Systematic characterization of stress-induced RNA granulation, Mol. Cell 70 (1) (2018) 175–187.e8, 10.1016/j.molcel.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ng CS, Jogi M, Yoo JS, Onomoto K, Koike S, Iwasaki T, et al. , Encephalomyocarditis virus disrupts stress granules, the critical platform for triggering antiviral innate immune responses, J. Virol 87 (17) (2013) 9511–9522, 10.1128/JVI.03248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Oberstadt M, Stieler J, Simpong DL, Römuß U, Urban N, Schaefer M, et al. , TDP-43 self-interaction is modulated by redox-active compounds auranofin, chelerythrine and riluzole, Sci. Rep 8 (1) (2018) 2248–2262, 10.1038/s41598-018-20565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ohn T, Kedersha N, Hickman T, Tisdale S, Anderson P, A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly, Nat. Cell Biol. 10 (10) (2008) 1224–1231, 10.1038/ncb1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Oi N, Yuan J, Malakhova M, Luo K, Ryu J, Zhang L, et al. , Resveratrol induces apoptosis by directly targeting Ras-GTPase-activating protein SH3 domain-binding protein 1, Oncogene (2014), 10.1038/onc.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Onomoto K, Jogi M, Yoo J-S, Narita R, Morimoto S, Takemura A, et al. , Critical role of an antiviral stress granule containing RIG-I and PKR in viral detection and innate immunity, PLoS One 7 (8) (2012) e43031, 10.1371/journal.pone.0043031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Panas MD, Ahola T, McInerney GM, The C-terminal repeat domains of nsP3 from the Old World alphaviruses bind directly to G3BP, J. Virol 88 (10) (2014) 5888–5893, 10.1128/JVI.00439-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Panas MD, Schulte T, Thaa B, Sandalova T, Kedersha N, Achour A,McInerney GM, Viral and cellular proteins containing FGDF motifs bind G3BP to block stress granule formation, PLoS Pathog 11 (2) (2015) e1004659, 10.1371/journal.ppat.1004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Piotrowska J, Hansen SJ, Park N, Jamka K, Sarnow P, Gustin KE, Stable formation of compositionally unique stress granules in virus-infected cells, J. Virol 84(7) (2010) 3654–3665, 10.1128/JVI.01320-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Protter DSW, Parker R, Principles and properties of stress granules, Trends Cell Biol. 26 (9) (2016) 668–679, 10.1016/j.tcb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Reineke LC, Lloyd RE, Diversion of stress granules and P-bodies during viral infection, Virology 436 (2) (2013) 255–267, 10.1016/j.virol.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Reineke LC, Lloyd RE, The stress granule protein G3BP1 recruits PKR to promote multiple innate immune antiviral responses, J. Virol 89 (5) (2014) 2575–2589, 10.1128/JVI.02791-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Reineke LC, Cheema SA, Dubrulle J, Neilson JR, Chronic starvation induces noncanonical pro-death stress granules, J. Cell Sci. 131 (19) (2018) 220244–220254, 10.1242/jcs.220244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Reineke LC, Dougherty JD, Pierre P, Lloyd RE, Large G3BP-induced granules trigger eIF2α phosphorylation, Mol. Biol. Cell 23 (18) (2012) 3499–3510, 10.1091/mbc.E12-05-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Reineke LC, Kedersha N, Langereis MA, van Kuppeveld FJM, Lloyd RE, Stress granules regulate double-stranded RNA-dependent protein kinase activation through a complex containing G3BP1 and Caprin1, mBio 6 (2) (2015) e02486, 10.1128/mBio.02486-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Reineke LC, Tsai W-C, Jain A, Kaelber JT, Jung