Abstract
Introduction:
Treat to target (TTT) is an accepted paradigm for care of patients with rheumatoid arthritis (RA). Since TTT can be associated with more medication switches, concerns arise whether implementing TTT may increase adverse events and/or resource use.
Methods:
We used data from six practices enrolled in an 18-month cluster-randomized controlled trial to compare adverse events and resource use before (months 1–9) and during (months 10–18) a TTT intervention. The outcomes of interest, adverse events and resource use, were based on medical record review of all rheumatology visits for RA patients before and during the intervention.
Results:
We examined records for 321 patients before the intervention and 315 during the intervention. An adverse event was recorded in 10.2% of visits before the intervention and 8.8% during the intervention (P = 0.41). Biologic DMARDs were used in 53.6% of patients before the intervention and 49.8% during the intervention (p = 0.73). Rheumatology visits were more frequent before the intervention (mean 4.0 ± 1.4) than during the intervention (mean 3.6 ± 1.2; p = 0.02). More visits were accompanied by monitoring laboratory tests before the intervention (90.0%) compared with during the intervention (52.7%; p <0.001). A greater percentage of visits before the intervention included diagnostic imaging (15.4%) versus during the intervention (8.9%; p<0.001).
Conclusions:
We observed similar rates of adverse events before and during the implementation of TTT for RA. Rheumatology visits, use of laboratory monitoring, and diagnostic imaging did not increase during the TTT intervention.
INTRODUCTION
Treat to target (TTT) has become a widely endorsed paradigm for treatment of rheumatoid arthritis (RA). This involves provider and patient setting a target disease activity, measurement of disease activity at each visit, adjustment of treatments until target disease activity is met. Shared decision-making is used to both set the target and determine treatments.(1) We worked with 11 rheumatology practice sites in the US in a cluster-randomized controlled trial to test a Learning Collaborative intervention for improving implementation of TTT.(2) During the trial, we measured the implementation of TTT using a standardized medical record review and each component was noted as present or absent and the percent of components at each visit was averaged. The intervention increased TTT implementation from a baseline of 11% to 57% after the 9-month LC.(3) A very similar level of improvement was seen in a second phase of the work among the sites originally randomized to wait-list control.(4)
In other clinical areas, the implementation of a TTT paradigm has been associated with an increase in adverse events. One trial that tested a TTT intervention for diabetes found an increased risk of hypoglycemia and death when using a target glycosylated hemoglobin level.(5) While some hypertension trials have shown improvements in clinical outcomes when targeting a goal blood pressure, a large meta-analysis demonstrated an increased risk of severe hypotension when targeting blood pressure.(6–8) One medical society has recently recommended a less aggressive target threshold for hemoglobin A1c.(9) There are also concerns that implementing a TTT paradigm might increase resource use.
We conducted a post-hoc analysis of the TTT intervention trial to examine adverse events and resource use during the pre-intervention and intervention periods of the trial.
METHODS
Study design:
The current analyses examined six rheumatology sites that were part of a cluster-randomized controlled trial testing a Learning Collaborative to improve implementation of TTT (see Figure describing overall study design). Details of the Learning Collaborative (LC) have been described (supplement).(2) Briefly, the LC expert faculty provided guidance to teams through learning sessions. Teams worked on process improvement through tests of small changes in their practice. They subsequently evaluated these changes and adopted those that worked. Tests of change were conducted over several-day cycles. The faculty reviewed results and provided feedback. Teams focused on routine metrics collected across all sites and attempted to spread successful interventions to the broader provider group. Implementing TTT required a modified RA treatment discussion for some providers and a change in documentation. Providers worked with patients to choose a disease target; typically, low disease activity or remission. The practices were required to select a measure and then use it routinely at all visits. Providers were asked to respond to the disease activity measure when the target had not been reached. This required adjustment of treatment or documentation of why no changes were made. The LC also involved learning sessions; the first one was a one-day face-to-face meeting. Subsequent learning sessions were conducted via webinar, approximately once per month. All team members from each site were expected to attend these calls but this was not always possible. All learning sessions were recorded and made available on the web-based collaborative tool.
