Abstract
Fragile X syndrome (FXS) is a devastating developmental disability that has profound effects on cognition, behavior and seizure susceptibility. There are currently no treatments that target the underlying cause of the disorder and recent clinical trials have been unsuccessful. In 2007, seminal work demonstrated that amyloid-beta protein precursor (APP) is dysregulated in Fmr1KO mice through a metabotropic glutamate receptor 5 (mGluR5)-dependent pathway. These findings raise the hypotheses that: (1) APP and/or APP metabolites are potential therapeutic targets as well as biomarkers for FXS, and (2) mGluR5 inhibitors may be beneficial in the treatment of Alzheimer’s disease. Herein, advances in the field over the past decade that have reproduced and greatly expanded upon these original findings are reviewed, and required experimentation to validate APP metabolites as potential disease biomarkers as well as therapeutic targets for FXS are discussed.
Keywords: Amyloid-beta protein precursor (APP), cellular prion protein (PrPC), fragile X mental retardation protein (FMRP), fragile X syndrome (FXS), metabotropic glutamate receptor 5 (mGluR5)
1. Background
Fragile X syndrome (FXS) is a neurodevelopmental disorder clinically characterized by intellectual disability (overall IQ<70), autistic-like behaviors and seizures [1]. FXS results from a mutation in a single gene on the X chromosome, FMR1, that is associated with transcriptional silencing of the FMR1 promoter and loss of expression of fragile X mental retardation protein (FMRP) [2]. FMRP is a multi-functional mRNA binding protein involved in the transport, localization and translational repression of a subset of dendritic mRNAs [3–6]. In dendrites, FMRP is predominantly found in the post-synaptic density associated with polysomes or nontranslating ribonucleoprotein (RNP) particles [7–9]. Hundreds of mRNA ligands that bind to FMRP have been identified with many having the potential to influence synapse formation and synaptic plasticity [10–12]. FMRP expression is absent or greatly reduced in FXS, and many FXS phenotypes are manifested in Fmr1KO mice, which lack expression of FMRP [13]. Fmr1KO mice exhibit many of the physical and behavioral characteristics of humans with FXS and are thus the most widely employed, non-human model system available for testing interventions. The leading drug target to date is the glutamate-activated, G-protein-coupled receptor metabotropic glutamate receptor 5 (mGluR5), which is widely expressed in the CNS, generally postsynaptic in location and signals through FMRP [14].
In 2007, seminal findings were published demonstrating that FMRP binds to the coding region of App mRNA at a guanine-rich, G-quartet-like sequence and regulates amyloid-beta protein precursor (APP) synthesis through a mGluR5-dependent pathway [15]. APP is processed by β- and γ-secretases to produce amyloid-beta (Aβ), the predominant peptide found in the senile plaques in Alzheimer’s disease (AD). Thus, these data suggest a possible link between AD and FXS, and evoke the hypothesize that APP and Aβ can be developed as biomarkers for disease severity and drug efficacy and that drugs under study for AD can be repurposed for FXS. Over the past decade, these original findings have been independently reproduced and advanced by many laboratories (Figure 1). Herein, those findings are reviewed and a vision for future FXS-APP research is proposed.
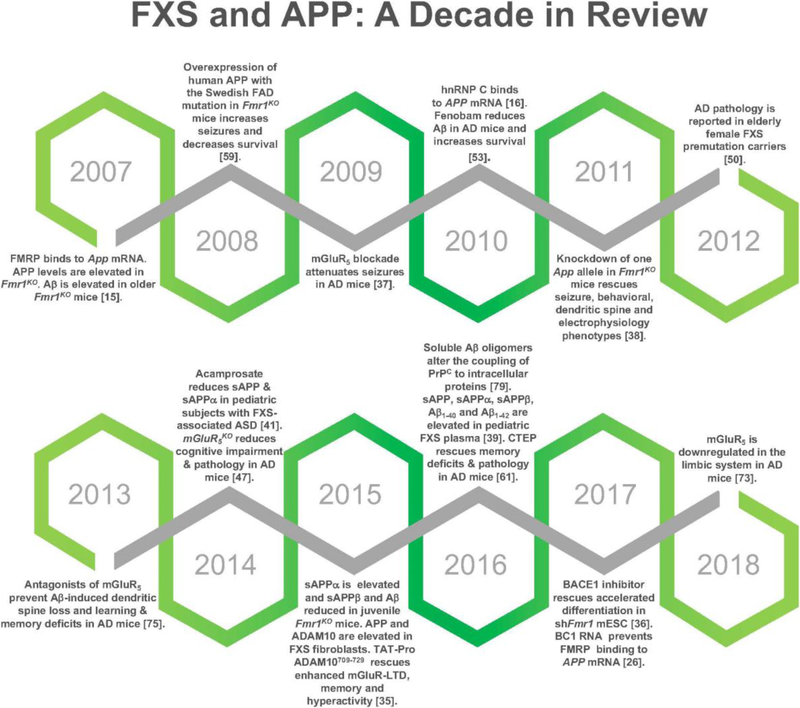
Fig 1.
FXS and APP: A Decade in Review. Major discoveries over the past decade span structural and functional relationships of FMRP and App mRNA to the effects of pharmaceutical interventions such as mGluR5 inhibitors on APP metabolite expression and function.
2. FMRP Represses APP Translation Through a Guanine-Rich Element in the Coding Region of App mRNA
FMRP monomer binds directly to a guanine-rich region (nucleotides 699–796) in the coding region of mouse App mRNA and is also part of a complex that protects a cis-regulatory element in the 3’-untranslated region (UTR) located approximately 200 bases downstream from the stop codon (nucleotides 2318–2416) (GenBank accession number X59379) [15]. Activation of mGluR5 rapidly increases translation of APP by displacing FMRP from the guanine-rich region of App mRNA [15]. In the absence of FMRP (Fmr1KO), APP levels are constitutively increased [15]. Of critical importance to this topic, in 2010, FMRP was found to bind to the guanine-rich region of human APP mRNA [16]. It was demonstrated that the RNA binding proteins (RBP) hnRNP C and FMRP associate with the guanine-rich coding region element in human APP mRNA to influence APP translation competitively in opposite directions [16]. Specifically, FMRP represses translation by recruiting APP mRNA to processing bodies (P-bodies). Another RBP, heterogeneous ribonucleoprotein C (hnRNP C), promotes APP translation by displacing FMRP [16]. FMRP does not repress APP translation in the absence of the processing-body proteins RCK, Ago1 or Ago2 [16]. The interaction of hnRNP C with mouse App mRNA is significantly more abundant in lysates prepared from Fmr1KO than from WT brain, and associates with higher expression of APP in Fmr1KO mice [16]. This work confirms the findings that FMRP binds to a guanine-rich region in the coding region of App mRNA and that APP levels are elevated in Fmr1KO mice, while utilizing a human neuroblastoma cell line, which generalizes the findings between species. Both laboratories found that steady state abundance of mouse App mRNA in whole brain is comparable between WT and Fmr1KO mice indicating a translational, as opposed to posttranscriptional mRNA stability, mechanism [15,16]. Overall, the findings suggest a paradigm whereby hnRNP C promotes translation of APP mRNA by competing with FMRP for interaction at the guanine-rich coding region element in APP mRNA, thereby preventing the localization of the message by FMRP to P-bodies.
Two independent laboratories employing crosslinking immunoprecipitation (CLIP) methodologies also found that FMRP binds to APP/App mRNA. First, App mRNA was identified using high throughput sequencing of RNAs isolated by CLIP to identify FMRP interactions with mouse brain polyribosomal mRNAs [17]. And second, FMRP binding to the guanine-rich region of APP mRNA was confirmed in vitro using electromobility shift assays (EMSA) with recombinant FMRP and radiolabeled fragments of APP mRNA [18]. Three photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP)-identified FMRP target sites within APP (see Supplementary Figure 7 within [18]) were tested. Site 1 in APP mRNA (nucleotides 888–948, NM_000484) overlaps with the guanine-rich region identified as binding to FMRP [15]. Site 2 (nucleotides 2169–2228, coding region) and site 3 (nucleotides 3337–3396; 3’-UTR) PAR-CLIP sites also bind to recombinant FMRP; however, binding is significantly stronger to site 1 [18]. Please note, the nucleotide citations differ for mouse (App) and human (APP) genes. GenBank accession numbers have been provided for alignment purposes. Site 1 of the human gene (nucleotides 888–948, NM_000484) overlaps with nucleotides 699–796 of the coding region of the mouse App gene (X59379).
Two additional laboratories using bioinformatics approaches provided complementary data supporting the importance of the guanine-rich coding region element in App/APP mRNA. First, the catRAPID method, which identifies potential interactions between protein and RNA molecules, predicted that FMRP binds to nucleotides 650–751 and 751–852, which is the experimentally validated region encompassing nucleotides 699–796 in the coding region of App mRNA [19]. Second, Quadparser found a putative G-quadruplex in the coding region of APP mRNA consistent with earlier findings [20]. The G-quadruplex is predicted to be relatively weak because the intervening loops are relatively long with four nucleotides each allowing a quadruplex with only two stacks of guanine tetrads [20]. These data corroborate the observation that FMRP monomer binds to the guanine-rich region immediately upstream from the predicted G-quadruplex and not to the G-quadruplex. In addition to the G-quadruplex in the coding region of APP mRNA, there is a relatively strong G-quadruplex in the 3’-UTR (739 bases downstream from the stop codon) that has no apparent role in regulating transcription or mRNA stability but negatively regulates APP levels [20,21]. It is interesting that both of the predicted G-quadruplexes occur immediately downstream of known cis-regulatory elements in App mRNA. There is an 81-nt cis-regulatory element in the 3’-UTR of APP mRNA that forms part of a 68 kD RNA-protein complex involved in TGFβ-induced stabilization of the message [22]. The last three bases (GGG) of the 81-nt cis-element overlap with the first three bases of the second G-quadruplex. These data suggest that the G-quadruplexes in APP mRNA may act as protein loading signals for upstream cis-regulatory elements. Overall, the bioinformatics approaches predict the experimental observation that FMRP binds to the guanine-rich coding region element in App mRNA.
The detailed mechanism(s) through which FMRP represses the translation of APP remain to be determined. Current findings suggest a competition between FMRP and hnRNP C for binding to the guanine-rich region in the coding region of APP mRNA [16]. FMRP interacts with Ago proteins and the microRNA pathway [23,24], and microRNAs have been shown to target APP mRNA and reduce APP expression [25]. Of significant importance to this topic, in 2017, findings were published indicating that inhibition of the noncoding RNA (ncRNA) BC1, or specifically the BC1-FMRP association in Tg2576 AD mice, blocks aggregation of Aβ in the brain and protects against spatial learning and memory deficits [26]. In contrast, expression of exogenous BC1 in excitatory pyramidal neurons of mice induces Aβ peptides accumulation and impairs spatial learning and memory [26]. The combination of in vitro and in vivo data indicates that when BC1 associates with FMRP, FMRP does not bind to APP mRNA, allowing for increased translation and processing of APP resulting in increased Aβ levels and spatial learning and memory deficits [26]. Conversely, inhibition of BC1 reduces full-length APP, Aβ peptides and Aβ plaques without affecting APP mRNA levels [26]. In total, these findings suggest a molecular mechanism involving FMRP, hnRNP C and BC1 in the regulation of APP synthesis and are of interest in the context of FXS.
