Abstract
Objectives:
Autoimmune encephalitis (AE) is increasingly recognized as an important cause of subacute cognitive decline, seizures, and encephalopathy, with an ever-broadening clinical phenotype. Sleep disturbances are reported in AE patients, including rapid eye movement sleep behavior disorder, hypersomnia, fragmented sleep, and sleep-disordered breathing; however, the prevalence of sleep disturbances and contributions to outcomes in AE patients remain unknown. There is a need to determine the prevalence of sleep disturbances in AE patients, and to clarify the relationship between specific autoantibodies and disruptions in sleep.
Methods:
Clinical history, results of serum and cerebrospinal fluid testing, electroencephalography, and neuroimaging were reviewed from 26 AE patients diagnosed and managed at our tertiary care hospital. Polysomnography was performed in patients with clinical indications, yielding data from 12 patients.
Results:
The median age of AE patients was 53 years (range, 18-83). Autoantibodies against intracellular antigens (including Ma and Hu autoantibodies) were identified in 6/26 (23%) patients, while autoantibodies against cell-surface neuronal antigens (including NMDAR and LGI1) were identified in 20/26 (77%) patients. New sleep complaints were reported by 19/26 (73%) AE patients, including gasping or snoring (9/19, 47%), dream enactment behavior (6/19, 32%), insomnia (5/19, 29%), hypersomnia (4/19, 21%), other parasomnias (4/19, 21%), and dream-wake confusional states (2/19, 11%). Dream enactment behaviors were particularly common in AE associated with LGI1 autoantibodies, reported in 4/7 (57%) patients. Polysomnography showed reduced total sleep time, stage 3 and rapid eye movement sleep, and prominent sleep fragmentation.
Conclusion:
Sleep disturbances are common in AE, warranting active surveillance in affected patients. Improved identification and treatment of sleep disorders may reduce morbidity associated with AE and improve long-term outcomes.
Keywords: autoimmune encephalitis, NMDAR encephalitis, LGI1 autoantibodies, REM-behavior disorder, restless legs, sleep apnea, polysomnography
Introduction
Autoimmune encephalitis (AE) is an important cause of subacute cognitive decline, seizures, and encephalopathy, with rising prevalence and a high potential for treatment-responsiveness [9, 11, 16]. As understanding of the clinical phenotype has expanded [30], so too has appreciation of the cognitive sequelae that persist in subsets of AE patients [12, 14, 18, 26, 37, 41]. The factors that contribute to persistent impairment are largely unknown, challenging efforts to develop efficacious treatments that improve long-term outcomes.
A myriad of sleep disturbances are reported in AE patients, including rapid eye movement (REM) sleep behavior disorder (in patients with antibodies against voltage-gated potassium channel complex antigens—now classified as antibodies against leucine-rich glioma-inactivated 1 [LGI1] or contactin-associated protein 2 [CASPR2] [6, 8, 19]), hypersomnia and fragmented sleep (in patients with Ma autoantibodies [22]), insomnia and periodic limb movements (in patients with dipeptidyl-peptidase-like protein-6 [DPPX] autoantibodies [42]), and sleep-disordered breathing (in patients with IgLON5 autoantibodies [15]). Although many sleep disorders manifest during the acute phase of the disease and resolve with appropriate treatment, others may persist long after inflammation is appropriately treated [4]. Whether persistent sleep disorders contribute to adverse outcomes following AE is unknown.
There is a clear need to define the prevalence and subtypes of sleep disturbances in AE patients, and to clarify the relationship between specific autoantibodies, sleep symptomatology, and outcomes. Recognizing this, we considered the frequency and subtypes of sleep disturbances in AE patients diagnosed and managed at our tertiary care center.
Methods
Standard Protocol Approvals, Registrations, Patient Consents
Clinical and diagnostic data, including serum and cerebrospinal fluid (CSF) test results, electroencephalography (EEG), and brain magnetic resonance imaging (MRI), were reviewed from 26 AE patients consecutively-encountered from July 2011 to May 2018 at our tertiary care center (Barnes-Jewish Hospital; Washington University School of Medicine; Saint Louis, Missouri). Patients were enrolled in prospective research studies, permitting continued collection and evaluation of active clinical symptoms and signs in patients with new diagnoses of AE, and retrospective review of existing medical records (including sleep symptomatology). Polysomnography (PSG) was performed in patients with active sleep complaints and a clinical indication. Study protocols were approved by the Washington University School of Medicine Human Research Protections Office. Written informed consent was obtained from all patients or their delegates.
Clinical Evaluation
Patients were admitted under the neurology inpatient service and thoroughly evaluated by experienced clinicians. Information concerning presentation and past history was obtained via interview of a reliable collateral source for all patients. All patients met criteria for probable or definite AE, including presentation with the subacute-onset of memory deficits, mental status change, or psychiatric symptoms; with at least one of new focal central nervous system findings, unexplained seizures, CSF pleocytosis, or MRI features suggestive of encephalitis; reasonable exclusion of alternative causes; and identification of disease-associated autoantibodies in CSF and/or serum [16]. Autoantibody testing was performed at the Mayo Clinic Neuroimmunology Laboratory (Rochester, Minnesota) using the proprietary Autoimmune or Paraneoplastic Autoantibody Evaluation panels. Both panels include indirect immunofluorescence assays, and are performed by applying specimen to frozen mouse composite tissue, washed and treated with fluorescein-conjugated IgG. Subsequent testing for specific disease-associated antibodies was performed when indicated (e.g., cell binding assays, Western blot, radioimmune assays).1 Ma1/Ma2 antibody testing was performed at Athena Diagnostics (Marlborough, Massachusetts) using a semi-quantitative automated nanoliter scale immunoassay.2 Investigations and treatments were ordered by treating clinicians, following discussion with the patient and collateral source.
