Abstract
Valproic acid (VPA), an agent that is used to treat epileptic seizures, can cause spatial memory impairment in adults and children. This effect is thought to be due to the ability of VPA to inhibit neurogenesis in the hippocampus, which is required for learning. We have previously used an animal model to show that VPA significantly impairs hippocampal-spatial working memory and inhibits neuronal generation in the sub-granular zone of the dentate gyrus. As there are patient reports of improvements in memory after discontinuing VPA treatment, the present study investigated the recovery of both spatial memory and hippocampal neurogenesis at two time points after withdrawal of VPA. Male Wistar rats were given intraperitoneal injections of 0.9% normal saline or VPA (300 mg/kg) twice a day for 10 d. At 1, 30, or 45 d after the drug treatment, the novel object location (NOL) test was used to examine spatial memory; hippocampal cell division was counted using Ki67 immunohistochemistry, and levels of brain-derived neurotrophic factor (BDNF) and Notch1 were measured using western immunoblotting. Spatial working memory was impaired 1 and 30 d after the final administration, but was restored to control levels by 45 d. Cell proliferation had increased to control levels at 30 and 45 d. Both markers of neurogenesis (BDNF and Notch1 levels) had returned to control levels at 45 d. These results demonstrate that memory recovery occurs over a period of six weeks after discontinuing VPA treatment and is preceded by a return of hippocampal neurogenesis to control levels.
Keywords: Hippocampus, Neurogenesis, Spatial memory, Valproic acid
1. Introduction
Valproic acid (VPA) is commonly used to treat patients for seizures (epilepsy) and mood disorders (bipolar disorder) (Henry, 2003; Buckley, 2008). It is also used as a medication for certain cancer and human immunodeficiency virus (HIV) therapies (Lehrman et al., 2005). VPA modulates neuronal activity by blocking sodium and calcium channels, increasing γ-aminobutyric acid (GABA)-mediated inhibitory neurotransmission and decreasing levels of brain aspartate (Kwan et al., 2001). In addition, it can function to stabilize mood by enhancing the extracellular signal-regulated kinase (ERK) pathway (Hao et al., 2004). Separate from its psychiatric effects, VPA is a potent blocker of cell proliferation. This action is mediated by the ability of VPA to inhibit histone deacetylase (HDAC) enzymes (Hsieh et al., 2004), which regulate the degree of binding between DNA and histone proteins. Down-regulation of HDACs induces the expression of growth arrest genes including the mitotic inhibitor p21 (Li et al., 2005; Das et al., 2007) and reduces brain-derived neurotrophic factor (BDNF) expression (Bredy et al., 2007).
Although VPA has low toxicity and a good safety profile, it causes mild to moderate cognitive impairment in over 20% of adult patients (Carpay et al., 2005; Cysique et al., 2006; Gualtieri and Johnson, 2006; Meador, 2007; Senturk et al., 2007; Bewernick and Schlaepfer, 2013; Quesseveur et al., 2013). Aside from its effects on humans, VPA can reduce spatial working memory in adult, but not neonatal, rats shortly after administration. A probable mechanism behind the cognitive changes found after VPA treatment is a decrease in adult neurogenesis in the hippocampus (Umka et al., 2010). Adult neurogenesis continually generates new granule cell neurons from proliferating neural stem cells in the sub-granular zone (SGZ) of the dentate gyrus, and levels of neurogenesis correlate with cognitive ability (Eriksson et al., 1998; Abrous et al., 2005; Kitabatake et al., 2007; Ehninger and Kempermann, 2008). VPA reduces the number of dividing cells in the SGZ, as measured by Ki67 expression (Kee et al., 2002; Jessberger et al., 2007; Umka et al., 2010). In addition, VPA reduces the levels of BDNF which is required for the survival, migration, and maturation of neural cells involved in neurogenesis, and the expression of Notch1, a receptor found in neural stem cells which regulates their proliferation (Hitoshi et al., 2002; Breunig et al., 2007; Jessberger et al., 2007; Bekinschtein et al., 2008; Chan et al., 2008). Both BDNF and Notch1 levels are associated with cognitive performance and provide markers of neurogenesis, which may correlate with cognitive changes (Wang et al., 2004; Costa et al., 2005; Cunha et al., 2010).
While memory improvement after the cessation of VPA treatment has been reported (Masmoudi et al., 2006; Hommet et al., 2007; Lossius et al., 2008), the time course and association with changes in hippocampal neurogenesis have not been investigated. A rat model used in the present study shows the consequences of VPA withdrawal on memory 30 and 45 d after the end of treatment as measured by the novel object location (NOL) test, which relates to human memory (Reed and Squire, 1997; Mumby et al., 2002). Behavior was compared to the expression of markers of hippocampal neurogenesis.
2. Materials and methods
2.1. Animals and drug administration
All subjects were male Wistar rats (the National Laboratory Animal Center, Mahidol University, Salaya, Nakorn Pathom, Thailand), which were 4–5 weeks old and 180–220 g at the beginning of the procedures. Four-to five-week-old rats, equivalent in maturity to 12-year-old humans, have a brief and accelerated childhood. Animals were housed three to a cage under a 12-h light/12-h dark cycle, and food and water were given ad libitum.
