Abstract
Objective
Calcification of the coronary arteries, aorta, and branch vessels can occur in both large-vessel vasculitis (LVV) and atherosclerosis. The study objective was to determine the location and amount of vascular calcification in patients with LVV versus hyperlipidemia (HLD) and to identify risk factors associated with vascular calcification in LVV.
Methods
Patients with giant cell arteritis (GCA), Takayasu’s arteritis (TAK), and HLD underwent non-contrast computed tomography of the aorta and branch vessels. Vascular calcification in 14 specific arterial territories (4 segments of the aorta, 9 branch arteries, and the coronary arteries) was quantified throughout the large arteries by a cumulative Agatston score. Multivariate linear regression analyses were used to identify associations between traditional and disease-specific risk factors and total Agatston score.
Results
A total of 88 subjects, including GCA (n=29); TAK (n=22); and HLD (n=37), participated.Prevalence of vascular calcification in the aorta and branch vessels significantly differed in the coronary arteries (HLD=67%, GCA=35%, TAK=9%, p<0.01). Total Agatston scores were higher in GCA (median 3260, range 25-18138) versus HLD (460, 19-17215) (p<0.01) but did not significantly differ between GCA and TAK (1944, 52-47520) (p=0.53). In multivariable regression analysis, age, type of vasculitis, and prednisone use was associated with vascular calcification in LVV.
Conclusion
The prevalence of coronary artery calcification is lower in LVV compared to HLD, but the amount of total vascular calcification throughout the large arteries is greater in LVV. Both traditional and disease-specific risk factors are associated with vascular calcification in LVV.
Keywords: large-vessel vasculitis, giant cell arteritis, Takayasu’s arteritis, vascular calcification, hyperlipidemia, cardiovascular disease
1.0. INTRODUCTION
Arterial wall calcification in the aorta, coronary, and peripheral arteries is a well-recognized complication in atherosclerosis and is a major cardiovascular risk factor (1). Coronary artery calcification, in particular, has been associated with increased cardiovascular morbidity and mortality (2, 3). Vascular calcification is also a known complication in patients with large-vessel vasculitis (LVV) (4-9), which is a group of disorders characterized by inflammation of the aorta and its primary branches. The two major forms of LVV are giant cell arteritis (GCA) and Takayasu’s arteritis (TAK), which are often differentiated based on the age of the patient at disease onset. GCA is a disease of older patients aged 50 and above, whereas TAK is typically diagnosed in patients aged 40 or younger (10).
Cardiovascular complications resulting from accelerated atherosclerosis and vascular calcification is a significant cause of morbidity and mortality in patients with LVV (4). Previous studies reported atherosclerotic changes and vascular calcification in TAK, particularly in areas of vascular inflammation (4). Older necropsy and biopsy studies in patients with GCA have demonstrated deposition of calcium in the large arteries (11-13). There is limited literature on the precise location and extent of vascular calcification in LVV. Studies have described calcification in the aorta, coronary arteries, and carotid plaques in patients with Takayasu’s arteritis (4, 14). There are few case reports and case series describing calcification in the subclavian and iliac arteries in Takayasu’s arteritis (7, 15). There are no reports on the prevalence and extent of vascular calcification in GCA.
Although there is literature on vascular calcification in patients with atherosclerosis, comparative data between LVV and atherosclerosis is limited. A higher prevalence of calcification was reported in patients with atherosclerosis compared to TAK (16). There are no reports comparing the location and burden of calcification in GCA versus atherosclerosis or TAK. Predisposing factors for vascular calcification in patients with LVV is also an area of active research. In addition to vascular inflammation, traditional cardiovascular risk factors, such as age and elevated systolic blood pressure, increase the risk of vascular calcification in TAK (4), and no studies have focused upon risk factors for vascular calcification in GCA. Although, cardiovascular complications are well described in patients with LVV, there are no guidelines for physicians to monitor these patients for cardiovascular risks. A recent study indicated that only 23% of patients with TAK were receiving statin therapy (17)
Understanding the pattern, burden, and risk factors for vascular calcification in patients with LVV compared to a population of patients who do not have vasculitis but are at high risk for atherosclerosis could inform cardiovascular risk assessment and management strategies in LVV. The study objectives were to compare the location and amount of vascular calcification in TAK, GCA and hyperlipidemia (HLD) and to determine the risk factors associated with vascular calcification in LVV.
2.0. PATIENTS AND METHODS
2.1. Patient population
Subjects with GCA, TAK, and HLD were recruited into observational cohorts at the National Institutes of Health (NIH) between October 2013 - February 2017. Patients with GCA or TAK fulfilled the 1990 American College of Rheumatology (ACR) Classification Criteria for these diseases (18, 19) or modified criteria for GCA (20). All subjects with LVV underwent detailed clinical evaluation and laboratory investigations with prospective data collection. Active disease was defined by presence of at least one clinical symptom directly attributed to ongoing vasculitis by the clinical investigative team. Clinical assessments were performed blinded to imaging data. Patients with hyperlipidemia over the age of 55 were prospectively enrolled in a parallel study as previously described (21).