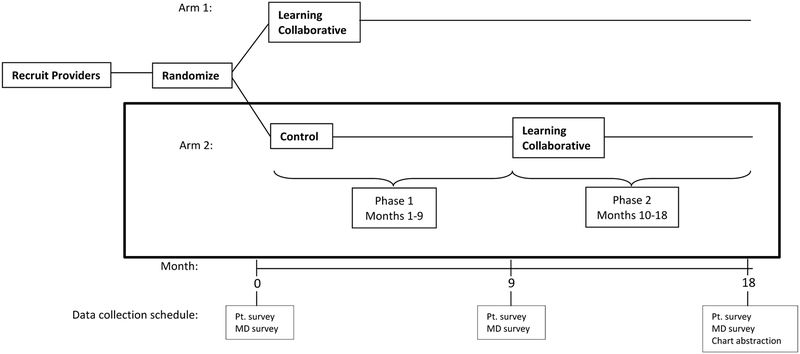
Figure:
TRACTION Trial Study Design
This figure shows the design of the TRACTION trial. Phase 1 was the cluster randomized controlled clinical trial comparing the Learning Collaborative intervention with a wait-list control group. Phase 2 provided the Learning Collaborative intervention to the Phase 1 control group while the Phase 1 intervention group is observed. The current paper compares the same six Control sites during Phase 1 to Phase 2 (outlined with the black box), with respect to adverse events and resource utilization.
For the current analyses, we used the before and during intervention data from these sites.(3) The original trial comprised two 9-month periods: during the first nine months, the six sites were in the wait-list control group (before intervention) and during the second nine months, they received the intervention. We used these two periods to examine adverse events and resource use before and during TTT implementation.
The appropriate institutional review boards approved all study activities.
Study population:
Each site chose at least 30 patients with RA seen during the periods of interest -- three months prior to intervention and the last three months of the intervention period. (Three months was chosen as the sampling frame since most patients with RA will have at least one visit every three months.) The intervention for these six sites occurred over nine months from November 2015 – July 2016. The patients were chosen randomly from these two three-month periods. The sites were all rheumatology practices from across the US. They all had at least two rheumatology providers and two included non-physician providers. Four sites had an academic affiliation.
Outcomes: adverse events and resource use:
We examined all visits for the selected patients during the two study periods described above, assessing for possible medication related adverse events and resource use. Adverse events of interest included: rashes, oral ulcers or mouth pain, alopecia, infections requiring antibiotics, liver toxicity as manifest by abnormal liver function tests (above the upper limits of normal) and/or abnormal liver imaging, cytopenias as manifest by complete blood counts below the lower limits of normal, renal insufficiency defined as a 50% decrease in creatinine clearance, a new cancer diagnosis, gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea, unexplained weight loss/gain, abdominal pain or dyspepsia), and other miscellaneous side effects. Three trained research assistants reviewed all the medical records using a standardized data abstraction form.
The same set of visits was assessed for resource use. We inspected visit notes, laboratory records, prescription lists, and imaging data for the following categories: prescription of biologic and non-biologic disease modifying anti-rheumatic drugs (DMARDs); orders and completion of monitoring laboratory tests, such as complete blood counts, liver function tests, serum creatinine, erythrocyte sedimentation rate, and C-reactive protein; and orders and completion of diagnostic imaging, such as plain radiographs, CT scans, magnetic resonance imaging, or dual energy x-ray absorptiometry. Most sites did not do routine joint ultrasounds and these were not included as part of the diagnostic imaging assessment.
Each aspect of the data analyzed was determined as absent or present based on a medical record review by trained study staff (inter-rater kappa = 94% (95% confidence interval 90–99%), and intra-rater reliability kappa = 98% (95% confidence interval 95–99%)).
