Abstract
Hesperetin, an abundant bioactive component of citrus fruits, is poorly water-soluble, resulting in low oral bioavailability. We developed new formulations to improve the water solubility, antioxidant activity, and oral absorption of hesperetin. Two nano-based formulations were developed, namely hesperetin-TPGS (D-α-tocopheryl polyethylene glycol 1000 succinate) micelles and hesperetin-phosphatidylcholine (PC) complexes. These two formulations were prepared by a simple technique called solvent dispersion, using US Food and Drug Administration (FDA)-approved excipients for drugs. Differential scanning calorimetry (DSC) and dynamic light scattering (DLS) were used to characterize the formulations’ physical properties. Cytotoxicity analysis, cellular antioxidant activity assay, and a pharmacokinetic study were performed to evaluate the biological properties of these two formulations. The final weight ratios of both hesperetin to TPGS and hesperetin to PC were 1:12 based on their water solubility, which increased to 21.5-and 20.7-fold, respectively. The hesperetin-TPGS micelles had a small particle size of 26.19 nm, whereas the hesperetin-PC complexes exhibited a larger particle size of 219.15 nm. In addition, the cellular antioxidant activity assay indicated that both hesperetin-TPGS micelles and hesperetin-PC complexes increased the antioxidant activity of hesperetin to 4.2-and 3.9-fold, respectively. Importantly, the in vivo oral absorption study on rats indicated that the micelles and complexes significantly increased the peak plasma concentration (Cmax) from 2.64 μg/mL to 20.67 and 33.09 μg/mL and also increased the area under the concentration–time curve of hesperetin after oral administration to 16.2-and 18.0-fold, respectively. The micelles and complexes increased the solubility and remarkably improved the in vitro antioxidant activity and in vivo oral absorption of hesperetin, indicating these formulations’ potential applications in drugs and healthcare products.
Keywords: Hesperetin, D-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS), Phosphatidylcholine, Antioxidant activity, Oral absorption
1. Introduction
Hesperetin (5,7,3'-trihydroxy-4'-methoxyflavanone; Fig. 1) is a natural bioflavonoid in citrus fruits, such as lemons and sweet oranges. In recent years, a number of biological activities have been attributed to hesperetin, including antioxidant and free-radical scavenging activities (de Souza et al., 2016), blood lipid and cholesterol-lowering effects (Kim et al., 2010), anti-inflammatory activities (Parhiz et al., 2015), cardiovascular protection (Testai and Calderone, 2017), and neuroprotective activities (Roohbakhsh et al., 2014). However, due to its poor water solubility and insufficient stability in the gastrointestinal tract (Liu and Chen, 2008), the oral bioavailability of hesperetin is extremely low (Kanaze et al., 2007), which restricts its application as an orally absorbed natural product that can protect against particular diseases.
Fig. 1.
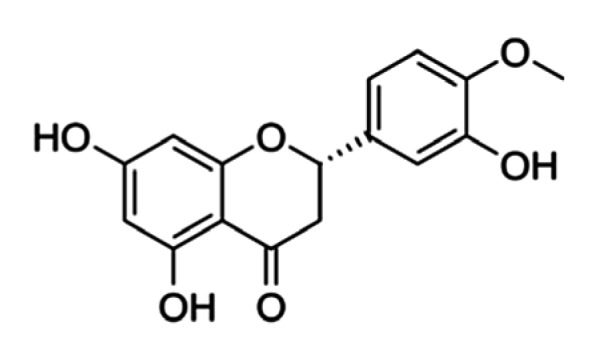
Structure of hesperetin
The oral route is the most popular method for administering drugs or dietary supplements because of its convenience and safety. There are now several formulations of hesperetin that have been developed to increase its oral absorption and maintain its concentration at a therapeutic level in plasma. These formulations include nanocrystals (Shete et al., 2015) and solid dispersions (Kanaze et al., 2007). However, these formulations can only moderately increase the oral absorption of hesperetin by 1.3‒2.5-fold. In addition, the preparation processes for these formulations are complicated and the excipients used are not approved by the US Food and Drug Administration (FDA) for oral administration. Thus, a novel and simple oral delivery system of hesperetin, using US FDA-approved and biocompatible excipients, is currently needed.