Sleep Assessment
All patients were specifically asked about sleep complaints and premorbid sleep diagnoses at presentation. Collateral sources (bed partners when appropriate; otherwise family members) were additionally queried concerning sleep symptoms, including restless sleep, snoring or gasping, witnessed apneas; insomnia or hypersomnia; uncomfortable urge to move legs or limb movements during sleep; and parasomnias, including somniloquy, somnambulism, and dream enactment behavior. Hypersomnia was defined as excessive daytime sleepiness or prolonged sleep duration (periods greater than 10 hours). Insomnia was characterized by reported atypical or disruptive difficulty falling or staying asleep. Restless legs syndrome (RLS) was clinically diagnosed using the International RLS Study Group criteria, requiring an urge to move limbs usually associated with paresthesias or dysesthesias, symptoms that start or worsen with rest, at least partial relief of symptoms with physical activity, and worsening of symptoms in the evening or night [3]. Limb movements in sleep were frequent stereotyped movements in sleep as described by a reliable bed partner. Parasomnias were defined as complex behaviors in sleep, including talking, walking, goal-directed behaviors, or clear dream enactment behavior with dream recall. In the subset of patients who underwent PSG, a detailed sleep history was obtained by a board-certified sleep specialist.
Polysomnography was completed in 13 patients with clinical indications (i.e., symptoms/signs suggesting sleep related breathing disorders, or potentially injurious sleep-related behaviors, such as dream enactment behavior). In one patient, only 5 minutes of sleep time was recorded; data from this PSG was excluded from subsequent analyses. For the 12 remaining patients, PSG was performed a median of eight months following symptom onset (range 1-67 months). Two PSGs were performed within a month of symptom onset. Prior to PSG completion, all patients were screened for neurologically active medications known to affect PSG results (Supplemental Table 1). PSG was recoded and scored by a board-certified sleep medicine physician (GDB), in accordance with the American Academy of Sleep Medicine (AASM) criteria for the scoring of sleep and associated events in place at the time of scoring (Versions 2.0 to 2.3) [1, 2]. A desaturation threshold of 4% was used for scoring hypopneas across all studies in accordance with acceptable parameters as outlined in Chapter VIII, Part 1: Rules for Adults, Category D, Section 1B [1, 2]. Predetermined PSG outcome measures included total sleep time, sleep efficiency, percentage of time in sleep stages, periodic limb movement index, presence of REM without atonia (defined according to visually scored AASM criteria using electromyography leads placed on the chin and the lower extremities), and the presence and severity of sleep-disordered breathing. Observed patient total sleep time was compared with normative data for the human sleep cycle, measured in cognitively normal, healthy adults and published in aggregate [32].
Statistical analysis
Clinical data were analyzed using SPSS Statistics (IBM Corp., Version 24.0. Armonk, NY). Continuous and categorical measures were compared using the Mann-Whitney Utest, and Fisher’s exact test, respectively. The relationship between the frequency of sleep disturbances and age was evaluated using univariate linear regression. Statistical significance was defined as p<0.05.
Results
Participant Characteristics and Clinical Findings
Aggregate demographic details, clinical characteristics, and results of investigations are presented in Table 1 for AE patients (see Supplemental Table 1 for case-by-case details). Disease severity varied across patients; 10 patients (38%) required admission to an intensive care unit, most often for management of respiratory failure (9/10, 90%). The median length of hospital stay was 15 days (range, 3-87 days). Sixteen patients (16/26, 62%) required readmission for resurgent or relapsing AE; of these, 12 were readmitted within 30 days of initial discharge.
Table 1:
Demographic features, clinical characteristics, and results of investigations in patients with autoimmune encephalitis.
