Abstract
Charcot-Marie-Tooth syndrome (CMT) is a clinically and genetically heterogeneous group of neuropathies affecting both peripheral motor and sensory nerves. Progressive sensorineural hearing loss, vestibular abnormalities, and dysfunction of other cranial nerves have been described. This is the second case report of otopathology in a patient with CMT syndrome. Molecular genetic testing of DNA obtained at autopsy revealed a missense variant in the MPZ gene (p.Thr65Ala), pathogenic for an autosomal dominant form of CMT1B. The temporal bones were also prepared for light microscopy by H & E and Gomori trichome stains, and immunostaining for anti-myelin protein zero. Pathology was consistent with a myelinopathy of the auditory, vestibular and facial nerves bilaterally. The pathophysiology of cranial nerve dysfunction in CMT is unknown. Findings in the current case suggested, at least in cranial nerves 7 and 8, that a myelinopathy may be causative.
Keywords: Charcot-Marie-Tooth Syndrome, myelinopathy, sensorineural hearing loss, cranial nerves, histopathology, myelin protein zero (MPZ) gene
INTRODUCTION
Charcot-Marie-Tooth syndrome (CMT) is a clinically and genetically heterogeneous group of neuropathies affecting both peripheral motor and sensory nerves, and is also known as Hereditary Motor and Sensory Neuropathy (HMSN) or peroneal muscular atrophy. These disorders are caused by genetically induced abnormalities of the myelin sheath or axon of the peripheral nerves. Thus, Charcot-Marie-Tooth type 1 (CMT1) is a group of disorders of the myelin sheath of peripheral nerves, the most common of which (CMT1A) is inherited as an autosomal dominant disorder of peripheral myelin protein 22 (PMP22), whereas CMT 1B is inherited as an autosomal dominant disorder of myelin protein 0 (MPZ), of which there are greater than 120 different mutations. In contrast, CMT 2 represents a group of disorders causing peripheral neuropathy in which the neuron itself is affected and of which there are several subtypes due to various mutations. CMT 3, also known as Dejerine-Sottas syndrome, is a demyelinating progressive neuropathy caused most commonly by a mutation in either the MPZ or PMP22 gene.
Progressive sensorineural hearing loss has been described as part of the clinical presentation in several genetic forms of CMT including various mutations in the MPZ gene [Chapon et al., 1999, deJonghe et al., 1999, Alcin et al., 2000, Misu et al., 2000, Starr et al., 2003, Kochanski et al., 2004, Seeman et al,. 2004, Leal et al., 2014, Tokuda et al., 2015, Duan et al., 2016], in kinships demonstrating mutations in the peripheral myelin protein (PMP22) gene [Alcin et al., 2000, Boerkoel et a.,l 2002, Kovach et al., 2002, Joo et al., 2004], in X-linked phosphoribosyl pyrophosphate synthetase-1 (PRPS-1) mutations [Synofzik et al., 2014, Gandia et al., 2015], and in CMT4C [Sivera et al., 2017].
The hearing loss that has been described in the CMT syndrome is progressive, often with poor word recognition scores [Alcin et al., 2000, Starr et al., 2003], consistent with auditory neuropathy.
To date, otopathology has been described in one other patient with Charcot-Marie-Tooth syndrome. That patient had a mutation (Tyr145 Ser) in the MPZ gene [Starr et al., 2003]. The findings included loss of spiral ganglion cells with beading of both peripheral and central auditory neurons; patchy atrophy of the stria vascularis; loss of large myelinated fibers and thinning of the myelin sheath in the proximal auditory nerve; and little change in the auditory and vestibular hair cell populations [Starr et al., 2003].
Vestibular test impairments have also been described in Charcot-Marie-Tooth syndrome [Poretti et al., 2013]. In the report of Starr et al (2003), the sensory epithelium of the vestibular system and the number of cell bodies in Scarpa’s ganglion were reported as normal.
In this report, we present the histopathology of the inner ear in a case of Charcot-Marie-Tooth syndrome caused by a missense variant (p.Thr65Ala) in the MPZ gene.
Case Report
This 70 year old man was diagnosed several years previously with Charcot-Marie-Tooth syndrome affecting his hands and feet. There was no family history of a similar disorder. Although audiology was not done, the patient claimed deterioration in hearing on a hospital questionnaire one year before death. The patient succumbed to chronic obstructive pulmonary disease. Autopsy revealed a peripheral demyelinating neuropathy with “onion bulb” formation involving both motor and sensory fibers consistent with Charcot-Marie-Tooth syndrome. Motor and sensory nerve roots showed severe demyelination with only scattered myelinated fibers. The anterior and posterior spinal cord root entry zones showed a transition from complete demyelination in the periphery to normal central myelination (Fig.1). The white and gray matter of the cord and brain were unremarkable. Electron microscopy of peripheral nerves confirmed nearly complete loss of myelination, and the few remaining myelinated fibers demonstrated no other abnormalities of the myelin sheath.
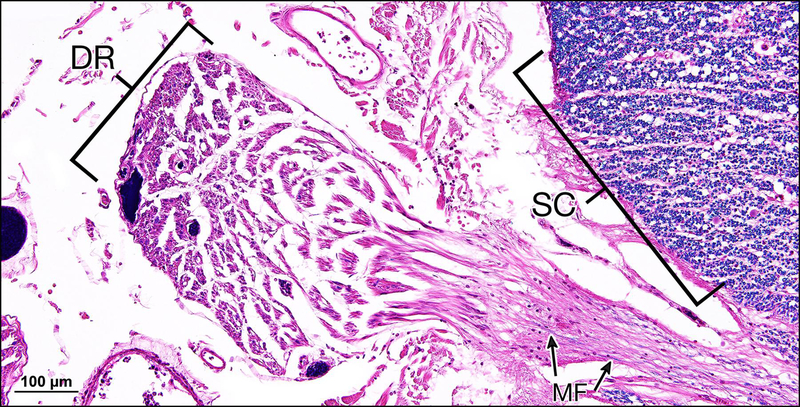
Figure 1.
Section through the spinal cord at the level of dorsal rootlets. Myelination of the spinal cord (SC) itself appears normal whereas that of the dorsal rootlet shown (DR) here is nearly totally devoid of myelinated fibers (MF). (Luxol fast blue, H&E counter stain)
Molecular testing was done at the Translational Genomics Core, Partners Health Care Personalized Medicine, Cambridge, MA. DNA was obtained from scalp tissue at autopsy. Analysis revealed a missense variant in the MPZ gene (p.Thr65Ala), identical to that previously reported by Kochanski et al (2004). This mutation was considered pathogenic for an autosomal dominant form of CMT 1B. The present case is only the second case of CMT syndrome in which peripheral otopathology is available.
