Abstract
Zinc has been described as the “calcium of the twenty-first century.” Zinc-based degradable biomaterials have recently emerged thanks to their intrinsic physiological relevance, biocompatibility, biodegradability, and pro-regeneration properties. Zinc-based biomaterials mainly include metallic zinc alloys, zinc ceramic nanomaterials, and zinc metal-organic frameworks (MOFs). Metallic zinc implants degrade at a desirable rate, matching the healing pace of local tissues, and stimulating remodeling and formation of new tissues. Zinc ceramic nanomaterials are also beneficial for tissue engineering and therapy thanks to their nanostructures and antibacterial properties. MOFs have large surface areas and are easily functionalized, making them ideal for drug delivery and cancer therapy. This review highlights recent developments in zinc-based biomaterials, discusses obstacles to overcome, and pinpoints directions for future research.
Keywords: Zinc, Biometal, Biodegradable, Bioresorbable, Nanomaterials, Tissue Engineering
Metallic Biomaterials for Regeneration and Therapy
Conventional metallic biomaterials, including titanium alloys, stainless steels, and cobalt-chromium alloys, have been widely used clinically for load bearing hard tissue reconstruction and regeneration. They have superior mechanical properties, machinability and durability, but are considered non-degradable, and long-term clinical complications may occur which necessitates a second removal surgery [1]. To overcome such drawbacks, novel biodegradable metals have been developed to degrade gradually in vivo while promoting complete healing of local tissues. Thus, the subsequent removal surgery is avoided [2].
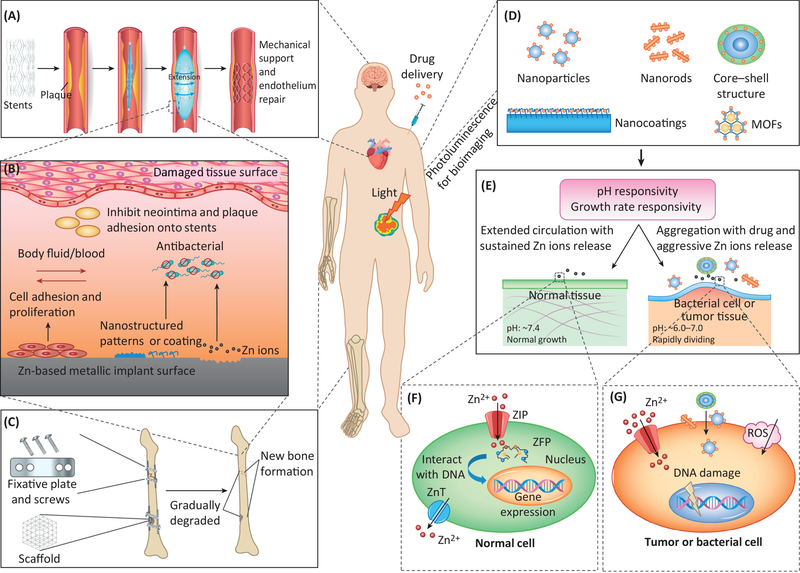
The three main biodegradable metals are magnesium (Mg), Zinc (Zn), and iron (Fe). Mg materials generally degrade too quickly (within 1–4 months) and are accompanied by harmful hydrogen gas evolution. Fe materials typically degrade too slowly (over 2–3 years), and the degradation products are retained in tissues for a long time. Zn materials have degradation rates between those of Mg and Fe, and their degradation products are fully bioresorbable without hydrogen gas evolution [3]. Ionic Zn has also been described as ‘the calcium of the twenty-first century’ because of the increasing awareness of its significant functional roles in physiological and biological systems [4]. Therefore, Zn is a better choice for biodegradable metallic materials than Mg and Fe, with a better in vivo biodegradation rate and biocompatibility for tissue regeneration and therapy (Figure 1, Key Figure).
Figure 1.
Key Figure: Zn-based biomaterials and their in vivo interactions with different tissues and cells for tissue regeneration and therapy. (A) Metallic Zn-based coronary stents provide mechanical support to the vascular wall and help repair the endothelium by removing plaque to avoid thrombosis and stent restenosis. (B) In vivo interactions of a Zn-based metallic implant surface with damaged tissues: an ideal nanostructured pattern or coating on the surface, and the release of Zn ions from the degradation process, may promote cellular adhesion and proliferation while inhibiting the adhesion of bacterial cells or other subjects (such as smooth muscle cells and plaque for a stent). (C) Metallic Zn-based orthopedic implants (fixative plates, screws and porous scaffolds) provide temporary mechanical support for bone tissue regeneration in a parallel process of implant biodegradation and new bone formation. (D) Nano-structured Zn-based ceramic and organic biomaterials provide high surface/volume ratios for drug delivery and excellent photoluminescence for in vivo bioimaging. (E) Nanostructured Zn-based ceramic and organic biomaterials have sensitive responses to pH and cell growth rate, differentiating their circulation or aggregation behaviors on normal tissues, bacterial cell or tumor tissues. (F) Relatively low concentrations of Zn ions have no adverse, and sometime beneficial, effects on normal cells. (G) High concentrations of Zn ions, aggressive Zn ion release, cellular surface aggregation of Zn-based nanomaterials, and induced ROS can damage the cell surface and DNA in tumors or bacterial cells.
Zn-based ceramic nanomaterials have been extensively applied in the emerging field of theranostics, including drug delivery, tissue targeting, bioimaging, and cancer therapy [5–7] (Figure 1). They have unique physical and chemical properties, such as large surface/volume ratio, photoluminescence, antibacterial activity, good biocompatibility, and pH-responsive nanostructure. Zn-based organic biomaterials, mainly metal-organic frameworks (MOFs) (see Glossary), also have large surface/volume ratios and pH responsiveness, making them promising materials for drug delivery, bioimaging, and cancer therapy [8].
Zn-based biomaterials have attracted increasing attention recently, but articles summarizing their features, applications, and potential are still infrequently published [9, 10]. We provide a timely overview on Zn-based biomaterials, briefly covering the biological roles of Zn in mammalian systems as well as describing the properties and biological performance of various Zn-based biomaterials and the challenges and perspectives in this rapidly developing field.
Physiological Role of Zn
Zn is the second most abundant micronutrient in living organisms [11] and is fundamental to cell biology, human anatomy, and physiology. It is necessary for hundreds of enzymatic reactions, affecting development, maturation, proper immune function [12], numerous disease states, and cancer. In humans, average daily zinc intake is 4–14 mg/day, and normal plasma levels range from 70–120 μg/dL, whereas plasma levels < 60 μg/dL are considered low [13]. Zn deficiency can be observed in growth failure, but Zn toxicity is rarely a concern as ingestion of 10 times the recommended daily dose leads to few symptoms [13].
Zinc finger proteins (ZFPs) and Zn receptors (Box 1), including Zn transporters (ZnTs) and Zrt/Irt-like proteins (ZIPs), are the predominant cellular transport mechanisms for Zn ions. ZFPs represent the largest and most diverse family of DNA binding transcription factors as they can bind to DNA, RNA, and other proteins with high specificity. ZnTs, ZIPs, and ZFPs have demonstrated relevance in important aspects of health ranging from epigenetic changes via DNA methylation [14, 15] to development [15–18], cancer progression/repression [19–27], bone remodeling/mineralization, and atherosclerosis.