| Participant characteristics (n=26) | Finding | |
|---|---|---|
| Demographic Features | ||
| Median age, y (range) | 53 (18-83) | |
| Female sex, n (%) | 12 (46) | |
| Education, y (range) | 12 (10-16) | |
| Race, n (%) | ||
| White, non-Hi spanic | 21 (81) | |
| Back or African-American | 4 (15) | |
| Hispanic | 1 (4) | |
| Clinical Findings, n (%) | ||
| Cognitive deficits (memory, confusion) | 23 (88) | |
| Psychosis, mood, or personality changes | 17 (65) | |
| Seizures | 11 (42) | |
| Ataxia | 7 (27) | |
| Involuntary movements (dystonia, tremor, myoclonus) | 6 (23) | |
| Results of Investigations | ||
| Autoantibody detected, n (%) | 25 (96) | |
| Intracellular | Ma1/Ma2 | 2 (8) |
| Yo | 1 (4) | |
| Hu | 3* (12) | |
| CRMP5 | 1* (4) | |
| Cell surface | LGI1 | 7 (27) |
| NMDAR | 9* (35) | |
| AMPA† | 2 (8) | |
| GABA | 1 (4) | |
| VGCC | 1 (4) | |
| Cerebrospinal fluid findings | ||
| Median nucleated cells (range) | 6.5 (0-619) | |
| >5 nucleated cells, n (%) | 13 (50) | |
| Median protein mg/dl (range) | 40 (17-484) | |
| >4 CSF-specific oligoclonal bands, n (%) | 8 (31) | |
| Disease-associated tumor detected, n (%) | 7 (27) | |
| T2 hyperintensities on MRI, n (%) | 18 (69) | |
| Temporal lobe/hippocampal, n (%) | 7 (39) | |
| Occipital lobe, n (%) | 2 (11) | |
| Frontal lobe, n (%) | 1 (6) | |
| Basal ganglia, n (%) | 3 (17) | |
| Cerebellum, n (%) | 2 (11) | |
| Brainstem, n (%) | 2 (11) | |
| Thalamus, n (%) | 1 (6) | |
Disease-associated autoantibodies were detected in the CSF (52%), serum (24%), or CSF and serum (24%) of 25 patients (25/26, 96%). Six patients (6/26, 23%) had antibodies to intracellular antigens, including Ma1/Ma2, Yo (Purkinje cell cytoplasmic antibody type 1), Hu (type 1 anti-neuronal nuclear antibody), and CRMP5 (collapsin response-mediator protein-5). Autoantibodies against cell-surface antigens were identified in 20 patients (20/26, 77%), including LGI1 (leucine-rich glioma-inactivated protein 1), NMDAR (N-Methyl-D-aspartate receptor), AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid— case details previously published [24]), and GABA (gamma-aminobutyric acid). One patient had antibodies against intracellular (Hu and CRMP5) and cell-surface antigens (NMDAR) [23]. Voltage-gated calcium channel antibodies were identified in the serum of one patient, with unclear pathogenic relevance (0.60 nmol/L; brain MRI with bilateral temporal lobe T2 hyperintense lesions). The remaining patient presented with subacute cognitive decline, seizures, and hallucinations (Case 26, Supplemental Table 1). Subsequent evaluation revealed CSF leukocytosis and unilateral occipital T2 hyperintensities on brain MRI Deficits resolved following treatment with steroids, with no alternative etiology identified, fulfilling diagnostic criteria for definite AE [16].
Magnetic resonance neuroimaging and CSF analyses were performed in all patients over the course of their admission. Brain MRI was abnormal in 18 patients (69%), with T2/FLAIR hyperintensities predominating. Contrast-enhancing lesions were detected in three patients (3/18, 17%). Other findings included diffusion restriction of the cortical ribbon (in one patient with anti-NMDAR encephalitis), mild generalized atrophy (in one patient with LGI1 antibody-associated encephalitis), and cerebellar atrophy (in one patient with cerebellar degeneration associated with Yo antibodies). Cerebrospinal fluid was abnormal in 21 patients (81%). Leukocytosis (>5 nucleated cells/HPF) was the most common finding. Greater than four CSF-specific oligoclonal bands were observed in the CSF of 8/26 (31%) patients.
Disease-associated malignancies were detected in seven patients (7/26, 27%), including ovarian teratomas in two patients with anti-NMDAR encephalitis, renal cell carcinoma and a regressed germ cell carcinoma in one patient each with encephalitis associated with Ma1 and Ma2 antibodies, ovarian carcinoma in a patient with paraneoplastic cerebellar degeneration (associated with Yo antibodies), and small cell lung cancer in two patients with paraneoplastic encephalitis associated with Hu antibodies.
Sleep Disturbances
Nineteen patients or their collateral sources (73%) complained of new or worsened sleep disturbances, most commonly gasping or snoring or witnessed apneas, followed by dream enactment behavior (Table 2). Two patients had pre-existing diagnoses, including RLS (Case 8) and obstructive sleep apnea (OSA; Case 7, Supplemental Table 1). Sleep disturbances emerged concurrent with symptoms of AE in all patients. No association was detected between the prevalence of new or worsened sleep disturbances and age (univariate linear regression, OR=1.03, [95% CI, 0.98-1.07], p=0.26), gender (female [8/12, 67%] vs. male [11/14, 79%]; p=0.67), or antibody class (cell-surface [13/19, 68%] vs. intracellular autoantibodies [5/6, 93%]; p=0.64). Table 3 summarizes new or worsened sleep symptoms, stratified by disease-associated autoantibodies. Dream enactment behavior was particularly common in AE associated with LGI1 autoantibodies, with complex nocturnal movements reported in 4/7 (57%) patients.
Table 2:
Reported new or worsened sleep symptoms in AE patients.
| Sleep disturbance (n=19) | Frequency, n (%) |
|---|---|
| Gasping or snoring | 9 (47) |
| Dream enactment | 6 (32) |
| Insomnia | 5 (26) |
| Restless sleep | 5 (26) |
| Witnessed apnea | 5 (26) |
| Hypersomnia | 4 (21) |
| Somnambulism, somniloquy | 4 (21) |
| Limb movements during sleep | 3 (16) |
| Restless legs symptoms* | 2 (11) |
| Dream-wake confusion | 2 (11) |
serum ferritin 8 ng/ml for one patient and results unavailable for another
Table 3:
Reported new or worsened sleep symptoms in AE patients, stratified by disease-associated autoantibody.