SY, Lloyd RE, Casein kinase 2 is linked to stress granule dynamics through phosphorylation of the stress granule nucleating protein G3BP1, Mol. Cell. Biol 37 (4) (2017), 10.1128/MCB.00596-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Remenyi R, Gao Y, Hughes RE, Curd A, Zothner C, Peckham M, et al. , Persistent replication of a chikungunya virus replicon in human cells is associated with presence of stable cytoplasmic granules containing non-structural protein 3, J. Virol (2018), 10.1128/JVI.00477-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sama RRK, Ward CL, Kaushansky LJ, Lemay N, Ishigaki S, Urano F,Bosco DA, FUS/TLS assembles into stress granules and is a prosurvival factor during hyperosmolar stress, J. Cell. Physiol 228 (11) (2013) 2222–2231, 10.1002/jcp.24395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sengupta MS, Boag PR, Germ granules and the control of mRNA translation, IUBMB Life 64 (7) (2012) 586–594, 10.1002/iub.1039. [DOI] [PubMed] [Google Scholar]
- [67].Shantanu S, Viljayalakshmi K, Shruthi S, Sagar CBK, Sathyaprabha TN,Nalini A, et al. , VEGF alleviates ALS-CSF induced cytoplasmic accumulations of TDP-43 and FUS/TLS in NSC-34 cells, J. Chem. Neuroanat 81 (2017) 48–52, 10.1016/j.jchemneu.2017.01.007. [DOI] [PubMed] [Google Scholar]
- [68].Shelkovnikova TA, Dimasi P, Kukharsky MS, An H, Quintiero A, Schirmer C, et al. , Chronically stressed or stress-preconditioned neurons fail to maintain stress granule assembly, Cell Death Dis. 8 (5) (2017) e2788, 10.1038/cddis.2017.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shim JH, Su ZY, Chae JI, Kim DJ, Zhu F, Ma WY, et al. , Epigallocatechin gallate suppresses lung cancer cell growth through Ras-GTPase-activating protein SH3 domain-binding protein 1, Cancer Prev. Res 3 (5) (2010) 670–679, 10.1158/1940-6207.CAPR-09-0185. [DOI] [PubMed] [Google Scholar]
- [70].Shorter J, Engineering therapeutic protein disaggregases, Mol. Biol. Cell 27 (10) (2016) 1556–1560, 10.1091/mbc.E15-10-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Smith HL, Mallucci GR, The unfolded protein response: mechanisms and therapy of neurodegeneration, Brain: J. Neurol 139 (Pt 8) (2016) 2113–2121, 10.1093/brain/aww101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Solomon S, Xu Y, Wang B, David MD, Schubert P, Kennedy D, Schrader JW, Distinct structural features of caprin-1 mediate its interaction with G3BP-1 and its induction of phosphorylation of eukaryotic translation initiation factor 2, entry to cytoplasmic stress granules, and selective interaction with a subset of mRNAs, Mol. Cell. Biol 27 (6) (2007) 2324–2342, 10.1128/MCB.02300-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Somasekharan SP, El-Naggar A, Leprivier G, Cheng H, Hajee S,Grunewald TGP, et al. , YB-1 regulates stress granule formation and tumor progression by translationally activating G3BP1, J. Cell Biol. 208 (7) (2015) 913–929, 10.1083/jcb.201411047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Soncini C, Berdo I, Draetta G, Ras-GAP SH3 domain binding protein (G3BP) is a modulator of USP10, a novel human ubiquitin specific protease, Oncogene 20 (29) (2001) 3869–3879, 10.1038/sj.onc.1204553. [DOI] [PubMed] [Google Scholar]
- [75].Standart N, Weil D, P-bodies: cytosolic droplets for coordinated mRNA storage, Trends Genet. 34 (8) (2018) 612–626, 10.1016/j.tig.2018.05.005. [DOI] [PubMed] [Google Scholar]
- [76].Thedieck K, Holzwarth B, Prentzell MT, Boehlke C, Kläsener K, Ruf S, et al. , Inhibition of mTORC1 by astrin and stress granules prevents apoptosis in cancer cells, Cell 154 (4) (2013) 859–874, 10.1016/j.cell.2013.07.031. [DOI] [PubMed] [Google Scholar]