Box 1. Cellular Handling of Zn.
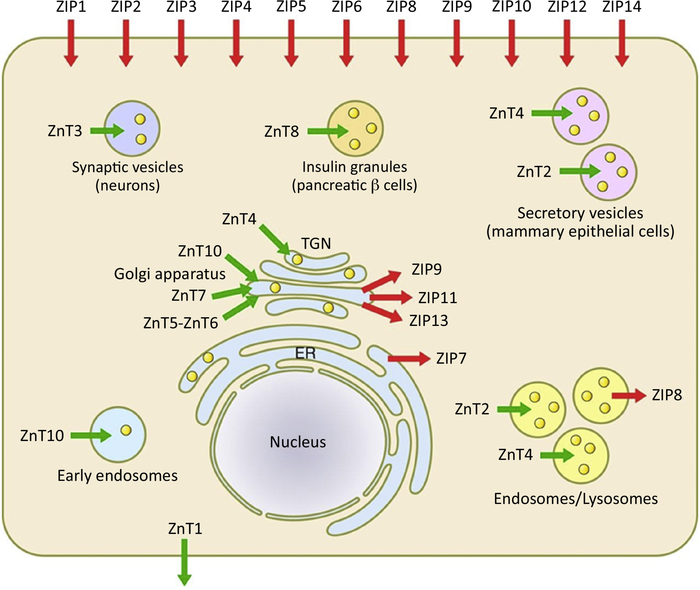
Zn is essential for all cellular functions and has been identified in over 23,000 human proteins [19]. Human adults contain approximately 2–3 grams of Zn; 60% is stored in skeletal muscle, 30% is stored in bone, 5% in the liver and skin, and the remaining 2–3% is located in other tissues [19]. ~80% of serum zinc is bound to albumin and 20% to α2-macroglobulin. Severe Zn deficiency manifests as anemia, growth retardation, hypogonadism, and mental exhaustion [19]. It does not require a redox reaction like iron or copper, so the precise localization of ZnTs and ZIPs is crucial to proper maintenance of Zn. 10 ZnTs [20], known collectively as the solute carrier family (SLC30), have been identified as responsible for transporting Zn out of the cytosol into the extracellular space or into lumens of intracellular structures such as the Golgi apparatus, endosomes, or synaptic/secretory vesicles (Figure I) [11]. ZnT1 is primarily localized to the plasma membrane. There are 14 ZIP transporters located at the phospholipid bilayer responsible for the cellular uptake of Zn. ZIP1 is expressed universally in the human body and closely regulates Zn hemostasis. In response to increased cytosolic Zn, metallothionein (MT) protein expression surges to lower the intracellular Zn concentration within appropriate bounds. ZnT and ZIPs are accountable for maintaining functional levels of Zn and protecting the cell from excess influx of Zn, such as during ischemia or during times of Zn deficiency.
Zn finger proteins (ZFPs) can bind to DNA, RNA, and, to a lesser extent, proteins with high specificity. They represent the largest and most diverse family of DNA binding transcription factors. The most characterized and well know is the C2H2 class, which comprises ~ 700 human proteins, 50% of which contain a Krüppel-associated box (KRAB) domain [21]. Their function is largely unknown, but a few have been shown to suppress transposable elements in embryonic stem cells (ES) through cofactor tripartite motif-containing 28 (TRIM28) [21, 22]. ZFP binding to DNA induces conformational and methylation changes in regulatory regions, which influence epigenetic mechanisms and lead to gene silencing or transcription. The eight classes of ZFP include Class 1 Cys2His2 (C2H2), Gag knuckle, Treble clef, Zn ribbon, Zn2/Cy6, TAZ2 domain like, Zn binding loops, and MT [23].
Zn plays a pivotal role in preventing and treating various pathophysiological conditions including cardiovascular, skeletal, and neurological diseases, as well as cancer. One example is atherosclerosis, the most prevalent cardiovascular disease; it is characterized by breakdown in endothelial cell integrity, and Zn can exert cardioprotection through preserving the endothelium [28, 29]. In addition, Zn is required for bone formation and mineralization, stimulating osteoblasts while inhibiting bone-resorbing osteoclasts [30]. To take advantage of the therapeutic features of Zn in various disorders, Zn can be functionalized and incorporated into numerous biomaterials (Figure 1), such as biodegradable stents or porous bone implants, to support, nourish, and stimulate regeneration of damaged blood vessels and new bone formation. Furthermore, Zn nanomaterials and Zn-MOFs can be manipulated to specifically target cancer cells or tissues with overexpressed cancer-specific proteins in a slightly acidic microenvironment, act as bioimaging contrasts, and deliver therapeutic agents (Figure 1). Given these impressive attributes, combined with its fundamental physiological roles, Zn is quickly becoming a primary choice for biomedical applications.
Zinc-based Biodegradable Metallic Biomaterials
Degradation and Mechanical Properties
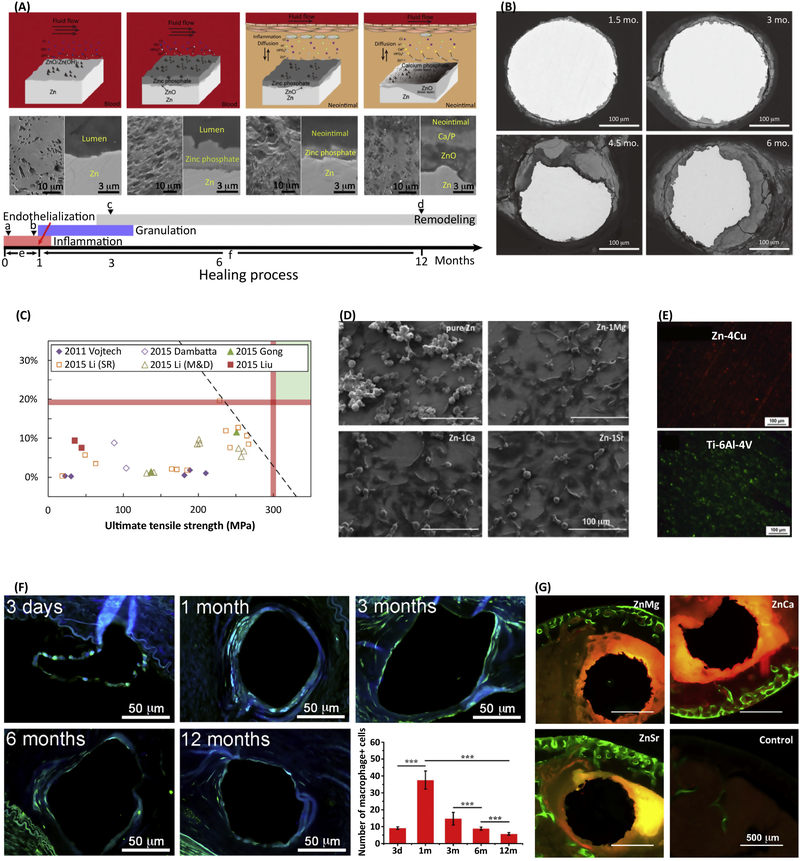
Zn has been recently proposed as a novel biodegradable metal thanks to its essential physiological and biological roles (as discussed above) and its promising in vivo degradation rate [3]. The standard electrode potential of Zn (−0.76 V/SCE) is between that of Mg (−2.37 V/SCE) and Fe (−0.44 V/SCE), so the degradation rate of Zn is slower than that of Mg but faster than that of Fe. It has been clinically demonstrated that Mg alloys degrade too quickly and Fe alloys degrade too slowly [2], but the behavior of Zn alloys is more likely in line with clinical demand. For example, the in vivo degradation behavior of a pure Zn stent in a rabbit abdominal aorta model showed localized corrosion (Figure 2A) [31]. In another study, implants into the abdominal aorta of a Sprague-Dawley rat (Figure 2B) exhibited uniform corrosion with degradation rates of ~0.05 mm/year after 6 months [3, 32], which is promising for biodegradable cardiac stents. The degradation products mainly included insoluble Zn-based compounds, including ZnO, Zn(OH)2, Zn3(PO4)2·4H2O [3, 31]. In addition, Ca2+ from body fluids could react with zinc phosphate and precipitate as calcium phosphate or Zn-doped calcium phosphate [31, 33]. These compounds have lower solubility in aqueous solution and may detached from implants together with substrate particles, which could be resolved or degraded in the physiological environment.