All study subjects provided written informed consent (NCT02257866, NCT0121900), and the study was approved by local ethics and radiation safety committees at the NIH. All procedures performed in studies involving human participants were in accordance with the declaration of Helsinki.
2.2. Imaging Technique
The same 128-slice computed tomography (CT) scanner was used to perform imaging on patients in both the HLD and LVV cohorts. However, the radiation dose as measured in milliampere seconds (mAs) was higher for the patients with HLD (56mAs) compared to the patients with LVV (17mAs). CT images for the patients with LVV were obtained from 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) studies performed for other research purposes, as previously described (22). The CT study for the patients with HLD was performed with 120 kVp, 1.5 mm slice thickness, helical mode and without intravenous contrast.
2.3. Quantification of Vascular Calcification
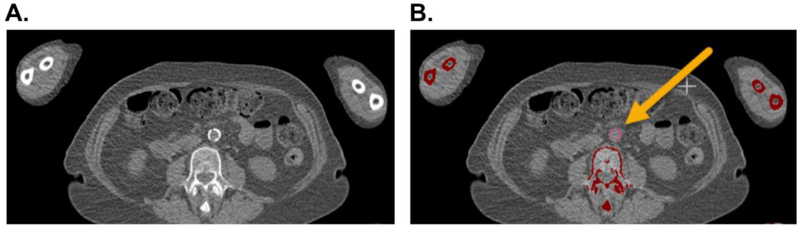
For this study, our group developed a semi-automated software to compute vascular calcification in 14 specific arterial territories (ascending aorta, aortic arch, descending thoracic aorta, abdominal aorta, carotids (right and left), subclavians (right and left), innominate, iliacs (right and left), femorals (right and left) and coronary arteries). In the software, the CT volume is first denoised using Gaussian smoothing and reformatted at 3mm thickness for standardized calcium score quantification. A threshold of 130 Hounsfield units (HU) is applied to generate all calcification candidates in the images. Through an interactive tool, an operator blinded to clinical information, selects true calcification candidates by either clicking at the centers or circling the regions and assigning them to specific arterial territories (Figure 1). Agatston score is a weighted score by HU of each pixel, score of 1 for 130–199 HU, 2 for 200–299 HU, 3 for 300–399 HU, and 4 for 400 HU and greater. This weighted score is then multiplied by the summed area (in square millimeters) of calcification of all the arteries. Agatston scores of 1000 or higher were considered to be high based on available literature on abdominal aortic calcification in diabetic subjects (23). Quantification of vascular calcification on all the scans was performed by two independent experts who were blinded to the clinical status of the subjects.
Figure 1.
Semi-Automated Software to Assess Vascular Calcification. Computed tomography of a study patient showing vascular calcification in the abdominal aorta on axial view (Panel A). The area of vascular calcification is highlighted (pink) and manually assigned to the abdominal aorta territory (Panel B). An Agatston score is calculated as a weighted score based upon Hounsfield units of each pixel multiplied by the summed area (in square millimeters) of calcification within each arterial territory.
2.4. Data Analysis
The prevalence of vascular calcification in specific arterial territories was compared across groups (GCA, TAK, HLD) using the chi-square test. Cumulative Agatston scores were compared across groups by the Kruskal-Wallis test with post-hoc Dunn’s test to account for multiple comparisons. To determine the associations between total Agatston score and traditional or disease-specific risk factors, multivariate linear regression analyses were performed only in patients with LVV. Agatston scores were log-transformed for the linear regression analyses, and the resultant effect estimates were back translated for ease of interpretation. Traditional risk factors included in the analysis were age, gender, body mass index, smoking history, statin use and hypertension. Hypertension was defined by a history of elevated blood pressure requiring anti-hypertensive medications. Disease-specific risk factors included type of vasculitis (GCA, TAK), disease duration from time of first symptom onset, treatment duration from time of diagnosis, clinical assessment (active disease, remission), daily prednisone dose, inflammatory markers (ESR, CRP, fibrinogen), and presence or absence of vascular disease activity as measured by positron emission tomography (FDG-PET). Two independent readers interpreted each FDG-PET scan blinded to all clinical data to determine whether the scan demonstrated active vasculitis based upon visual assessment, as previously described (22). Apart from type of vasculitis, only variables with p<0.10 in univariate analyses were included in the multivariate models.
3.0. RESULTS
3.1. Study Population
The study included 29 patients with GCA (median age = 72, %female = 79), 22 patients with TAK (median age = 37, %female = 73), and 37 patients with HLD (median age = 63, %female = 41). Patient characteristics are detailed in Table 1.
Table 1.