The role of BC1 in FXS has been hotly debated as there is contradictory evidence regarding if FMRP associates directly with dendritic BC1 RNA [27,28]. One set of evidence suggests that FMRP binds directly to BC1 RNA and that blocking BC1 inhibits the interaction of FMRP with its target mRNAs, which elicits the hypothesis that BC1 RNA acts as a bridge between FMRP and its target mRNAs [29,30] (Model 1). Other laboratories independently set out to test the BC1-FMRP model and could not document specific BC1-FMRP interactions in vitro or in vivo [31]. Their data suggest that the interactions between BC1 RNA and FMRP target mRNAs are nonspecific and support a model in which BC1 RNA and FMRP are translational repressors that operate independently (Model 2). Neither study tested BC1 binding to App mRNA. While subsequent work studying BC1 in the context of FMRP and APP mRNA, does not resolve the controversy [26], it does shed light on the mechanism underlying BC1 regulation of APP translation, which appears to support aspects of both previously published models. The subsequent study employed in vitro EMSA conditions containing physiological salt concentrations and competitor RNA and showed BC1 binding to truncated recombinant FMRP (amino acids 1–60) [26]. Nonetheless, recombinant RBPs can be notoriously “sticky” causing inherent specificity problems in EMSA. However, there is elegant in vivo work directly assessing BC1 effects on APP in Tg2576 mice through over- and underexpression of BC1 and in vivo competition assays with Tat-FMRP peptides that strongly supports a model whereby BC1 binds to FMRP, which then cannot bind to and repress APP mRNA, thus promoting APP synthesis [26] (Model 3). Thus, Models 1 and 3 concur in supporting the binding of BC1 and FMRP. Model 3 also indicates that FMRP binding is mutually exclusive in that it only binds to APP mRNA or BC1 but not both at the same time, which concurs with Model 2 that FMRP binds directly to its mRNA targets. BC1KOFmr1KO mice exhibit exacerbated synaptic hyperexcitability and epileptogenesis as well as deficits in place learning supporting a sequential-independent modus operandi for BC1 and FMRP [32]. Model 3 is congruent with Model 2 in that BC1 and FMRP appear to act in a sequential-independent manner; however, in the case of APP mRNA, BC1 activates rather than represses translation. It has been hypothesized that there could be two pools of mRNA that are in competition for the translational machinery and repression of pool 1 by FMRP would allow for efficient translation of pool 2 [14]. Likewise, pools of mRNA could be differentially regulated by BC1, which in the case of APP mRNA, appears to bind to and compete FMRP away from APP mRNA leading to increased production of APP and Aβ.
Model 3 employs the Tg2576 AD mouse model, which harbors a human APP cDNA with the Swedish familial AD mutation (APPSWE) but no UTR sequences [33]. Thus, the effects of over- and under-expression of BC1 RNA on APP translation in this model can be attributed to the coding region of APP mRNA. FMRP also protects at least one region of the 3’-UTR known to bind to other RBP including nucleolin and hnRNP C [15]. It is not known how interactions between multiple cis-regulatory elements, RBP, ncRNA and APP mRNA affect mRNA localization, stability and protein synthesis.
3. APP and Aβ Levels are Elevated in Mouse Fmr1KO Models
Steady state levels of APP are substantially higher in Fmr1KO synaptoneurosomes (SN) prepared from juvenile mouse cortices (postnatal day 14–17; P14–17) and in primary cultured Fmr1KO neurons compared to WT controls [15]. This work has been partially substantiated by two other laboratories by western blot analysis, which indicates an approximately 1.7-fold increase in APP in Fmr1KO versus WT SN prepared from P14 mouse cortices [34], as well as a significant upregulation of APP expression in whole brain lysates in Fmr1KO at P21, P30 and P90 [35]. The last study found no difference in APP expression in whole brain lysates comparing WT and Fmr1KO at P7 and P14 [35]. In total, three independent studies in SN or whole brain lysates prepared from postnatal mice demonstrate increased APP levels in Fmr1KO mice albeit there were varied results at P14. These results indicate that the absence of FMRP leads to increased APP expression in the brain during a period that is critical for the stabilization of synapses.
Regarding APP levels in primary cultured Fmr1KO neurons, SILAC (stable isotope labeling by amino acids in cell culture) experiments indicate decreased APP expression in Fmr1KO [34]. The opposite results between western blot analysis of the synaptic fractions (1.7-fold increase) and SILAC analysis of cultured neurons (2–3.4-fold decrease) may be attributed to differences in protein stability in different assay buffers and/or to differential regulation of APP at varied developmental stages [34]. As two independent studies both prepared primary cultured neurons from embryonic day 18 mice [15,34], lower detection of APP by SILAC versus immunofluorescence could be due to a stability or solubility issue with the SILAC or to differences in local protein concentration with immunofluorescence. SILAC requires harvesting total protein from the neurons prior to digesting the protein and analysis by mass spectrometry whereas immunofluorescence involves fixing primary cultured neurons and screening APP expression in dendrites by confocal microscopy after staining with an APP-specific antibody. Of the 10 SILAC-identified proteins that underwent validation by western blot, 8 proteins exhibited good correlation between the two methods [34]. The two proteins with opposite responses were APP and α-synuclein, which are high molecular weight membrane bound proteins that would be expected to have solubility issues [34]. Alternatively, the proteins could have been diluted in the whole cell lysates or there could be differences in distribution between dendritic puncta and cell bodies. Further support implicating FMRP in the regulation of APP during embryogenesis comes from studies in mouse embryonic stem cells (mESC) where APP levels increase as FMRP is downregulated [36]. In the final analysis, the majority of the data support higher APP levels in Fmr1KO, and there are numerous possible explanations for the discrepancy with the SILAC data.
There appears to be an age-dependent transition in APP levels and/or processing in Fmr1KO mice. In adult animals, one study finds no difference in brain APP or Aβ levels between WT and Fmr1KO young adult mice (3.5 months of age) in hippocampus, cortex or cerebellum by western blotting using an anti-mouse antibody against the amino-terminus of APP [37]. Another study reports increased APP at P90 in Fmr1KO whole brain lysates and transient overexpression of sAPP α in the Fmr1KO at P21 and P30 but not P14 and P90 [35]. Regardless of whether this variance in APP results is due to the small difference in age of the mice (3 versus 3.5 months) or utilizing brain-specific regions versus whole brain lysates, there appears to be an age-dependent shift in APP levels between postnatal and young adult Fmr1KO mice. Two laboratories find elevated Aβ levels in older adult Fmr1KO mice [15,35]. In addition, there is a significant decrease in Aβ in juvenile Fmr1KO mice compared to WT controls as well as increased soluble APPalpha (sAPPα) and decreased soluble APPbeta (sAPPβ) at P21, suggesting increased α-secretase processing in juvenile Fmr1KO mice [35]. In total, these studies suggest that dominant APP processing may change as a function of development resulting in varied ratios of APP metabolites.
4. APP and Aβ Levels are Dysregulated in Human Fragile X Models
In human studies, there is conflicting data regarding APP metabolite levels, which may vary dependent on tissue, subject age, and sample collection methods. One study finds decreased levels of Aβ1–42 in blood plasma from full-mutation FXS adult males with no change in APP/sAPPα or Aβ1–40 levels [38]. Due to a limited number of FXS autopsy brain samples available for analysis, there were only trends for decreased APP/sAPPβ and increased Aβ1–40 in FXS brain (neocortex and hippocampus). Another study finds increased levels of APP and ADAM10 (a disintegrin and metalloproteinase domain-containing protein 10; aka α-secretase) in primary fibroblasts from adolescent and adult FXS patients and in frontal cortex of adult FXS [35]. A third study finds elevated levels of sAPP, sAPPα, sAPPβ, Aβ1–40 and Aβ1–42 in pediatric FXS plasma as well as elevated sAPP, sAPPα and Aβ1–40 in FXS brain [39,40]. Interestingly, acamprosate treatment significantly reduced sAPP and sAPPα in pediatric subjects with FXS-associated autism spectrum disorder (ASD) [41]. There are potentially confounding issues with these studies. For example, blood levels of APP metabolites may not correlate with brain levels, or inversely correlate if the brain acts as sink for Aβ. Also, the anticoagulant used to collect blood can have large effects on APP metabolites, which may explain the varied results in Aβ levels in plasma (lower Aβ levels in blood collected in lithium heparin versus higher Aβ levels with EDTA) [42]. In addition, subject age varied between studies (adult versus pediatric), and similar to the Fmr1KO mouse studies, APP processing may change as a function of age. In total, these findings support a role for dysregulated APP production and processing in FXS and indicate that the APP metabolites may be viable therapeutic targets and/or biomarkers if consistent testing conditions can be determined.
5. FMRP Expression Varies in AD Models
The flipside of APP metabolite expression in FXS models is FMRP expression in AD models. FMRP levels decrease with age in mice [43,44], supporting the hypothesis that loss of this translational regulator could contribute to increased APP synthesis and Aβ processing with aging and thus the development of AD. There are some complicated data from the AD field in regard to this hypothesis, which is summarized (Table 1). One laboratory finds no difference in FMRP expression in AD model mice (APPSWE/PS1ΔE9; 2- and 20-months old) in cerebellum or cortex compared to littermate controls albeit there was a non-significant increase in FMRP in cerebellum in the 2-month old AD mice [45]. In addition, FMRP expression is similar in frontal cortex and cerebellum samples from sporadic AD and control patient autopsy samples [45]. A second laboratory finds reduced FMRP in Tg2576 compared to WT mice (1- and 3-months old) [46]. FMRP levels are equivalent in 6-month old mice; however, FMRP levels drop dramatically with age resulting in low levels in both WT and Tg2576 [46]. This group provides complementary evidence that hnRNP C expression is elevated in 1- and 3- month old Tg2576 but not 6-month old mice compared to WT controls [46]. Furthermore, FMRP is decreased and hnRNP C increased in hippocampal SN prepared from human sporadic AD compared to healthy donors [46]. A third group finds that FMRP expression is increased in APPSWE/PS1ΔE9 mice (12-months old) [47]. They propose that increased FMRP levels are a compensatory mechanism to reduce ectopic/transgenic translation of APP although that compensatory mechanism appears ineffective [47].
Table 1:
Summary of Cited Alzheimer’s Disease Mouse Model Data
| Phenotype | 3XTg | 5XFAD | APPE693Q | APPSWE/PS1ΔE9 | FRAXAD | J20 | R1.40 | Tg2576 | ArcSWE | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mutated Genes* | APPSWE MAPTP301L PSEN1M146V | APPSWE APPFLOR APPLOND PSEN1M146L PSEN1L286V | APPDUTCH | APP PSEN1ΔE9 | APPSWE Fmr1KO | APPSWE APPIND | APPSWE | APP | APPSWE APPArc | |
| APP Gene Contains UTR Sequences | No [133] | Yes [134] | No [59] | Yes [135] | Yes [136] | No [33] | Yes [137] | |||
| Early Mortality | Yes [110] | Yes [59] | Yes [59] | |||||||
| AGS Susceptibility | Yes [114] | Yes [111] | No [113] | No [113] | Yes [111] | |||||
| Anti-Aβ Rescues | Seizures | Yes [114] | Yes [111] | |||||||
|
Epilepti form Discharge Duration |
Yes [114] | |||||||||
| mGlu R5 Inhibitors or Genetic Knockout Rescues | Seizures | Yes [114] | Yes [37] | Yes [37,111] | ||||||
| Aβ Levels or Plaques | Yes [61] | Yes [47,61] | Yes [53] | Yes & No** [53] | ||||||
| Dendritic spine or synapse loss | Yes [75,86] | |||||||||
| Early Mortality | Yes [110] | |||||||||
| Epilepti form | Yes [114] | |||||||||
| Discharge Duration | ||||||||||
|
Learning / Memory |
Yes [75] | Yes [47,61,75,86] | ||||||||
| Altered Expression of mGluR5 | Yes [73] | Yes [47] | ||||||||
| Fmr1 gene Differentially Spliced | Yes [103] | Yes [103] | ||||||||
| FMRP Differentially Expressed | No [45] Yes [47] | Yes [46] | ||||||||
APPSWE = APP KM670/671NL (APP with Swedish familial AD mutation); APPFLOR = APP I716V (APP with Florida familial AD mutation); APPLOND = APP V717I (APP with London familial AD mutation); APPDUTCH = APP E693Q (APP with Dutch familial AD mutation); APPIND = APP V717F (APP with Indiana familial AD mutation); APPARC = APP E693G (APP with Arctic familial AD mutation); MAPT P301L (tau familial AD mutation); PSEN1 (presenilin 1 familial AD mutation).
Human Aβ produced from the transgene is not reduced. Endogenous mouse Aβ is reduced.