Statistical analyses:
To compare adverse events across periods, we assessed the following metrics: the percent of visits during the period with any of the above adverse events, the percent of patients with any of the above adverse events, and the mean number of any of the above adverse events per patient. We also calculated similar metrics for resource use: the percent of visits with each resource used, the percent of patients with each resource used, and the mean number of resources used per patient.
The samples of patients at each site across the two periods were different. We described and compared the baseline characteristics of patients during the three months prior to the control period and the three months prior to the intervention using two sample t tests, Chi-square tests or non-parametric tests when applicable. Then, the metrics for adverse events and resource use were compared across the two periods. Due to hierarchical structure of data, we used generalized linear mixed models (GLMM) to adjust for site effect and within-provider correlation. GLMM for binary, Poisson, or negative binomial outcomes were used based on distributions of adverse events and resource use in the analysis. All analyses were performed using SAS 9.4 (Cary, NC).
RESULTS
We examined records for 321 patients included in the assessment for the 9 months before the intervention and 315 patients during the 9 months of the intervention (see Table 1); this included 1284 visits before and 1134 visits during the intervention. Patients were similar in all respects across the two periods: mean (SD) age 60 (14) years, 81% female, and 76% seropositive. There was a similar proportion of patients using biologic DMARDs in the period before the intervention (40.8%) compared with during the intervention (38.7%) (p = 0.59). The percentage with joint erosions was also similar: 53.4% before and 52.6% during the intervention (p = 0.86).
Table 1:
Characteristics of Patients Before and During the Treat to Target Intervention
| Before Intervention (n = 321) |
During Intervention (n = 315) |
P-value | |
|---|---|---|---|
| N (%) unless noted | |||
| Age, years, mean (SD) | 59.7 (14.3) | 61.0 (13.5) | 0.28 |
| BMI*, kg/m2, mean (SD) | 30.1 (7.5) | 30.0 (8.1) | 0.90 |
| Female sex | 250 (77.9) | 260 (82.5) | 0.14 |
| RA duration*, years | 0.47 | ||
| ≤ 2 | 22 (16.1) | 19 (11.3) | |
| 2–5 | 39 (28.5) | 52 (31.0) | |
| 6–10 | 30 (21.9) | 31 (18.5) | |
| >10 | 46 (33.6) | 66 (39.3) | |
| Serologic status* | 0.94 | ||
| Positive | 193 (76.3) | 180 (76.6) | |
| Negative | 60 (23.7) | 55 (23.4) | |
| Use of synthetic DMARDs | 248 (77.3) | 248 (78.7) | 0.65 |
| Use of biologic DMARDs | 131 (40.8) | 122 (38.7) | 0.59 |
| Comorbidity index, mean (SD) | 1.33 (0.6) | 1.31 (0.7) | 0.62 |
| Joint erosion | 0.86 | ||
| Yes | 109 (53.4) | 103 (52.6) | |
| No | 95 (46.6) | 93 (47.5) | |
| Total medications | 0.14 | ||
| 0 | 0 (0.0) | 0 (0.0) | |
| 1–5 | 42 (13.1) | 26 (8.3) | |
| 6–10 | 104 (32.4) | 105 (33.2) | |
| 10+ | 175 (54.5) | 184 (58.4) | |
Notes:
Data were missing for the following variables: age, missing = 64; body mass index, missing = 150; rheumatoid arthritis duration, missing = 331; serologic status, missing = 148; and joint erosions, missing = 236. Abbreviations: BMI, body mass index; RA, rheumatoid arthritis; DMARD, disease modifying anti-rheumatic drug.