Rats were randomly divided into six groups: control 10 d (n=10), control withdrawal 30 d (n=10), and control withdrawal 45 d (n=12) groups received 0.9% saline (9 g/L NaCl) injections, while VPA 10 d (n=10), VPA withdrawal 30 d (n=11), and VPA withdrawal 45 d (n=12) groups received VPA (300 mg/kg, dissolved in 0.9% normal saline; Sigma-Aldrich, Inc., St. Louis, USA) at 1 mL/kg for 10 consecutive days (Fig. 1). The saline and VPA were administered by two daily intraperitoneal injections at about 10:00 and 14:00. This treatment regime was used as it is within the range which diminishes seizure prevalence in uncontrollably epileptic rats (Nissinen and Pitkänen, 2007) and has been used in previous studies (Hsieh et al., 2004; Umka et al., 2010). After arrival, rats were weighed every day and acclimatized for one week before drug treatment.
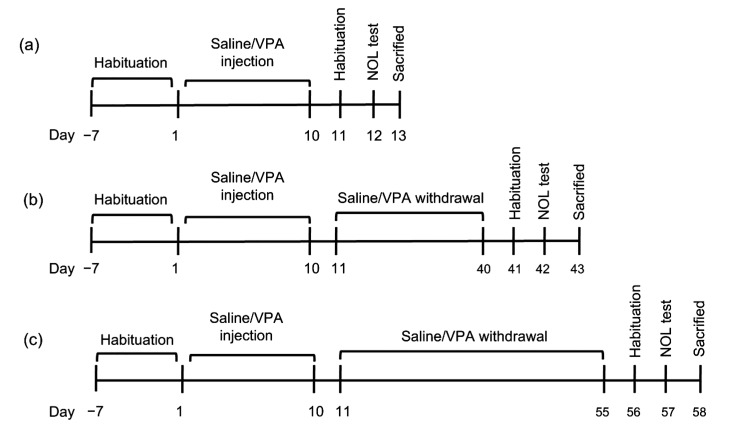
Fig. 1.
Timeline showing protocol of saline/VPA administration and experimental paradigm
Saline/VPA was administrated twice daily for 10 d (a). The animals were allowed to recover for 30 (b) or 45 (c) d after saline/VPA injection. Their behavior was tested using the NOL test
2.2. Behavioral testing (NOL test)
The modified NOL test (Dix and Aggleton, 1999) was carried out as described previously (Umka et al., 2010). The test made use of the rats’ inquisitiveness and required the animals to recognize distinct objects in familiar and novel locations. The test was performed between 9:00 and 16:00 under an illumination of 350–400 lx. The test apparatus consisted of an arena (an open square opaque Perspex box, 36 cm wide×50 cm long×30 cm high). The animals were acclimated for 30 min in the arena 24 h before the test. The following day, two identical weighted water bottles (19 cm height, 6.5 cm diameter) were placed in different random locations in the arena and the rats were permitted to investigate for 3 min (familiarization trial). The rats were then returned to their cages for a 5-min inter-trial interval. During this period, the arena and the objects were wiped with 20% ethanol. In the choice trial, the rats were put back into the arena to explore for 3 min after one object had been placed in its original location whereas the other was placed in a new location. Exploratory activity such as sniffing, licking, chewing or directing their noses at the object from a distance of less than 2 cm was monitored (Dix and Aggleton, 1999). The preference index (PI) is defined as the percentage of time spent exploring an object in the novel location over the sum of exploration time of novel and familiar locations in the choice trial (Sirichoat et al., 2015; Welbat et al., 2016a, 2016c; Chaisawang et al., 2017). A PI higher than 50% chance reveals that the animals prefer the object in the novel position and were able to remember the original object locations. The time spent exploring objects in all trials was scored twice a random time and averaged from digitized recordings using a stopwatch. During the test period, the observer was not present in the room.
2.3. Brain tissue preparation
The hippocampus is a prime candidate for the site of action of VPA in causing cognitive decline as this structure is required for spatial working memory (Carrozzo et al., 2005) and recognition (Reed and Squire, 1997). It is also one of the few sites in the adult brain where the production of new neurons continues. These newly formed granule cell neurons, which integrate into the circuitry of the dentate gyrus, are required for spatial memory consolidation (Zhao et al., 2006; Imayoshi et al., 2008). After behavioral testing, animals were put down using rapid stunning and cervical decapitation. After removal of the brain, one half was kept for immunofluorescence staining and the hippocampus was removed from the other half and snap-frozen for western blotting. For immunofluorescence staining, the half brains were immersed in a cryoprotectant (30% sucrose) for 3 h at 4 °C before being embedded in optimum cutting temperature (OCT; Thermo Fisher Scientific, Germany), and snap-frozen in liquid nitrogen-cooled isopentane. They were then stored at −80 °C before being cut and analyzed. Frozen brains were cut into 20-μm thick consecutive sections through the whole dentate gyrus (between Bregma −2.3 to 6.3 mm) in the coronal plane using a cryostat (Cryostat series HM550 Microm international, A.S. Science, Co., Ltd., Walldorf, Germany). Sections were prepared on 3-aminopropyl-triethoxysilane (APES)-coated slides and kept at −20 °C until needed for immunofluorescence staining (Welbat et al., 2016a).