Figure 2.
In vitro and in vivo performance and potential clinical applications of Zn-based biodegradable metals. (A) Schematic diagrams showing the degradation mechanism of zinc stents associated with the conversion of degradation microenvironments during the healing process, including the formation of zinc phosphate under dynamic flow conditions in blood fluid and its conversion to ZnO and calcium phosphate [31]. (B) Representative backscattered electron images of cross-sectional areas from a pure Zn wire explant after 1.5, 3, 4.5, and 6 months in the abdominal aorta of an adult male Sprague-Dawley rat [3]. (C) A comparison of experimental Zn-based biodegradable metals with approximate mechanical benchmarks (red lines) [10]. (D) Cell morphologies adhered on pure Zn and Zn-1X (X = Mg, Ca, Sr) alloy after one day of culture [41]. (E) Biofilm formation on Zn-4Cu alloy compared to Ti-6Al-4V after incubation with S. aureus for 1 day, illustrated by live/dead staining [36]. (F) Representative immunofluorescence staining images of macrophage antibody during implantation and the corresponding number of macrophages per strut [31]. (G) Representative histology of cross-sections of a mouse distal femoral shaft from Zn-1Mg, Zn-1Ca, and Zn-1Sr implanted pins and a sham control group observed under fluorescent microscopy at week 8 post-implant, with green fluorescence indicating new bone formation [41]. Images reproduced with permission from the indicated references.
The mechanical properties of Zn and its alloys in comparison to biodegradable stent benchmarks are summarized in Figure 2C. Compared to Mg alloys and Fe alloys, one of the main factors limiting the extensive clinical application of Zn and its alloys is their lower mechanical strength [10]. While it can be improved through alloying with different elements (such as Mg, Ca, Sr, Mn, Cu, or Li) and plastic deformation processing techniques [34], low mechanical strength still remains an important challenge, and more effort is required to obtain a series of alloys with reproducible mechanical strength and ductility. These techniques can also affect the degradation behavior of Zn-based alloys [35, 36].
Localized corrosion is easily induced at the interface of the surrounding Zn matrix because of galvanic coupling. Due to homogenization, wrought alloys exhibit more uniform corrosion behavior than cast alloys and thus superior corrosion resistance. Nevertheless, the effects of various post-treatments for specific alloys on their mechanical and degradation behaviors are still largely unknown. The mechanical integrity during the in vivo degradation process should be closely monitored.
In vitro Biocompatibility
The in vitro cytocompatibility of pure Zn shows interesting results [32, 37, 38], which could possibly result from biphasic cellular responses of different Zn ion concentrations [39, 40]. This is a concentration-dependent phenomenon whereby low concentrations of Zn ion are beneficial to cells, while high concentrations are harmful. Zn-1X (X = Ca, Sr, Mg) alloys significantly improved cell viability and proliferation compared to pure Zn (Figure 2D) [4143]. To test the in vitro biocompatibility of biodegradable metallic materials, an extraction medium diluted by a factor of 6–10 for is recommended to obtain reliable and consistent results [44]. A surface oxide film is important for the degradation and biocompatibility behavior of Zn and its alloys [45]. Surface modifications with collagen [37], pre-incubation in simulated body fluid (SBF) [46] or cell culture medium [47] was also suggested for designing in vitro experiments. Therefore, surface modification could be a new strategy to provide programmable biocompatibility and tunable degradation rates [48]. Moreover, the surfaces of orthopedic and dental implants should ideally inhibit bacterial colonization and concomitantly promote osteoblast functions through its interactions with proteins, bacteria, and cells. For example, a Zn-Cu alloy inhibits biofilm formation better than pure Zn or pure Ti (Figure 2E) [36].
In vivo Biocompatibility
When biodegradable metallic implants corrode, the released degradation products ideally should not induce local or systemic toxicity, but instead promote remodeling and healing of local tissues. A recent study demonstrated there was no severe inflammation (Figure 2F), platelet aggregation, thrombosis formation, or obvious intimal hyperplasia observed during the first 12 months of implantation of Zn in the abdominal aorta of rabbits [31]. In another study, Zn-1X (X = Mg, Ca, Sr) alloys were implanted in femoral shaft from the distal femur of 3-month old C57BL/6 mice to evaluate the tissue compatibility [41]. Figure 2G shows representative histological cross-sections of implanted pins under fluorescent microscopy. Compared to the control group, the Zn-1X alloys promoted more new bone formation at the periosteum, with the Zn-1Sr alloy exhibiting the most promising osteogenic potential [41]. In contrast, the Zn-Al alloys induced intense inflammatory response due to a high density of mononuclear cells present at the tissue interface surrounding the implants in the abdominal aorta wall of adult Sprague-Dawley rats [49].
Cardiovascular Therapy
Zn plays critical roles in maintaining cardiac function, and Zn deficiency is associated with cardiovascular disease [50]. As described previously, the mechanical integrity and good biocompatibility of pure zinc wire provide strong evidence for its promising potential in cardiovascular stents [3, 31]. Although the mechanical strength of pure Zn might be insufficient for a biodegradable stent (Figure 2C) [3, 9, 10], extruded Zn-Cu alloys and hot rolled Zn-Mn-Cu and Zn-Li alloys have sufficient mechanical strength and elongation to meet clinical requirements [43, 51, 52]. Another issue is potentially unstable behavior during in vivo degradation, which was assumed to be influenced by the reendothelialization process, leading to potentially harmful localized degradation (Figure 2A). However, uniform degradation was also observed during 6 months of implantation in vivo (Figure 2B) [3]. Clearly, more in vivo studies in larger animals for a longer term are needed to fully ascertain the potential of zinc biomaterials for cardiovascular stent applications.