Materials and Methods
Exome library preparation and sequencing
Exome capture and library construction was performed at the Translational Genomics Core at Partners HealthCare Personalized Medicine using the SureSelect XT Exome V5 kit (Agilent Technologies, Santa Clara, CA) following the manufacturer’s standard protocol. 1 ug of DNA extracted from fresh frozen scalp tissue was used as input. The Exome V5+ kit uses biotinylated RNA baits to enrich for exons and splice regions of genes, with a total target size of ~50Mb. The kit utilizes standard library construction and hybrid capture methodology (see http://www.agilent.com/cs/library/usermanuals/Public/G7530-90000.pdf). The final exome library was then run on the Illumina HiSeq 2500 instrument in Rapid mode to generate 100bp paired end sequencing reads.
Post-sequencing processing and variant calling
The sequencing yielded roughly 70 million paired reads, which were then aligned to the GRCh37 (hg19) reference genome by using Burrows-Wheeler Aligner followed by SNV and Indel variant detection with the use of the Genome Analysis Toolkit Unified software (GATK v 1.0.4705, Broad Institute, Cambridge MA). The sequencing yielded an average read depth of 141.52X across the target bases, with 96.7% of target bases having 15X coverage or higher, and a total of 72,356 variants were called. Variants were annotated using an in-house annotation tool that curates variant and gene specific data from several disease databases including ClinVar, HGMD, and OMIM, general population frequencies from databases such as dbSNP, ExAC, and 1000 Genomes, and conservation and computational prediction data (PolyPhen2, SIFT, AlignGVGD).
Histopathology
Both temporal bones were removed and immersed in 10% formalin solution approximately 12 hours after death and subsequently decalcified in ethylene diamine tetra-acetic acid (EDTA). Following decalcification, both specimens were dehydrated in graded alcohols and embedded in celloidin. Serial sections with a thickness of 20 micrometers were cut in the horizontal (axial) plane, and every tenth section was stained with hematoxylin and eosin and mounted on glass slides. The cochlear duct and Rosenthal’s canal were reconstructed in two dimensions by a method described by Merchant (2010). Special stains included Gomori trichrome stain, Luxol fast blue, and immunostaining for anti-MPZ.
Celloidin sections containing the modiolus and cochlea from the subject patient and from a normal hearing adult human as a control were chosen for immunostaining. Sections were mounted on gelatin subbed glass slides. The celloidin was removed with changes of a solution of sodium hydroxide and methanol followed by 100% methanol rinses. The tissue was rehydrated from 100 % methanol to 70 % methanol and then distilled water. The sections were rinsed in phosphate buffered saline (PBS) and incubated for one hour in 5 % normal horse serum. A chicken polyclonal primary antibody against myelin protein zero (AB39375, Abcam, Cambridge, MA) at a dilution of 1:1000 was applied and incubated overnight in a humid chamber at room temperature.
On the following morning, sections were rinsed in 3 washes of PBS. Secondary antibody (made against chicken) diluted 1:200 was applied for one hour, and sections were then rinsed 3 times with PBS. Avidin-Biotin-horseradish peroxidase (standard ABC kit, Vector Labs, Burlingame, CA) was applied for one hour and rinsed in PBS. Colorization was accomplished with 0.01 % diaminobenzidine and 0.01 % hydrogen peroxide (H2O2) for 5 to 10 minutes. The sections were then rinsed in PBS, dehydrated in graded alcohols, and cover slips were applied.
RESULTS
Exome sequencing
To identify candidate variants, we employed analysis filtration steps that removed variants with a high minor allele frequency (MAF) in general population databases (MAF ≥ 0.01) and prioritized variants matching the following criteria: 1. Predicted functional impact on coding (missense, nonsense, frameshift), or conserved splice regions (+/− 1–2 splice site positions), 2. Variants in 152 genes having an association with Charcot-Marie-Tooth Disease (CMT) in the OMIM database (https://www.omim.org). Variants passing these filters were then assessed for pathogenicity based on ACMG guidelines [Richards et al 2015].
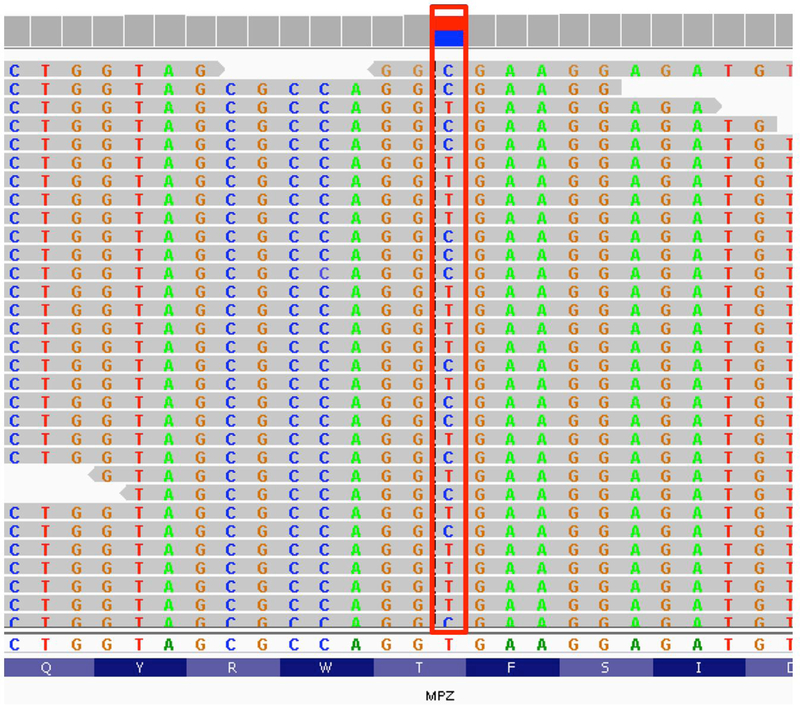
Our filtration and variant prioritization approach identified a heterozygous missense variant in exon 2 of the MPZ gene (c.193A>G; p.Thr65Ala) (Fig. 2). The MPZ gene is associated with autosomal dominant Charcot-Marie-Tooth (CMT) type 1B disease (MIM #118200), and this particular variant was previously reported in a sporadic CMT type1B case presenting with early-onset polyneuropathy case [Kochanski et al., 2004]. Two different missense variants that impact the same amino acid position, p.Thr65Ile and p.Thr65Asn, have also been reported in CMT-related neuropathy cases [Namakura 2002, Brozkova 2010]. All three missense variants at this position were absent in over 200,000 alleles from a broad multi-ethnic population in the Genome Aggregation Database (http://gnomad.broadinstitute.org/). This evidence suggests that the p.Thr65 position in the MPZ gene is intolerant to variation, and supports a causative role for the p.Thr65Ala variant.