2.4. Immunofluorescence staining
A technique for systematic random selection (Mayhew and Burton, 1988) was employed to choose every 20th section from the entire dentate gyrus to obtain eight sections per brain. Ki67 staining was performed as described earlier (Umka et al., 2010). The procedure was carried out at room temperature in a light-protected, humid environment. Briefly, sections were defrosted and preserved in 0.5% paraformaldehyde (pH 7.4) for 3 min, followed by 1 h incubation with monoclonal mouse Ki67 primary antibody (1:100, Vector Laboratory, Inc., USA). They were then washed, incubated with Alexa fluor 488 Rabbit Anti-mouse IgG (1:300, Invitrogen, USA), and counterstained with propidium iodide (Sigma-Aldrich, USA). Sections were examined at ×40 using a Nikon ECLIPSE 80i fluorescence microscope. Ki67-positive cells were counted within the SGZ, defined as cells within three cell diameters of the dentate gyrus (Umka et al., 2010). The total number of positive cells was calculated by averaging the positive cell numbers per section and multiplying by 20 (Welbat et al., 2016a, 2016b, 2016c; Chaisawang et al., 2017).
2.5. Western immunoblotting
Hippocampal tissue was used for western immunoblotting as previously described (Mustafa et al., 2008). The primary antibodies used as reference markers were polyclonal rabbit anti-BDNF N-20 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), polyclonal goat anti-Notch1 C20 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), or monoclonal mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:20000; Abcam, Cambridge, UK). The secondary antibodies were polyclonal goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:2000; Cruz Biotechnology, Santa Cruz, CA, USA), polyclonal mouse anti-goat horseradish peroxidase-conjugated (1:2000; Cruz Biotechnology, Santa Cruz, CA, USA), and polyclonal mouse anti-rabbit horseradish peroxidase-conjugated (1:2000; Cruz Biotechnology, Santa Cruz, CA, USA). Examination of protein bands was performed using a chemiluminescence kit (Amersham GE Healthcare, Buckinghamshire, UK) and ImageQuant 400 camera system (GE Healthcare). The optical density of bands was quantified using ImageJ software. Protein expression is presented as the percentage of GAPDH expression.
2.6. Statistical analysis
All statistical parameters were calculated using GraphPad Prism 5.0 software and SPSS (V13.0), and significance was regarded as P<0.05. The normality of distributions of all data was tested using the Kolmogorov-Smirnov test. Student’s unpaired t-test was used to compare exploration time of animals in familiarization and choice trials. One-way analysis of variance (ANOVA) was used to analyze PI, distance moved, proliferating cell count, and protein expression. Bonferronni’s post-hoc test was performed when ANOVA was significant.
3. Results
3.1. Effect of VPA withdrawal on spatial working memory
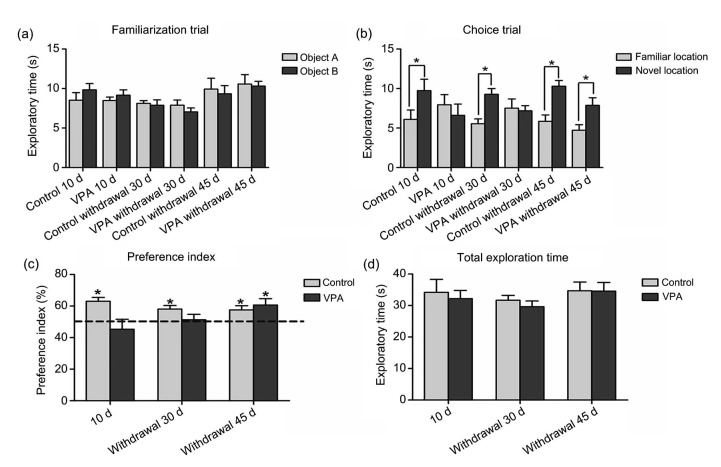
The effect of VPA withdrawal on hippocampal-spatial working memory was measured with the NOL test. In the familiarization trial, all the treatment groups showed no significant difference in results for the two objects in different locations, indicating that all animals showed no preference for either replica or different locations of the object (P>0.05, Fig. 2a). Differentiation of exploratory activity in the choice trial indicated that the animals in all saline groups inspected the object in the novel location to a significantly greater degree than they did the object in the familiar position, suggesting unaffected spatial working memory (P<0.05, Fig. 2b). However, rats tested soon after VPA administration and 30 d after VPA treatment were unable to distinguish between objects in familiar and novel locations during the choice trial (P>0.05, Fig. 2b), but rats in the VPA withdrawal 45 d group were able to make such distinctions. This shows that a deficit in spatial working memory had persisted after VPA withdrawal in these groups, but spatial memory had improved by 45 d after VPA treatment. Further analysis using the preference for objects in familiar or novel locations (PI) in the choice trial showed significantly less preference in VPA 10 d or VPA withdrawal 30 d rats compared to 50% chance (mean±standard error of mean (SEM); control 10 d: (63.04±2.45)%; VPA 10 d: (45.29±6.34)%; control withdrawal 30 d: (58.11±2.24)%; VPA withdrawal 30 d: (51.29±3.51)%; P<0.01, Fig. 2c), but not in the VPA withdrawal 45 d group (mean±SEM; control withdrawal 45 d: (57.58±2.67)%; VPA withdrawal 45 d: (60.65±4.07)%). VPA treatment did not affect the overall motor activity of rats (mean±SEM; control 10 d: (34.17±11.71) s; VPA 10 d: (32.18±6.35) s; control withdrawal 30 d: (31.67±4.87) s; VPA withdrawal 30 d: (29.62±6.03) s; control withdrawal 45 d: (34.70±7.74) s; VPA withdrawal 45 d: (34.53±7.88) s; P>0.05, Fig. 2d).