Study Participant Characteristics
| TAK | GCA | HLD | |
|---|---|---|---|
| Number of Patients | 22 | 29 | 37 |
| Age in years, median (range) | 34 (18-52) | 71 (54-85) | 63 (59-67) |
| BMI, Kg/m2 | 27.4 (18.5-49.7) | 26.9 (20.9-39.2) | 27.1 (25.2-29.8) |
| SBP, mmHg, median (range) | 115.5 (83-132) | 133 (98-164) | 130 (121.5-136) |
| DBP, mmHg, median (range) | 63.5 (44-78) | 62 (48-81) | 73 (65-79.5) |
| Smoker, n (%) | 0 | 0 | 16 (43) |
| HTN requiring antihypertensive medication, n (%) | 12 (55) | 17 (59) | NA |
| Statin, n (%) | 5 (23) | 7 (24) | 32 (86) |
| Anti- Diabetic medication n (%) | 1 (4) | 1 (3.4) | 0 (0) |
| Glucocorticoid, n (%) | 13 (59) | 19 (66) | NA |
| DMARD, n (%) | 14 (64) | 13 (45) | NA |
| Fasting serum glucose, mg/dl | 89.5 (68-154) | 93 (76-140) | 89 (84-97) |
| C-Reactive protein, mg/L | 4.9 (0.6-89.1) | 6.2 (0.2-127.9) | NA |
| ESR, mm/hr | 18.5 (2-55) | 16 (2-105) | NA |
| Active vascular FDG PET uptake, n (%) | 10 (45) | 19 (66) | NA |
| Physician assessment of active disease, n (%) | 10 (45) | 10 (35) | NA |
| Disease duration in years, median (range) | 12 (1.5- 38) | 1.5 (0.4-10.5) | NA |
N = number; SBP = systolic blood pressure; DBP = diastolic blood pressure; htn = hypertension; DMARD = disease modifying antirheumatic drug; FDG-PET = fluorodeoxyglucose positron emission tomography; NA = not assessed.
Among the 22 patients with TAK included in the study, 10 (45%) had clinically active disease at the time of imaging. Increased vascular FDG-PET uptake was noted in 10 patients (45%). Daily prednisone use was reported in 13 patients (59%) with a median dose of 15 mg (range 2.5-60 mg). Fourteen patients (64%) were on disease modifying antirheumatic drug (DMARD) therapy, and methotrexate was the most commonly used immunosuppressive agent (7 out of 22 patients, 32%). Among the other conventional DMARDS, mycophenolate mofetil was used in three patients, azathioprine in two patients and cyclophosphamide in one patient. Two patients were on infliximab in addition to methotrexate.
At the time of imaging assessment, out of 29 patients (34.5%) with GCA had clinically active disease. Increased arterial FDG uptake consistent with active vasculitis by PET was observed in 19 patients (66%) with GCA. Eleven (38%) patients had coexisting PMR and 13 (45%) patients had disease involving the aorta and primary branches. Most patients with GCA (19 of 29, 66%) were taking daily prednisone at a median dose of 10 mg (range 1- 60 mg), and 13 patients (45%) were taking DMARD therapy, typically methotrexate.
3.2. Prevalence of Vascular Calcification
Overall prevalence of vascular calcification was found to be high in all 3 groups of patients (Table 2). Presence of vascular calcification in at least one arterial territory was observed in the majority of patients with HLD (97%), GCA (93%) and TAK (73%). Coronary artery calcification was significantly more prevalent in HLD compared to LVV (67 vs 24%, p<0.01). Between the LVV subgroups, GCA patients had higher prevalence of coronary artery calcification than TAK (35% vs 9%, p<0.01). There was significantly greater prevalence of calcification in the aortic arch in GCA (79%) compared to TAK (36%) and HLD (51%), p<0.01. Calcification in arteries below the diaphragm (abdominal aorta, iliac, and femoral arteries) was less prevalent in TAK compared to GCA and HLD.
Table 2.
Prevalence of Vascular Calcification by Arterial Territory
| Vascular Territories | LVV N=51 | HLD N=37 | P value | GCA N=29 | TAK N=22 | HLD N=37 | P value |
|---|---|---|---|---|---|---|---|
| Ascending Aorta | 12 (23.5%) | 9 (24.3%) | 1 | 5 (17.2%) | 7 (31.8%) | 9 (24.3%) | 0.4 |
| Aortic Arch | 31 (61%) | 19 (51.4%) | 0.3 | 23 (79.3%) | 8 (36.3%) | 19 (51.4%) | 0.006 |
| Descending Thoracic Aorta | 27 (53%) | 16 (43.2%) | 0.3 | 18 (62%) | 9 (41%) | 16 (43.2%) | 0.2 |
| Abdominal Aorta | 35 (68.6%) | 35 (94.6%) | 0.002 | 24 (82.8%) | 11 (50%) | 35 (94.6%) | 0.0002 |
| BL Carotid Arteries | 17 (33.3%) | 9 (24.3%) | 0.4 | 13 (45%) | 4 (18%) | 9 (24.3%) | 0.07 |
| Innominate Artery | 3 (6%) | 4 (11%) | 0.4 | 3 (10.3%) | 0 | 4 (11%) | |
| BL Subclavian Arteries | 12 (23.5%) | 8 (21.6%) | 1 | 8 (27.6%) | 4 (18%) | 8 (21.6%) | 0.6 |
| BL Iliac Arteries | 28(55%) | 29 (78.4%) | 0.02 | 22 (76%) | 6 (27.3%) | 29 (78.4%) | 0.0001 |
| BL Femoral Arteries | 14 (27.4%) | 16 (43.2%) | 0.01 | 11 (38%) | 3 (13.6%) | 16 (43.2%) | 0.05 |
| Coronary Arteries | 12 (23.5%) | 25 (67.6%) | 0.0001 | 10 (34.5%) | 2 (9%) | 25 (67.6%) | <0.0001 |
| Presence of calcification in at least one territory | 43 (84.3%) | 36 (97.3%) | 0.07 | 27 (93%) | 16 (72.7%) | 36 (97.3%) | 0.008 |
LVV = large-vessel vasculitis; HLD = hyperlipidemia; GCA = giant cell arteritis; TAK=Takayasu’s arteritis; BL = bilateral.