Figure 2.
Representative sequencing reads from exome sequencing showing a heterogenous T>C transition (red box) on the genomic reference sequence (hg19), which corresponds to a c.193A>G (p.Thr65Ala) missense variant in exon 2 of the MPZ gene.
Histopathology
The findings were similar in both ears and hence will be described together.
There was minor scattered loss of both inner and outer auditory hair cells with mild atrophy of the stria vascularis (Fig.3). The spiral ligament was atrophied in the middle and apical turns, but less so in the basal turn. On the right side, the corrected spiral ganglion cell count for segment 1 was 2747 cells; segment 2, 7058 cells; segment 3, 4032 cells; segment 4, 2822 cells; total corrected spiral ganglion cell count was 16,659 which represented 73% of normal for age. On the left side, the corrected spiral ganglion count in segment 1 was 2400; segment 2, 6888 cells; segment 3, 3114 cells; segment 4, 3869 cells; total corrected spiral ganglion cell count was 16, 271 representing 71% of normal for age-matched controls.
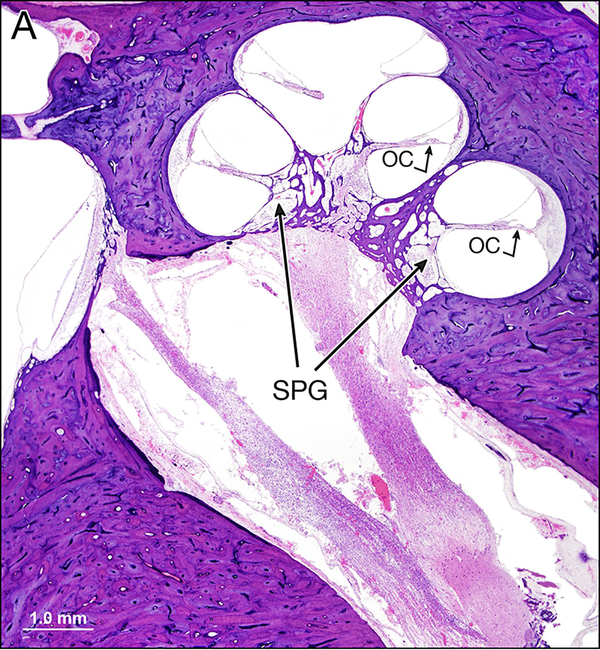
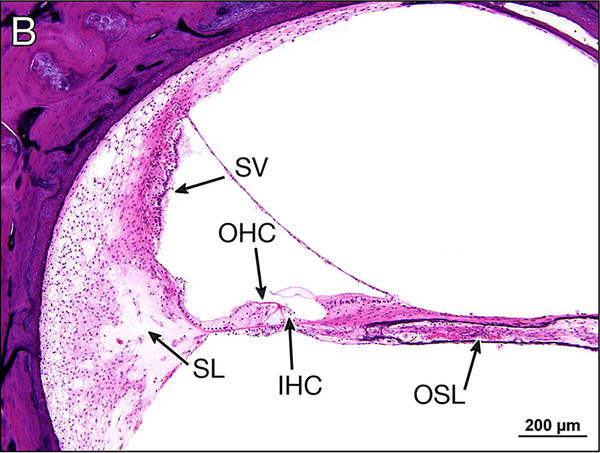
Figure 3.
a: Mid-modiolar section of the left cochlea. The organ of Corti (OC) is present in all three turns of the cochlea. The spiral ganglion cell density (SPG) is reduced in the basal turn.
b Basal turn of the right cochlea. Both inner (IHC) and outer (OHC) hair cells are present. The cochlear neurons in the osseous spiral lamina (OSL) are intact. Note the mild loss of cellular elements within the spiral ligament (SL) and the normal stria vascularis (SV).
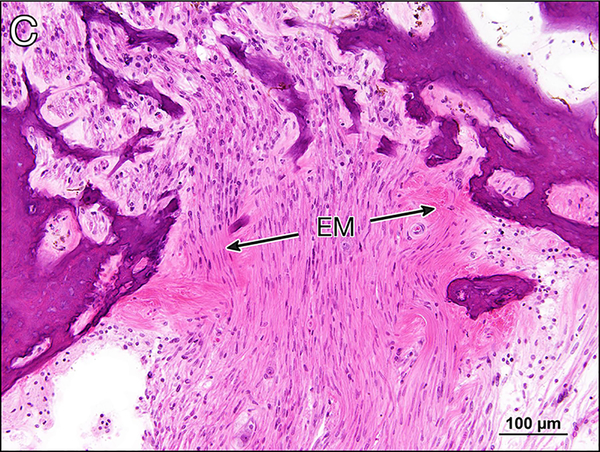
c Cribrose area seen in the mid-modiolar section of the right cochlea. There is deposition of eosinophilic amorphous extracellular material (EM) between the cochlear nerve fibers.
There was moderate degeneration of the ampullae of the superior and posterior semicircular canals. The ampullae of the lateral semi-circular canals were normal. The maculae sacculi and utriculi were normal.