Fig. 2.
Exploration time of the novel object location task after treatment
(a) In the familiarization trial, objects A and B are the objects placed in separate locations in the arena. Each animal was allowed to explore the objects for 3 min. No significant difference was found in the exploration time of either object for any group (P>0.05). (b) There was a significant difference comparing the replica objects between familiar and novel locations in the control 10 d, control withdrawal 30 d, control withdrawal 45 d, and VPA withdrawal 45 d groups (* P<0.05) in the choice trial. In contrast, the rats receiving VPA and which were tested shortly after treatment and 30 d after treatment, did not show a significant difference (P>0.05). (c) For the preference index (PI) of control and VPA-treated rats performing the novel object location test shortly after VPA treatment and 30 and 45 d after treatment, a significant difference was found in comparison of all experimental groups (P=0.0185). The PI of rats showed a significant difference compared to 50% chance (dashed line) in the control 10 d, control withdrawal 30 d, control withdrawal 45 d, and VPA withdrawal 45 d groups (* P<0.05); however, there were no significant differences in the VPA 10 d or VPA withdrawal 30 d group (P>0.05). (d) Total exploration time of control, VPA 10 d, VPA withdrawal 30 d, and VPA withdrawal 45 d groups did not show a significant difference among groups (P>0.05). Data are expressed as mean±SEM (n=10)
3.2. Effect of VPA withdrawal on the number of proliferating cells in the SGZ
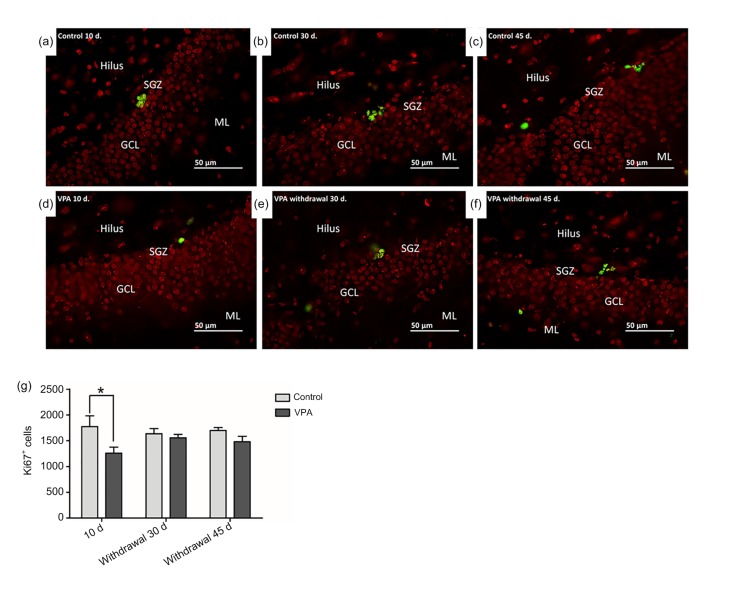
Quantification of Ki67-positive cells in the SGZ alongside the internal border of the hippocampal dentate gyrus (Figs. 3a–3c) showed significant differences among groups (P<0.05, Fig. 3g). Rats receiving VPA for 10 d had fewer Ki67-positive cells than saline-treated rats (mean±SEM; control 10 d: 1775±208; VPA 10 d: 1260±115; P<0.05, Fig. 3g). Conversely, no significant difference was found between saline-and VPA-treated rats that were killed at either 30 or 45 d after treatment (mean±SEM; control withdrawal 30 d: 1637±101; VPA withdrawal 30 d: 1557±67; control withdrawal 45 d: 1700±56; VPA withdrawal 45 d: 1480±105; P>0.05, Fig. 3g).
Fig. 3.