3.3. Total Agatston Scores
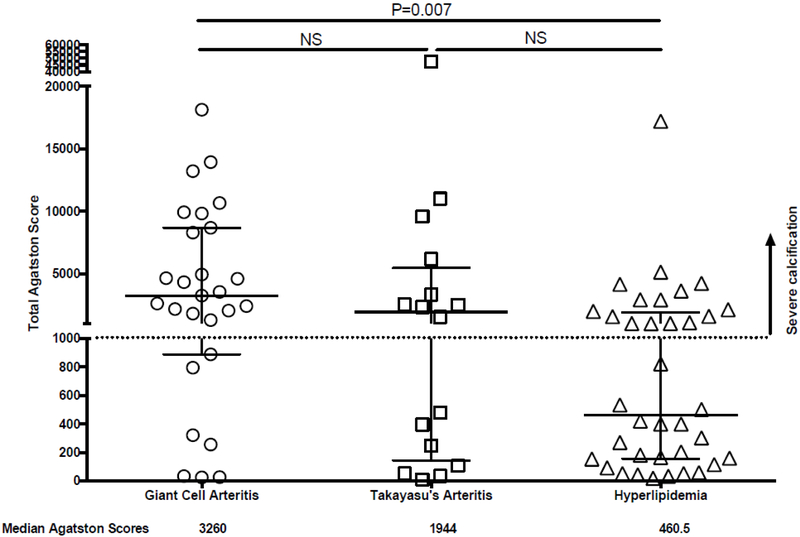
Median Agatston scores from cumulative vascular calcification in 14 arterial territories was greatest in patients with GCA (3260, interquartile range: 887-8691) compared to TAK (1994, IQR: 131-5330) and HLD (460.5, IQR: 165-1776) (Figure 2). Agatston scores were significantly higher in patients with GCA compared to HLD (p<0.01). There were no statistically significant differences in scores between GCA and TAK and between TAK and HLD. An Agatston score >1000, consistent with severe vascular calcification burden, was observed in many patients with GCA (74%), TAK (56%), and HLD (42%) (Figure 2). For each condition, a subset of patients had minimal vascular calcification, and few patients had extremely high Agatston scores (i.e. > 10,000). Representative images of severe vascular calcification in LVV are shown in Figure 3.
Figure 2.
Scatter plot of total Agatston scores throughout the large arteries. Total Agatston scores are shown for patients with giant cell arteritis, Takayasu’s arteritis, and hyperlipidemia. Median Agatston scores were significantly greater in patients with giant cell arteritis compared to patients with hyperlipidemia. Agatston scores > 1000 (dotted line) were considered representative of severe vascular inflammation. Median values and interquartile ranges are depicted. NS = not significant.
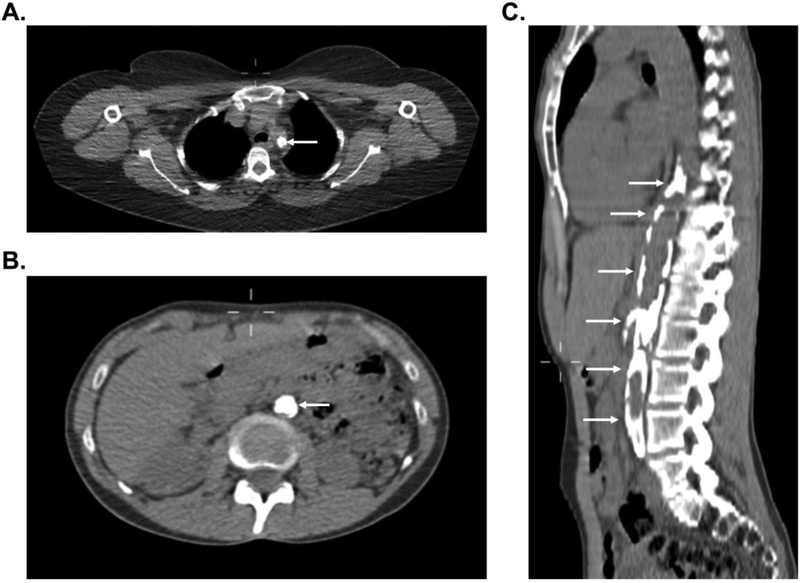
Figure 3.