There are some caveats associated with these studies that could explain the different outcomes. For example, different strains and ages of AD mice were employed. Examining the entirety of the data, FMRP levels are reduced in AD mice at 1- and 3-months of age (Tg2576), equivalent at 2- (APPSWE/PS1ΔE9) and 6-months of age (Tg2576), elevated at 12-months of age (APPSWE/PS1ΔE9), and equivalent at 20-months of age (APPSWE/PS1ΔE9) compared to WT controls suggesting a bell-shaped, age-dependent expression pattern of FMRP in the context of greatly exacerbated APP and Aβ production in AD mice with a shift in the curve dependent on the AD mouse model. The double transgenic AD mice (APPSWE/PS1ΔE9) begin to develop Aβ deposits by 6 months of age compared to 11–13 months in the Tg2576 [33,48]. In the APPSWE/PS1ΔE9, there is a progressive increase in plaque number up to at least 12-months of age [49]. Overall, the data support a model in which FMRP production increases with accumulating APP and Aβ followed by reduced levels upon Aβ sequestration into plaques. The early decrease in FMRP in the 1- to 3-month of age period in the Tg2576 could indicate that the compensatory mechanism does not kick in until after a threshold level of APP or metabolites is produced. These results have important implications for therapeutic timing in the treatment of AD. Regarding the varied results assessing FMRP levels in human sporadic AD tissue, the studies analyzed different brain regions (frontal cortex and cerebellum [45] versus hippocampus [46]).
Interestingly, there is reduced FMRP expression in cortex and cerebellum tissue of two of three patients with fragile X-associated tremor/ataxia syndrome (FXTAS) compared to control and AD brains, with a corresponding increase in APP in cerebellar but not cortical samples [45]. Alzheimer’s disease-type pathologies (plaques and tangles) have been reported in elderly female fragile X premutation carriers [50]. APP is a deregulated gene in FXTAS and the corresponding mouse model, where APP/App mRNA is upregulated 1.87-fold in peripheral blood of FXS premutation male carriers as well as 1.38-fold in the prefrontal cortex and 1.18-fold in the brainstem of FXTAS mice [51]. These data deserve further evaluation in a larger population.
6. APP Translation is Regulated through FMRP and mGluR5
Stimulation of cortical SN or primary neuronal cells with the mGluR agonist (S)-3,5-diydroxyphenylglycine (DHPG) increases APP levels in wild type (WT) but not Fmr1KO samples [15]. The increase in APP in SN can be blocked with the translational inhibitor anisomycin or the mGluR5 antagonist 2-Methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) [15]. App mRNA co-immunoprecipitates with FMRP in resting SN but the interaction is lost after DHPG treatment [15]. App mRNA levels remain constant irrespective of genotype or treatment [15]. And, treatment of primary neuronal cells with mGluR5 antagonists reduces steady state levels of APP [15,52]. In combination, these data demonstrate that the production of APP is regulated through a FMRP- and mGluR5-dependent signaling pathway, that mGluR-dependent APP synthesis is already at maximal capacity in the Fmr1KO and cannot be further stimulated, and that APP translation is independent of mRNA decay. The finding that App mRNA levels are not affected by FMRP dosage has been replicated by two laboratories [35,36]. In addition, evidence is provided that APPα affects the overall translation of APP, genetic reduction of App in Fmr1KO mice returns synaptic de novo protein synthesis to WT levels, sAPPα increases de novo protein synthesis in primary cortical neurons, genetic reduction of ADAM10 lowers de novo protein synthesis in Fmr1KO SN, and SN prepared from APPKO mice exhibit reduced protein synthesis [35]. These data strongly support a role for sAPPα in translational activation. sAPPα acts though mGluR5 as MPEP blocks the sAPPα-mediated increase in phosphorylated extracellular signal-regulated kinase 1/2 (ERK1/2). Overall, these findings implicate mGluR5, FMRP and sAPPα in a feedback loop that regulates protein synthesis.
Verification of an mGluR5-dependent signaling pathway that regulates APP and Aβ comes from the AD field. Aβ levels were quantitated in AD mice after chronic dosing with the mGluR5 inhibitor fenobam [53]. The drug was incorporated into the feed at 0.2g fenobam per kg feed for an anticipated dose of 24–30 mg drug/kg body weight/day [53]. Drug delivery via feed is advantageous because daily oral gavage or intraperitoneal injections can be stressful to the animals. Phase I dose escalation trials show safety and a lack of cognitive dysfunction in humans receiving up to 8–9 mg/kg/day fenobam for 4 weeks albeit there were psychostimulant side effects [54–57]. The mouse dose was 3-fold higher than that safely tested in humans, but far less than that safely tested in rats [58]. As there are no reports of toxicity with the drug, an err on the side of over-dosing facilitates determining if inhibition of mGluR5 affects Aβ levels or behavior. Two AD models, Tg2576 and R1.40 mice, which both overexpress the human APP gene with the familial Swedish mutation (APPSWE, K670N/M671L) were employed. There was a large decrease in mouse Aβ levels in the Tg2576 in response to fenobam with no change in transgenic human Aβ levels [53]. In the R1.40 mice, there was a small, statistically significant decrease in Aβ [53]. The inability of fenobam to reduce human Aβ in the Tg2576 is likely due to the nature of the hAPPSWE transgene. In the Tg2576, only the coding region of hAPP695 is inserted into the mouse genome (no 3’-UTR sequences) whereas the R1.40 transgene contains 250 kB of flanking sequence. FMRP binds to the 3’-UTR of App mRNA [15]. It is not known at this time if FMRP binds directly to the 3’-UTR or as part of a RNP complex, but it appears that regions outside of the coding region are vital for FMRP regulation of APP synthesis. The mouse App gene in the Tg2576 and the human APP transgene in the R1.40 both contain coding and noncoding FMRP-dependent cis-regulatory elements. No adverse side effects were observed in response to chronic dosing nor any premature deaths [53]. In the Tg2576, there is a 40% death rate by 60 days of age [59]. Thus, chronic treatment with the mGluR5 inhibitor fenobam reduces mouse Aβ in Tg2576 mice and human Aβ in R1.40 mice and improves survival in the Tg2576 [53].
The lack of 3’-UTR cis-regulatory elements in App transgenes may explain other unexpected findings. First, it was hypothesized that the absence of FMRP in FRAXAD mice (cross of Tg2576 and Fmr1KO that overexpresses both human and mouse APP in the absence of FMRP) would result in increased synthesis of APP and generation of Aβ, which would accumulate with aging [37]. No significant differences were observed in APP or Aβ levels in young adult Tg2576 and FRAXAD mice (2-months old) albeit there was an increase in APP in aged FRAXAD mice (16–18 months old) suggesting differential regulation of the endogenous murine versus transgenic human App/APP genes [37]. Immunofluorescence analysis of cultured Tg2576 neurons reveals that APPSWE is predominantly in the soma rather than the dendrites [37]. Thus, FMRP binding to the 3’-UTR of the APP transgene may be required for dendritic targeting. Second, increased expression of APP would be expected in response to siRNA knockdown of FMRP in Neuro-2a cells stably expressing wild type (WT) human APP695 [60]. Surprisingly, FMRP siRNA does not cause increased APP levels in the control lanes (see Figure 4 within [60]). Information is not available regarding if the APP695 construct used contained App 3’-UTR cis-regulatory elements [60]. Third, mGluR5 knockout in APPSWE/PS1ΔE9 AD mice reduces spatial learning deficits, Aβ oligomer formation, Aβ plaque number, mTOR phosphorylation, and FMRP expression [47]. This suggests that a positive feedback loop involving mGluR5 increases Aβ formation and AD pathology in APPSWE/PS1ΔE9 mice. However, the genetic deletion of mGluR5 had no apparent effect on the transgenic expression of APP in APPSWE/PS1ΔE9 suggesting that Aβ levels were altered through increased cleavage and not overall synthesis. These data support the hypothesis that APP flanking sequences are required for mGluR5/FMRP-mediated regulation of APP synthesis.
The genetic knockdown study complemented findings that blockade of mGluR5 with 2-chloro-4-((2,5-dimethyl-1-(4-(trifluoromethoxy)phenyl)-1H-imidazol-4-yl) ethynyl) pyridine (CTEP) improves memory and cognitive function in APPSWE/PS1ΔE9 mice while reducing Aβ oligomers and plaques [61]. Chronic administration of CTEP is required to rescue memory deficits in novel object recognition and the Morris water maze [61]. Aβ oligomer concentrations and plaque formation are also reduced in the 3xTg-AD mice [61]. In addition, selective blockade of mGluR5 is neuroprotective in cortical cultures challenged with toxic concentrations of Aβ [62]. Thus, mGluR5 is a viable drug target to modulate Aβ levels and ensuing phenotypes.
7. An mGluR5 Signaling Nexus Includes APP, Aβ, PrPC and FMRP
The feedback regulation between mGluR5 and APP/Aβ is complex (Figure 2). While mGluR5 activation increases APP production, APP metabolites also appear to influence mGluR5 expression and localization. mGluR5 transcript and protein levels increase significantly in cells treated with Aβ [63–67]. Reactive astrocytes that surround Aβ plaques in AD mice and patients overexpress mGluR5 [64,65,68]. Although there is an age-related decrease in mGluR5 in WT mice, the decrease is less apparent in Tg-ArcSWE AD mice resulting in a significant increase in mGluR5 expression in 16-month old Tg-ArcSWE mice compared to WT [69]. And, cell surface expression of mGluR5 increases by 4.4-fold in APPSWE/PS1ΔE9 mice compared to WT without a change in total cellular expression of mGluR5 [47]. These data fit with an earlier finding that Aβ oligomers bind to and aggregate on neuronal and astrocytic plasma membranes and cause redistribution of mGluR5 to synapses resulting in clusters that partially co-localize with Aβ oligomers [68,70]. In contrast, there are reported tissue and model-specific effects. For example, mGluR5 protein expression increases in astrocytes from the hippocampus but not the entorhinal cortex and selectively in non-transgenic mice but not 3XTg-AD mice [71]. In hippocampus, mGluR5 mRNA expression levels decrease in 9-month old but not 6-month old APPSWE/PS1ΔE9 mice compared to controls, but the decrease does not result in altered mGluR5 hippocampal protein levels [72]. In cortex, there is no significant difference in mGluR5 mRNA or protein expression in APPSWE/PS1ΔE9 versus control mice. And, mGluR5 density is lower in the limbic system in 5xFAD mice [73]. In human subjects, mGluR5 is expressed by 40% of striatal neurons in young individuals with significant intensity variations among neurons, which increases to 80% and 92% of striatal neurons in elderly individuals and AD patients, respectively [74]. Overall, the synergy between mGluR5 and APP/Aβ is complex and may vary dependent on tissue and disease model.
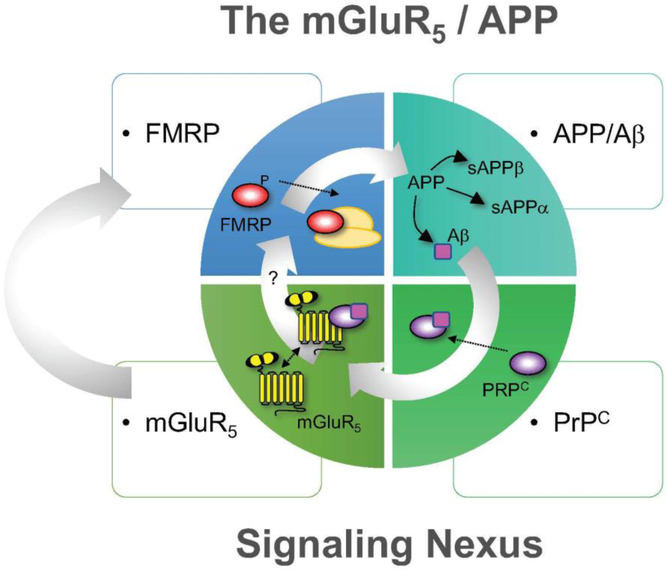
Fig 2.
The mGluR5 / APP Signaling Nexus: The feedback regulation between mGluR5 and APP/Aβ is complex and complicated by the involvement of FMRP and PrPC. APP synthesis is regulated through a FMRP- and mGluR5-dependent signaling pathway [15]. The phosphorylation status of FMRP regulates its association with actively translating polyribosomes [94]. The generation of APP provides template for cleavage of α-, β- and γ-secretases to produce a multitude of APP metabolites, which have varied functions at the synapse [116]. One of those metabolites, A β, associates with PrPC, which acts as a bridge between mGluR5 and A β to form a complex that modulates neuronal signaling [75–82]. The potential role of PrPC in FXS has not been studied. Moving forward, it will be important to explore the temporal and spatial expression and function of APP, APP metabolites, APP secretases, PrPC, mGluR5 and FMRP in control and FXS models.