- [77].Thomas MG, Tosar LJM, Desbats MA, Leishman CC, Boccaccio GL, Mammalian Staufen1 is recruited to stress granules and impairs their assembly, J. Cell Sci. 122 (4) (2009) 563–573, 10.1523/JNEUROSCI.3537-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tourriere H, The RasGAP-associated endoribonuclease G3BP assembles stress granules, J. Cell Biol. 160 (6) (2003) 823–831, 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [79].Tsai N-P, Wei L-N, RhoA/ROCK1 signaling regulates stress granule formation and apoptosis, Cell. Signal 22 (4) (2010) 668–675, 10.1016/j.cellsig.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tsai W-C, Gayatri S, Reineke LC, Sbardella G, Bedford MT, Lloyd RE, Arginine demethylation of G3BP1 promotes stress granule assembly, J. Biol. Chem 291 (43) (2016) 22671–22685, 10.1074/jbc.M116.739573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tsai W-C, Gayatri S, Reineke LC, Sbardella G, Bedford MT, Lloyd RE, Arginine demethylation of G3BP1 promotes stress granule assembly, J. Biol. Chem (2016), 10.1074/jbc.M116.739573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tsai WC, Reineke LC, Jain A, Jung SY, Lloyd RE, Histone arginine demethylase JMJD6 is linked to stress granule assembly through demethylation of the stress granule-nucleating protein G3BP1, J. Biol. Chem 292 (46) (2017) 18886–18896, 10.1074/jbc.M117.800706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Van Treeck B, Protter DSW, Matheny T, Khong A, Link CD, Parker R, RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome, Proc. Natl. Acad. Sci 115 (11) (2018) 2734–2739, 10.1073/pnas.1800038115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Vanderweyde T, Yu H, Varnum M, Liu-Yesucevitz L, Citro A, Ikezu T, et al. , Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies,J. Neurosci.: Off. J. Soc. Neurosci 32 (24) (2012) 8270–8283, 10.1523/JNEUROSCI.1592-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wheeler JR, Matheny T, Jain S, Abrisch R, Parker R, Distinct stages in stress granule assembly and disassembly, eLife 5 (2016) 875, 10.7554/eLife.18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].White JP, Cardenas AM, Marissen WE, Lloyd RE, Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase, Cell Host Microbe 2 (5) (2007) 295–305, 10.1016/j.chom.2007.08.006. [DOI] [PubMed] [Google Scholar]
- [87].Yagi R, Miyazaki T, Oyoshi T, G-quadruplex binding ability of TLS/FUS depends on the β-spiral structure of the RGG domain, Nucl. Acids Res. 46 (12) (2018)5894–5901, 10.1093/nar/gky391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Yang X, Hu Z, Fan S, Zhang Q, Zhong Y, Guo D, et al. , Picornavirus 2A protease regulates stress granule formation to facilitate viral translation, PLoS Pathog. 14 (2) (2018) e1006901, 10.1371/journal.ppat.1006901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yoo J-S, Takahasi K, Ng CS, Ouda R, Onomoto K, Yoneyama M, et al. , DHX36 enhances RIG-I signaling by facilitating PKR-mediated antiviral stress granule formation, PLoS Pathog. 10 (3) (2014) e1004012, 10.1371/journal.ppat.1004012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Youn J-Y, Dunham WH, Hong SJ, Knight JDR, Bashkurov M, Chen GI, et al. , High-density proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies, Mol. Cell 69 (3) (2018) 517–532.e11, 10.1016/j.molcel.2017.12.020. [DOI] [PubMed] [Google Scholar]
- [91].Zhang Y, Gaetano CM, Williams KR, Bassell GJ, Mihailescu MR, FMRP interacts with G-quadruplex structures in the 3’-UTR of its dendritic target Shank1 mRNA, RNA Biol. 11 (11) (2014) 1364–1374, 10.1080/15476286.2014.996464. [DOI] [PMC free article] [PubMed] [Google Scholar]