| Autoantibody | Reported Sleep Symptom, n (%) |
|---|---|
| AMPA | Any symptom, 2 (100) |
| Hypersomnia, 1 (50) | |
| Insomnia, 1 (50) | |
| Snoring or gasping, 1 (50) | |
| CRMP5* | Any symptom, 1 (100) |
| Insomnia | |
| GABA | Any symptom, 1 (100) |
| Hypersomnia | |
| Snoring or gasping, witnessed apnea | |
| Hu* | Any symptom, 3 (100) |
| Insomnia, 1 (33) | |
| Witnessed apnea, 2 (66) | |
| LGI1 | Any symptom, 6 (86) |
| Dream enactment 4 (57) | |
| Restless sleep, 3 (43) | |
| Somniloquy, 3 (43) | |
| Snoring or gasping, 2 (29) | |
| Insomnia, 2 (29) | |
| Limb movements during sleep, 2 (29) | |
| Somnambulism, 1 (14) | |
| Dream-wake confusion, 1 (14) | |
| Restless legs symptoms, 1 (14) | |
| Vivid dreams, 1 (14) | |
| Ma1/Ma2 | Any symptom, 1 (50) |
| Hypersomnia | |
| Insomnia | |
| Limb movements during sleep | |
| Restless legs symptoms | |
| Snoring or gasping | |
| NMDAR* | Any symptom, 5 (56) |
| Dream enactment, 2 (22) | |
| Insomnia, 2 (22) | |
| Snoring or gasping, witnessed apnea 2 (22) | |
| Dream-wake confusion, 1 (11) | |
| Restless sleep, 1 (11) | |
| Somniloquy, 1 (11) | |
| VGCC | Any symptom, 0 |
| Yo | Any symptom, 1 (100) |
| Hypersomnia |
One patient presented with AE associated with Hu, CRMP5, and NMDAR autoantibodies [23]
Polysomnography
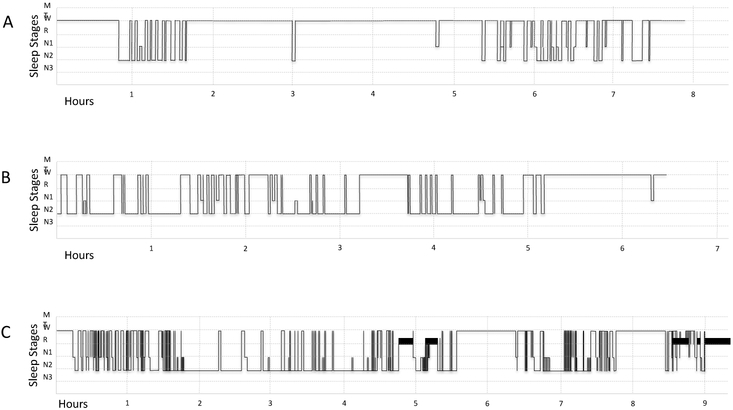
Overnight PSG results are reported in Table 4 for the twelve AE patients who underwent formal evaluation. Total recorded sleep time was highly variable, ranging from 78 to 395 minutes. Sleep fragmentation was frequently observed (Figure 1). Seven participants (7/12, 58%) had total sleep time below the range of values reported in age-matched healthy sleepers [32]. Identifiable sleep architecture, including sleep spindles and K complexes, was present in 11/12 patients (one patient clinically appeared to be sleeping). Stage I and II sleep predominated in the majority of participants. Stage III sleep was absent in 10/12 (83%) patients, while REM sleep was absent in 4/12 (33%) patients (Supplemental Table 2). These findings distinguished patients with AE from normal adults, in whom stage III and REM sleep was absent in less than 5% of age-similar reference populations [32].
Table 4:
Polysomnography in AE patients. Values are median and range of all available data.
| PSG Findings, n=12 | Median (range) | |
|---|---|---|
| AE Cohort | Healthy Sleepers | |
| Median Total Sleep Time, minutes | 223 (78-395) | 403 (165-458) |
| Median % N1 sleep | 3.85 (2-14.1) | 9.7 (3.4-35.4) |
| Median % N2 sleep | 44.95 (6.8-69.1) | 45.5 (17.7-59.3) |
| Median % N3 sleep | 0 (0-11.2) | 17.4 (0-37.5) |
| Median % REM sleep | 10.55 (0-25.1) | 13.6 (1.0-25.6) |
| Median Sleep Efficiency % | 60.7 (17.7-96.9) | 83.7 (34.4-95.3) |
| Median Apnea-Hypopnea Index | 3 (0.2-41.6) | 1.7 (0-25) |
| Frequency, n (%) | ||
| Elevated Apnea-Hypopnea Index | 5 (42) | |
| Mild (AHI 5-15) | 3 (25) | |
| Mod (AHI 15-30) | 0 | |
| Severe (AHI >30)* | 2 (17) | |
| REM without atonia | 3 (25) | |
| Dream enactment behavior | 2 (17) | |
| Periodic limb movement >15/hour | 3 (25) | |
median body mass index (BMI) for patients with OSA 30.4 (range 26.7-39.9) and without OSA 33.9 (range 21.3-38.6)
AHI= apnea hypopnea index; N1=stage I sleep, N2=stage II sleep, N3=stage III sleep, and REM= rapid eye movement sleep. Normative values from healthy sleepers as reported in Mitterling et al., (2014) [32].