Representative images of severe vascular calcification in large-vessel vasculitis. Complete occlusion with associated vascular calcification of the proximal left subclavian artery (white arrow) in a patient with Takayasu’s arteritis (Panel A). Near complete occlusion of the abdominal aorta with associated vascular calcification (white arrows) in a patient with Takayasu’s arteritis in axial view (Panel B) and sagittal view (Panel C).
3.4. Risk Factors for Vascular Calcification
In univariable regression, older age (β estimate=0.06, p=0.01), hypertension β=2.43, p<0.01), and female gender β=0.85, p=0.04) were associated with higher total Agatston score among patients with LVV. Acute phase reactants (ESR, CRP), PET scan findings, statin use, disease duration, clinical assessment, and BMI were not associated with global burden of vascular calcification as measured by the total Agatston score. In a multivariable model, older age (β=0.14, p<0.01), a diagnosis of TAK versus GCA (β=4.02, p=0.03), and daily prednisone dose(β=0.05, p=0.04) were associated with increased total Agatston score, adjusting for hypertension and gender which were no longer significantly associated with Agatston score in the multivariable model (Table 3).
Table 3.
Association of Total Agatston Score for Vascular Calcification and Clinical Variables
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| Estimate (SE) | P Value | Estimate (SE) | P Value | |
|
Age (per year) |
0.06 (1.06) | 0.01 | 0.14 (1.15) | <0.01 |
|
Type of Vasculitis (TAK vs GCA) |
−0.96 (0.38) | 0.30 | 4.02 (55.70) | 0.03 |
|
Prednisone dose (per 1mg/day) |
0.05 (1.16) | 0.08 | 0.05 (1.05) | 0.04 |
|
Anti-hypertensive Medication (Yes vs No) |
2.43 (11.35) | <0.01 | 1.16 (3.19) | 0.19 |
|
Gender (Female vs Male) |
0.85 (2.3) | 0.04 | 0.12 (1.12) | 0.90 |
|
C-reactive Protein (per 1mg/L) |
−0.03 (0.97) | 0.11 | Not included in multivariable model. | |
|
Statin Medication (Yes vs No) |
1.49 (4.43) | 0.17 | ||
|
FDG-PET interpretation (Active vs Inactive) |
0.99 (2.69) | 0.29 | ||
|
Treatment duration (per year) |
0(1) | 0.53 | ||
|
Clinical Assessment (Active vs remission) |
0.44(1.55) | 0.65 | ||
|
Disease duration (per year) |
0 (1) | 0.74 | ||
|
Body mass index (per Kg/m2) |
0.02 (1.02) | 0.77 | ||
|
Erythrocyte sedimentation rate (per 1 mm/hr) |
0.003 (1.0) | 0.89 | ||
SE = standard error; TAK = Takayasu’s arteritis; GCA = giant cell arteritis, FDG-PET = 18F-fluorodeoxyglucose positron emission tomography.
4.0. DISCUSSION
Vascular calcification is a known complication of atherosclerosis and LVV (4, 6, 24). In this study, vascular calcification in at least one arterial territory was observed in the majority of patients with hyperlipidemia (97%) and LVV (84%). Similarly, Sharma et al. reported a high prevalence of vascular calcification in patients with atherosclerosis (n=12) and patients with TAK (n=24) (100% vs 54%) (16). Location of vascular calcification was similar between patients with LVV versus hyperlipidemia, with the notable exception of the coronary arteries. This study, the first to quantify the burden of vascular calcification throughout the large arteries in patients with LVV compared to patients with hyperlipidemia, revealed a significantly higher burden of vascular calcification in patients with LVV compared to patients with hyperlipidemia.
The prevalence of coronary artery calcification was significantly greater in patients with hyperlipidemia (67%) compared to patients with GCA (35%) or TAK (9%). Coronary arteries are not commonly directly affected by vasculitis in patients with LVV, and the relative absence of local inflammation could explain the disparity between coronary calcification and major arterial calcification in GCA and TAK (25). Age may also explain the low prevalence of coronary artery calcification in TAK. Similar to our study, Seyahi et al. reported an 11% prevalence of coronary calcification in TAK and found no differences in coronary calcification in these patients compared to healthy volunteers (4). Our study also demonstrated that coronary calcification was significantly lower in patients with GCA compared to patients with hyperlipidemia, despite similarities in age among these groups. Only 27% of patients with GCA in this study were males, compared to 57% in the hyperlipidemia group. In addition, none of our GCA patients were smokers in contrast to the HLD group, where 59% reported smoking history, underscoring the importance of traditional cardiovascular risk factors like hyperlipidemia, male sex and smoking in the development of coronary calcification (26-28). Previous studies have shown conflicting results about whether patients with GCA are at increased risk for myocardial infarction. (29-32). Large population-based studies found an increased risk for myocardial infarction in patients with GCA compared to age-and sex-matched controls that was greatest within the first year after GCA diagnosis (30, 32). This finding, coupled with the observation that patients with GCA have less coronary artery calcification than patients with hyperlipidemia, suggests that myocardial infarction risk in GCA may be mediated more strongly by disease-specific rather than traditional cardiovascular risk factors.