Orthopedic Regeneration
Zn ion is well-known for its roles in bone growth through promoting osteoblast and chondrocyte differentiation (cells responsible for new bone generation) while inhibiting osteoclast (cells responsible for bone absorbance) differentiation and resorption [30]. Pure Zn has insufficient strength to act as a load-bearing orthopedic implant (natural bone has a compressive yield strength of 130 – 180 MPa), but its strength can be significantly improved through alloying. There are many options for fabricating wrought Zn-based alloys, with different alloying elements, including Mg, Ca, Sr, Li, and Cu, beneficial to improve the mechanical properties and bone health [41, 53, 54]. Nonetheless, there are so far few reports on the in vivo implantation of Zn-based alloys in orthopedic applications. The feasibility of novel Zn-hydroxyapatite (HA) composites was explored recently in the form of orthopedic implants with tunable degradation rates, enhanced bone formation ability, and effective antibacterial properties [55]. Compared with Mg and its alloys, the higher density and slower degradation rates of Zn make it more suitable as porous scaffolds for bone, tooth, cartilage, tendon, and spinal regeneration, with potentially more functionalization possibilities (e.g., for drug loading and delivery).
Zinc-based Ceramic Biomaterials
The richness of structures and properties of the Zn-based nanostructure family endows these materials with diverse functionalities. Zn-based ceramic materials, including zinc oxide (ZnO), zinc sulphide (ZnS), zinc selenide (ZnSe), zinc phosphate (Zn3(PO4)2), and zinc aluminate (ZnAl2O4), have been extensively explored in a variety of biomedical applications [7, 56]. The major methods, characteristics, and biomedical applications of Zn based ceramic biomaterials are summarized (Table 1) and discussed in the following sections [57–75].
Table 1.
Major methods, characteristics and biomedical applications of Zn based ceramic biomaterials.
| Category | Methods | Characteristics | Potential biomedical applications | Refs |
|---|---|---|---|---|
| ZnO | Hydrothermal Sol-gel Vapor–liquid–solid Physical vapor deposition Chemical vapor deposition Biosynthesis |
Easy fabrication Antibacterial pH-responsive Large surface/volume ratio Wide band gap Efficient excitonic blue and near-UV emission Phototoxic effect Good biocompatibility |
Orthopedic regeneration Drug and gene delivery Bioimaging Cancer therapy |
[57] [58, 59] [60–63] [64] |
| ZnS | Hydrothermal/ solvothermal One pot synthesis Sol-gel Ultrasonic assisted Microwave assisted |
Antibacterial Wide band gap Highly luminescent High transmittance in the visible region High thermal and photochemical stabilities Large surface/volume ratio Good biocompatibility |
Drug delivery Bioimaging Cancer therapy |
[65, 66] [67, 68] [69] |
| ZnSe | Hydrothermal/ solvothermal One pot synthesis Solid state reaction Ultrasonic assisted Microwave assisted Chemical vapor deposition |
Wide band gap Characteristic emission profile in the UV-blue region High thermal and photochemical stabilities Good biocompatibility |
Bioimaging | [70, 71]. |
| Zn3(PO4)2 | Hydrothermal Biomimetic Solid state reaction Chemical conversion coating |
Good corrosion resistance Excellent biocompatibility |
Orthopedic regeneration | [72, 73] |
| ZnAl2O4 | Hydrothermal Sol-gel Ultrasonic assisted Microwave assisted |
Wide band gap High thermal and chemical stability Low sintering temperature High quantum yields Excellent biocompatibility |
Orthopedic regeneration Bioimaging | [74] [75] |
Antibacterial and Biocompatibility
The Zn2+ ions released from Zn-based ceramic materials could potentially interact with bacterial surfaces, altering charge balance and inducing cell deformation and bacteriolysis [76]. Theoretically, all Zn-based biodegradable materials can potentially have antibacterial abilities. Apart from these antibacterial mechanisms, photocatalytic and nano-antibiotic mechanisms predominate through generation of reactive oxygen species (ROS) and specific nanostructures, respectively [56]. Therefore, nano-structured Zn-based ceramic materials, especially the photocatalytic ZnO and ZnS, exhibit unique antibacterial properties. The cytotoxicity of ZnO nanoparticles to various mammalian cell types is mainly due to increased intracellular Zn2+ ions as a result of ZnO solubility. Other Zn-based nanoparticles or quantum dots may also have similar issues under certain physicochemical and environmental conditions [77]. However, other nanostructures (e.g. nanowires, nanorods) or thin films have been reported with significantly improved cytocompatibility [78, 79], especially when composited with other inorganic and organic agents [80], which is another potential direction for Zn-based nanomaterials.
Skeletal Repair and Regeneration
Due to their inherent antibacterial properties, Zn-based nanomaterials have attracted much attention, but there are few reports for tissue regeneration applications so far. However, incorporating Zn in various biomaterials, including calcium phosphates [81], bioactive glasses [82], and titanium dioxide (TiO2) [30, 57] could greatly enhance bone formation through inhibiting osteoclasts while stimulating osteoblastic differentiation. TiO2 has been extensively studied as a biocompatible coating on Ti implants for orthopedic regeneration [83], but TiO2 nanoparticles can induce significant oxidative DNA damage and apoptosis in human liver cells even at a concentration of 1 μg/ml [84]. Nanostructured ZnO/TiO2 hybrid nanofibers show enhanced cell adhesion, proliferation, and spreading behavior (Figure 3B) [57]. This provides a new promising direction for biodegradable coatings with Zn-based ceramic biomaterials. For example, Zn3(PO4)2 and ZnAl2O4 coatings have demonstrated excellent cytocompatibility [73, 74]. In addition, because of the released Zn ions, Zn-containing composites could potentially provide antibacterial properties for orthopedic implants to decrease or eliminate bacterial infections and the subsequent complications in the orthopedic surgery.
Figure 3.