Figure 1:
Sleep fragmentation in AE. Tracings of representative sleep stages from three AE patients. A: 52 year-old woman with AE associated with NMDAR antibodies (Case 20), B: 66 year-old man with Hu antibodies (Case 6), C: 83 year-old woman with LGI1 antibodies (Case 8). The x-axis depicts sleep study duration (hours). The y-axis depicts sleep stages: movement time (MT), wake (W), rapid eye movement sleep (R), stage I sleep (N1), stage II sleep (N2), and stage III sleep (N3).
Other abnormal findings included OSA in five patients (5/12, 42%) and elevated periodic limb movement index (>15/hour) in three patients (3/12, 25%), representing new diagnoses in all cases. In patients with an elevated AHI, no seizure activity was detected on EEG leads during PSG. Four of six patients with suspected dream enactment behaviors underwent PSG, including 2/4 patients with LGI1 autoantibodies. PSG demonstrated REM without atonia in three patients: two with LGI1 autoantibodies, and one with NMDAR autoantibodies, establishing the diagnosis of REM sleep behavior disorder. Multiple sleep-onset latency testing was consistent with narcolepsy in one patient with Ma1/Ma2 antibodies (mean sleep latency 3.74 minutes, sleep onset REM periods 2/5). Another patient with autoantibodies against Hu, NMDAR, and CRMP5 had a clinical presentation suggestive of agrypnia excitata with absence of normal sleep architecture on PSG (previously reported [23]).
Patient Outcome
All patients were treated with immunomodulatory therapies, including steroids, IVIg, plasmapheresis, and/or rituximab. Four patients (4/19, 21%) died of complications of their illness (median time from symptom onset to death, 5.5 months; range, 4-57), with three patients undergoing post-mortem neuropathological assessment (Cases 4, 6, and 8; Supplemental Table 2). Of the 15 surviving patients, 14 completed at least one outpatient follow-up visit, providing an opportunity to assess outcomes (median duration of follow-up, 27 months; range 9-76). Sleep complaints completely resolved following treatment in 10/14 (71%) patients (Supplemental Table 2). Four of six (67%) patients with dream enactment behavior reported improvement following treatment. Patients also reported improvement in snoring or gasping and witness apneas (5/9, 56%), insomnia (3/5, 60%), restless sleep (3/5, 60%), hypersomnia (2/4, 50%), somniloquy or somnambulism (2/4, 50%), limb movements during sleep (1/3, 33%), and dream-wake confusion (1/2, 50%). The remaining four patients (29%) endorsed persistent sleep disturbances of varying intensity, including three patients with excessive daytime sleepiness (of these, one patient with OSA was non-compliant with recommendations for continuous positive airway pressure, and one patient was actively receiving chemotherapy for the treatment of ovarian cancer).
Discussion
Seventy-three percent of patients diagnosed with AE at our center endorsed new or worsened sleep disturbances: most commonly gasping or snoring, dream enactment behavior, and insomnia. This finding suggests that sleep disruptions are common in AE patients. Dream enactment behaviors were especially common in patients with AE associated with LGI1 autoantibodies, while narcolepsy was diagnosed in one patient with Ma1/Ma2 autoantibodies. Taken together with prior reports [10, 15, 19, 36], these findings suggest that specific autoantibodies may connote increased risk of specific sleep disturbances. If confirmed in larger cohorts, these observations would provide insight into both the clinical manifestations of AE, and the neurological underpinnings that mediate sleep function and dysfunction.
The importance of consolidated stage III sleep to memory and attentional function is well recognized in animals [17, 33] and humans [5, 7, 39], with impaired stage III sleep predicting impaired memory and attention. Additionally, emergent literature suggests that sleep disruption may contribute to the clinical and pathological manifestations of neurodegenerative diseases, including Alzheimer disease [20, 28, 34], Parkinson disease [13, 27], and human prionopathies [21]. Disruptions in sleep architecture were apparent in AE patients who underwent PSG in our cohort, with prominent decreases in sleep efficiency, loss of stage III and REM sleep, and sleep fragmentation compared to age-similar healthy adults. This finding raises the possibility that sleep disturbances may contribute to the impairments in memory and attention that are increasingly reported in recovering AE patients [18, 26, 31, 41, 44].
In addition to abnormalities in sleep architecture, treatable sleep disorders were frequently recognized in AE patients, including OSA and RLS. Beyond cognitive deficits seen in untreated OSA [43], sleep disordered breathing adversely affects cardiac and respiratory function [25, 29, 38]. Thus, untreated OSA could contribute to autonomic dysfunction that is common in several subtypes of AE, complicating efforts to wean from cardiorespiratory support in mechanically ventilated patients, prolonging requirement for sedating medications and hospital stay, and exposing patients to additional risk. This cohort was not large enough to compare the relative frequency of RLS and OSA with that expected in other populations; thus, it is unknown whether RLS and OSA is more common in AE patients than would be expected in cognitively normal healthy individuals, and patients with other neurological disorders [40].