Cell proliferation in the sub-granular zone (SGZ) of the dentate gyrus using Ki67-positive staining
(a–f) In the SGZ, the nuclei of Ki67-positive cells were stained green and cells of the dentate gyrus were stained red. (g) Total numbers of Ki67-positive cells (mean±SEM, n=10) in the dentate gyrus were determined from cell counts. The number of Ki67-positive cells in the SGZ of rats receiving VPA was not significantly different from that of rats receiving saline (P>0.05) in both the VPA withdrawal 30 and 45 d groups. However, rats administered with VPA for 10 d showed a significantly lower number of Ki67-positive cells in comparison with the control 10 d group (* P<0.05) (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
3.3. Effect of VPA withdrawal on Notch1 and BDNF expression
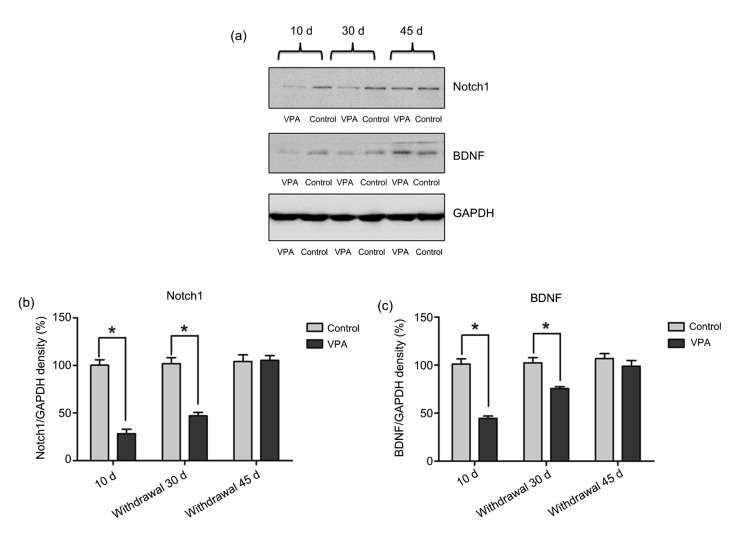
Notch1 and BDNF protein expression was determined using western blotting (Fig. 4a). The results showed significant differences in Notch1 protein expression between rats in the control 10 d and VPA-treated 10 d groups and also between the control withdrawal 30 d and VPA withdrawal 30 d groups (mean±SEM; control 10 d: (100.20±14.08)%; VPA 10 d: (28.19±17.64)%; control withdrawal 30 d: (101.80±15.44)%; VPA withdrawal 30 d: (47.02±9.73)%; P<0.05, Fig. 4b). However, Notch1 protein expression was not significantly different from the controls after treatment for 45 d (mean±SEM; control withdrawal 45 d: (104.20±18.88)%; VPA withdrawal 45 d: (105.40±12.01)%; P>0.05, Fig. 4b). Similar to Notch1, there were significant differences in BDNF between rats treated with saline and VPA for 10 d (mean±SEM; control 10 d: (101.00±13.39)%; VPA 10 d: (44.61±6.01)%; P<0.001, Fig. 4c). Levels of BDNF tested 30 d after the final treatment of VPA were also significantly different (mean±SEM; control withdrawal 30 d: (102.30±13.08)%; VPA withdrawal 30 d: (75.62±4.89)%; P<0.05, Fig. 4c). Levels of BDNF had increased to control levels 45 d after VPA treatment (mean±SEM; control withdrawal 45 d: (106.90±12.31)%; VPA withdrawal 45 d: (98.80±15.75)%; P>0.05, Fig. 4c).
Fig. 4.