The complexity of mGluR5/Aβ signaling is increased by involvement of the cellular prion protein (PrPC). mGluR5 does not bind directly to Aβ, but physically associates with PrPC, and this complex plays important roles in modulating Aβ binding and neuronal activity [75,76]. The Aβ/PrPC/mGluR5 signaling complex allows PrPC to associate with the intracellular protein mediators such as Homer1b/c, calcium/calmodulin-dependent protein kinase II (CaMKII) and protein tyrosine kinase 2 beta (Pyk2) to affect synaptic plasticity [77–82]. Coupling of PrPC to these intracellular proteins is modified by soluble Aβ oligomers [79]. Aβ oligomer treatment dissociates Homer1b/c and Pyk2 from the PrPC complex while enhancing the association between PrPC and CamKII as well as between PrPC and mGluR5. The Aβ/PrPC/mGluR5 complex also interacts with Fyn kinase, which is a tyrosine-specific phosphotransferase in the Src family of non-receptor tyrosine protein kinases [75]. A screen of 61-transmembrane postsynaptic density-enriched proteins in HEK293T cells identified mGluR5 as the only candidate to mediate Aβ-induced Fyn phosphorylation in a PrPC-dependent manner [75].
Altered Aβ/PrPC/mGluR5 complex interactions affect downstream signaling. For example, a peptide mimicking the binding site of laminin onto PrPC (Ln-γ1) binds to PrPC and induces intracellular Ca2+ increase in neurons via the PrPC-mGluR5 complex [76]. Ln-γ1 promotes internalization of PrPC and mGluR5 (decreased surface expression) and transiently decreases Aβ binding to neurons [76]. Aβ binding to the neuronal surface is also decreased in mGluRKO5 [70,76]. Amino acids 91–153 of PrPC mediate its interaction with mGluR5 [83]. Agonists of mGluR5 or synthetic Aβ increase the mGluR5-PrPC interaction and mGluR5 antagonists suppress the association. The mGluR5-PrPC interaction is enhanced dramatically in the brains of familial AD transgenic mice, but expression and activity remain to be determined in FXS models. These data suggest that alterations in the PrPC/mGluR5/Aβ complex control downstream mGluR5 signaling. Also in support of this hypothesis, (1) cortical exposure of neuronal cultures to Aβ oligomers upregulated mGluR and PrPC α-cleavage [72]; (2) Aβ-PrPC generates mGluR5-mediated increases of intracellular calcium [75]; (3) Aβ prevents alterations in protein-protein interactions induced by group 1 mGluR activation [84]; (4) repeat treatment with the mGluR5 antagonist basimglurant or an antibody that prevents Aβ oligomer binding to PrPC causes a strong but transient reversal of the long-term potentiation (LTP) deficit in 5-month old transgenic AD rats that express human APP751 with the Swedish and Indiana mutations [85]; and (5) binding of BMS-984923, a potent mGluR5 silent allosteric modulator, does not change glutamate signaling but strongly reduces mGluR5 interaction with PrPC bound to Aβ, which prevents Aβ-induced signal transduction in brain slices and in APPSWE/PS1ΔE9 mice resulting in rescue of memory deficits and synaptic depletion [86].
Aβ affects calcium responses, long-term depression (LTD), and learning & memory through mGluR5- dependent pathways. There appear to be two separate Aβ-dependent signaling cascades that affect calcium mobilization [84]. One cascade is dependent on extracellular Ca2+ and Fyn kinase activation and the other is dependent on the release of Ca2+ from intracellular stores. Aβ increases intracellular calcium mobilization in a time and concentration-dependent fashion, which is associated with a significant increase in astrocytic mGluR5 mRNA and protein expression, and can be blocked by MPEP [64]. Aβ, given exogenously or by altering ADAM10 /synapse-associated protein 97 (SAP97) interactions, induces Ca2+ transients in astrocytes and alters Ca2+ release from neuronal stores through an mGluR5-mediated pathway [64]. Aβ potentiates DHPG-induced Ca2+ responses in a cell-specific manner where DHPG significantly increases the response in Aβ-treated hippocampal but not entorhinal cortex astrocytes [71]. And, a mGluR5 antagonist rescues Ca2+ signaling impairment in APP knockin (KI) neurons [87]. To examine the effects of Aβ on mGluR-LTD, DHPG was applied to hippocampal slices pre-treated with Aβ1–42. While no significant difference in mGluR-LTD between slices treated with vehicle and Aβ were found, mGluRLTD was blocked in aged APPSWE/PS1ΔE9 mice (12–15 months old) [88]. Antagonists of mGluR5 prevent Aβ-induced dendritic spine loss and learning and memory deficits in 9-month old APPSWE/PS1ΔE9 mice and 8–9-month old 3XTg-AD mice [75]. Overall, these data suggest that PrPC couples Aβ with mGluR5 allowing Aβ to stimulate mGluR5 activities, but that Aβ can trap the PrPC-mGluR5 complex in a state that does not allow glutamate-induced regulation of the complex.
There are likely multiple feedback loops facilitating mGluR5 and APP/Aβ effects. The addition of Aβ1–42 oligomers to primary cortical neurons induces a transient increase in α-secretase activity and secreted sAPPα [89]. Preventing the generation of sAPPα with siRNAs increases Aβ1–42 oligomer-induced cell death [89]. These data suggest that neurons respond to stress by generating sAPPα for survival. Additional feedback loops may act at the transcriptional level. Aβ binds to promoter regions of the APP and BACE1 genes and may function as a transcription factor to regulate its own production and/or processing [90].
Additional evidence supporting synergy between APP and mGluR5 comes from other neurological disorders. Subjects with autism exhibit altered levels of APP, FMRP and mGluR5 dependent on age and brain region. There is a significant increase in APP (120 and 88 kDA proteins) in the superior frontal cortex (Brodmann Area 9, BA9) of children with autism versus healthy controls [91], which correlates with increased levels of mGluR5 [92]. This data supports the theory that elevated mGluR5 signaling increases APP synthesis. Conversely, there is no change in APP (120 kDa) expression in the cerebral vermis of children with autism although mGluR5 is also elevated in this brain region [91,93]. Of note, APP expression is much lower in the vermis compared to the BA9 and the antibody epitope of APP was not identified. Thus, APP expression and processing may vary with brain region. Adults with autism have significantly decreased APP (120 kDa) in vermis compared to healthy controls [91,93]. FMRP levels do not differ between control and autism BA9 child samples while there is reduced FMRP in the vermis and BA9 of adults with autism [92,93]. These data appear to contradict the model that mGluR5 activation increases APP production through a FMRP-dependent pathway. However, FMRP activity is modulated by posttranslational modification status. Unphosphorylated FMRP associates with actively translating polyribosomes and a fraction of phosphorylated FMRP associates with stalled ribosomes [94]. Group 1 mGluR-induced dephosphorylation of FMRP facilitates its ubiquitination and degradation [95]. The ratios of phosphorylated serine-499 (S499)-FMRP to neuronal specific enolase (NSE) are reduced in the vermis of adults and children with autism and in BA9 of adults with autism but not in the BA9 of children with autism [96]. These data indicate a positive correlation between FMRP phosphorylation status and APP levels in autism.
In addition to autism, other neurological disorders provide complementary evidence regarding dysregulated expression of mGluR5, FMRP and/or APP in brain. Patients with Down syndrome (DS) are trisomic for chromosome 21, which carries the APP gene. mGluR5 is upregulated in astrocytes of DS brain [97,98]. FMRP is decreased in major psychiatric disorders [99], which is accompanied by altered expression of FMRP-regulated proteins [100]. Regarding APP, levels are reduced in the BA9 of patients with schizophrenia or bipolar disorder, but there are no group differences in the lateral cerebella. Interestingly, subcellular localization studies of BA9 homogenates indicate trends for reduced APP with a significant reduction in FMRP and phosphorylated FMRP in whole homogenates; elevated APP with trends for increased FMRP and phosphorylated FMRP in nuclear fractions; and reduced APP with trends for increased FMRP and phosphorylated FMRP in the rough endoplasmic reticulum (rER) [101]. Thus, in the ribosome-rich rER, there is a correlation between elevated FMRP and reduced expression of APP. mGluR5 density and expression are increased in the prefrontal cortex of patients or carriers of FXS compared to healthy controls [102]. Data is not available regarding APP levels. Overall, substantial evidence suggests that dysregulated mGluR5/FMRP signaling modulates APP levels in multiple neurological disorders. Of note, total FMRP levels are drastically lower in adults than in children (see Figure 2 control lanes within [93]). Much remains to be learned regarding the priority of the mGluR5/FMRP/APP signaling pathway as a function of cell type and compartment, brain region, age and disease status.
Much also remains to be learned regarding the role of FMRP in mGluR5/APP signaling and AD. Transcriptome analysis of brains of 2 AD mouse strains and postmortem gene expression profiles from individuals diagnosed with late onset AD (LOAD) converge on FMR1 [103]. The mouse lines under study are APPE693Q (Dutch mutation) and APPSWE/PS1ΔE9. The APPE693Q mice accumulate Aβ oligomers and behavioral impairment but do not develop parenchymal fibrillary amyloid deposits [103]. The APPSWE/PS1ΔE9 mice accumulate fibrillar Aβ and plaques accompanied by neuritic dystrophy and behavioral impairment [103]. The LOAD tissue included profiles for 6 brain regions across 34 individuals diagnosed with LOAD along with 14 age-matched non-demented controls [103]. The FMR1 gene is differentially spliced in fibrillogenic APPSWE/PS1ΔE9, oligomerogenic APPE693Q dentate gyrus, and multiple LOAD brain regions [103]. Of note, FMR1 is differentially spliced in the frontal pole and superior temporal gyrus in LOAD [103]. There was no evidence of altered cortical or cerebellar expression of FMRP in APPSWE/PS1ΔE9 nor in the frontal cortex or cerebellum of human AD postmortem samples [45]. Likewise, there was not differential expression of FMR1, but rather overexpression of FMR1 exon 5 suggesting a role for alternate splicing of FMR1 in mediating AD pathology [103]. Early work in the field indicated that the FMR1 gene is alternatively spliced although the first splice site commenced at amino acid 282 (nucleotides 844–868) [104]. Interestingly, exon 5 of the FMR1 gene codes for amino acids 90–139 of the amino-terminal domain of FMRP (GenBank accession number L29074.1, nucleotides 30441–30589), which overlaps with the nuclear localization signal (NLS) (amino acids 115–150) [105] and occurs upstream of the BC1 binding region (amino acids 180–217) [30,106]. A rare genetic mutation in exon 5 (arginine to glutamine at amino acid 138, R138Q) has been reported to cause FXS in 2 patients [107]. In Drosophila, alternative splicing of the dfmr1 gene produces 2 isoforms that have different roles in mediating neural development and behavior [108]. The isoform lacking the glutamine/asparagine (Q/N)-rich protein interaction domain in the C-terminus of dFMRP results in defects for a subset of neural development phenotypes associated with the dfmr1 null allele [108]. Thus, alternative splicing as well phosphorylation, ubiquitination and degradation mechanisms likely plays a role in FMRP-mediated regulation of APP. Overall, APP, FMRP and mGluR5 are linked at the molecular level in activity-dependent relationships [109]
8. Functional Consequences of Altered APP Metabolite Levels in Fragile X Models
There is significant premature mortality in Tg2576 mice that overexpress human APP and Aβ (97% survival at 30 days of age; 60% at 60 days), which occurs earlier in FRAXAD mice that overexpress human APPSWE in an Fmr1KO background (77% survival at 30 days of age; 60% at 60 days), compared to WT and Fmr1KO controls (100% survival at 60 days) [59]. Interestingly, an early mortality phenotype is also observed in APPSWE/PS1ΔE9 mice, which exhibit a 70% survival rate at 100 days, 40% at 200 days, and 20% at 300 days of age [110]. Full genetic deletion of mGluR5 or PrPC significantly improves survival in APPSWE/PS1ΔE9 to near WT levels [110].Removal of a single Grm5 (mGluR5) or Prnp (PrPC) allele did not benefit survival [110]. Genetic removal of one allele of both Grm5 and Prnp improves survival midway between WT and APPswe/PS1ΔE9 mice [110]. These data strongly suggest that overexpression of Aβ contributes to early mortality in AD mice and is dependent on the interaction of PrPC and mGluR5.