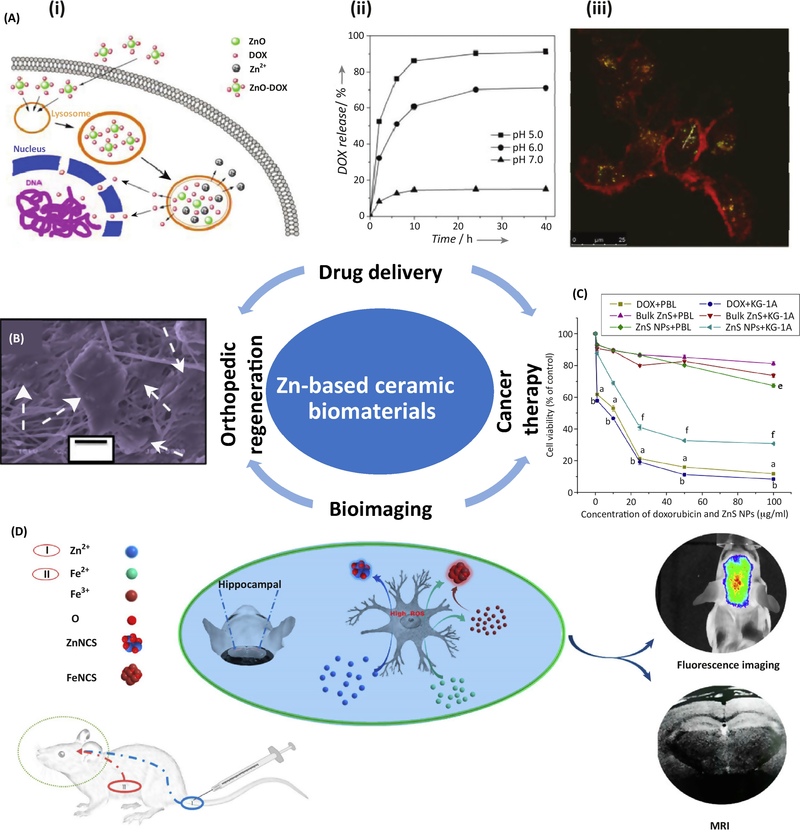
Potential clinical applications of Zn-based ceramic biomaterials. (A) Multifunctional ZnO@polymer–DOX composites for drug delivery: (i) schematic delivery process into the cells, (ii) drug release profiles at different pH values, and (iii) CLSM images of U251 cells after 3 h incubation with the composites and lysotracker. The green emission is from the lysotracker; the red emission is from the composites [58]. (B) SEM images of the cellular spread pattern (white arrows) of C2C12 cells after 72h incubation on electrospun ZnO/TiO2 nanofibers [57]. (C) Cell viability assay of peripheral blood lymphocytes (PBL) and KG-1A cell (leukemic cells) when cultured with DOX, bulk zinc sulfide powder, and ZnS nanoparticles for 24 h [69]. (D) Scheme illustration of in vivo dual-modality bioimaging of modeled Alzheimer’s mice brains through biosynthesized zinc and iron oxide via the combined injection of ferrous chloride solution post-stomach and zinc gluconate solution post-tail vein [63]. Images reproduced with permission from the indicated references
Drug Delivery
Nanoparticles have been studied extensively as drug delivery systems due to their outstanding merits (e.g. high stability, big surface area, and easy fabrication and incorporation) to target specific cells and control drug release in different microenvironments [85]. ZnO and ZnS nanoparticles have been studied as a type of pH-responsive drug carrier to target tumor cells, which have significantly lower pH values than normal cells and tissues [5, 58]. Traditionally, ZnO and ZnS nanoparticles or quantum dots have been used as cappers to cover the pores of mesoporous silica nanoparticles (MSNs) or one component in the nanocomposites [58, 65, 86]. Because of the adsorption and non-degradability of MSNs, nanocomposites are preferred for drug delivery systems. In vitro and in vivo active tumor targeting is possible using microwave-assisted synthesized water-dispersed NIR CdTe/CdS/ZnS CSS QDs with a novel core-shell-shell structure [86]. Figure 3A shows the performance of a core-shell-structured ZnO@polymer-DOX nanocomposite with degradability, rapid pH response, and stable luminescence in an aqueous solution. This system ensures the stability and accuracy of the drug release, is safe for normal tissues, and can be monitored during the drug delivery process (Figure 3A) [58].
Bioimaging
The inherent photoluminescent property and good compatibility of Zn-based ceramic nanomaterials make them superior bioimaging agents over traditional organic fluorescent dyes and commonly used CdSe and CdTe QDs. Therefore, most of the Zn-based nanomaterials in Table 1, including ZnO, ZnS, ZnSe, and ZnAl2O4, have been studied for their bioimaging applications. Surface modification using certain polymeric or silica ligands to form a core– shell or core-shell-shell structure is a key method to achieve chemical stability and high photoluminescent quantum yield in aqueous solutions, especially circulating blood [56]. In in vivo animal imaging, because the animal body cannot be penetrated by UV excitation when using ZnO QDs, a dual modal imaging ZnO-based nanoprobe incorporated with radionuclide (e.g. 64Cu) or rare-earth elements (e.g. Gd3+ and Yb3+) was used to achieve good tumor targeting and image contrast [56]. Similar results were found with ZnS, ZnSe, and ZnAl2O4 QDs doped with Ag, In, and Cr [67, 68], but the biosafety of these doping elements is a concern. Thus, Fe and Mn could be alternatives with improved biocompatibility [63, 71]. In vivo bioimaging of Alzheimer’s disease was also achieved through targeted bio-labeling fluorescent ZnO nanoclusters, which are biosynthesized and specifically accumulate in the hippocampus [62]. Similar results with high spatiotemporal dual-modality bioimaging (i.e., magnetic resonance and fluorescence imaging) were obtained in a subsequent study through the combined injection of ferrous chloride solution post-stomach and Zn gluconate solution post-tail vein into Alzheimer’s model mice, as shown in Figure 3D [63].
Cancer Therapy
One of the timeliest clinical applications for drug delivery and bioimaging is cancer therapy. A key goal is to construct a synergistic nanoplatform for combined cancer targeting, bioimaging, and responsive drug delivery [87]. Multifunctional ZnO@polymer-DOX nanocomposites (Figure 3A) [58], including surface modifications with targeting moieties (e.g. folic acid [88]) are typical examples. In addition to smart drug delivery nanocarriers and bioimaging agents, ZnO and ZnS based composite nanoparticles show a strong preference to kill tumor cells [64, 69]. Different cell types and their manner of proliferation could influence the cytotoxic and genotoxic properties of Zn-based ceramic nanoparticles which induce generation of higher levels of ROS in rapidly dividing cells compared to non-cancerous cells [89]. The selective cytotoxic effect of ZnS nanoparticles was observed in leukemic cells and human normal lymphocytes, resulting from the different internalization of Zn2+ ions in these two cells, as shown in Figure 3C [69]. Similar selective anti-proliferative performance of ZnO nanoparticles were observed in a coculture mode of C2C12 myoblastoma cancer cells and 3T3-L1 normal cells [64].
Zinc Organic Biomaterials
Metal organic frameworks (MOFs) are being utilized in many biotech applications, including drug delivery systems, bioimaging, and cancer therapy [8, 90]. High surface area and pore volume in combination with facile modification and chemical functionalization have made them attractive tools in biomedical engineering. Zeolithic imidazolate MOF-8 (ZIF-8) formulated for cancer therapy and tested on HepG2 cells exhibited slow release profiles (< 15% over 24 hours) under neutral conditions and accelerated release (>50%) when under pH 6.0 [91]. Similarly, temperature and pH responsive Zn-MOFs increase drug release with increasing temperature and pH. MOFs can also be used as coatings to regenerate bone in tissue engineering applications. For example nano ZIF-8 thin films have been applied to surfaces of porous titanium to improve osteogenic activity and antibacterial effect [92].
Concluding Remarks and Future Perspectives
The significant physiological and biochemical roles of zinc have been illustrated by a great number of studies describing its biological functions, health implications, and pharmacological targets. The progressive understanding of multifunctional Zn could facilitate the development of Zn-based biomaterials. Compared to Mg and Fe, Zn-based alloys have more desirable degradation characteristics for cardiovascular therapy and orthopedic regeneration. Zn-based ceramic biomaterials, in the form of nanoparticles, coatings, or other nanomaterials, are promising in the emerging field of theranostics, including drug delivery, tissue targeting, bioimaging, and cancer therapy. In addition, other Zn-containing nanomaterials and organic materials (e.g., MOFs) show various biofunctional properties and have great potential as “smart materials” for drug delivery, bioimaging, and cancer therapy.
Inspired by the promising results of Zn alloys in bone fixation and vascular stents, more Zn-based implants or devices could be developed for other clinical applications. For example, Zn wire seems to be suitable for fixation of comminuted fractures and tension fixation in intercondylar fractures as well as medical sutures in wound closure. Zn-based staples/clips are also good candidates for gastrointestinal anastomosis applications. Additionally, highly porous Zn-based scaffolds filled with different soft materials containing growth factors and cells could be useful as bone graft substitutes for segmental or large bone defects.