Effects of VPA withdrawal on BDNF and Notch1 expression in the hippocampus
(a) GAPDH was used as a loading control. Western blotting was used to measure Notch1 (120 kDa) and BDNF (15 kDa) protein expression. (b) Notch1 expression showed a significant difference among groups (P<0.0001). There was no significant difference between the control and VPA-treated rats at 45 d after treatment (P>0.05). However, Notch1 levels in both time scales after treatment showed significantly lower expression than controls (* P<0.05). (c) BDNF protein expression showed significant differences between groups (P<0.0001). Levels of BDNF in both the VPA-treated 10 d and VPA withdrawal 30 d groups revealed a significant decrease after treatment compared to controls (* P<0.05), but not in the VPA withdrawal 45 d group (P>0.05). Data are expressed as mean±SEM (n=10)
4. Discussion
VPA, which is frequently administered as an anti-seizure and mood stabilizing drug, has been reported to produce mild to moderate learning and memory deficits in humans (Carpay et al., 2005; Senturk et al., 2007). The present study confirmed our previous results, which show that clinically relevant doses of VPA decreased spatial working memory. This behavioral effect was linked to a decline in the cell division needed for neurogenesis in the hippocampus (Umka et al., 2010). Moreover, VPA induces dendritic morphological alteration by decreasing dendritic length, branch nodes, and spine density on both apical and basal dendrites. VPA was also found to cause hippocampal-dependent memory loss in an animal study (Sgobio et al., 2010). The hippocampus is a prime candidate for the site of action of VPA in causing cognitive decline as this structure is required for spatial working memory (Carrozzo et al., 2005) and recognition (Reed and Squire, 1997). New neurons continue to be generated in the hippocampus. These newly formed granule cell neurons merge into the circuitry of the dentate gyrus, and are essential for spatial memory consolidation (Zhao et al., 2006; Imayoshi et al., 2008). Some reports have revealed that VPA stimulates spatial learning and memory deficits, which are related to induced hippocampal neuronal apoptosis. These impairments are associated with up-regulation of the sphingosine 1-phosphate (S1P) signaling pathway and down-regulation of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and cAMP-responsive element binding protein (CREB) signaling pathway (Wu et al., 2018). The NOL test was selected as a measure of spatial memory, as successful performance of this test requires the hippocampus and exploits the natural habit of rats to prefer novelty. As such it does not require positive or negative reinforcement (Mumby et al., 2002). This newness-preference model is similar to recognition memory in humans as detected in normal life (Ennaceur and Delacour, 1988). In rodents and humans, the dentate gyrus within the hippocampus is required for spatial working memory. Lesion of this structure prevents animals from successfully carrying out the NOL test (Dix and Aggleton, 1999). VPA is thought to inhibit cell proliferation via the epigenetic mechanism of DNA hyperacetylation which causes cell cycle arrest due to increased expression of genes which inhibit mitosis (Kostrouchová et al., 2007). Changes in cell division in the SGZ of the dentate gyrus correlate with changes in spatial memory ability (Seigers et al., 2008; Sirichoat et al., 2015; Welbat et al., 2016b), making alterations in hippocampal neurogenesis a likely cause of the behavioral changes brought about by VPA.
Some studies have reported that even after taking VPA for long periods, patients can show improvements in memory after ceasing to take it (Louissaint et al., 2002; Masmoudi et al., 2006; Hommet et al., 2007). This study was designed to see if this change could be replicated in an animal model in which the cognitive deficits of VPA treatment have previously been demonstrated, and to determine the time course of any improvements in cognition. Also, we wished to ascertain whether changes in spatial memory were correlated with changes in markers of hippocampal neurogenesis and neurotrophin levels which have been demonstrated to show a decline after VPA treatment.
Our results show that hippocampal-dependent spatial memory, as evaluated by the NOL test, was impaired after 10 d of VPA treatment, which is in line with our previous report (Umka et al., 2010). This memory deficit remained at 30 d, but had returned to control levels within 45 d of the end of VPA treatment. This demonstrates that the memory deficit shown shortly after VPA treatment (Umka et al., 2010) can be reversed by withdrawal of VPA.
The level of proliferating cells in the sub-granular zone of the dentate gyrus had returned to the control level within 30 d after discontinuing VPA administration, indicating that cell proliferation recovers faster than improvements in spatial memory. This reflects the time taken for new born neurons to become integrated into hippocampal circuitry, a process known to take one to two months (Zhao et al., 2006). Similar to changes in cell proliferation, control values in the expression of BDNF and Notch1 in the hippocampus were present by 45 d post treatment.
Newly produced neurons in the SGZ develop into mature granule cells within four weeks of progenitor cell division (Zhao and Daley, 2008). Recently formed (4-to 8-week-old) neurons are specifically recruited to form hippocampal circuits contributing to spatial memory (Kee et al., 2007). This would suggest that discontinuation of VPA allows up-regulation of hippocampal neurogenesis which is followed by cognitive improvement after six weeks. It is at present unclear whether changes in BDNF precede or follow increases in cell proliferation in the SGZ. Up-regulation of BDNF levels could improve cell proliferation and survival with a subsequent reduction in memory deficits. Notch1 levels reflect the numbers and activation of neural stem cells. Levels of this receptor were similar to controls reflecting the normal levels of neurogenesis occurring after discontinuation of VPA treatment.
The present study shows that a period of recovery after discontinuation of VPA produces a significant improvement in cognitive performance compared to that found shortly after VPA treatment. This behavioral change is preceded by an increase in hippocampal neurogenesis and neurotrophin levels to control values. For patients who can stop taking VPA, these results indicate that this could have relatively rapid beneficial effects on memory.
Footnotes
Project supported by the Invitation Research of the Faculty of Medicine, Neuroscience Research and Development Group, Khon Kaen University, Thailand (No. I54119)
Contributors: Wanassanun PANNANGRONG and Jariya Umka WELBAT performed the complete experimental research and data analysis, wrote and revised the manuscript. Peter WIGMORE and Apiwat SIRICHOAT contributed to the study design, data analysis, and manuscript writing. Trai WONGSIRI checked the statistical methods.
Compliance with ethics guidelines: Wanassanun PANNANGRONG, Apiwat SIRICHOAT, Trai WONGSIRI, Peter WIGMORE, and Jariya Umka WELBAT declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed. The animal investigation was accepted by the Animal Ethics Committee of Khon Kaen University, Thailand, based on the Ethics of the Animal Experiment of National Research Council of Thailand under permit number AEKKU 36/2557.