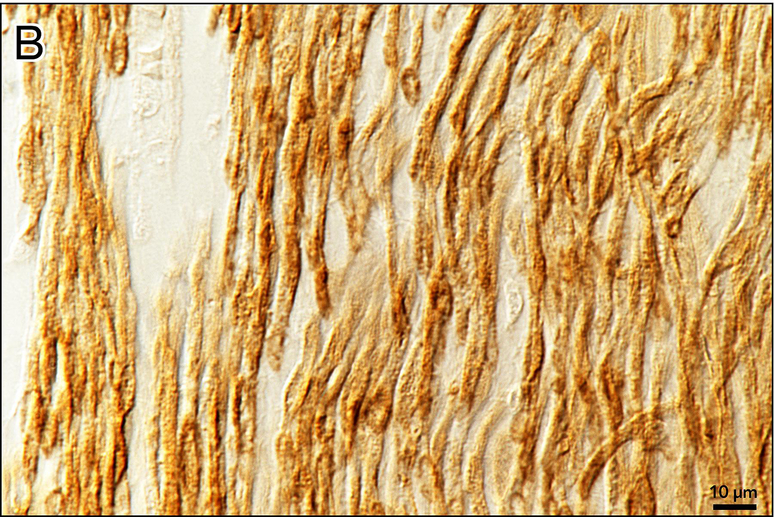
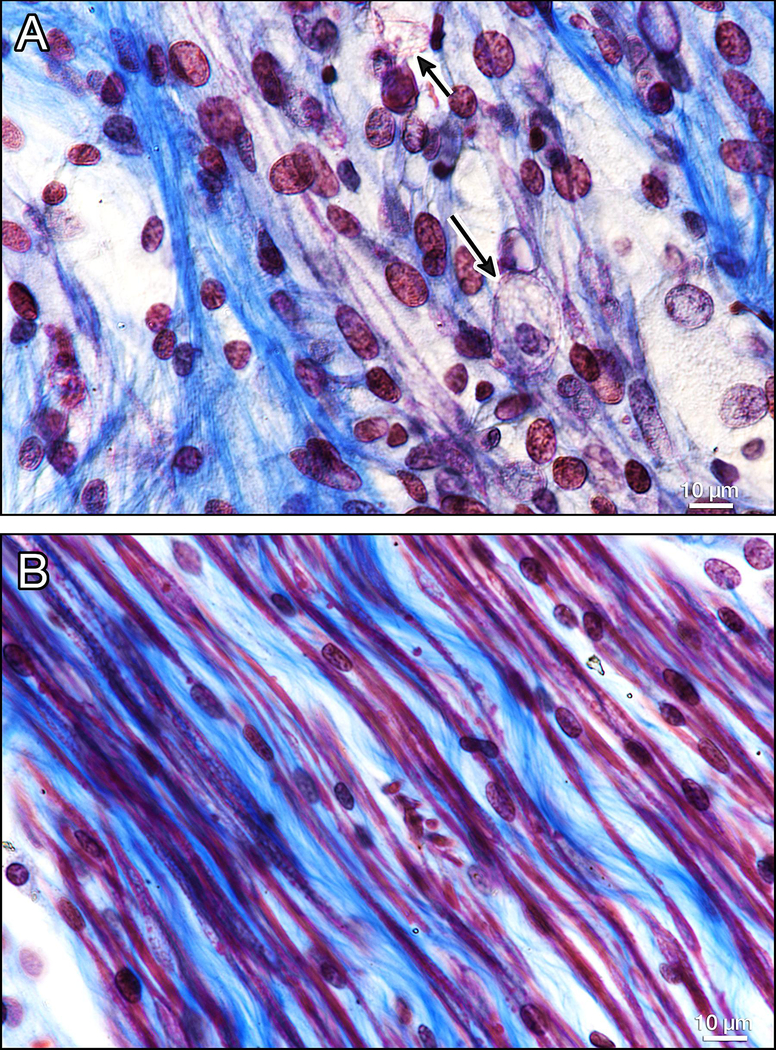
Special stains demonstrated loss of myelinated fibers and increased collagen deposition in the auditory (Figs 4,5), vestibular (Fig. 6), and facial (Figs. 7,8) nerves.
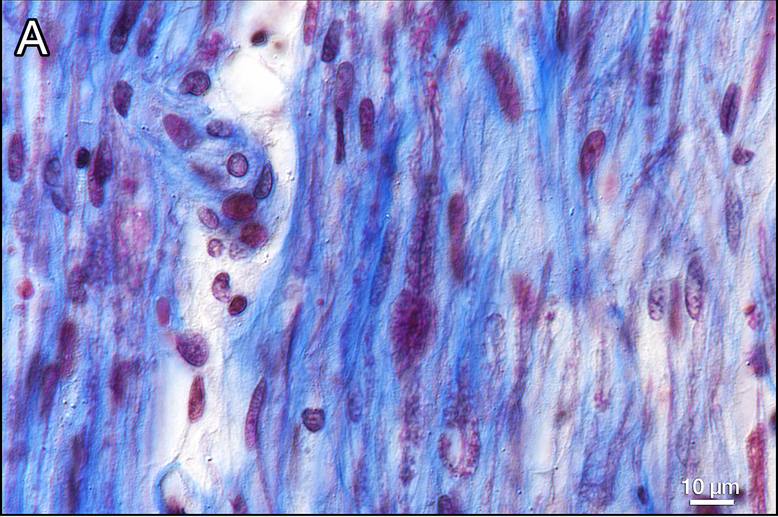
Figure 4. Gomori trichrome stain of auditory nerves in the internal auditory canal.
a Distal internal auditory canal near cribrose area of left ear. Myelin is stained red and connective tissue blue. Only a few remaining myelinated fibers are present within a fibrillar deposit of connective tissue between residual axons. Schwann cell nuclei are also depleted compared to the normal.
b Control from a normal hearing adult human. The density of myelinated axons (red) is considerably greater than that seen in Fig. 4a.
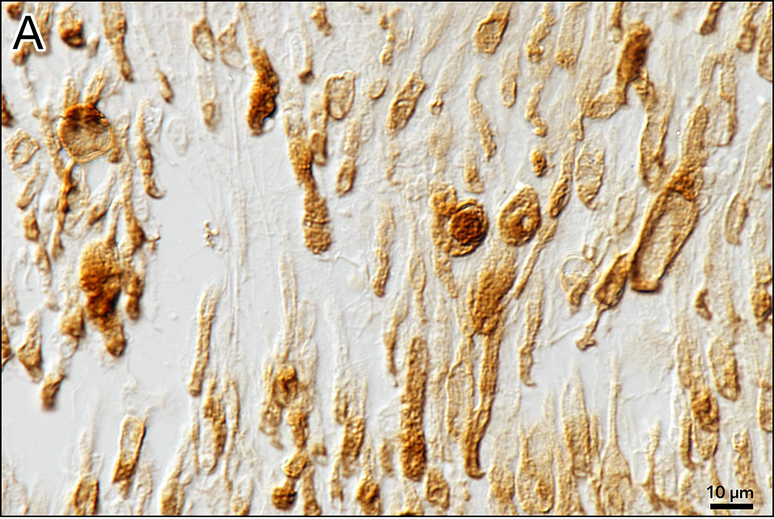
Figure 5. Anti-MPZ immunostaining of the proximal auditory nerve in the internal auditory canal.
a The myelin is sheath stained brown. The number of myelinated axons is decreased. The caliber of the residual myelinated neurons and the thickness of the myelin sheath are more varied than normal.
b Control from a normal hearing adult human. The caliber of the myelinated axons is much more uniform than those in Figure 5a.