Based on the clinical requirements for controllable degradation rate, prolonged stability and excellent biocompatibility, discovering and implementing new strategies to improve the targeting, mechanical features, degradation pace, or biocompatibility will enhance the usefulness of Zn-based biomaterials for regeneration and therapy (see Outstanding Questions). Reproducible and improved mechanical profiles of Zn-based alloys could be obtained by proper alloying and post-treatments, and changes to the mechanical integrity in vivo during degradation could be modeled by in vitro tests in appropriate simulated body fluids under dynamic conditions. Surface modifications, composites, or porous structures could be applied to create multifunctional designs for Zn-based nanomaterials. This approach will help to construct a synergistic nanoplatform for combined cancer targeting, bioimaging, and responsive drug delivery, or obtain a selectively cytotoxic or cytocompatible effect on bacterial cells, tumor cells, or normal mammalian cells.
Outstanding Questions.
What properties and functions of Zn-based biomaterials are optimal for the regeneration and therapy of specific tissues?
How to functionalize Zn-based biomaterials for the regeneration and therapy of specific tissues?
How to tune the biodegradation rate of Zn-based implants in vivo and maintain the mechanical and biofunctional support long enough for complete healing during degradation?
How to minimize the unwanted toxicity of Zn-based biomaterials, while maximizing their antimicrobial, anticancer and other desirable features, for applications in tissue regeneration and therapy?
How feasible are therapeutic Zn-based microscale or nanoscale medical devices in the future?
Figure I.
Cellular Receptors and Channels of Zn. Image reproduced with permission from [11].
Highlights.
Zn-based biomaterials have promising applications in tissue regeneration, theranostics and treatments.
Zn-based biomaterials have desirable biological features for regeneration and therapy, including biocompatibility, osteogenesis, and antibacterial, antifungal, and anticancer properties.
Zn-based biodegradable metals have good degradation rates and biocompatibility, and their mechanical strength and ductility can be enhanced through alloying, thus making them promising for cardiovascular and orthopedic applications.
Zn-based ceramic biomaterials are being developed as synergistic nanocomposite platforms capable of combined cancer targeting, bioimaging and responsive drug delivery.
Strategies for controlled release of degradation products from biodegradable Zn-based biomaterials are necessary to ensure their biosafety when optimizing their therapeutic and treatment effects.
Acknowledgements
This work was supported by National Institutes of Health [Grant number R01HL140562]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary:
- α2-macroglobulin
a plasma protein with a wide variety of functions produced by macrophages, fibroblasts, and liver and adrenocortical cells
- Albumin
a transport protein in human blood plasma that also regulates osmotic pressure
- Alzheimer’s disease
the most common cause of dementia; it affects memory, cognition, and behavior
- Bacteriolysis
the rupture of a bacterial cell by chemical or physical interactions
- Biodegradable metal
a metal or alloy that degrades in the body
- Biphasic cellular responses
two separate and distinct responses of cells to different signals
- Cell viability assay
an assessment of the ability of a cell to remain viable in the presence of a foreign material
- Chondrocyte differentiation
the differentiation of mesenchymal stem cells (MSCs) to chondroblasts with the secretion of cartilage extracellular matrix
- DNA methylation
the process of adding a methyl group to DNA, which serves as an important regulatory mechanism in epigenetic repression or activation of target genes
- Luminescence
cold-body emission of light from a source
- Metal organic frameworks (MOF)
organic-inorganic hybrid materials formed by organic ligands linked to metal ions or clusters
- Osteoclast
a type of multinucleated bone cell that breaks down bone tissue by secreting acid to facilitate maintenance, restoration, and remodeling of bone
- Photoluminescent
emission of light due to absorption of photons
- Photoluminescent quantum yield
emission efficiency from a given photon absorption
- Quantum dots
nanoscale semiconductor particles that have unique electrical and optical properties
- Reactive oxygen species (ROS)
chemically reactive species of oxygen, such as hydroxyl radical, superoxide, or singlet oxygen
- Reendothelialization
the migration of endothelial cells and attraction of endothelial progenitor cells to repair a damaged blood vessel following stent implantation
- Standard electrode potential
the electric potential difference measured between a metal and the standard hydrogen electrode, which is set to 0 volts. It tells how easily an element can be oxidized or reduced, and it is associated with the likelihood for electrochemical corrosion/degradation reactions to occur
- Thrombosis formation
restriction of blood through a blood vessel resulting from a blood clot
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen Q and Thouas GA (2015) Metallic implant biomaterials. Materials Science and Engineering: R: Reports 87, 1–57 [Google Scholar]
- 2.Zheng YF, et al. (2014) Biodegradable metals. Materials Science and Engineering R: Reports 77, 1–34 [Google Scholar]
- 3.Bowen PK, et al. (2013) Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater 25, 2577–2582 [DOI] [PubMed] [Google Scholar]
- 4.Frederickson CJ, et al. (2005) The neurobiology of zinc in health and disease. Nat. Rev. Neurosci 6, 449–462 [DOI] [PubMed] [Google Scholar]
- 5.Xiong HM (2013) ZnO Nanoparticles Applied to Bioimaging and Drug Delivery. Adv. Mater 25, 5329–5335 [DOI] [PubMed] [Google Scholar]
- 6.Nasajpour A, et al. (2017) Nanostructured Fibrous Membranes with Rose Spike-Like Architecture. Nano Lett. 17, 6235–6240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, et al. (2015) Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application. Chem. Rev 115, 11669–11717 [DOI] [PubMed] [Google Scholar]
- 8.Furukawa H, et al. (2013) The chemistry and applications of metal-organic frameworks. Science 341, 1230444. [DOI] [PubMed] [Google Scholar]
- 9.Mostaed E, et al. (2018) Zinc-based alloys for degradable vascular stent applications. Acta Biomater. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowen PK, et al. (2016) Biodegradable Metals for Cardiovascular Stents: from Clinical Concerns to Recent Zn-Alloys. Adv Healthc Mater 5, 1121–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kambe T, et al. (2015) The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiological reviews 95, 749–784 [DOI] [PubMed] [Google Scholar]
- 12.Haase H (2017) Chapter 21 - Zinc Signals and Immune Function In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals (Collins JF, ed), pp. 261–271, Academic Press [Google Scholar]
- 13.Little PJ, et al. (2010) Zinc and cardiovascular disease. Nutrition 26, 1050–1057 [DOI] [PubMed] [Google Scholar]