References
- 1.Abrous DN, Koehl M, le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85(2):523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 2.Bekinschtein P, Cammarota M, Katche C, et al. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci USA. 2008;105(7):2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bewernick BH, Schlaepfer TE. Chronic depression as a model disease for cerebral aging. Dialogues Clin Neurosci. 2013;15(1):77–85. doi: 10.31887/DCNS.2013.15.1/bbewernick. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bredy TW, Wu H, Crego C, et al. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14(4):268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breunig JJ, Silbereis J, Vaccarino FM, et al. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci USA. 2007;104(51):20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley PF. Update on the treatment and management of schizophrenia and bipolar disorder. CNS Spectr. 2008;13(2 Suppl 1):1–10; quiz 11-12. doi: 10.1017/s1092852900028212. [DOI] [PubMed] [Google Scholar]
- 7.Carpay JA, Aldenkamp AP, van Donselaar CA. Complaints associated with the use of antiepileptic drugs: results from a community-based study. Seizure. 2005;14(3):198–206. doi: 10.1016/j.seizure.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Carrozzo M, Koch G, Turriziani P, et al. Integration of cognitive allocentric information in visuospatial short-term memory through the hippocampus. Hippocampus. 2005;15(8):1072–1084. doi: 10.1002/hipo.20126. [DOI] [PubMed] [Google Scholar]
- 9.Chaisawang P, Sirichoat A, Chaijaroonkhanarak W, et al. Asiatic acid protects against cognitive deficits and reductions in cell proliferation and survival in the rat hippocampus caused by 5-fluorouracil chemotherapy. PLoS ONE. 2017;12(7):e0180650. doi: 10.1371/journal.pone.0180650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan JP, Cordeira J, Calderon GA, et al. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 2008;39(3):372–383. doi: 10.1016/j.mcn.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa RM, Drew C, Silva AJ. Notch to remember. Trends Neurosci. 2005;28(8):429–435. doi: 10.1016/j.tins.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci, 3:1. 2010 doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cysique LA, Maruff P, Brew BJ. Valproic acid is associated with cognitive decline in HIV-infected individuals: a clinical observational study. BMC Neurol, 6:42. 2006 doi: 10.1186/1471-2377-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das CM, Aguilera D, Vasquez H, et al. Valproic acid induces p21 and topoisomerase-II (α/β) expression and synergistically enhances etoposide cytotoxicity in human glioblastoma cell lines. J Neurooncol. 2007;85(2):159–170. doi: 10.1007/s11060-007-9402-7. [DOI] [PubMed] [Google Scholar]
- 15.Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99(2):191–200. doi: 10.1016/S0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- 16.Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331(1):243–250. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- 17.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-X. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 19.Gualtieri CT, Johnson LG. Comparative neurocognitive effects of 5 psychotropic anticonvulsants and lithium. MedGenMed. 2006;8(3):46. [PMC free article] [PubMed] [Google Scholar]
- 20.Hao YL, Creson T, Zhang L, et al. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci. 2004;24(29):6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37(Suppl 2):5–16. [PubMed] [Google Scholar]
- 22.Hitoshi S, Alexson T, Tropepe V, et al. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16(7):846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hommet C, Mondon K, de Toffol B, et al. Reversible cognitive and neurological symptoms during valproic acid therapy. J Am Geriatr Soc. 2007;55(4):628. doi: 10.1111/j.1532-5415.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh J, Nakashima K, Kuwabara T, et al. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA. 2004;101(47):16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imayoshi I, Sakamoto M, Ohtsuka T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 26.Jessberger S, Nakashima K, Clemenson GD, et al. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci. 2007;27(22):5967–5975. doi: 10.1523/JNEUROSCI.0110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kee N, Sivalingam S, Boonstra R, et al. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115(1):97–105. doi: 10.1016/S0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 28.Kee N, Teixeira CM, Wang AH, et al. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10(3):355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 29.Kitabatake Y, Sailor KA, Ming GL, et al. Adult neurogenesis and hippocampal memory function: new cells, more plasticity, new memories? Neurosurg Clin North Am. 2007;18(1):105–113. doi: 10.1016/j.nec.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kostrouchová M, Kostrouch Z, Kostrouchová M. Valproic acid, a molecular lead to multiple regulatory pathways. Folia Biol (Praha) 2007;53(2):37–49. [PubMed] [Google Scholar]