Early mortality may be due to lower seizure threshold. Juvenile Tg2576, FRAXAD and Ts65Dn (Down syndrome model that expresses 3 copies of murine App) mice (P21) are highly susceptible to audiogenic-induced seizures (AGS), which are attenuated with mGluR5 antagonists or by passive immunization with anti-Aβ antibody in Tg2576 [111]. Adult Tg2576 and FRAXAD mice are highly susceptible to pentylenetetrazole-induced seizures, which are attenuated with MPEP [37,59]. Thus, seizures are associated with increased APP/Aβ levels and are reduced by inhibition of mGluR5. Of note, AGS are exacerbated in both Fmr1KO/APPKO and BC1KOFmr1KO mice [32,112]. At first, it seems contradictory that seizures would be exacerbated in these models as one would predict decreased expression of APP and Aβ and rescue of phenotypes; however, the data strongly suggest that both over- and underexpression of APP and BC1 increases hyperexcitability.
Restoring homeostatic APP levels in Fmr1KO mice rescues many FXS phenotypes including AGS, marble burying, open field, the ratio of mature to immature dendritic spines, and mGluR-LTD in Fmr1KO mice in response to knockdown of a single App allele [38]. An alternative approach, knockdown of APP in Fmr1KO neurons with a lentiviral vector carrying a short hairpin RNA directed specifically against App mRNA, reduces APP and spine density [35]. Exogenous sAPPα increases the number of immature spines while modulation of ADAM10 activity re-establishes physiological sAPPα levels and ultimately ameliorates FXS molecular, synaptic, and behavioral deficits [35]. Fmr1KO/APPHET brain slices exhibit complete rescue of UP states in a neocortical hyperexcitability model and reduced duration of ictal discharges in a CA3 hippocampal model [113]. Thus, APP plays a pivotal role in maintaining an appropriate balance of excitation and inhibition in neural circuits. Based on these data, a model was proposed whereby mGluR5 inhibitors act as a circuit breaker, FMRP as an automatic transfer switch and APP as a rheostat in a circuit that controls hyperexcitability where: (1) excess levels of APP in the Fmr1KO appear to cause a short circuit through overload of the APP rheostat resulting in hyperexcitability, (2) complete loss of APP bypasses the APP rheostat, and (3) the APP rheostat provides a graded response to mGluR5 activation through feedback loops involving amyloidogenic and non-amyloidogenic secretase processing [113].
In support of a role for APP in hyperexcitability, 3xTg-AD mice, which harbor mutated APP, tau and presenilin (PS1) genes, were tested prior to Aβ plaque deposition, neurofibrillary pathology and cognitive impairment (3-weeks old). Results indicated that 3xTg-AD mice exhibit AGS that can be attenuated by passive immunization with anti-human APP/Aβ antibody (6E10) or blockade of mGluR5 with MPEP [114]. These data confirm similar studies performed in a different AD mouse model (Tg2576) using an alternate APP/Aβ antibody (sc-28365LS) and mGluR5 inhibitors (MPEP, fenobam) [111]. It is interesting to note that Tg2576 and 3xTg-AD mice exhibit a strong AGS phenotype, but J20 and R1.40 AD mice do not [111,113,114]. All of these strains overexpress human APP and Aβ; however, the J20 and R1.40 transgenes contain flanking sequences in addition to the coding sequence, which has been previously discussed in terms of the role of FMRP in regulating APP synthesis.
In addition to seizures, measuring ictal-like activity in the CA3 region of the hippocampus in 3xTg-AD mice demonstrates that epileptiform discharge duration positively correlates with intraneuronal human APP/Aβ expression in the CA3 region, which can be suppressed with 6E10 or MPEP [114]. Specifically, in contrast to saline injected 3xTg-AD mice, none of the slices from 6E10-treated 3xTg-AD mice exhibit prolonged epileptiform discharges 60 min after bicuculline application [114]. By 90 min after bicuculline application, 34% of slices from 6E10-treated 3xTg-AD mice develop prolonged epileptiform discharges [114]. Thus, the overall incidence of bicuculline-induced prolonged epileptiform discharges is significantly reduced by passive immunization with 6E10 [114], And in slices that develop prolonged epileptiform discharges, passive immunization with 6E10 significantly increases the latency to the occurrence of these discharges [114]. Prolonged epileptiform discharges are suppressed after MPEP treatment [114]. These data strongly support roles for APP and mGluR5 in network hyperexcitability and suggest that hyperexcitability is independent of plaque load. Likewise, epileptiform activity, as measured by electroencephalography (EEG) in AD mice, exhibits abnormal EEG discharges independent of plaque load [115].
The dysregulated expression of APP and metabolites in multiple FXS models elicits the hypothesis that drugs under development for AD could be repurposed for FXS [116]. In support of that hypothesis, inhibition of beta-secretase 1 (aka beta-site APP cleaving enzyme 1; BACE1) reduces AGS in juvenile Fmr1KO mice and rescues altered morphology of shFmr1 mouse neural progenitor cells [36,113].
9. Moving Forward
Substantial evidence has been reviewed supporting pivotal roles of APP and metabolites in FXS [117,118]. Thus, evolving AD therapies directed at modulating APP synthesis and/or processing may be applicable to FXS, and APP and metabolites may be viable blood-based biomarkers to test therapeutic efficacy and disease severity. A critical question remains regarding what preclinical studies need to be performed to further validate APP as a drug target and biomarker for FXS and help propel this target into clinical trials. Several areas of research that would increase experimental rigor and further validate the role of APP in FXS include:
Study the expression and roles of APP metabolites in the brain as a function of development in FXS models. Constitutive knockdown of APP levels in Fmr1KO mice rescues seizures, behavior, dendritic spine, mGluR-LTD and hyperexcitability phenotypes [35,38,113]. APP metabolites are also associated with the kinetics of neurogenesis [36]. While APP expression remains stable during the differentiation of human induced pluripotent stem cells (iPSC) towards cortical neurons, APP processing changes [119]. Specifically, secretion of sAPPα is detected from day 10 of differentiation and peaks on day 75; sAPPβ is detected from day 45 and remains stable from day 75; Aβx-38 is detected from day 45 and increases thereafter; and Aβx-40 and Aβx-42 are detected at low levels from day 10 but increase significantly between days 45 and 75 [119]. Thus, there is a shift in APP processing during cortical neuronal differentiation. It will be important to determine the expression level and function of APP metabolites and secretases throughout development in FXS. This could be accomplished through conditional knockdown of App in Fmr1KO flies, mice, rats and cells as well as in FXS iPSC lines. Data to date suggest that APP processing changes as a function of age; thus; conditional expression studies will identify therapeutic windows to target specific APP metabolites.
Further elucidate the protein-RNA interactions that regulate APP synthesis and their function. Alternate models have been proposed regarding how RBP affect translation: (a) direct ribosomal recruitment, (b) inhibition of repressor binding, (c) long-range structural remodeling to remove inhibitory features, (d) biogenesis of a translation-competent mRNP, and (e) the influence of mRNA localization [120]. Binding activity and associated functions for the RBP FMRP and hnRNP C in relation to APP mRNA have been identified to date [15,16], but it is probable that the complexity of the protein complexes that assemble on APP mRNA to regulate translation resembles the more multifactorial protein-nucleic acid interactions found in transcription complexes. Much remains to be learned regarding the identity, location and function of RBP that regulate APP mRNA synthesis. FMRP is a known binding partner for nucleolin and YB1 [121,122]. Nucleolin, hnRNPC, RCK/p54, PAI-RBP1, autoantigen La, EF1α and YB1 bind to cis-regulatory elements in the 3’-UTR of App mRNA [123,124]. Iron regulatory protein 1 (IRP1) binds to an iron response element (IRE) in the 5’-UTR of APP mRNA [125]. These protein/RNA interactions could bring multiple cis-regulatory elements in APP mRNA into close proximity to regulate APP synthesis.
Determine the role of BC1 RNA and other ncRNAs in modulating APP in FXS. Inhibiting the BC1-FMRP association in Tg2576 mice blocks Aβ aggregation and protects against spatial learning and memory deficits whereas overexpression of BC1 induces Aβ peptide accumulation and impairs spatial learning and memory [26]. BC1 RNA has two dendritic targeting codes [126]. It will be of interest to define the potential role of BC1 RNA and other ncRNA in the dendritic localization and translation of App mRNA.
Investigate PrPC in FXS models. Despite a substantial quantity of work to date regarding PrPC/mGluR5/Aβ signaling, PrPC has not yet been studied in FXS models. It will be important to examine PrPC in FXS models and to consider the effects of mGluR5 and APP-related therapeutics on PrPC, which plays a pivotal role in the signaling pathway.
Quantitate mGluR5 in multiple brain regions and cell types. Substantial evidence suggests that mGluR5 and downstream signaling molecules are differentially expressed dependent on disease status, brain region and cell type. It would be informative to perform positron emission tomography (PET imaging) in preclinical mouse models and in patients with FXS to determine mGluR5 binding and density in critical brain regions. A mGluR5 tracer has been used to quantitate higher mGluR5 binding potential by PET in postcentral gyrus and cerebellum, but not in other brain regions, of individuals with autism [127]. There was a positive correlation with the lethargy subscale score on the Aberrant Behavior Checklist (ABC) in the precuneus [127]. In the cerebellum, there were significant negative correlations between binding potential and ABC total score, ABC hyperactivity subscale score, and the ABC inappropriate speech subscale score [127]. PET has also been employed to examine in vivo changes in mGluR5 in an Aβ pathology model (Tg-ArcSWE mice) [69]. PET with [11C]ABP688, which is highly specific to mGluR5, resulted in similar levels of tracer in mice regardless of genotype or age and identified receptor-dense brain regions (hippocampus, thalamus, and striatum) [69]. PET with the mGluR5 specific radiotracer ([18F]FPEB) in 5xFAD AD model mice indicated significantly lower radioactivity and binding activity in the hippocampus and striatum of 5xFAD mice (APP transgene with Swedish, Florida and London AD mutations and PS-1 with the M146L and L286V mutations) compared to control animals indicating that mGluR5 is downregulated in the limbic system [73]. It is of interest to quantitate how mGluR5 expression, localization, density and association with PrPC and Aβ change with development, cell type and disease model
Understand the synergy between FMRP and APP. For example, J20 AD mice exhibit elevated APP expression but are not very sensitive to AGS [113]. The inclusion of flanking sequences in the human APP gene in this model likely alters the temporal and spatial expression of APP and metabolites resulting in a higher seizure threshold. Genetically crossing J20 with Fmr1KO mice produces offspring that overexpress human APP in the context of the Fmr1KO background. Male offspring (Fmr1KO/J20) do not exhibit an exacerbated AGS phenotype in comparison to Fmr1KO and unlike FRAXAD mice [111,113]. However, Fmr1Het/J20 female mice exhibit 50% wild running and 40% AGS rate in response to audiogenic stimulation, which is significantly higher than WT, Fmr1HET and J20 controls,supporting a dosage effect and the assertion that FMRP works in synergy with APP to regulate hyperexcitability [113].
Study APP synthesis and cleavage in response to environmental stressors. To date, studies have not been published indicating whether environmental stressors can alter the binding or activities of RBPs or microRNAs that interact with APP mRNA and thus affect protein production. For example, seizures are associated with the consumption of soy-based diets in mouse and human models [112,128]. Gastrointestinal problems are common in FXS and these infants may be more likely to consume alternative diets such as soy-based infant formula. FXS is not part of the newborn screening panel; thus, most children are 2–3 years of age before diagnosis and well past the period of infant diet selection. Thus, it is important to determine if infant diet can affect disease outcomes. The soy phytoestrogen daidzein increases APP expression in primary cultured neurons and increases wild running in response to audiogenic stimulation in WT mice [112].
Investigate mGluR5/FMRP/APP signaling in other FXS models. Elevated APP causes accelerated generation of neural progenitors and neurons in shFmr1 mESC [129]. It will be of interest to study this signaling pathway in other FXS models such as Drosophila and iPSC derived from FXS patients versus controls. Flies that express a 50% reduction in presenilin 1 (PSEN1; a core protein in the gamma secretase complex involved in APP cleavage) display age related-onset impairment in learning and memory, which can be prevented with MPEP [130].