Adverse events were similar across periods under consideration (see Table 2). Any adverse event was recorded in 10.2% of visits before the intervention and 8.8% during the intervention (adjusted p = 0.41). The percent of patients with an abnormal liver function test was slightly greater in the period before the intervention (0.8%) than during the intervention (0.3%) (adjusted p = 0.12). Mucocutaneous adverse events (alopecia, oral ulcers or any rash) were also slightly more common in the period before the intervention (1.7%) than during the intervention (0.8%) (adjusted p = 0.07). The percent of patients who experienced an infection trended higher during the intervention (12.1%) compared with before the intervention (9.4%) (adjusted p = 0.18). Gastrointestinal symptoms were experienced by a similar percentage of patients in both periods: 2.2% before and 2.2% during the intervention (adjusted p = 0.79).
Table 2:
Adverse Events Before and During the Treat to Target Intervention
| Before intervention | During intervention | P-value ǂ | Difference (95% CI) | ||
|---|---|---|---|---|---|
| Any adverse event* | |||||
| Percent of visits | 10.2% | 8.8% | 0.41 | −1.4% (−3.7,1.0%) | |
| Percent of patients | 29.6% | 25.7% | 0.45 | −3.9% (−10.8, 3.1%) | |
| Number per patient, mean | 0.43 | 0.35 | 0.18 | −0.1% (−0.2, 0.1%) | |
| Abnormal liver function tests | |||||
| Percent of visits | 0.8% | 0.3% | 0.12 | −0.5% (−1.1, 0.1%) | |
| Percent of patients | 2.8% | 1.0% | 0.11 | −1.8% (−4.0, 0.3%) | |
| Number per patient, mean | 0.03 | 0.01 | 0.08 | −0.02% (−0.1, 0.1%) | |
| Rash/oral ulcers/alopecia | |||||
| Percent of visits | 1.7% | 0.8% | 0.07 | −0.9% (−1.7, 0.1%) | |
| Percent of patients | 5.9% | 2.9% | 0.07 | −3.0% (−6.2, 0.1%) | |
| Number per patient, mean | 0.07 | 0.03 | 0.04 | −0.04% (−0.1, 0.00%) | |
| Infections | |||||
| Percent of visits | 2.7% | 3.8% | 0.07 | 1.1% (−0.3, 2.6%) | |
| Percent of patients | 9.4% | 12.1% | 0.18 | 2.7% (−2.1, 7.5%) | |
| Number per patient, mean | 0.11 | 0.14 | 0.13 | 0.03% (0.0, 0.1%) | |
| Gastrointestinal symptoms | |||||
| Percent of visits | 2.2% | 2.2% | 0.79 | 0.0% (−1.2, 1.2%) | |
| Percent of patients | 6.5% | 7.9% | 0.37 | 1.4% (−2.6, 5.4%) | |
| Number per patient, mean | 0.09 | 0.08 | 0.96 | 0.01% (−0.1, 0.1%) | |
| Rheumatology visits | |||||
| Number per patient, mean (± SD) | 4.0 (1.4) | 3.6 (1.2) | 0.02 | −0.4 (−0.6, −0.2) | |
| Biologic DMARDs used | |||||
| Percent of visits | 46.5% | 42.5% | 0.76 | −3.9% (−7.9, 0.0%) | |
| Percent of patients | 53.6% | 49.8% | 0.73 | −3.7% (−11.5, 4.0%) | |
| Monitoring laboratory tests¶ | |||||
| Percent of patients | 90.0% | 52.7% | <0.001 | −37% (−44, −31%) | |
| Number per patient, mean (± SD) | 10.6 (6.3) | 5.1 (6.3) | <0.001 | −5.5 (−6.5, −4.5) | |
| Diagnostic imaging¶ | |||||
| Percent of visits | 15.4% | 8.9% | 0.005 | −6.5% (−9.7, −3.2%) | |
| Percent of patients | 38.6% | 12.4% | <0.001 | −26% (−33, −20%) | |
| Number per patient, mean (± SD) | 1.0 (2.0) | 0.3 (1.3) | <0.001 | −0.7 (−1.0, −0.4) | |
Adverse events included rashes, oral ulcers, alopecia, infections requiring antibiotics, liver toxicity as manifest by abnormal liver function tests and/or abnormal liver imaging, cytopenias as manifest by complete blood counts below the lower limits of normal, renal insufficiency defined as a 50% decrease in creatinine clearance, cancer, and gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea, unexplained weight loss/gain, abdominal pain or dyspepsia).