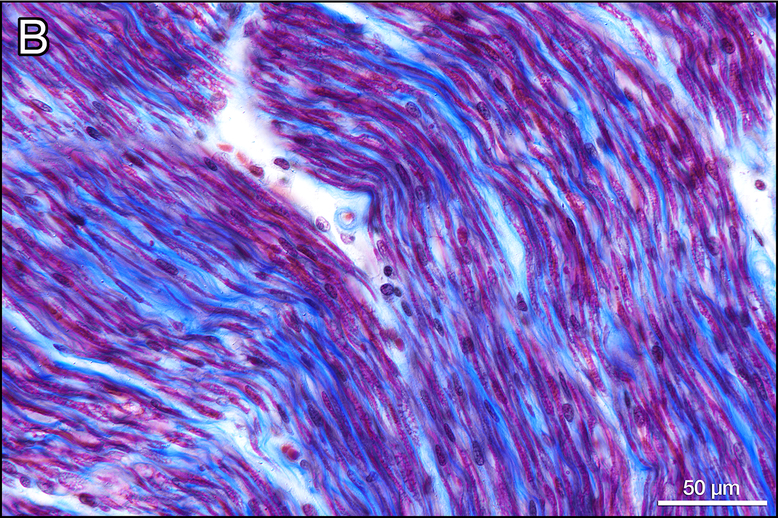
Figure 6. Vestibular neurons in the IAC (Gomori trichrome stain).
In Figure 6a, there are only a few remaining myelinated nerve fibers (red) as compared to the control ear (6b). The presence of macrophages (arrows, Fig. 6a) suggest ongoing degeneration.
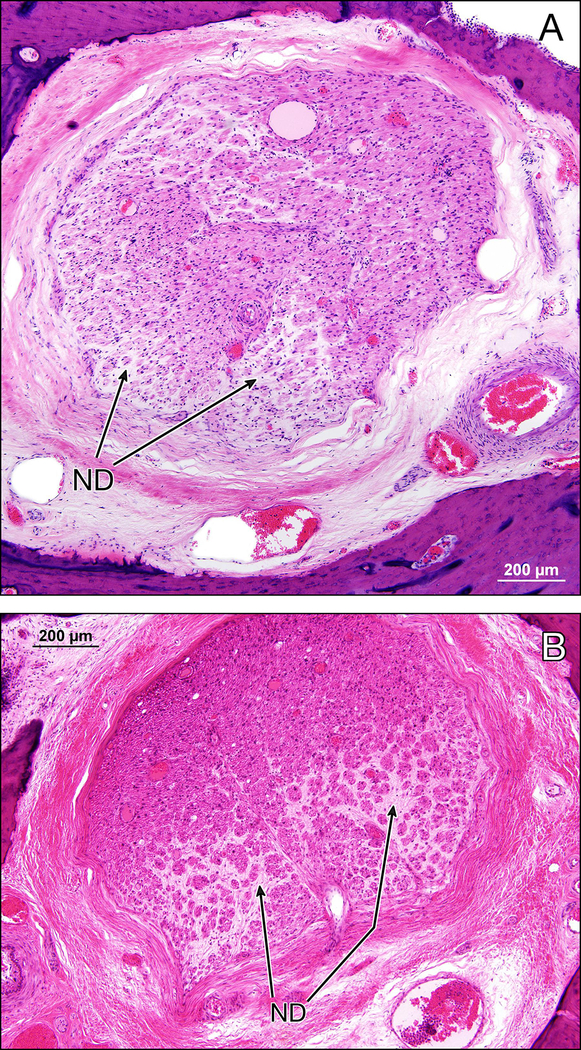
Figure 7.
Facial nerve trunk in the left (7a) and right (7b) temporal bones in the proximal descending segment. There is axonal degeneration with onion bulb formation and interstitial fibrosis (ND) indicative of ongoing demyelination and remyelination bilaterally. (H&E stain).
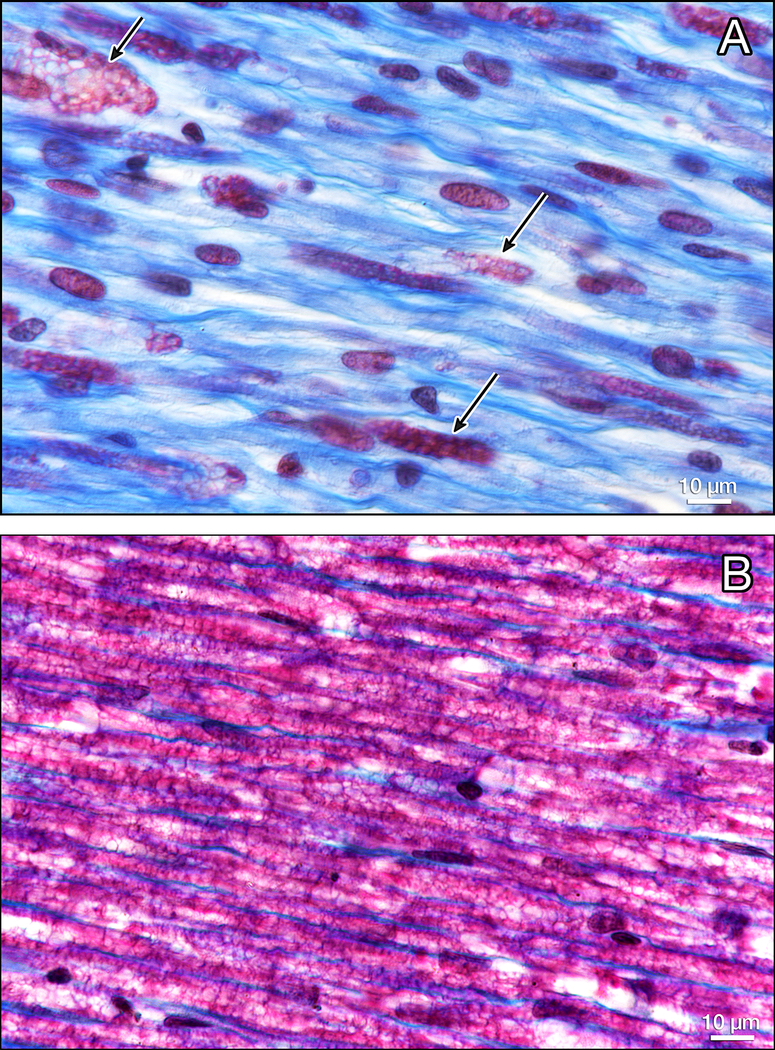
Figure 8.