Employ APP metabolites as exploratory biomarkers in clinical trials. Drugs under study for FXS such as acamprosate, AFQ056, Donepezil, Ganaxolone, Lithium, Lovastatin, Memantine, Minocycline and Sertraline exhibit on- and/or off-site effects expected to modulate APP, Aβ, BACE1 and/or ADAM10 [116]. In addition, many of the identified receptor and signaling molecules with established roles in FXS are regulated by APP and/or Aβ. A clinical study indicates that acamprosate is associated with a significant reduction in plasma soluble APP and sAPPα in youth with ASD [41]. Youth with FXS-associated ASD exhibited increased sAPPα-processing compared to age-, gender- and IQ-matched youth with idiopathic ASD [41]. Thus, APP metabolites may be promising biomarkers for FXS clinical trials.
Elucidate the connection between FMRP, APP and autism. There are high levels of APP in children with severely autistic behavior and aggression, and it has been proposed that sAPPα contributes to neuronal overgrowth in autistic brain [131,132]. It will be important to elucidate the connection between FXS, autism and APP.
7. Conclusions
In conclusion, the past decade has produced over 125 papers citing and advancing the 2007 report that mGluR5/FMRP signaling mediates the synthesis of APP. Much remains to be learned in terms of cell, circuit, tissue and disease-specific regulation of APP in FXS models to support development of this potential biomarker and therapeutic target.
Acknowledgements.
The author was funded by FRAXA Research Foundation, University of Wisconsin ADRC (P50 AG033514) and ICTR (NCATS 9U54TR000021) programs, NIA (R21 AG044714), and the Department of Defense (W81XWH-16–1–0082). The funding bodies played no role in the preparation of the manuscript or the decision to submit for publication. The author thanks Dr. Pamela Westmark for critical review of the manuscript.
Footnotes
Conflict of Interest Disclosures.
CJW has received research funding from Lundbeck Research USA, Inc. and Merz Pharmaceuticals GmbH to test novel mGluR5 inhibitors in Fmr1KO mice.
References.
- 1.Hagerman RJ and Hagerman PJ (2002) Physical and behavioral phenotype, John Hopkins University Press, Baltimore, pp. 3–109. [Google Scholar]
- 2.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP and et al. , (1991) Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–914. [DOI] [PubMed] [Google Scholar]
- 3.Bagni C and Greenough WT, (2005) From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat. Rev. Neurosci 6, 376–387. [DOI] [PubMed] [Google Scholar]
- 4.Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A and Fischer U, (2001) Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum. Mol. Genet 10, 329–338. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST and Feng Y, (2001) The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res 29, 2276–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazroui R, Huot ME, Tremblay S, Filion C, Labelle Y and Khandjian EW, (2002) Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Hum. Mol. Genet 11, 3007–3017. [DOI] [PubMed] [Google Scholar]
- 7.Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J and Greenough WT, (1997) Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc. Natl. Acad. Sci. U. S. A 94, 5395–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Y, Absher D, Eberhart DE, Brown V, Malter HE and Warren ST, (1997) FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol. Cell 1, 109–118. [DOI] [PubMed] [Google Scholar]
- 9.Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST and Hersch SM, (1997) Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J. Neurosci 17, 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB and Warren ST, (2001) Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 107, 477–487. [DOI] [PubMed] [Google Scholar]
- 11.Darnell JC, Jensen KB, Jin P, Brown V, Warren ST and Darnell RB, (2001) Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 107, 489–499. [DOI] [PubMed] [Google Scholar]
- 12.Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT and Eberwine J, (2003) RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron 37, 417–431. [DOI] [PubMed] [Google Scholar]
- 13.The Dutch-Belgian Fragile X Consortium, (1994) Fmr1 knockout mice: a model to study fragile X mental retardation. Cell 78, 23–33. [PubMed] [Google Scholar]
- 14.Bear MF, Huber KM and Warren ST, (2004) The mGluR theory of fragile X mental retardation. Trends Neurosci 27, 370–377. [DOI] [PubMed] [Google Scholar]
- 15.Westmark CJ and Malter JS, (2007) FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol 5, e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EK, Kim HH, Kuwano Y, Abdelmohsen K, Srikantan S, Subaran SS, Gleichmann M, Mughal MR, Martindale JL, Yang X, Worley PF, Mattson MP and Gorospe M, (2010) hnRNP C promotes APP translation by competing with FMRP for APP mRNA recruitment to P bodies. Nat. Struct. Mol. Biol 17, 732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD and Darnell RB, (2011) FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ascano M Jr, Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, Langlois C, Munschauer M, Dewell S, Hafner M, Williams Z, Ohler U and Tuschl T, (2012) FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature 492, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cirillo D, Agostini F, Klus P, Marchese D, Rodriguez S, Bolognesi B and Tartaglia GG, (2013) Neurodegenerative diseases: quantitative predictions of protein-RNA interactions. RNA 19, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crenshaw E, Leung BP, Kwok CK, Sharoni M, Olson K, Sebastian NP, Ansaloni S, Schweitzer-Stenner R, Akins MR, Bevilacqua PC and Saunders AJ, (2015) Amyloid Precursor Protein Translation Is Regulated by a 3’UTR Guanine Quadruplex. PLoS One 10, e0143160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian M, Rage F, Tabet R, Flatter E, Mandel JL and Moine H, (2011) G-quadruplex RNA structure as a signal for neurite mRNA targeting. EMBO Rep 12, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amara FM, Junaid A, Clough RR and Liang B, (1999) TGF-beta(1), regulation of Alzheimer amyloid precursor protein mRNA expression in a normal human astrocyte cell line: mRNA stabilization. Brain Res. Mol. Brain Res 71, 42–49. [DOI] [PubMed] [Google Scholar]
- 23.Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, Johnson N, Howlett IC, Geng C, Ueda R, Brand AH, Newbury SF, Wilhelm JE, Levine RB, Nakamura A, Parker R and Ramaswami M, (2006) Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron 52, 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenny PJ, Zhou H, Kim M, Skariah G, Khetani RS, Drnevich J, Arcila ML, Kosik KS and Ceman S, (2014) MOV10 and FMRP regulate AGO2 association with microRNA recognition elements. Cell. Rep 9, 1729–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Idda ML, Munk R, Abdelmohsen K and Gorospe M, (2018) Noncoding RNAs in Alzheimer’s disease. Wiley Interdiscip. Rev. RNA 9, 10–1002/wrna..1463. Epub 2018. January 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T, Pang P, Fang Z, Guo Y, Li H, Li X, Tian T, Yang X, Chen W, Shu S, Tang N, Wu J, Zhu H, Pei L, Liu D, Tian Q, Wang J, Wang L, Zhu LQ and Lu Y, (2017) Expression of BC1 Impairs Spatial Learning and Memory in Alzheimer’s Disease Via APP Translation. Mol. Neurobiol 55, 6007–6020. [DOI] [PubMed] [Google Scholar]
- 27.Bagni C, (2008) On BC1 RNA and the fragile X mental retardation protein. Proc. Natl. Acad. Sci. U.S. A 105, E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, Brosius J, Denman RB, Khandjian EW, Kindler S and Tiedge H, (2008) Reply to Bagni: On BC1 RNA and the fragile X mental retardation protein. Proc. Natl. Acad. Sci. U. S. A 105, E29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B and Bagni C, (2003) The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell 112, 317–327. [DOI] [PubMed] [Google Scholar]
- 30.Zalfa F, Adinolfi S, Napoli I, Kuhn-Holsken E, Urlaub H, Achsel T, Pastore A and Bagni C, (2005) Fragile X mental retardation protein (FMRP) binds specifically to the brain cytoplasmic RNAs BC1/BC200 via a novel RNA-binding motif. J. Biol. Chem 280, 33403–33410. [DOI] [PubMed] [Google Scholar]
- 31.Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schutt J, Brosius J, Denman RB, Khandjian EW, Kindler S and Tiedge H, (2008) On BC1 RNA and the fragile X mental retardation protein. Proc. Natl. Acad. Sci. U. S. A 105, 734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong J, Chuang SC, Bianchi R, Zhao W, Paul G, Thakkar P, Liu D, Fenton AA, Wong RK and Tiedge H, (2010) Regulatory BC1 RNA and the fragile X mental retardation protein: convergent functionality in brain. PLoS One 5, e15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F and Cole G, (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274, 99–102. [DOI] [PubMed] [Google Scholar]
- 34.Liao L, Park SK, Xu T, Vanderklish P and Yates JR 3rd., (2008) Quantitative proteomic analysis of primary neurons reveals diverse changes in synaptic protein content in fmr1 knockout mice. Proc. Natl. Acad. Sci. U. S. A 105, 15281–15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasciuto E, Ahmed T, Wahle T, Gardoni F, D’Andrea L, Pacini L, Jacquemont S, Tassone F, Balschun D, Dotti CG, Callaerts-Vegh Z, D’Hooge R, Muller UC, Di Luca M, De Strooper B and Bagni C, (2015) Dysregulated ADAM10-Mediated Processing of APP during a Critical Time Window Leads to Synaptic Deficits in Fragile X Syndrome. Neuron 87, 382–398. [DOI] [PubMed] [Google Scholar]
- 36.Khalfallah O, Jarjat M, Davidovic L, Nottet N, Cestele S, Mantegazza M and Bardoni B, (2017) Depletion of the Fragile X Mental Retardation Protein in Embryonic Stem Cells Alters the Kinetics of Neurogenesis. Stem Cells 35, 374–385. [DOI] [PubMed] [Google Scholar]
- 37.Westmark CJ, Westmark PR and Malter JS, (2009) MPEP reduces seizure severity in Fmr-1 KO mice over expressing human Abeta. Int. J. Clin. Exp. Pathol 3, 56–68. [PMC free article] [PubMed] [Google Scholar]
- 38.Westmark CJ, Westmark PR, O’Riordan KJ, Ray BC, Hervey CM, Salamat MS, Abozeid SH, Stein KM, Stodola LA, Tranfaglia M, Burger C, Berry-Kravis EM and Malter JS, (2011) Reversal of Fragile X Phenotypes by Manipulation of AbetaPP/Abeta Levels in Fmr1 Mice. PLoS One 6, e26549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray B, Sokol DK, Maloney B and Lahiri DK, (2016) Finding novel distinctions between the sAPPalpha-mediated anabolic biochemical pathways in Autism Spectrum Disorder and Fragile X Syndrome plasma and brain tissue. Sci. Rep 6, 26052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westmark CJ, Sokol DK, Maloney B and Lahiri DK, (2016) Novel roles of amyloid-beta precursor protein metabolites in fragile X syndrome and autism. Mol. Psychiatry 21, 1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erickson CA, Ray B, Maloney B, Wink LK, Bowers K, Schaefer TL, McDougle CJ, Sokol DK and Lahiri DK, (2014) Impact of acamprosate on plasma amyloid-beta precursor protein in youth: A pilot analysis in fragile X syndrome-associated and idiopathic autism spectrum disorder suggests a pharmacodynamic protein marker. J. Psychiatr. Res 59, 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westmark CJ, Hervey CM, Berry-Kravis EM and Malter JS, (2011) Effect of Anticoagulants on Amyloid beta-Protein Precursor and Amyloid Beta Levels in Plasma. Alzheimer’s Disease and Parkinsonism 1, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh K, Gaur P and Prasad S, (2007) Fragile x mental retardation (Fmr-1) gene expression is down regulated in brain of mice during aging. Mol. Biol. Rep 34, 173–181. [DOI] [PubMed] [Google Scholar]
- 44.Singh K and Prasad S, (2008) Differential expression of Fmr-1 mRNA and FMRP in female mice brain during aging. Mol. Biol. Rep 35, 677–684. [DOI] [PubMed] [Google Scholar]