Six patients reported complex movements in sleep consistent with dream enactment. REM behavior disorder typically occurs in older patients, and may herald the emergence of neurodegenerative diseases associated with pathological aggregation of alpha synuclein (i.e., idiopathic Parkinson disease, Dementia with Lewy bodies, and multiple systems atrophy) [35]. In our cohort, dream enactment behaviors were reported in patients across the age spectrum (median 49.5 years; range 19-83), including three patients younger than 40 years. Symptoms resolved in 4/6 of these patients following AE treatment (two patients with persistent sleep disturbances following treatment had AE associated with LGI1 autoantibodies). Although the duration of follow-up was not sufficient to exclude the possibility that surviving patients may yet present with symptoms and signs characteristics of disorders associated with aggregates of alpha synuclein, parkinsonian signs were not detected at follow-up in any patient. Furthermore, no alpha-synuclein aggregates were reported on post-mortem examination in the brains of any of the three AE patients who died and underwent autopsy (including an 88-year-old patient with prior history of AE associated with LGI1 autoantibodies and dream enactment behavior; Case 8, Supplemental Table 2). These findings add to a growing literature suggesting that REM sleep behavior disorder may arise as a consequence of AE [6, 8, 15, 19]. The mechanisms underlying this phenomenon are poorly understood.
Taken together, these observations justify active screening for sleep symptoms in AE patients, acknowledging that prompt recognition and treatment of sleep disturbances may improve acute and long-term outcomes. In addition, the high prevalence of disruptions in sleep architecture on PSG suggests that clinicians should maintain a low threshold for requesting formal PSG, in the interest of better defining sleep disorders in patients recovering from AE (including the prevalence of asymptomatic disruptions in sleep architecture).
Although sleep complaints were prevalent amongst AE patients, access to a relatively small cohort limited our ability to consider the association between sleep disturbances and specific autoantibodies. In addition, PSG was completed only in individuals with clinical indications, and was performed a median of 8 months following diagnosis (range 1-67)— after initiation of immunotherapy. As a result, our findings likely underestimated the prevalence of sleep disruption in AE patients, particularly in the acute period. Immunotherapy itself may also modify PSG findings. Finally, as clinically available antibody testing was completed in patients, emergent antibodies, evaluated only in research settings, may have gone undetected. The clinical expression of sleep disturbances are expected to vary with AE subtype, with the frequency and type of sleep disturbance dictated by the specificity and end-results of antibody-antigen interactions, and distribution of the antigenic target within the central nervous system. Prospective studies enrolling larger cohorts of newly diagnosed AE patients with active screening for sleep complaints and routine PSG are needed to clarify the prevalence, spectrum of sleep dysfunction, association between specific sleep disturbances and subtypes of AE, and indications for PSG in this unique patient population. Additional studies should delineate the evolution of sleep disorders in this population over time. Indeed longitudinal follow-up will be required to assess the effect of sleep dysfunction on rates of recovery, and to evaluate the effects of treatment of associated sleep dysfunction on meaningful longer-term outcomes in recovering AE patients.
Conclusions
Sleep disturbances were commonly encountered in AE patients assessed at our center, including dream enactment behavior and poor sleep efficiency. These findings emphasize the importance of screening for sleep disturbances in AE patients. Improved detection and treatment of sleep disorders may reduce morbidity associated with AE, promoting better long-term outcomes in recovering patients.
Supplementary Material
Acknowledgements
Funding was provided by the American Academy of Neurology/American Brain Foundation (Clinical Research Training Fellowship to GSD), and via philanthropic contributions from patients and family members to promote research and education into Autoimmune Encephalitis (GSD).
Abbreviations:
- AASM
American Academy of Sleep Medicine
- AE
autoimmune encephalitis
- AHI
apnea hypopnea index
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CRMP5
collapsin response-mediator protein-5
- CSF
cerebrospinal fluid
- EEG
electroencephalography
- GABA
gamma-aminobutyric acid
- Hu
type 1 anti-neuronal nuclear antibody
- LGI1
leucine-rich glioma-inactivated protein 1
- MRI
magnetic resonance imaging
- N1
stage I sleep
- N2
stage II sleep
- N3
stage III sleep
- NMDAR
N-methyl-D-aspartate receptor
- PSG
polysomnography
- REM
rapid eye movement
- RLS
restless legs syndrome
- VGCC
voltage-gated calcium channel
- Yo
Purkinje cell cytoplasmic antibody type 1
Footnotes
Compliance with ethical standards: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflicts of interest: On behalf of all authors, the corresponding author states that there are no conflicts of interest. Dr. Blattner has no disclosures to report. Dr. de Bruin has equity in Neuroquestions, LLC. Dr. Bucelli receives an annual gift from a patient's family for Parsonage-Turner research; served on an advisory board for MT Pharma; and has equity in Neuroquestions, LLC. Dr. Day has served as a topic editor on dementia for DynaMed Plus (EBSCO Industries, Inc) and as clinical director for the Anti-NMDA Receptor Encephalitis Foundation (uncompensated). He receives research/grant support from The American Academy of Neurology/American Brain Foundation, Avid Radiopharmaceuticals, the Foundation for Barnes Jewish Hospital, and the National Institutes of Health (P01AG03991, R56AG057195, U01AG057195) and holds stock in ANI Pharmaceuticals, Inc. Dr. Day has provided record review and expert medical testimony on legal cases pertaining to management of Wernicke encephalopathy.
Details available via the Mayo Clinic website:https://www.mayocliniclabs.com/test-catalog/Overview.