While there were few differences in the location of vascular calcification between patients with hyperlipidemia versus LVV, there were significant differences in the total burden of vascular calcification, with the greatest burden in patients with GCA followed by patients with TAK, and least in patients with hyperlipidemia. Generalized atherosclerosis and vascular calcification in GCA has been previously described (11, 12). A high burden of vascular calcification in GCA relative to TAK and HLD is likely attributable to a combination of disease-specific and traditional cardiovascular risk factors in these patients. Interestingly, total calcification burden was also higher in the patients with TAK compared to the HLD group, although the patients with TAK in our cohort were mostly women (73%) and significantly younger than the patients with HLD. This observation underscores the role of vascular inflammation in the development of atherosclerosis and vascular calcification (4). Prevalence of vascular calcification has been compared in patients with TAK and other systemic inflammatory diseases affecting younger populations. Compared to patients with SLE, patients with TAK have an increased prevalence of aortic calcification (45% vs 23%) and a lower prevalence of coronary artery calcification (11% vs 21%) (4).
Specific traditional and disease-specific cardiovascular risk factors were identified in association with vascular calcification in patients with LVV. Associations between traditional cardiovascular risk factors like age, male sex, hypertension, hyperlipidemia, obesity and smoking with vascular calcification are well established in the general population (33). In this cohort, advanced age was found to be associated with vascular calcification in patients with LVV. No associations were observed between vascular calcification and gender, BMI, hypertension, or statin use in patients with LVV. High systolic blood pressure has been previously reported to be associated with coronary and thoracic aortic calcification in TAK patients (4). In this study, hypertension was associated with burden of vascular calcification in univariable models; however, this association was no longer significant in models that adjusted for potential confounders, including age and glucocorticoid use.
Disease-specific risk factors were also observed in patients with LVV in association with vascular calcification. No previous studies have compared vascular calcification in TAK versus GCA. In univariable models, a diagnosis of GCA compared to TAK was associated with increased burden of vascular calcification. However, in multivariable models that adjusted for age, TAK compared to GCA was significantly associated with increased burden of vascular calcification. The higher burden of vascular calcification in TAK versus GCA after adjusting for age could be attributable to the predominant cranial artery involvement in many patients with GCA with sparing of the large vessels, whereas all patients with TAK have inflammation in the aorta and its major branches.
A higher dose of prednisone at the time of imaging was also associated with higher burden of vascular calcification in patients with LVV. This association may be attributable to multiple factors. Prednisone dose could be a surrogate marker for disease activity and/or treatment-refractory disease. Alternatively, the association between prednisone and vascular calcification could be mediated by known adverse cardiovascular effects of chronic glucocorticoid use, including development of hyperglycemia, hypertension, and obesity.
There were no associations between vascular calcification and markers of ongoing vascular inflammation, including C-reactive protein, erythrocyte sedimentation rate, metabolic activity on FDG-PET, and clinical assessment of disease activity. This is perhaps not unexpected as vascular calcification in vasculitis is typically a sequela of prior inflammation (34-36). Previous studies on patients with atherosclerosis showed only 2-7% overlapping areas of increased vascular FDG uptake and vascular calcification (35, 36). Longitudinal assessment of patients with TAK have demonstrated worsening vascular calcification over time as the disease becomes inactive. Serial CT scans in patients with TAK show decreased mural thickening suggesting improvement in vascular inflammation and corresponding increase in mural calcification over time (37, 38). There were no associations between vascular calcification and disease or treatment duration. Chronic disease in most of our patients (median duration 12 years) and frequent delays in diagnosis are probably responsible for this observation. Similarly, Seyahi et al. did not find any association between disease duration and calcification in patients with TAK (4).
This study has a few potential limitations to consider. Patients with LVV were not age-or gender-matched to patients with hyperlipidemia, and lipid testing was not performed in patients with LVV. The cohort of patients with LLV was small and did not contain smokers and/or diabetic patients. Thus additional risks of those factors in patients with LVV could not be estimated. Although differences in the prevalence of traditional cardiovascular risk factors (e.g. age, gender, hyperlipidemia, diabetes mellitus, and smoking) between patients with LVV and HLD is a study limitation, the high burden of vascular calcification in absence of traditional cardiovascular risk factors in the LVV cohort, particularly the TAK subgroup, is an important finding. Compared to patients with HLD, patients with LVV underwent a low radiation CT scan as part of an FDG-PET evaluation which may be less sensitive to detect vascular calcification compared to CT scans that do not use the low radiation protocol that was applied in this study.