Gomori trichrome staining of the facial nerve. There are only a few remaining myelinated fibers staining red (arrows)(Fig. 8a) as compared to a normal control from an adult human (8b).
DISCUSSION
The MPZ gene encodes myelin protein-zero, a major structural adhesion glycoprotein of peripheral myelin expressed in Schwann cells, but not in the CNS. Variants impacting its function result in peripheral myelin defects that cause several types of neurological disease with varying severity, including progressive late-onset Charcot-Marie-Tooth type 1 B (CMT1B; OMIM 118200), and the more severe Dejerine-Sottas syndrome (DSS; OMIM 145900) and congenital hypomyelinating neuropathy (CHN; OMIM 605253). The genotype-phenotype correlation is unclear across the different MPZ related neuropathies; however the more severe forms appear to result from variants having a dominant-negative mechanism, while variants resulting in a loss-of-function of MPZ protein are associated with milder disease [Inoue 2004, reviewed in Houlden 2006]. Missense variants can result in either type of disease mechanism depending on the position and function of the amino acid residue affected by the variant [Boerkoel 2002].
The family history for our patient was negative suggesting the variant occurred de novo, but parental testing was unavailable. However, roughly 30–40% of causative MPZ variants occur de novo in sporadic cases of CMT-related neuropathy [Boerkoel 2002, Brozkova 2010]. In addition, the previously reported CMT case with the same variant as our patient was also sporadic [Kochanski et al., 2004], and the two cases with causative missense variants impacting the same codon as our patient (p.Thr65Ile and p.Thr65Asn) were also sporadic, with de novo occurrence confirmed for one of these cases [Numakura 2002, Brozkova 2010]. This data suggest that the p.Thr65 position is a mutation hotspot susceptible to variation arising de novo.
This is the second case report of otopathology in a patient with CMT syndrome, here with a mutation in the MPZ gene. In the first case, reported by Starr et al (2003), a missense mutation in the MPZ gene (p.Tyr45Ser) was inherited in an autosomal dominant fashion. This case was consistent with CMT1B. Audiology done nine years before death showed moderate to severe sensorineural loss and a speech discrimination of 4 and 8% on the right and left sides respectively. The principal histopathologic correlate of the hearing loss seemed to be a severe loss of spiral ganglion cells whereas the sensory epithelium of the organ of Corti was nearly normal. This was interpreted as consistent with a neuropathy of the auditory nerve. Both the sural and proximal auditory nerves showed a loss of nerve fibers, which was interpreted as consistent with a primary axonal disorder, and thinning of the myelin sheath and remaining axons consistent with incomplete remyelination.
The current case with a missense mutation in the MPZ gene (Thr65Ala) is also consistent with a CMT 1B disorder. Unfortunately, no audiogram was done, although by the patient’s own report a mild hearing loss may have been present at the time of death. Histopathology in this case was consistent with a myelinopathy of the auditory nerve as well as the vestibular and facial nerves bilaterally, similar to the neuropathological findings at autopsy demonstrating severe demyelination of motor and sensory roots and nearly complete demyelination in the periphery compared to near normal central myelination.
Hearing loss has been described in CMT 1A (PMP22 gene), [Alcin et al., 2000, Boerkoel et al., 2002, Kovach et al., 2002, Joo et al., 2004], in CMT 1B (MPZ gene) [Starr et al., 2003], in CMTX (X-linked PRPS-1 mutations) [Synofzik et al., 2014, Gandia et al., 2015], in CMT 4C [Yger et al., 2012, Silvera et al., 2017], and in CMT 4B3, [Manole et al., 2017]. Vestibular abnormalities detected by electrophysiology have been described in CMT [Poretti et al., 2013].
In addition to vestibular and auditory cranial nerve involvement, other cranial nerves have been described as being affected in various genetic subtypes of CMT, including electrophysiologic involvement of cranial nerves VII, IX, XII in CMT 1A [Kumagai-Eto et al., 2004], subclinical abnormalities in cranial nerves III and V and unilateral cranial nerve III palsy in CMT 1A [Posa et al., 2017], abnormalities in cranial nerves V and VII as detected by enlargement of the cranial foramina using MRI and CT, but without clinical symptoms in CMT 1A [Das et al., 2017], involvement of cranial nerves III, V, and VII as detected by radiographic analysis of cranial nerve foramina by CT and MRI in CMT 1A [Aho et al., 2004], cranial nerve X involvement in CMT of undefined subtype causing bilateral abductor vocal cord paralysis [Hollinger et al., 1979], electrophysiologic abnormalities of cranial nerves II, VII, VIII in CMT 1A [Triantafyllou et al., 1989], and electrophysiologic abnormalities of cranial nerve VII in CMT 1A and in CMT 3 [Glocker et al., 1999]. In addition, involvement of cranial nerves III, VIII, and X has been described in CMT 1D [Pareyson et al., 2000], vocal cord paresis and dysfunction of cranial nerve III in CMT 2C [Chen et al 2010], involvement of cranial nerve VII, IX, X, in CMT 3 [Boerkoel et al., 2001], vocal cord paresis in 8 out of 9 patients, and phrenic nerve dysfunction in eight of eight patients in CMT 4A [Sevilla et al., 2008], facial weakness in CMT 4B3 [Manole et al., 2017], and involvement of cranial nerves VII, VIII, IX, X in CMT 4C [Gooding et al., 2005, Colomer et al., 2006, Yger et al., 2012].
Despite electrophysiologic or radiologic evidence of cranial nerve involvement, in many cases there was no clinical evidence of dysfunction [Glocker et al., 1999, Kumagi-Eto et al., 2004, Das et al., 2017]. This is consistent with the findings in the current case in which there was no clinical evidence of vestibular or facial dysfunction, despite histologic evidence of a myelinopathy of both vestibular and facial nerves.