Details available via the Athena Diagnostics website: https://www.athenadiagnostics.com/view-full-catalog/r/recombx-trade;-mata-autoantibody-test-(1)
References:
- 1.The American Academy of Sleep Medicine (2012) The AASM Manual for the Scoring of Sleep and Associated Events. Version 2.0. [Google Scholar]
- 2.The American Academy of Sleep Medicine (2016) The AASM Manual for the Scoring of Sleep and Associated Events. Version 2.3. [Google Scholar]
- 3.Allen R, Picchietti D, Garcia-Borreguero D, Ondo W, Walters A, Winkelman J, Zucconi M, Ferri R, Trenkwalder C, Lee H, Group IRLSS (2014) Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Medicine 15:860–873 [DOI] [PubMed] [Google Scholar]
- 4.Anderson KN, Kelly TP, Griffiths TD (2013) Primary sleep disorders can cause long-term sleep disturbance in patients with autoimmune mediated limbic encephalitis. Clin Neurol Neurosurg 115:1079–1082 [DOI] [PubMed] [Google Scholar]
- 5.Barnes DC, Wilson DA (2014) Slow-wave sleep-imposed replay modulates both strength and precision of memory. J Neurosci 34:5134–5142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barone DA, Krieger AC (2016) Sleep disturbances in voltage-gated potassium channel antibody syndrome. Sleep Med 21:171–173 [DOI] [PubMed] [Google Scholar]
- 7.Cairney SA, Ashton JE, Roshchupkina AA, Sobczak JM (2015) A Dual Role for Sleep Spindles in Sleep-Dependent Memory Consolidation? J Neurosci 35:12328–12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelius JR, Pittock SJ, McKeon A, Lennon VA, Aston PA, Josephs KA, Tippmann-Peikert M, Silber MH (2011) Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol 68:733–738 [DOI] [PubMed] [Google Scholar]
- 9.Dalmau J, Graus F (2018) Antibody-Mediated Encephalitis. N Engl J Med 378:840–851 [DOI] [PubMed] [Google Scholar]
- 10.Dauvilliers Y, Bauer J, Rigau V, Lalloyer N, Labauge P, Carlander B, Liblau R, Peyron C, Lassmann H (2013) Hypothalamic immunopathology in anti-Ma-associated diencephalitis with narcolepsy-cataplexy. JAMA Neurol 70:1305–1310 [DOI] [PubMed] [Google Scholar]
- 11.Dubey D, Pittock SJ, Kelly CR, McKeon A, Lopez-Chiriboga AS, Lennon VA, Gadoth A, Smith CY, Bryant SC, Klein CJ, Aksamit AJ, Toledano M, Boeve BF, Tillema JM, Flanagan EP (2018) Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol 83:166–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finke C, Pruss H, Heine J, Reuter S, Kopp UA, Wegner F, Then Bergh F, Koch S, Jansen O, Munte T, Deuschl G, Ruprecht K, Stocker W, Wandinger KP, Paul F, Bartsch T (2017) Evaluation of Cognitive Deficits and Structural Hippocampal Damage in Encephalitis With Leucine-Rich, Glioma-Inactivated 1 Antibodies. JAMA Neurol 74:50–59 [DOI] [PubMed] [Google Scholar]
- 13.Fyfe I (2015) Sleep disorder deficits suggest signature for early Parkinson disease. Nature Reviews Neurology 12:3–3 [DOI] [PubMed] [Google Scholar]
- 14.Gadoth A, Pittock SJ, Dubey D, McKeon A, Britton JW, Schmeling JE, Smith A, Kotsenas AL, Watson RE, Lachance DH, Flanagan EP, Lennon VA, Klein CJ (2017) Expanded phenotypes and outcomes among 256 LGI1/CASPR2-IgG-positive patients. Ann Neurol 82:79–92 [DOI] [PubMed] [Google Scholar]
- 15.Gaig C, Graus F, Compta Y, Hogl B, Bataller L, Bruggemann N, Giordana C, Heidbreder A, Kotschet K, Lewerenz J, Macher S, Marti MJ, Montojo T, Perez-Perez J, Puertas I, Seitz C, Simabukuro M, Tellez N, Wandinger KP, Iranzo A, Ercilla G, Sabater L, Santamaria J, Dalmau J (2017) Clinical manifestations of the anti-IgLON5 disease. Neurology 88:1736–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Hoftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Pruss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostasy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15:391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hars B, Hennevin E (1987) Impairment of learning by cueing during postlearning slow wave sleep in rats. Neurosci Lett 79:290–294 [DOI] [PubMed] [Google Scholar]
- 18.Hebert J, Day GS, Steriade C, Wennberg RA, Tang-Wai DF (2018) Long-Term Cognitive Outcomes in Patients with Autoimmune Encephalitis. Can J Neurol Sci 45:540–544 [DOI] [PubMed] [Google Scholar]
- 19.Iranzo A, Graus F, Clover L, Morera J, Bruna J, Vilar C, Martinez-Rodriguez JE, Vincent A, Santamaria J (2006) Rapid eye movement sleep behavior disorder and potassium channel antibody-associated limbic encephalitis. Ann Neurol 59:178–181 [DOI] [PubMed] [Google Scholar]
- 20.Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, Fagan AM, Mignot E, Zempel JM, Claassen J, Holtzman DM (2017) Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain 140:2104–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang P, de Bruin GS, Wang LH, Ward BA, Ances BM, Lim MM, Bucelli RC (2016) Sleep Pathology in Creutzfeldt-Jakob Disease. J Clin Sleep Med 12:1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura N, Kawajiri M, Ohyagi Y, Minohara M, Murai H, Kira J (2005) [A patient with paraneoplastic limbic encephalitis induced by breast cancer presenting with hypersomnia]. Rinsho Shinkeigaku 45:575–578 [PubMed] [Google Scholar]