- 14.Kessels JE, et al. (2016) Influence of DNA-methylation on zinc homeostasis in myeloid cells: Regulation of zinc transporters and zinc binding proteins. J. Trace Elem. Med Biol 37, 125–133 [DOI] [PubMed] [Google Scholar]
- 15.Yang P, et al. (2017) The Role of KRAB-ZFPs in Transposable Element Repression and Mammalian Evolution. Trends in Genetics 33, 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang P, et al. (2017) A placental growth factor is silenced in mouse embryos by the zinc finger protein ZFP568. Science 356, 757–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lomniczi A, et al. (2015) Epigenetic regulation of puberty via Zinc finger protein-mediated transcriptional repression. Nature communications 6, 10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibata M, et al. (2011) TRIM28 is required by the mouse KRAB domain protein ZFP568 to control convergent extension and morphogenesis of extra-embryonic tissues. Development 138, 5333–5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krężel A and Maret W (2016) The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys 611, 3–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bafaro E, et al. (2017) The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduction And Targeted Therapy 2, 17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imbeault M, et al. (2017) KRAB zinc-finger proteins contribute to the evolution of gene regulatory networks. Nature 543, 550. [DOI] [PubMed] [Google Scholar]
- 22.Najafabadi HS, et al. (2015) C2H2 zinc finger proteins greatly expand the human regulatory lexicon. Nat. Biotechnol 33, 555. [DOI] [PubMed] [Google Scholar]
- 23.Jen J and Wang Y-C (2016) Zinc finger proteins in cancer progression. Journal of biomedical science 23, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matijasevic Z, et al. (2016) The Zn-finger domain of MdmX suppresses cancer progression by promoting genome stability in p53-mutant cells. Oncogenesis 5, e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, et al. (2015) Myeloid zinc-finger 1 (MZF-1) suppresses prostate tumor growth through enforcing ferroportin-conducted iron egress. Oncogene 34, 3839. [DOI] [PubMed] [Google Scholar]
- 26.Divisato G, et al. (2016) ZNF687 Mutations in Severe Paget Disease of Bone Associated with Giant Cell Tumor. The American Journal of Human Genetics 98, 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang T, et al. (2017) Overexpression of zinc finger protein 687 enhances tumorigenic capability and promotes recurrence of hepatocellular carcinoma. Oncogenesis 6, e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z, et al. (2014) Zinc plays a critical role in the cardioprotective effect of postconditioning by enhancing the activation of the RISK pathway in rat hearts. Journal of molecular and cellular cardiology 66, 12–17 [DOI] [PubMed] [Google Scholar]
- 29.Zhu D, et al. (2017) Zinc regulates vascular endothelial activities through zinc-sensing receptor ZnR/GPR39. American Journal of Physiology-Cell Physiology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao Y, et al. (2014) Stimulation of bone growth following zinc incorporation into biomaterials. Biomaterials 35, 6882–6897 [DOI] [PubMed] [Google Scholar]
- 31.Yang H, et al. (2017) Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 145, 92–105 [DOI] [PubMed] [Google Scholar]
- 32.Bowen PK, et al. (2015) Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Materials Science and Engineering: C 56, 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, et al. (2018) Initial formation of corrosion products on pure zinc in simulated body fluid. Journal of Materials Science & Technology [Google Scholar]
- 34.Mostaed E, et al. (2016) Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater 60, 581–602 [DOI] [PubMed] [Google Scholar]
- 35.Liu X, et al. (2016) Micro-alloying with Mn in Zn-Mg alloy for future biodegradable metals application. Mater Design 94, 95–104 [Google Scholar]
- 36.Niu J, et al. (2016) Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Mater Sci Eng C Mater Biol Appl 69, 407–413 [DOI] [PubMed] [Google Scholar]
- 37.Shearier ER, et al. (2016) In Vitro Cytotoxicity, Adhesion, and Proliferation of Human Vascular Cells Exposed to Zinc. Acs Biomater Sci Eng 2, 634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu D, et al. (2017) Biological Responses and Mechanisms of Human Bone Marrow Mesenchymal Stem Cells to Zn and Mg Biomaterials. ACS Appl Mater Interfaces 9, 27453–27461 [DOI] [PubMed] [Google Scholar]
- 39.Ma J, et al. (2016) Bioabsorbable zinc ion induced biphasic cellular responses in vascular smooth muscle cells. Scientific reports 6, 26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma J, et al. (2015) Endothelial Cellular Responses to Biodegradable Metal Zinc. Acs Biomater Sci Eng 1, 1174–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li HF, et al. (2015) Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Scientific reports 5, 10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li HF, et al. (2015) Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr. Mater Design 83, 95–102 [Google Scholar]
- 43.Tang Z, et al. (2017) Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater 72, 182–191 [DOI] [PubMed] [Google Scholar]
- 44.Wang J, et al. (2015) Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomaterialia 21, 237–249 [DOI] [PubMed] [Google Scholar]
- 45.Drelich AJ, et al. (2016) Importance of oxide film in endovascular biodegradable zinc stents. Surface Innovations 4, 133–140 [Google Scholar]
- 46.Jablonska E, et al. (2016) Influence of surface pre-treatment on the cytocompatibility of a novel biodegradable ZnMg alloy. Mat Sci Eng C-Mater 68, 198–204 [DOI] [PubMed] [Google Scholar]
- 47.Li Y, et al. (2018) Additively manufactured biodegradable porous magnesium. Acta Biomater. 67, 378–392 [DOI] [PubMed] [Google Scholar]
- 48.Su Y, et al. (2018) Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater 77, 90–105 [DOI] [PubMed] [Google Scholar]
- 49.Guillory RJ, et al. (2016) Corrosion Characteristics Dictate the Long-Term Inflammatory Profile of Degradable Zinc Arterial Implants. Acs Biomater Sci Eng 2, 2355–2364 [DOI] [PubMed] [Google Scholar]
- 50.Prasad AS (2013) Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease–. Advances in nutrition 4, 176–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao S, et al. (2017) Structural characteristics and in vitro biodegradation of a novel Zn-Li alloy prepared by induction melting and hot rolling. Metallurgical and Materials Transactions A 48, 1204–1215 [Google Scholar]
- 52.Shi Z-Z, et al. (2018) Fabrication and characterization of novel biodegradable Zn-Mn-Cu alloys. Journal of materials science & technology 34, 1008–1015 [Google Scholar]
- 53.Zhao S, et al. (2017) Zn-Li alloy after extrusion and drawing: Structural, mechanical characterization, and biodegradation in abdominal aorta of rat. Materials Science and Engineering: C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang Z, et al. (2016) Design and characterizations of novel biodegradable Zn-Cu-Mg alloys for potential biodegradable implants. Mater Design [Google Scholar]
- 55.Yang H, et al. (2018) In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 71, 200–214 [DOI] [PubMed] [Google Scholar]