- 45.Renoux AJ, Carducci NM, Ahmady AA and Todd PK, (2014) Fragile X mental retardation protein expression in Alzheimer’s disease. Front. Genet 5, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borreca A, Gironi K, Amadoro G and Ammassari-Teule M, (2016) Opposite Dysregulation of Fragile-X Mental Retardation Protein and Heteronuclear Ribonucleoprotein C Protein Associates with Enhanced APP Translation in Alzheimer Disease. Mol. Neurobiol 53 3227–3234. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton A, Esseltine JL, DeVries RA, Cregan SP and Ferguson SS, (2014) Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer’s disease. Mol. Brain 7, 40–6606–7–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG and Borchelt DR, (2004) Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet 13, 159–170. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ and Frosch MP, (2006) Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis 24, 516–524. [DOI] [PubMed] [Google Scholar]
- 50.Tassone F, Greco CM, Hunsaker MR, Seritan AL, Berman RF, Gane LW, Jacquemont S, Basuta K, Jin LW, Hagerman PJ and Hagerman RJ, (2012) Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mateu-Huertas E, Rodriguez-Revenga L, Alvarez-Mora MI, Madrigal I, Willemsen R, Mila M, Marti E and Estivill X, (2014) Blood expression profiles of fragile X premutation carriers identify candidate genes involved in neurodegenerative and infertility phenotypes. Neurobiol. Dis 65, 43–54. [DOI] [PubMed] [Google Scholar]
- 52.Westmark PR, Dekundy A, Gravius A, Danysz W and Westmark CJ, (2018) Rescue of Fmr1KO Phenotypes with mGluR5 Inhibitors: MRZ-8456 versus AFQ-056. Neurobiol. Dis doi: 10.1016/j.nbd.2018.08.008 (ahead of print). [DOI] [PubMed] [Google Scholar]
- 53.Malter JS, Ray BC, Westmark PR and Westmark CJ, (2010) Fragile X Syndrome and Alzheimer’s Disease: Another story about APP and beta-amyloid. Curr. Alzheimer Res 7, 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L and Allan T, (1982) Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J. Clin. Psychopharmacol 2, 129–133. [PubMed] [Google Scholar]
- 55.Friedmann C, Davis L, Ciccone P and Rubin R, (1980) Phase II double blind controlled study of a new anxiolytic, fenobam (McN-3377) vs placebo. Curr Ther Res 274, 144–144–151. [Google Scholar]
- 56.Itil TM, Seaman PA, Huque M, Mukhopadhyay S, Blascucci D, Nq KT and Ciccone PE, (1978) The clinical and quantitative EEG effects and plasma levels of fenobam (McN-3377) in subjects with anxiety: an open rising dose tolerance and efficacy study. Curr. Ther. Res 24, 708–724. [Google Scholar]
- 57.Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV and Hagerman R, (2009) A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J. Med. Genet 46, 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porter RH, Jaeschke G, Spooren W, Ballard TM, Buttelmann B, Kolczewski S, Peters JU, Prinssen E, Wichmann J, Vieira E, Muhlemann A, Gatti S, Mutel V and Malherbe P, (2005) Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 recepto antagonist with inverse agonist activity. J. Pharmacol. Exp. Ther 315, 711–721. [DOI] [PubMed] [Google Scholar]
- 59.Westmark CJ, Westmark P, Beard A, Hildebrandt S and Malter JS, (2008) Seizure susceptibility and mortality in mice that over-express amyloid precursor protein. Int J Clin Exp Pathol 1, 157–168. [PMC free article] [PubMed] [Google Scholar]
- 60.Rivera D, Fedele E, Marinari UM, Pronzato MA and Ricciarelli R, (2015) Evaluating the role of hnRNP-C and FMRP in the cAMP-induced APP metabolism. Biofactors 41, 121–126. [DOI] [PubMed] [Google Scholar]
- 61.Hamilton A, Vasefi M, Vander Tuin C, McQuaid RJ, Anisman H and Ferguson SS, (2016) Chronic pharmacological mGluR5 inhibition prevents cognitive impairment and reduces pathogenesis in an Alzheimer disease mouse model. Cell. Rep 15, 1859–1865. [DOI] [PubMed] [Google Scholar]
- 62.Bruno V, Ksiazek I, Battaglia G, Lukic S, Leonhardt T, Sauer D, Gasparini F, Kuhn R, Nicoletti F and Flor PJ, (2000) Selective blockade of metabotropic glutamate receptor subtype 5 is neuroprotective. Neuropharmacology 39, 2223–2230. [DOI] [PubMed] [Google Scholar]
- 63.Grolla AA, Fakhfouri G, Balzaretti G, Marcello E, Gardoni F, Canonico PL, DiLuca M, Genazzani AA and Lim D, (2013) Abeta leads to Ca(2)(+) signaling alterations and transcriptional changes in glial cells. Neurobiol. Aging 34, 511–522. [DOI] [PubMed] [Google Scholar]
- 64.Casley CS, Lakics V, Lee HG, Broad LM, Day TA, Cluett T, Smith MA, O’Neill MJ and Kingston AE, (2009) Up-regulation of astrocyte metabotropic glutamate receptor 5 by amyloid-beta peptide. Brain Res 1260, 65–75. [DOI] [PubMed] [Google Scholar]
- 65.Lim D, Iyer A, Ronco V, Grolla AA, Canonico PL, Aronica E and Genazzani AA, (2013) Amyloid beta deregulates astroglial mGluR5-mediated calcium signaling via calcineurin and Nf-kB. Glia 61, 1134–1145. [DOI] [PubMed] [Google Scholar]
- 66.Ronco V, Grolla AA, Glasnov TN, Canonico PL, Verkhratsky A, Genazzani AA and Lim D, (2014) Differential deregulation of astrocytic calcium signalling by amyloid-beta, TNFalpha, IL-1beta and LPS. Cell Calcium 55, 219–229. [DOI] [PubMed] [Google Scholar]
- 67.Overk CR, Cartier A, Shaked G, Rockenstein E, Ubhi K, Spencer B, Price DL, Patrick C,Desplats P and Masliah E, (2014) Hippocampal neuronal cells that accumulate alpha-synuclein fragments are more vulnerable to Abeta oligomer toxicity via mGluR5--implications for dementia with Lewy bodies. Mol. Neurodegener 9, 18–1326–9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shrivastava AN, Kowalewski JM, Renner M, Bousset L, Koulakoff A, Melki R, Giaume C and Triller A, (2013) beta-amyloid and ATP-induced diffusional trapping of astrocyte and neuronal metabotropic glutamate type-5 receptors. Glia 61, 1673–1686. [DOI] [PubMed] [Google Scholar]
- 69.Fang XT, Eriksson J, Antoni G, Yngve U, Cato L, Lannfelt L, Sehlin D and Syvanen S, (2017) Brain mGluR5 in mice with amyloid beta pathology studied with in vivo [(11)C]ABP688 PET imaging and ex vivo immunoblotting. Neuropharmacology 113, 293–300. [DOI] [PubMed] [Google Scholar]
- 70.Renner M, Lacor PN, Velasco PT, Xu J, Contractor A, Klein WL and Triller A, (2010) Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron 66, 739–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grolla AA, Sim JA, Lim D, Rodriguez JJ, Genazzani AA and Verkhratsky A, (2013) Amyloid-beta and Alzheimer’s disease type pathology differentially affects the calcium signalling toolkit in astrocytes from different brain regions. Cell. Death Dis 4, e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ostapchenko VG, Beraldo FH, Guimaraes AL, Mishra S, Guzman M, Fan J, Martins VR, Prado VF and Prado MA, (2013) Increased prion protein processing and expression of metabotropic glutamate receptor 1 in a mouse model of Alzheimer’s disease. J. Neurochem 127, 415–425. [DOI] [PubMed] [Google Scholar]
- 73.Lee M, Lee HJ, Park IS, Park JA, Kwon YJ, Ryu YH, Kim CH, Kang JH, Hyun IY, Lee KC and Choi JY, (2018) Abeta pathology downregulates brain mGluR5 density in a mouse model of Alzheimer. Neuropharmacology 133, 512–517. [DOI] [PubMed] [Google Scholar]
- 74.Tsamis KI, Mytilinaios DG, Njau SN and Baloyannis SJ, (2013) Glutamate receptors in human caudate nucleus in normal aging and Alzheimer’s disease. Curr. Alzheimer Res 10, 469–475. [DOI] [PubMed] [Google Scholar]
- 75.Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, Kerrisk ME, Vortmeyer A, Wisniewski T, Koleske AJ, Gunther EC, Nygaard HB and Strittmatter SM, (2013) Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron 79, 87–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beraldo FH, Ostapchenko VG, Caetano FA, Guimaraes AL, Ferretti GD, Daude N, Bertram L, Nogueira KO, Silva JL, Westaway D, Cashman NR, Martins VR, Prado VF and Prado MA, (2016) Regulation of amyloid beta oligomer binding to neurons and neurotoxicity by the prion protein-mGluR5 complex. J. Biol. Chem 291, 21945–21955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kostylev MA, Kaufman AC, Nygaard HB, Patel P, Haas LT, Gunther EC, Vortmeyer A and Strittmatter SM, (2015) Prion-protein-interacting amyloid-beta oligomers of high molecular weight are tightly correlated with memory impairment in multiple Alzheimer mouse models. J. Biol. Chem 290, 17415–17438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu NW, Nicoll AJ, Zhang D, Mably AJ, O’Malley T, Purro SA, Terry C, Collinge J, Walsh DM and Rowan MJ, (2014) mGlu5 receptors and cellular prion protein mediate amyloid-beta-facilitated synaptic long-term depression in vivo. Nat. Commun 5, 3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haas LT, Salazar SV, Kostylev MA, Um JW, Kaufman AC and Strittmatter SM, (2016) Metabotropic glutamate receptor 5 couples cellular prion protein to intracellular signalling in Alzheimer’s disease. Brain 139, 526–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salazar SV and Strittmatter SM, (2017) Cellular prion protein as a receptor for amyloid-beta oligomers in Alzheimer’s disease. Biochem. Biophys. Res. Commun 483, 1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sivanesan S, Tan A and Rajadas J, (2013) Pathogenesis of Abeta oligomers in synaptic failure. Curr. Alzheimer Res 10, 316–323. [DOI] [PubMed] [Google Scholar]
- 82.Brody AH and Strittmatter SM, (2018) Synaptotoxic signaling by amyloid beta oligomers in Alzheimer’s disease through prion protein and mGluR5. Adv. Pharmacol 82, 293–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haas LT, Kostylev MA and Strittmatter SM, (2014) Therapeutic molecules and endogenous ligands regulate the interaction between brain cellular prion protein (PrPC) and metabotropic glutamate receptor 5 (mGluR5). J. Biol. Chem 289, 28460–28477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haas LT and Strittmatter SM, (2016) Oligomers of Amyloid beta Prevent Physiological Activation of the Cellular Prion Protein-Metabotropic Glutamate Receptor 5 Complex by Glutamate in Alzheimer Disease. J. Biol. Chem 291, 17112–17121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang D, Qi Y, Klyubin I, Ondrejcak T, Sarell CJ, Cuello AC, Collinge J and Rowan MJ, (2017) Targeting glutamatergic and cellular prion protein mechanisms of amyloid beta-mediated persistent synaptic plasticity disruption: Longitudinal studies. Neuropharmacology 121, 231–246. [DOI] [PubMed] [Google Scholar]
- 86.Haas LT, Salazar SV, Smith LM, Zhao HR, Cox TO, Herber CS, Degnan AP, Balakrishnan A, Macor JE, Albright CF and Strittmatter SM, (2017) Silent allosteric modulation of mGluR5 maintains glutamate signaling while rescuing Alzheimer’s mouse phenotypes. Cell. Rep 20, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang H, Wu L, Pchitskaya E, Zakharova O, Saito T, Saido T and Bezprozvanny I, (2015) Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of Alzheimer’s disease. J. Neurosci 35, 13275–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang W, Zhou X, Zimmermann HR, Cavener DR, Klann E and Ma T, (2016) Repression of the eIF2alpha kinase PERK alleviates mGluR-LTD impairments in a mouse model of Alzheimer’s disease. Neurobiol. Aging 41, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rose C, Dorard E, Audrain M, Gorisse-Hussonnois L, Cartier N, Braudeau J and Allinquant B, (2018) Transient increase in sAPPalpha secretion in response to Abeta1–42 oligomers: an attempt of neuronal self-defense? Neurobiol. Aging 61, 23–35. [DOI] [PubMed] [Google Scholar]