The pathophysiology of cranial nerve dysfunction in CMT syndrome as evidenced by either electrophysiologic or clinical abnormalities, or both, is unknown. Findings in the current case suggest that in at least cranial nerves VII and VIII, a myelinopathy may have been causative.
Acknowledgments
Supported by grant #5R01DC000152-35, National Institute on Deafness and Other Communication Disorders, Bethesda, MD
Footnotes
There are no conflicts of interest reported by the authors of this manuscript.
All patients consented to the sharing of clinical information and pathologic findings.
The study protocol was approved by the Human Studies Committee under exemption #4.
REFERENCES
- 1.Aho TR, Wallace RC, Pitt AM, Sivakumar K. Charcot-Marie-Tooth disease: Extensive cranial nerve involvement on CT and MR imaging. Am J NR. 2004;25:494–497. [PMC free article] [PubMed] [Google Scholar]
- 2.Alcin B, Vatovec J, Zargi M. Pure tone audiogram and speech audiometry in patients with hereditary motor and sensory neuropathy. Pflugers Arch. 2000;439(3 Suppl):R202–203. [PubMed] [Google Scholar]
- 3.Boerkoel CF, Takashima H, Bacino CA, Daentl D, Lupski JR. EGR2 mutation R259W causes a spectrum of Dejerine-Sottas neuropathy. Neurogenetics. 2001. July;3(3):153–157. [DOI] [PubMed] [Google Scholar]
- 4.Boerkoel CF, Takashima H, Garcia CA, et al. Charcot-Marie-Tooth disease and related neuropathies; mutation distribution and genotype-phenotype correlation. Ann Neurol 2002;51:190–201. [DOI] [PubMed] [Google Scholar]
- 5.Brozková D, Mazanec R, Haberlová J, Sakmaryová I, Seeman P. Clinical and in silico evidence for and against pathogenicity of 11 new mutations in the MPZ gene. Clin Genet 2010. July;78(1):81–87. [DOI] [PubMed] [Google Scholar]
- 6.Chapon F, Latour P, Diraison P, et al. Axonal phenotype of Charcot-Marie-Tooth disease associated with a mutation in the myelin protein zero gene. J Neurol Neurosurg Psychiatry. 1999;66:779–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen DH, Sul Y, Weiss M, Hillel A, Lipe H, Wolff J, Matsushita M, Raskind W, Bird T. CMT2 with vocal cord paresis associated with short stature and mutations in the TRPV4 gene. Neurology. November 30;75(22):1968–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colomer J, Gooding R, Angelicheva D, King RH, Guillen-Navarro E, Parman Y, Nascimento A, Conill J, Kalaydjieva L. Clinical spectrum of CMT4C disease in patients homozygous for the p.Arg1109X mutation in SH3TC2. Neuromuscul Disord. 2006. July;16(7):449–453. [DOI] [PubMed] [Google Scholar]
- 9.Das N, Kandalaft S, Wu X, Malhotra A. Cranial nerve involvement in Charcot-Marie-Tooth disease. J Clin Neurosci. 2017. March;37:59–62. [DOI] [PubMed] [Google Scholar]; DeJonghe P, Timmerman V, Ceuterick C, et al. The Thr124Met mutation in the peripheral myelin protein zero (MPZ) gene is associated with a clinically distinct Charcot-Marie-Tooth phenotype. Brain. 1999;122:281–290. [DOI] [PubMed] [Google Scholar]
- 10.Duan X, Gu W, Hao Y, Wang R, Wen H, Sun S, Jiao J, Fan D. A novel Asp121Asn mutation of myelin protein zero is associated with late-onset axonal Charcot-Marie-Tooth disease, hearing loss and pupil abnormalities. Front Aging Neurosci. 2016. September22;8:222 ECollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandia M, Fernández-Toral J, Solanellas J, Dominguez-Ruiz M, Gómez-Rosas E, Del Castillo FJ, Villamar M, Moreno-Pelayo MA, Del Castillo I. Mutations in PRPS1 causing syndromic or nonsyndromic hearing impairment: intrafamilial phenotypic variation complicates genetic counseling. Pediatr Res. 2015. July;78(1):97–102. [DOI] [PubMed] [Google Scholar]
- 12.Glocker FX, Rosler KM, Linden D, Heinen F, Hess CW, Lucking CH. Facial nerve dysfunction in hereditary motor and sensory neuropathy type I and III. Muscle Nerve. 1999. September;22(9):1201–1208. [DOI] [PubMed] [Google Scholar]
- 13.Gooding R, Colomer J, King R, Angelicheva D, Marns L, Parman Y, Chandler D, Bertrapenpetit J, Kalaydjieva L. A novel gypsy founder mutation, p.Arg1109X in the CMT4C gene, causes variable peripheral neuropathy phenotypes. J. Med Genet 2005. December;42(12):e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holinger PC, Vuckovich DM, Holinger LD, Holinger PH. Bilateral abductor vocal cord paralysis in Charcot-Marie-Tooth disease. Ann Otol Rhinol Laryngol. 1979. Mar-Apr;88(2 Pt 1):205–209. [DOI] [PubMed] [Google Scholar]
- 15.Houlden H, Reilly MM. Molecular genetics of autosomal-dominant demyelinating Charcot-Marie-Tooth disease. Neuromolecular Med 2006;8(1–2):43–62. [DOI] [PubMed] [Google Scholar]
- 16.Inoue K, Khajavi M, Ohyama T, Hirabayashi S, Wilson J, Reggin JD, Mancias P, Butler IJ, Wilkinson MF, Wegner M, Lupski JR. Molecular mechanism for distinct neurological phenotypes conveyed be allelic truncating mutations. Nat Genet 2004. April;36(4):361–369. [DOI] [PubMed] [Google Scholar]
- 17.Joo IS, Ki CS, Joo SY, Huh K, Kim JW. A novel point mutation in PMP22 gene associated with a familial case of Charcot-Marie-Tooth disease type 1A with sensorineural deafness. Neuromuscul Disord. 2004. May;14(5):325–328. [DOI] [PubMed] [Google Scholar]