- 56.Zhu P, et al. (2016) Biomedical Applications of Functionalized ZnO Nanomaterials: from Biosensors to Bioimaging. Advanced Materials Interfaces 3 [Google Scholar]
- 57.Amna T, et al. (2014) Electrospun nanofibers of ZnO‐TiO2 hybrid: characterization and potential as an extracellular scaffold for supporting myoblasts. Surf. Interface Anal 46, 72–76 [Google Scholar]
- 58.Zhang ZY, et al. (2013) Biodegradable ZnO@polymer core-shell nanocarriers: pH-triggered release of doxorubicin in vitro. Angew Chem Int Ed Engl 52, 4127–4131 [DOI] [PubMed] [Google Scholar]
- 59.Tripathy N, et al. (2015) Enhanced anticancer potency using an acid-responsive ZnO-incorporated liposomal drug-delivery system. Nanoscale 7, 4088–4096 [DOI] [PubMed] [Google Scholar]
- 60.Hong H, et al. (2015) Red fluorescent zinc oxide nanoparticle: a novel platform for cancer targeting. ACS Appl. Mat. Interfaces 7, 3373–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin Q, et al. (2014) Biocompatible folate-modified Gd 3+/Yb 3+-doped ZnO nanoparticles for dualmodal MRI/CT imaging. RSC Advances 4, 53561–53569 [Google Scholar]
- 62.Lai L, et al. (2016) In vivo target bio-imaging of Alzheimer’s disease by fluorescent zinc oxide nanoclusters. Biomater Sci 4, 1085–1091 [DOI] [PubMed] [Google Scholar]
- 63.Lai L, et al. (2017) In Vivo Biosynthesized Zinc and Iron Oxide Nanoclusters for High Spatiotemporal Dual-Modality Bioimaging of Alzheimer’s Disease. Langmuir 33, 9018–9024 [DOI] [PubMed] [Google Scholar]
- 64.Chandrasekaran M and Pandurangan M (2016) In vitro selective anti-proliferative effect of zinc oxide nanoparticles against co-cultured C2C12 myoblastoma cancer and 3T3-L1 normal cells. Biol. Trace Elem. Res 172, 148–154 [DOI] [PubMed] [Google Scholar]
- 65.Pathania D, et al. (2015) Fabrication of ZnS–cellulose nanocomposite for drug delivery, antibacterial and photocatalytic activity. Materials & Design 87, 1056–1064 [Google Scholar]
- 66.Gupta D, et al. (2015) Synthesis of chitosan-g-poly (acrylamide)/ZnS nanocomposite for controlled drug delivery and antimicrobial activity. International journal of biological macromolecules 74, 547–557 [DOI] [PubMed] [Google Scholar]
- 67.Song J, et al. (2016) Bandgap and Structure Engineering via Cation Exchange: From Binary Ag2S to Ternary AgInS2, Quaternary AgZnInS alloy and AgZnInS/ZnS Core/Shell Fluorescent Nanocrystals for Bioimaging. ACS Appl. Mat. Interfaces 8, 24826–24836 [DOI] [PubMed] [Google Scholar]
- 68.Deng T, et al. (2017) Water-Solubilizing Hydrophobic ZnAgInSe/ZnS QDs with Tumor-Targeted cRGD-Sulfobetaine-PIMA-Histamine Ligands via a Self-Assembly Strategy for Bioimaging. ACS Appl. Mat. Interfaces 9, 11405–11414 [DOI] [PubMed] [Google Scholar]
- 69.Dash SK, et al. (2014) Zinc sulfide nanoparticles selectively induce cytotoxic and genotoxic effects on leukemic cells: involvement of reactive oxygen species and tumor necrosis factor alpha. J. Appl. Toxicol 34, 1130–1144 [DOI] [PubMed] [Google Scholar]
- 70.Moura IMR, et al. (2018) Highly fluorescent positively charged ZnSe quantum dots for bioimaging. J. Lumin 201, 284–289 [Google Scholar]
- 71.Selvaraj J, et al. (2017) Phosphine-Free, Highly Emissive, Water-Soluble Mn: ZnSe/ZnS Core–Shell Nanorods: Synthesis, Characterization, and in Vitro Bioimaging of HEK293 and HeLa Cells. ACS Applied Nano Materials 1, 371–383 [Google Scholar]
- 72.Herschke L, et al. (2006) Zinc phosphate as versatile material for potential biomedical applications Part 1. Journal of Materials Science: Materials in Medicine 17, 81–94 [DOI] [PubMed] [Google Scholar]
- 73.Zhao X. c., et al. (2014) Ultrasonic induced rapid formation and crystal refinement of chemical conversed hopeite coating on titanium. The Journal of Physical Chemistry C 118, 1910–1918 [Google Scholar]
- 74.Suárez-Franco JL, et al. (2013) Effects of surface morphology of ZnAl 2 O 4 ceramic materials on osteoblastic cells responses. Journal of Nanomaterials 2013, 2 [Google Scholar]
- 75.Kamińska I, et al. (2014) Synthesis of ZnAl 2 O 4:(Er 3+, Yb 3+) spinel-type nanocrystalline upconverting luminescent marker in HeLa carcinoma cells, using a combustion aerosol method route. RSC Advances 4, 56596–56604 [Google Scholar]
- 76.Wang Y-W, et al. (2014) Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl. Mat. Interfaces 6, 2791–2798 [DOI] [PubMed] [Google Scholar]
- 77.Oh E, et al. (2016) Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol 11, 479. [DOI] [PubMed] [Google Scholar]
- 78.Agnihotri S, et al. (2015) Arginine-assisted immobilization of silver nanoparticles on ZnO nanorods: an enhanced and reusable antibacterial substrate without human cell cytotoxicity. Nanoscale 7, 7415–7429 [DOI] [PubMed] [Google Scholar]
- 79.Lewinski NA, et al. (2018) Influence of ZnO thin film crystallinity on in vitro biocompatibility. Toxicology Research [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramanery FP, et al. (2013) One-step colloidal synthesis of biocompatible water-soluble ZnS quantum dot/chitosan nanoconjugates. Nanoscale Res. Lett 8, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Surmenev RA, et al. (2014) Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis--a review. Acta Biomater. 10, 557–579 [DOI] [PubMed] [Google Scholar]
- 82.Hoppe A, et al. (2013) Therapeutic inorganic ions in bioactive glasses to enhance bone formation and beyond. Biomaterials Science 1, 254–256 [DOI] [PubMed] [Google Scholar]
- 83.Jia Z, et al. (2016) Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 75, 203–222 [DOI] [PubMed] [Google Scholar]
- 84.Shukla RK, et al. (2013) TiO2 nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology 7, 48–60 [DOI] [PubMed] [Google Scholar]
- 85.Sahoo SK, et al. (2017) Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging In Nanomedicine in Cancer, pp. 73–124, Pan Stanford; [DOI] [PubMed] [Google Scholar]
- 86.Wang J, et al. (2013) Photostable water-dispersible NIR-emitting CdTe/CdS/ZnS core–shell–shell quantum dots for high-resolution tumor targeting. Biomaterials 34, 9509–9518 [DOI] [PubMed] [Google Scholar]
- 87.Wang H, et al. (2018) Carbon-based hybrid nanogels: a synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev 47, 4198–4232 [DOI] [PubMed] [Google Scholar]
- 88.Bwatanglang IB, et al. (2016) Folic acid targeted Mn: ZnS quantum dots for theranostic applications of cancer cell imaging and therapy. Int. J. Nanomed 11, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mishra PK, et al. (2017) Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today 22, 1825–1834 [DOI] [PubMed] [Google Scholar]
- 90.Wu MX and Yang YW (2017) Metal – Organic Framework (MOF) ‐ Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 29, 1606134. [DOI] [PubMed] [Google Scholar]
- 91.Song M-R, et al. (2018) Zeolitic imidazolate metal organic framework-8 as an efficient pH-controlled delivery vehicle for zinc phthalocyanine in photodynamic therapy. Journal of Materials Science 53, 2351–2361 [Google Scholar]
- 92.Chen J, et al. (2017) Osteogenic activity and antibacterial effect of porous titanium modified with metal‐organic framework films. Journal of Biomedical Materials Research Part A 105, 834–846 [DOI] [PubMed] [Google Scholar]