- 23.Kim AE, Kang P, Bucelli RC, Ferguson CJ, Schmidt RE, Varadhachary AS, Day GS (2018) Autoimmune Encephalitis With Multiple Autoantibodies: A Diagnostic and Therapeutic Challenge. Neurologist 23:55–59 [DOI] [PubMed] [Google Scholar]
- 24.Laurido-Soto O, Brier MR, Simon LE, McCullough A, Bucelli RC, Day GS (2018) Patient characteristics and outcome associations in AMPA receptor encephalitis. J Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CH, Khoo SM, Tai BC, Chong EY, Lau C, Than Y, Shi DX, Lee LC, Kailasam A, Low AF, Teo SG, Tan HC (2009) Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest 135:1488–1495 [DOI] [PubMed] [Google Scholar]
- 26.Long JM, Day GS (2018) Autoimmune Dementia. Seminars in Neurology 38:303–315 [DOI] [PubMed] [Google Scholar]
- 27.Louter M, Aarden WC, Lion J, Bloem BR, Overeem S (2012) Recognition and diagnosis of sleep disorders in Parkinson's disease. J Neurol 259:2031–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucey BP, McCullough A, Landsness EC, Toedebusch CD, McLeland JS, Zaza AM, Fagan AM, McCue L, Xiong C, Morris JC, Benzinger TLS, Holtzman DM (2019) Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer's disease. Sci Transl Med 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marin JM, Carrizo SJ, Vicente E, Agusti AGN (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–1053 [DOI] [PubMed] [Google Scholar]
- 30.McKeon A (2016) Autoimmune Encephalopathies and Dementias. Continuum (Minneap Minn) 22:538–558 [DOI] [PubMed] [Google Scholar]
- 31.McKeon GL, Robinson GA, Ryan AE, Blum S, Gillis D, Finke C, Scott JG (2018) Cognitive outcomes following anti-N-methyl-D-aspartate receptor encephalitis: A systematic review. J Clin Exp Neuropsychol 40:234–252 [DOI] [PubMed] [Google Scholar]
- 32.Mitterling T, Hogl B, Schonwald SV, Hackner H, Gabelia D, Biermayr M, Frauscher B (2015) Sleep and Respiration in 100 Healthy Caucasian Sleepers--A Polysomnographic Study According to American Academy of Sleep Medicine Standards. Sleep 38:867–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molle M, Eschenko O, Gais S, Sara SJ, Born J (2009) The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci 29:1071–1081 [DOI] [PubMed] [Google Scholar]
- 34.Musiek ES, Xiong DD, Holtzman DM (2015) Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med 47:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson EJ, Boeve BF, Silber MH (2000) Rapid eye movement sleep behaviour disorder: Demographic, clinical and laboratory findings in 93 cases. Brain 123:331–339 [DOI] [PubMed] [Google Scholar]
- 36.Overeem S, Dalmau J, Bataller L, Nishino S, Mignot E, Verschuuren J, Lammers GJ (2004) Hypocretin-1 CSF levels in anti-Ma2 associated encephalitis. Neurology 62:138–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peer M, Pruss H, Ben-Dayan I, Paul F, Arzy S, Finke C (2017) Functional connectivity of large-scale brain networks in patients with anti-NMDA receptor encephalitis: an observational study. Lancet Psychiatry 4:768–774 [DOI] [PubMed] [Google Scholar]
- 38.Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342:1378–1384 [DOI] [PubMed] [Google Scholar]
- 39.Saxvig IW, Lundervold AJ, Gronli J, Ursin R, Bjorvatn B, Portas CM (2008) The effect of a REM sleep deprivation procedure on different aspects of memory function in humans. Psychophysiology 45:309–317 [DOI] [PubMed] [Google Scholar]
- 40.Sivathamboo S, Farrand S, Chen Z, White EJ, Pattichis AA, Hollis C, Carino J, Roberts CJ, Minogue T, Jones NC, Yerra R, French C, Perucca P, Kwan P, Velakoulis D, O'Brien TJ, Goldin J (2019) Sleep-disordered breathing among patients admitted for inpatient video-EEG monitoring. Neurology 92 [DOI] [PubMed] [Google Scholar]
- 41.Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J (2013) Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 12:157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobin WO, Lennon VA, Komorowski L, Probst C, Clardy SL, Aksamit AJ, Appendino JP, Lucchinetti CF, Matsumoto JY, Pittock SJ, Sandroni P, Tippmann-Peikert M, Wirrell EC, McKeon A (2014) DPPX potassium channel antibody: Frequency, clinical accompaniments, and outcomes in 20 patients. Neurology 83:1797–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verstraeten E (2007) Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep 7:161–166 [DOI] [PubMed] [Google Scholar]
- 44.Yeshokumar AK, Gordon-Lipkin E, Arenivas A, Cohen J, Venkatesan A, Saylor D, Probasco JC (2017) Neurobehavioral outcomes in autoimmune encephalitis. J Neuroimmunol 312:8–14 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.