- 18.Kochanski A, Drac H, Kabzinska D, Hausmanowa-Petrusewicz I. A novel mutation, Thr65Ala, in the MPZ gene in a patient with Charcot-Marie-Tooth type 1B disease with focally folded myelin. Neuromusc Disord. 2004;14:229–232. [DOI] [PubMed] [Google Scholar]
- 19.Kovach MJ, Campbell KC, Herman K, et al. Anticipation in a unique family with Charcot-Marie-Tooth syndrome and deafness; delineation of the clinical features and review of the literature. Am J Med Genet. 2002;108:295–303. [DOI] [PubMed] [Google Scholar]
- 20.Kumagai-Eto R, Kaseda Y, Tobimatsu S, Uozumi T, Tsuji S, Nakamura S. Subclinical cranial nerve involvement in hereditary motor and sensory neuropathy: a combined conduction study with electrical and magnetic stimulation. Clin Neurophysiol. 2004. July;115(7):1689–1696. [DOI] [PubMed] [Google Scholar]
- 21.Leal A, Berghoff C, Berghoff M, Rojas-Araya M. Carolina O, Heuss D, Del Valle G, Rautenstrauss B. A Costa Rican family affected with Charcot-Marie-Tooth disease due to the myelin protein zero (MPZ)p.Thr124Met mutation shares the Belgian haplotype. Rev Biol Trop. 2014. December;62(4):1285–1293. [DOI] [PubMed] [Google Scholar]
- 22.Manole A, Horga A, Gamez J, Raguer N, Salvado M, San Millan B, Navarro C, Pittmann A, Reilly MM, Houlden H. SBF1 mutations associated with autosomal recessive axonal neuropathy with cranial nerve involvement. Neurogenetics 2017. January;18(1):63–67. [DOI] [PubMed] [Google Scholar]
- 23.Merchant SN. Quantitative cochlear otopathology. pp 34–43. In: Merchant SN, Nadol JB Jr: Schuknecht’s Pathology of the Ear Third edition. People’s Medical Publishing House; USA, Shelton, CT: 2010. [Google Scholar]
- 24.Misu K, Yoshihara T, Shikama Y, et al. An axonal form of Charcot-Marie-Tooth disease showing distinctive features in association with mutations in the peripheral myelin protein zero gene (Thr124Met or Asp75Val). J Neurol Neurosurg Psychiatry. 2000;69:806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Numakura C, Lin C, Ikegami T, Guldberg P, Hayasaka K. Molecular analysis in Japanese patients with Charcot-Marie-Tooth disease: DGGE analysis for PMP2, MPZ, and Cx32/GJB1 mutations. Hum Mutat 2002. November;20(5):392–398. [DOI] [PubMed] [Google Scholar]
- 26.Pareyson D, Taroni F, Botti S, Morbin M, Baratta S, Lauria G, Ciano C, Sghirlanzoni A. Cranial nerve involvement in CMT disease type 1 due to early growth response 2 gene mutation. Neurology. 2000;54:1696–1698. [DOI] [PubMed] [Google Scholar]
- 27.Poretti A, Palla A, Tarnutzer AA, Petersen JA, Weber KP, Straumann D, Jung HH. Vestibular impairment in patients with Charcot-Marie-Tooth disease. Neurology. 2013: June 4; 80(23):2099–2105. [DOI] [PubMed] [Google Scholar]
- 28.Posa A, Emmer A, Kornhuber ME. Unilateral oculomotor palsy in Charcot-Marie-Tooth disease 1A (CMT 1A). clin Neurol Neurosurg. 2017. April;155:20–21. [DOI] [PubMed] [Google Scholar]
- 29.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015. 2015. May;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeman P, Mazanec R, Huehne K, Suslikova P, Keller O, Rautenstrauss B. Hearing loss as the first feature of late-onset axonal CMT disease due to a novel P0 mutation. Neurology. 2004. August 24;63(4):733–735. [DOI] [PubMed] [Google Scholar]
- 31.Sevilla T, Jaijo T, Nauffal D, Collado D, Chumillas MJ, Vilchez JJ, Muelas N, Bataller L, Domenech R, Espinos C, Palau F. Vocal cord paresis and diaphragmatic dysfunction are severe and frequent symptoms of GDAP1-associated neuropathy. Brain. 2008. November;131(Pt11):3051–3061. [DOI] [PubMed] [Google Scholar]
- 32.Sivera R, Cavalle L, Vilchez JJ, Espinos C, Perez Garrigues H, Sevilla T. Audiological findings in charcot-Marie-Tooth disease type 4c. J Int Adv Otol. 2017. April;13(1);93–99. [DOI] [PubMed] [Google Scholar]
- 33.Starr A, Michalewski HJ, Zeng F-G, Fujikawa-Brooks S, Linthicum F, Kim CS, Winnier D, Keats B. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145→Ser). Brain. 2003;126:1604–1619;2003. [DOI] [PubMed] [Google Scholar]
- 34.Synofzik M, Müller von Hagen J, Haack TB, Wilhelm C, Lindig T, Beck-Wödl S, Nabuurs SB, van Kuilenburg AB, de Brouwer AP, Schöls L. X-linked Charcot-Marie-Tooth disease, Arts syndrome, and prelingual non-syndromic deafness form a disease continuum: evidence from a family with a novel PRPS1 mutation. Orphanet J. Rare Dis 2014; February 14:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokuda N, Noto Y, Kitani-Morii F, Hamano A, Kasai T, Shiga K, Mizuta L, Niwa F, Nakagawa M, Mizuno T. Parasympathetic dominant autonomic dysfunction in Charcot-Marie-Tooth Disease type 2J with the MPZ Thr124Met mutation. Intern Med. 2015;54(15):1919–1922. [DOI] [PubMed] [Google Scholar]
- 36.Triantafyllou N, Rombos A, Athanasopoulou H, Siafakas A, Loulakaki SM. Electrophysiological study (EEG, VEPs, BAEPs) in patients with Charcot-Marie-Tooth (type HMSN 1) disease. Electromyogr Clin Neurophysiol. 1989. Jul-Aug;29(5):259–263. [PubMed] [Google Scholar]
- 37.Yger M, Stojkovic T, Tardieu S, Maisonobe T, Brice A, Echaniz-Laguna A, Alembik Y, Girard S, Cazeneuve C, Leguern E, Dubourg, O. Characteristics of clinical and electrophysiological pattern of Charcot-Marie-Tooth 4C. J Peripher Nerv Syst. 2012. March;17(1):112–222 [DOI] [PubMed] [Google Scholar]