Nevertheless, both groups were imaged on the same 128-slice CT scanner at the same institution, and the minor differences in imaging technique were not expected to have a significant effect on calcium scores. High pitch or electrocardiography-gating was not used to minimize respiratory and cardiac motion. While these technical issues likely limit the ability to detect small foci of calcification in the coronary arteries, they are unlikely to negate the differences observed in the prevalence of coronary artery calcification in LVV versus HLD as nondifferential misclassification tends to bias study results towards the null. The study design was cross-sectional precluding the ability to assess the prognostic value of vascular calcification in LVV to predict future cardiovascular events.
5.0. CONCLUSION
In conclusion, this study demonstrated a high prevalence of vascular calcification in patients with GCA and TAK. Traditional and disease-specific risk factors are associated with vascular calcification in LVV. These findings provide indirect support for aggressive cardiovascular risk management in these patients. Prospective, longitudinal studies to determine the relative contributions of traditional versus disease-specific risk factors for future cardiovascular events in LVV are needed.
STATEMENT OF CLINICAL SIGNIFICANCE.
Large-vessel vasculitis (LVV) is associated with increased cardiovascular morbidity and mortality due to atherosclerosis. Vascular calcification is a marker for atherosclerosis risk, but the extent and distribution of vascular calcification is not well defined in LVV. This study details the amount, location, and clinical factors associated with arterial calcification in LVV. In comparison to patients with hyperlipidemia, patients with LVV have a greater total amount of vascular calcification throughout the aorta and branch vesels, with the greatest amount in patients with giant cell arteritis compared to Takayasu’s arteritis. However, patients with hyperlipidemia have significantly greater coronary artery calcification than patients with LVV. Traditional and disease-specific risk factors are associated with vascular calcification in LVV. Prospective, longitudinal studies to determine the relative contributions of traditional versus disease-specific risk factors for future cardiovascular events in LVV are needed.
Acknowledgments
FUNDING
This research was supported through the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jayalath RW, Mangan SH, Golledge J. Aortic calcification. Eur J Vasc Endovasc Surg. 2005;30(5):476–88. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulos D, Toulgaridis T, Davlouros P, Christodoulou J, Sitafidis G, Hahalis G, et al. Prognostic significance of coronary artery calcium in asymptomatic subjects with usual cardiovascular risk. Am Heart J. 2003;145(3):542–8. [DOI] [PubMed] [Google Scholar]
- 3.O'Malley PG, Taylor AJ, Jackson JL, Doherty TM, Detrano RC. Prognostic value of coronary electron-beam computed tomography for coronary heart disease events in asymptomatic populations. Am J Cardiol. 2000;85(8):945–8. [DOI] [PubMed] [Google Scholar]
- 4.Seyahi E, Ucgul A, Cebi Olgun D, Ugurlu S, Akman C, Tutar O, et al. Aortic and coronary calcifications in Takayasu arteritis. Semin Arthritis Rheum. 2013;43(1):96–104. [DOI] [PubMed] [Google Scholar]
- 5.Lupi E, Horwitz S, Sanchez G. Calcifications in Takayasu's arteritis. Vasc Surg 1973;7(5):259–64. [DOI] [PubMed] [Google Scholar]
- 6.Gujadhur A, Smith ER, McMahon LP, Spanger M, Chuen J, Holt SG. Large vessel calcification in Takayasu arteritis. Intern Med J. 2013;43(5):584–7. [DOI] [PubMed] [Google Scholar]
- 7.Yamato M, Lecky JW, Hiramatsu K, Kohda E. Takayasu arteritis: radiographic and angiographic findings in 59 patients. Radiology. 1986;161(2):329–34. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama A, Matsubara S, Toyama J. Takayasu arteritis with multiple cardiovascular complications. Heart Vessels. 2001;16(1):23–7. [DOI] [PubMed] [Google Scholar]
- 9.Yamada I, Nakagawa T, Himeno Y, Numano F, Shibuya H. Takayasu arteritis:evaluation of the thoracic aorta with CT angiography. Radiology. 1998;209(1):103–9. [DOI] [PubMed] [Google Scholar]
- 10.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. [DOI] [PubMed] [Google Scholar]
- 11.Lie JT. Aortic and extracranial large vessel giant cell arteritis: a review of 72 cases with histopathologic documentation. Semin Arthritis Rheum. 1995;24(6):422–31. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson IM, Russell RW. Arteries of the head and neck in giant cell arteritis. A pathological study to show the pattern of arterial involvement. Arch Neurol. 1972;27(5):378–91. [DOI] [PubMed] [Google Scholar]