P-values from generalized linear mixed models adjusting for site effect, and within provider clustering.
Monitoring laboratory tests included complete blood count, liver function tests, serum creatinine, and acute phase reactants. Diagnostic imaging included DXA, plain radiographs, computed tomography (CT) scans, and magnetic resonance imaging. We did not calculate the percent of visits with laboratory tests because many tests took place between visits.
Finally, we compared resource use across the two periods (see Table 2). Rheumatologist visits during the 9-months before the intervention were slightly more frequent, mean 4.0 (1.4), than during the intervention, 3.6 (1.2) (p = 0.02). Biologic DMARDs were used in 53.6% of patients before the intervention and 49.8% during the intervention (p = 0.73). Rheumatology visits were more frequent before the intervention (mean 4.0 ± 1.4) than during the intervention (mean 3.6 ± 1.2; p = 0.02). More visits were accompanied by monitoring laboratory tests before the intervention (90.0%) compared with during the intervention (52.7%; p <0.001). A greater percentage of visits before the intervention included diagnostic imaging (15.4%) versus during the intervention (8.9%; p<0.001).
DISCUSSION
Ample evidence supports TTT as an effective paradigm for managing RA.(10) While there appear to be opportunities for enhancing use of TTT in rheumatology practice,(11, 12) understanding the potential for excess adverse events and resource use is required to optimize implementation. Using data from a recently completed randomized controlled trial, we examined adverse event rates and resource use in six rheumatology practices prior to, and during, implementation of TTT. We did not observe clinically important or statistically significant increases in adverse events during the intervention. We saw no increase in the use of biologic DMARDs, laboratory monitoring, diagnostic imaging, or overall rheumatology visits during the intervention.
The lack of an increase in adverse events should provide some reassurance to providers and patients. Treating to target in other chronic disease areas, such as diabetes and hypertension, has been associated with excess adverse events. (5, 6, 8) Our results are consistent with other trials of TTT which did not observe an increase in drug toxicity.(10)
Resource use differed across time periods, but appeared to be slightly reduced during the TTT intervention. It may be that using a more systematic TTT approach reduced the need for the use of the resources we measured. We believe that the important result is that no increase in resource use was observed. However, it may be that the slight improvement in disease activity that we observed during TTT was associated with a reduced need for resources. It is also possible that this finding was based on chance because of a relatively small sample size or some degree of misclassification that differed across time periods producing a biased result; this seems unlikely.
We acknowledge several strengths and limitations. The fact that the same six sites were examined before and during the TTT intervention limit the possible confounding bias. However, secular trends over the 18-months of the study period could have impacted the results. Further, six rheumatology practices may not be representative of national trends. A standardized review of medical records was performed centrally to reduce inter-observer variability, but medical records may not perfectly represent all adverse events and resources used during the two periods studied. A larger sample size may have yielded a statistically significant increase in infections.
In conclusion, we did not observe an increase in overall adverse events or resource use associated with TTT. Prior work demonstrates the clinical benefits of TTT, which may translate into a reduction in resource use. But, most importantly, patients treated with a TTT approach do not appear to be at risk of increased adverse events.
Supplementary Material
SIGNIFICANCE AND INNOVATION.
Among six rheumatology practices enrolled in a prospective randomized trial, we observed no increase in adverse events when the sites implemented treat to target for rheumatoid arthritis.
No increase was observed in health care resource use when implementing treat to target for rheumatoid arthritis.