- 31.Kwan P, Sills GJ, Brodie MJ. The mechanisms of action of commonly used antiepileptic drugs. Pharmacol Ther. 2001;90(1):21–34. doi: 10.1016/S0163-7258(01)00122-X. [DOI] [PubMed] [Google Scholar]
- 32.Lehrman G, Hogue IB, Palmer S, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366(9485):549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XN, Shu Q, Su JMF, et al. Valproic acid induces growth arrest, apoptosis, and senescence in medulloblastomas by increasing histone hyperacetylation and regulating expression of p21Cip1, CDK4, and CMYC. Mol Cancer Ther. 2005;4(12):1912–1922. doi: 10.1158/1535-7163.MCT-05-0184. [DOI] [PubMed] [Google Scholar]
- 34.Lossius MI, Hessen E, Mowinckel P, et al. Consequences of antiepileptic drug withdrawal: a randomized, double-blind study (Akershus study) Epilepsia. 2008;49(3):455–463. doi: 10.1111/j.1528-1167.2007.01323.x. [DOI] [PubMed] [Google Scholar]
- 35.Louissaint A, Jr, Rao S, Leventhal C, et al. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34(6):945–960. doi: 10.1016/S0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 36.Masmoudi K, Gras-Champel V, Masson H, et al. Parkinsonism and/or cognitive impairment with valproic acid therapy: a report of ten cases. Pharmacopsychiatry. 2006;39(1):9–12. doi: 10.1055/s-2006-931471. [DOI] [PubMed] [Google Scholar]
- 37.Mayhew TM, Burton GJ. Methodological problems in placental morphometry: apologia for the use of stereology based on sound sampling practice. Placenta. 1988;9(6):565–581. doi: 10.1016/0143-4004(88)90001-X. [DOI] [PubMed] [Google Scholar]
- 38.Meador MJ. The basic science of memory as it applies to epilepsy. Epilepsia. 2007;48(Suppl 9):23–25. doi: 10.1111/j.1528-1167.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 39.Mumby DG, Gaskin S, Glenn MJ, et al. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mustafa S, Walker A, Bennett G, et al. 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. 2008;28(2):323–330. doi: 10.1111/j.1460-9568.2008.06325.x. [DOI] [PubMed] [Google Scholar]
- 41.Nissinen J, Pitkänen A. Effect of antiepileptic drugs on spontaneous seizures in epileptic rats. Epilepsy Res. 2007;73(2):181–191. doi: 10.1016/j.eplepsyres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Quesseveur G, David DJ, Gaillard MC, et al. BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl Psychiatry. 2013;3(4):e253. doi: 10.1038/tp.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav Neurosci. 1997;111(4):667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- 44.Seigers R, Schagen SB, Beerling W, et al. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186(2):168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Senturk V, Goker C, Bilgic A, et al. Impaired verbal memory and otherwise spared cognition in remitted bipolar patients on monotherapy with lithium or valproate. Bipolar Disord. 2007;9(Suppl 1):136–144. doi: 10.1111/j.1399-5618.2007.00481.x. [DOI] [PubMed] [Google Scholar]
- 46.Sgobio C, Ghiglieri V, Costa C, et al. Hippocampal synaptic plasticity, memory, and epilepsy: effects of long-term valproic acid treatment. Biol Psychiatry. 2010;67(6):567–574. doi: 10.1016/j.biopsych.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Sirichoat A, Chaijaroonkhanarak W, Prachaney P, et al. Effects of asiatic acid on spatial working memory and cell proliferation in the adult rat hippocampus. Nutrients. 2015;7(10):8413–8423. doi: 10.3390/nu7105401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umka J, Mustafa S, ElBeltagy M, et al. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience. 2010;166(1):15–22. doi: 10.1016/j.neuroscience.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 49.Wang HD, Dunnavant FD, Jarman T, et al. Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology. 2004;29(7):1230–1238. doi: 10.1038/sj.npp.1300449. [DOI] [PubMed] [Google Scholar]
- 50.Welbat JU, Sirichoat A, Chaijaroonkhanarak W, et al. Asiatic acid prevents the deleterious effects of valproic acid on cognition and hippocampal cell proliferation and survival. Nutrients. 2016;8(5):303. doi: 10.3390/nu8050303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welbat JU, Sangrich P, Sirichoat A, et al. Fluoxetine prevents the memory deficits and reduction in hippocampal cell proliferation caused by valproic acid. J Chem Neuroanat. 2016;78:112–118. doi: 10.1016/j.jchemneu.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Welbat JU, Chaisawang P, Chaijaroonkhanarak W, et al. Kaempferia parviflora extract ameliorates the cognitive impairments and the reduction in cell proliferation induced by valproic acid treatment in rats. Ann Anat. 2016;206:7–13. doi: 10.1016/j.aanat.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 53.Wu HM, Zhang QZ, Gao JQ, et al. Modulation of sphingosine 1-phosphate (S1P) attenuates spatial learning and memory impairments in the valproic acid rat model of autism. Psychopharmacology (Berl) 2018;235(3):873–886. doi: 10.1007/s00213-017-4805-4. [DOI] [PubMed] [Google Scholar]
- 54.Zhao CM, Teng EM, Summers RG, Jr, et al. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao R, Daley GQ. From fibroblasts to iPS cells: induced pluripotency by defined factors. J Cell Biochem. 2008;105(4):949–955. doi: 10.1002/jcb.21871. [DOI] [PubMed] [Google Scholar]