- 90.Maloney B and Lahiri DK, (2011) The Alzheimer’s amyloid beta-peptide (Abeta) binds a specific DNA Abeta-interacting domain (AbetaID) in the APP, BACE1, and APOE promoters in a sequence-specific manner: characterizing a new regulatory motif. Gene 488, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fatemi SH, Folsom TD, Kneeland RE, Yousefi MK, Liesch SB and Thuras PD, (2013) Impairment of fragile X mental retardation protein-metabotropic glutamate receptor 5 signaling and its downstream cognates ras-related C3 botulinum toxin substrate 1, amyloid beta A4 precursor protein, striatal-enriched protein tyrosine phosphatase, and homer 1, in autism: a postmortem study in cerebellar vermis and superior frontal cortex. Mol. Autism 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fatemi SH and Folsom TD, (2011) Dysregulation of fragile x mental retardation protein and metabotropic glutamate receptor 5 in superior frontal cortex of individuals with autism: a postmortem brain study. Mol. Autism 2, 6–2392–2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fatemi SH, Folsom TD, Kneeland RE and Liesch SB, (2011) Metabotropic glutamate receptor 5 upregulation in children with autism is associated with underexpression of both Fragile X mental retardation protein and GABAA receptor beta 3 in adults with autism. Anat. Rec. (Hoboken) 294, 1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ceman S, O’Donnell WT, Reed M, Patton S, Pohl J and Warren ST, (2003) Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum. Mol. Genet 12, 3295–3305. [DOI] [PubMed] [Google Scholar]
- 95.Nalavadi VC, Muddashetty RS, Gross C and Bassell GJ, (2012) Dephosphorylation-induced ubiquitination and degradation of FMRP in dendrites: a role in immediate early mGluR-stimulated translation. J. Neurosci 32, 2582–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rustan OG, Folsom TD, Yousefi MK and Fatemi SH, (2013) Phosphorylated fragile X mental retardation protein at serine 499, is reduced in cerebellar vermis and superior frontal cortex of subjects with autism: implications for fragile X mental retardation protein-metabotropic glutamate receptor 5 signaling. Mol. Autism 4, 41–2392–4–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oka A and Takashima S, (1999) The up-regulation of metabotropic glutamate receptor 5 (mGluR5) in Down’s syndrome brains. Acta Neuropathol. (Berl) 97, 275–278. [DOI] [PubMed] [Google Scholar]
- 98.Iyer AM, van Scheppingen J, Milenkovic I, Anink JJ, Lim D, Genazzani AA, Adle-Biassette H, Kovacs GG and Aronica E, (2014) Metabotropic glutamate receptor 5 in Down’s syndrome hippocampus during development: increased expression in astrocytes. Curr. Alzheimer Res 11, 694–705. [DOI] [PubMed] [Google Scholar]
- 99.Fatemi SH, Kneeland RE, Liesch SB and Folsom TD, (2010) Fragile X mental retardation protein levels are decreased in major psychiatric disorders. Schizophr. Res 124, 246–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Folsom TD, Thuras PD and Fatemi SH, (2015) Protein expression of targets of the FMRP regulon is altered in brains of subjects with schizophrenia and mood disorders. Schizophr. Res 165, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fatemi SH, Folsom TD and Thuras PD, (2017) Altered subcellular localization of fragile X mental retardation signaling partners and targets in superior frontal cortex of individuals with schizophrenia. Neuroreport 28, 1066–1070. [DOI] [PubMed] [Google Scholar]
- 102.Lohith TG, Osterweil EK, Fujita M, Jenko KJ, Bear MF and Innis RB, (2013) Is metabotropic glutamate receptor 5 upregulated in prefrontal cortex in fragile X syndrome? Mol. Autism 4, 15–2392–4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Readhead B, Haure-Mirande JV, Zhang B, Haroutunian V, Gandy S, Schadt EE, Dudley JT and Ehrlich ME, (2016) Molecular systems evaluation of oligomerogenic APP(E693Q) and fibrillogenic APP(KM670/671NL)/PSEN1(Deltaexon9) mouse models identifies shared features with human Alzheimer’s brain molecular pathology. Mol. Psychiatry 21, 1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Verkerk AJ, de Graaff E, De Boulle K, Eichler EE, Konecki DS, Reyniers E, Manca A, Poustka A, Willems PJ and Nelson DL, (1993) Alternative splicing in the fragile X gene FMR1. Hum. Mol. Genet 2, 1348. [DOI] [PubMed] [Google Scholar]
- 105.Bardoni B, Sittler A, Shen Y and Mandel JL, (1997) Analysis of domains affecting intracellular localization of the FMRP protein. Neurobiol. Dis 4, 329–336. [DOI] [PubMed] [Google Scholar]
- 106.Lacoux C, Di Marino D, Boyl PP, Zalfa F, Yan B, Ciotti MT, Falconi M, Urlaub H, Achsel T, Mougin A, Caizergues-Ferrer M and Bagni C, (2012) BC1-FMRP interaction is modulated by 2’-O-methylation: RNA-binding activity of the tudor domain and translational regulation at synapses. Nucleic Acids Res 40, 4086–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sitzmann AF, Hagelstrom RT, Tassone F, Hagerman RJ and Butler MG, (2018) Rare FMR1 gene mutations causing fragile X syndrome: A review. Am. J. Med. Genet. A 176, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Banerjee P, Schoenfeld BP, Bell AJ, Choi CH, Bradley MP, Hinchey P, Kollaros M, Park JH, McBride SM and Dockendorff TC, (2010) Short- and long-term memory are modulated by multiple isoforms of the fragile X mental retardation protein. J. Neurosci 30, 6782–6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sokol DK, Maloney B, Long JM, Ray B and Lahiri DK, (2011) Autism, Alzheimer disease, and fragile X: APP, FMRP, and mGluR5 are molecular links. Neurology 76, 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haas LT, Salazar SV, Kostylev MA, Um JW, Kaufman AC and Strittmatter SM, (2016) Metabotropic glutamate receptor 5 couples cellular prion protein to intracellular signalling in Alzheimer’s disease. Brain 139, 526–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Westmark CJ, Westmark PR and Malter JS, (2010) Alzheimer’s disease and Down syndrome rodent models exhibit audiogenic seizures. J. Alzheimers Dis 20, 1009–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Westmark CJ, Westmark PR and Malter JS, (2013) Soy-based diet exacerbates seizures in mouse models of neurological disease. J. Alzheimers Dis 33, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Westmark CJ, Chuang SC, Hays SA, Filon MJ, Ray BC, Westmark PR, Gibson JR, Huber KM and Wong RK, (2016) APP Causes Hyperexcitability in Fragile X Mice. Front. Mol. Neurosci 9, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kazim SF, Chuang SC, Zhao W, Wong RK, Bianchi R and Iqbal K, (2017) Early-Onset Network Hyperexcitability in Presymptomatic Alzheimer’s Disease Transgenic Mice Is Suppressed by Passive Immunization with Anti-Human APP/Abeta Antibody and by mGluR5 Blockade. Front. Aging Neurosci 9, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Born HA, Kim JY, Savjani RR, Das P, Dabaghian YA, Guo Q, Yoo JW, Schuler DR, Cirrito JR, Zheng H, Golde TE, Noebels JL and Jankowsky JL, (2014) Genetic suppression of transgenic APP rescues Hypersynchronous network activity in a mouse model of Alzheimer’s disease. J. Neurosci 34, 3826–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Westmark CJ, Berry-Kravis EM, Ikonomidou C, Yin JC and Puglielli L, (2013) Developing BACE-1 inhibitors for FXS. Front. Cell. Neurosci 7, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Westmark CJ, (2013) What’s hAPPening at synapses? The role of amyloid beta-protein precursor and beta-amyloid in neurological disorders. Mol. Psychiatry 18, 425–434. [DOI] [PubMed] [Google Scholar]
- 118.Westmark CJ, (2017) Commentary: Depletion of the fragile X mental retardation protein in embryonic stem cells alters the kinetics of neurogenesis. Front. Mol. Neurosci 10, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bergstrom P, Agholme L, Nazi FH, Satir TM, Toombs J, Wellington H, Strandberg J, Bontell TO, Kvartsberg H, Holmstrom M, Borestrom C, Simonsson S, Kunath T, Lindahl A, Blennow K, Hanse E, Portelius E, Wray S and Zetterberg H (2016) Amyloid precursor protein expression and processing are differentially regulated during cortical neuron differentiation. Sci. Rep 6, 29200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee JE and Wilusz J, (2010) Translational symphony in (hnRNP) C major for APP. Nat. Struct. Mol. Biol 17, 675–676. [DOI] [PubMed] [Google Scholar]
- 121.Ceman S, Brown V and Warren ST, (1999) Isolation of an FMRP-associated messenger ribonucleoprotein particle and identification of nucleolin and the fragile X-related proteins as components of the complex. Mol. Cell. Biol 19, 7925–7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ceman S, Nelson R and Warren ST, (2000) Identification of mouse YB1/p50 as a component of the FMRP-associated mRNP particle. Biochem. Biophys. Res. Commun 279, 904–908. [DOI] [PubMed] [Google Scholar]
- 123.Broytman O, Westmark PR, Gurel Z and Malter JS, (2009) Rck/p54 interacts with APP mRNA as part of a multi-protein complex and enhances APP mRNA and protein expression in neuronal cell lines. Neurobiol. Aging 30, 1962–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Westmark CJ and Malter JS, (2012) The regulation of AbetaPP expression by RNA-binding proteins. Ageing Res. Rev 11, 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cho HH, Cahill CM, Vanderburg CR, Scherzer CR, Wang B, Huang X and Rogers JT, (2010) Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1. J. Biol. Chem 285, 31217–31232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muslimov IA, Iacoangeli A, Brosius J and Tiedge H, (2006) Spatial codes in dendritic BC1 RNA. J. Cell Biol 175, 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fatemi SH, Wong DF, Brasic JR, Kuwabara H, Mathur A, Folsom TD, Jacob S, Realmuto GM, Pardo JV and Lee S, (2018) Metabotropic glutamate receptor 5 tracer [(18)F]-FPEB displays increased binding potential in postcentral gyrus and cerebellum of male individuals with autism: a pilot PET study. Cerebellum Ataxias 5, 3–018–0082–1. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Westmark CJ, (2014) Soy infant formula and seizures in children with autism: a retrospective study. PLoS One 9, e80488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bardoni B, Capovilla M and Lalli E, (2017) Modeling Fragile X syndrome in neurogenesis: An unexpected phenotype and a novel tool for future therapies. Neurogenesis (Austin) 4, e1270384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McBride SM, Choi CH, Schoenfeld BP, Bell AJ, Liebelt DA, Ferreiro D, Choi RJ, Hinchey P, Kollaros M, Terlizzi AM, Ferrick NJ, Koenigsberg E, Rudominer RL, Sumida A, Chiorean S, Siwicki KK, Nguyen HT, Fortini ME, McDonald TV and Jongens TA, (2010) Pharmacological and genetic reversal of age-dependent cognitive deficits attributable to decreased presenilin function. J. Neurosci 30, 9510–9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sokol DK, Chen D, Farlow MR, Dunn DW, Maloney B, Zimmer JA and Lahiri DK, (2006) High levels of Alzheimer beta-amyloid precursor protein (APP) in children with severely autistic behavior and aggression. J. Child Neurol 21, 444–449. [DOI] [PubMed] [Google Scholar]
- 132.Lahiri DK, Sokol DK, Erickson C, Ray B, Ho CY and Maloney B, (2013) Autism as early neurodevelopmental disorder: evidence for an sAPPalpha-mediated anabolic pathway. Front. Cell. Neurosci 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y and LaFerla FM, (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39, 409–421. [DOI] [PubMed] [Google Scholar]
- 134.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R and Vassar R, (2006) Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. Neurobiol. Dis 26, 10129–10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Games D, Adams D, Alessandrinl R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillesple F and et al. , (1995) Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373, 523–527. [DOI] [PubMed] [Google Scholar]
- 136.Lamb BT, Call LM, Slunt HH, Bardel KA, Lawler AM, Eckman CB, Younkin SG, Holtz G, Wagner SL, Price DL, Sisodia SS and Gearhart JD, (1997) Altered metabolism of familial Alzheimer’s disease-linked amyloid precursor protein variants in yeast artificial chromosome transgenic mice. Hum. Mol. Genet 6, 1535–1541. [DOI] [PubMed] [Google Scholar]
- 137.Lord A, Kalimo H, Eckman C, Zhang X-Q., Lannfelt L. and Nilsson LNG, (2006) The Artic Alzheimer mutation facilitates early intraneuronal A β aggregation and senile plaque formation in transgenic mice. Neurobiol. Aging 27, 67–77. [DOI] [PubMed] [Google Scholar]