- 13.Ostberg G. Temporal arteritis in a large necropsy series. Ann Rheum Dis. 1971;30(3):224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SH, Kim WH, Ko JK. Vascular signs of Takayasu's arteritis: porcelain aorta and the 'macaroni sign'. Eur Heart J. 2014;35(29):1981. [DOI] [PubMed] [Google Scholar]
- 15.Garland BT, Boehm M, Grayson PC, Hilaire CS, Brofferio A, Starnes BW. Abnormal molecular response to Takayasu arteritis causing extensive large-vessel calcification. J Vasc Surg Cases Innov Tech. 2016;2(4):190–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma S, Sharma S, Taneja K, Gupta AK, Rajani M. Morphologic mural changes in the aorta revealed by CT in patients with nonspecific aortoarteritis (Takayasu's arteritis). AJR Am J Roentgenol. 1996;167(5):1321–5. [DOI] [PubMed] [Google Scholar]
- 17.Barra L, Liang P, Benseler SM, Cabral DA, Fifi-Mah A, Li Y, et al. Variations in the clinical practice of physicians managing Takayasu arteritis: a nationwide survey. Open Access Rheumatol 2017;9:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33(8):1122–8. [DOI] [PubMed] [Google Scholar]
- 19.Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–34. [DOI] [PubMed] [Google Scholar]
- 20.Sait MR, Lepore M, Kwasnicki R, Allington J, Balasubramanian R, Somasundaram SK, et al. The 2016 revised ACR criteria for diagnosis of giant cell arteritis – Our case series: Can this avoid unnecessary temporal artery biopsies? International Journal of Surgery Open. 2017;9:19–23. [Google Scholar]
- 21.Sandfort V, Lai S, Ahlman MA, Mallek M, Liu S, Sibley CT, et al. Obesity Is Associated With Progression of Atherosclerosis During Statin Treatment. J Am Heart Assoc. 2016;5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ, et al. Positron Emission Tomography as an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients with Large Vessel Vasculitis. Arthritis Rheumatol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reaven PD, Sacks J, Investigators for the Veterans Affairs Cooperative Study of Glycemic C, Complications in Diabetes Mellitus T. Reduced coronary artery and abdominal aortic calcification in Hispanics with type 2 diabetes. Diabetes Care. 2004;27(5):1115–20. [DOI] [PubMed] [Google Scholar]
- 24.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117(22):2938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godoy P, Araujo Sde A, Paulino E Jr., Lana-Peixoto MA. Coronary giant cell arteritis and acute myocardial infarction. Arq Bras Cardiol. 2007;88(4):e84–7. [DOI] [PubMed] [Google Scholar]
- 26.Oei HH, Vliegenthart R, Hofman A, Oudkerk M, Witteman JC. Risk factors for coronary calcification in older subjects. The Rotterdam Coronary Calcification Study. Eur Heart J. 2004;25(1):48–55. [DOI] [PubMed] [Google Scholar]
- 27.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115(21):2722–30. [DOI] [PubMed] [Google Scholar]
- 28.Udayakumar PD, Chandran AK, Crowson CS, Warrington KJ, Matteson EL. Cardiovascular risk and acute coronary syndrome in giant cell arteritis: a population-based retrospective cohort study. Arthritis Care Res (Hoboken). 2015;67(3):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ungprasert P, Koster MJ, Warrington KJ. Coronary artery disease in giant cell arteritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2015;44(5):586–91. [DOI] [PubMed] [Google Scholar]
- 30.Tomasson G, Peloquin C, Mohammad A, Love TJ, Zhang Y, Choi HK, et al. Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: a cohort study. Ann Intern Med. 2014;160(2):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray JG, Mamdani MM, Geerts WH. Giant cell arteritis and cardiovascular disease in older adults. Heart. 2005;91(3):324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amiri N, De Vera M, Choi HK, Sayre EC, Avina-Zubieta JA. Increased risk of cardiovascular disease in giant cell arteritis: a general population-based study. Rheumatology (Oxford). 2016;55(1):33–40. [DOI] [PubMed] [Google Scholar]
- 33.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(2):331–6. [DOI] [PubMed] [Google Scholar]
- 34.Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging. 2013;6(5):747–54. [DOI] [PubMed] [Google Scholar]
- 35.Dunphy MP, Freiman A, Larson SM, Strauss HW. Association of vascular 18F-FDG uptake with vascular calcification. J Nucl Med. 2005;46(8):1278–84. [PubMed] [Google Scholar]
- 36.Ben-Haim S, Kupzov E, Tamir A, Israel O. Evaluation of 18F-FDG uptake and vasc Ima arterial wall calcifications using 18F-FDG PET/CT. J Nucl Med. 2004;45(11):1816–21. [PubMed] [Google Scholar]
- 37.Kim SY, Park JH, Chung JW, Kim HC, Lee W, So YH, et al. Follow-up CT evaluation of the mural changes in active Takayasu arteritis. Korean J Radiol. 2007;8(4):286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul JF, Fiessinger JN, Sapoval M, Hernigou A, Mousseaux E, Emmerich J, et al. Follow-up electron beam CT for the management of early phase Takayasu arteritis. J Comput Assist Tomogr. 2001;25(6):924–31. [DOI] [PubMed] [Google Scholar]