Acknowledgment/Support:
NIH-P60-AR047782. Research reported in this publication was also supported by NIH-K24-AR060231 (DHS), NIH-K24-AR060231 (LF), a Rheumatology Research Foundation Innovative Research Grant (LF)
Footnotes
Potential Conflicts:
DHS receives research support from grants to his institution from Amgen, Pfizer, BMS, Genentech, and Abbvie. As well, he serves as an epidemiology consultant to Corrona. ZY: none. JNK: none. AB: none. CC: none. LF: none. LH: employee of UMass Medical School and Corrona, LLC; consultant to BMS and Roche (both <$10,000). EL: statistical consultant to TissueGene, consultant to Samumed. BL: none.
Clinical Trials Registration: NCT02260778 for the original TRACTION trial.
Contributor Information
Daniel H. Solomon, Email: dsolomon@bwh.harvard.edu.
Zhi Yu, Email: zhy872@mail.harvard.edu.
Jeffrey N. Katz, Email: jnkatz@bwh.harvard.edu.
Asaf Bitton, Email: abitton@bwh.harvard.edu.
Cassandra Corrigan, Email: ccorrigan2@bwh.harvard.edu.
Liana Fraenkel, Email: liana.fraenkel@yale.edu.
Leslie R. Harrold, Email: leslie.harrold@umassmed.edu.
Josef S. Smolen, Email: josef.smolen@wienkav.at.
Elena Losina, Email: elosina@bwh.harvard.edu.
Bing Lu, Email: blu@bwh.harvard.edu.
CITATIONS
- 1.Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon DH, Lee SB, Zak A, Corrigan C, Agosti J, Bitton A, et al. Implementation of treat-to-target in rheumatoid arthritis through a Learning Collaborative: Rationale and design of the TRACTION trial. Semin Arthritis Rheum 2016;46(1):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon DH, Losina E, Lu B, Zak A, Corrigan C, Lee SB, et al. Implementation of Treat-to-Target in Rheumatoid Arthritis Through a Learning Collaborative: Results of a Randomized Controlled Trial. Arthritis Rheumatol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon DH, Lu B, Yu Z, Corrigan C, Harrold LR, Smolen JS, et al. Benefits and Sustainability of a Learning Collaborative for Implementation of Treat to Target in Rheumatoid Arthritis: Results of the TRACTION Trial Phase II. Arthritis Care Res (Hoboken) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr., et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364(9):818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogihara T Practitioner’s Trial on the Efficacy of Antihypertensive Treatment in the Elderly Hypertension (The PATE-Hypertension Study) in Japan. Am J Hypertens 2000;13(5 Pt 1): 461–7. [DOI] [PubMed] [Google Scholar]
- 7.Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res 2009;32(1):3–107. [PubMed] [Google Scholar]
- 8.Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet 2016;387(10017):435–43. [DOI] [PubMed] [Google Scholar]
- 9.Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA. Hemoglobin A1c Targets for Glycemic Control With Pharmacologic Therapy for Nonpregnant Adults With Type 2 Diabetes Mellitus: A Guidance Statement Update From the American College of Physicians. Ann Intern Med 2018. [DOI] [PubMed] [Google Scholar]
- 10.Schoels M, Knevel R, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas DT, et al. Evidence for treating rheumatoid arthritis to target: results of a systematic literature search. Ann Rheum Dis 2010;69(4):638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrold LR, Reed GW, Harrington JT, Barr CJ, Saunders KC, Gibofsky A, et al. The rheumatoid arthritis treat-to-target trial: a cluster randomized trial within the Corrona rheumatology network. BMC Musculoskelet Disord 2014;15:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tymms K, Zochling J, Scott J, Bird P, Burnet S, de Jager J, et al. Barriers to optimal disease control for rheumatoid arthritis patients with moderate and high disease activity. Arthritis Care Res (Hoboken) 2014;66(2):190–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.