D-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS) is an amphiphilic molecule with a hydrophile/lipophile balance (HLB) value of 13.2 and a relatively low critical micelle concentration (CMC) of 0.02% (w/w) (Zhang et al., 2012). It is a safe excipient for oral administration or intravenous injection. Phosphatidylcholine (PC) is a class of phospholipid that has been widely used in the pharmaceutical and food industries. It is the major component of biological membranes and can be obtained from a variety of readily available sources, such as egg yolk and soybeans. Due to the amphipathic property of phospholipids, PC has been used to enhance the water solubility and therapeutic efficacy of some hydrophobic molecules (Qin et al., 2018; Wang et al., 2018). The aim of this study is to develop simple techniques for preparing oral formulations of hesperetin with high oral bioavailability using TPGS and PC. The resulting two formulations were evaluated comparatively for their solubility enhancement, cellular antioxidant activity, and pharmacokinetic behaviors.
2. Materials and methods
2.1. Materials
Hesperetin (purity 98%) was obtained from Shaanxi Huike Botanical Development Co., Ltd. (Shaanxi, China). TPGS was purchased from Aladdin Reagent, Ltd. (Shanghai, China). PC (purity ≥80%) was purchased from Shanghai Advanced Vehicle Technology Pharmaceutical Co., Ltd. (Shanghai, China). Hanks’ balanced salt solution (HBSS) was purchased from Sangon Biotech (Shanghai, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH), and 2',7'-dichlorofluorescin diacetate (DCFH-DA) were obtained from Sigma-Aldrich (Shanghai, China).
2.2. Determination of hesperetin in aqueous solution
High-performance liquid chromatography (HPLC) was used to determine the concentration of hesperetin. The HPLC system was equipped with a Waters 1525 binary HPLC pump, a 2998 Photodiode Array Detector (Waters, Singapore), and a Phenomenex C18 (250 mm×4.6 mm, 5 µm) column. Data acquisition and analysis were performed by a Waters Breeze 2 System. Samples were analyzed using the mobile phase of methanol/water at the ratio of 7:3 (v/v). The flow rate was set at 1.0 mL/min and the column temperature was set at 40 °C. The detection wavelength was set to 288 nm and 20 μL of sample was injected into the HPLC system.
2.3. Determination of hesperetin in plasma
After absorption and metabolism, several forms of hesperetin exist in plasma, including free hesperetin and its glucuronic acid and sulfate conjugates (Németh et al., 2003). Total hesperetin was measured as previously described (Maiti et al., 2009) with minor modifications. Briefly, a mixture of plasma (50 μL) and crude digestive juice (5 μL) of Helix pomatia (type HP-2, containing β-glucuronidase and sulfatase, Sigma Chemical Co., USA) was incubated at 37 °C for 4 h. Then 250 μL of methanol was added to each sample to precipitate proteins. After vortex mixing for 15 s, the samples were centrifuged at 18 800g for 5 min. The supernatants were transferred into centrifuge tubes and stored at −20 °C until HPLC analysis. The same HPLC condition used to analyze hesperetin in aqueous solution was employed to determine the hesperetin concentrations in plasma, except for the gradient elution starting at 70% methanol and ending at 50% methanol.
2.4. Formulation preparation
Hesperetin-TPGS micelles and hesperetin-PC complexes were prepared by the solvent dispersion technique. Briefly, hesperetin (5 mg) and TPGS or hesperetin and PC at different weight ratios (1:3, 1:6, 1:9, 1:12, and 1:15) were dissolved in 10 mL of methanol by ultrasound in a 50-mL round bottom flask. After ultrasonic homogenization, the mixture was stirred for 30 min at 40 °C and methanol was evaporated using a rotary evaporator at a negative pressure of 0.095 MPa. The final product was dried under vacuum (50 Pa) to remove traces of solvents and kept in a glass desiccator.
2.5. Solubility evaluation
For solubility study, a series of standard hesperetin-methanol solutions (50–500 µg/mL) were prepared for the calibration curve. The solubility of hesperetin was determined by adding excess hesperetin or hesperetin formulations into 1 mL of deionized water. The suspensions were sonicated for 5 min and shaken for 24 h to reach equilibrium and then centrifuged at 1500g for 15 min. A total of 50 μL of supernatant liquid was diluted to 1 mL with methanol and the concentration of hesperetin was determined by HPLC.
2.6. Differential scanning calorimetry
Differential scanning calorimetry (DSC) analyses for hesperetin, TPGS, PC, hesperetin-TPGS micelles, and hesperetin-PC complexes were carried out using a differential scanning calorimeter (DSC1/400, METTLER-TOLEDO, China). Samples were heated at a rate of 10 °C/min from 30 to 300 °C under a nitrogen atmosphere.
2.7. Dynamic light scattering
Size distribution was measured using a Zetasizer Nano-ZS (Malvern Zen3600, UK). Samples were dissolved in deionized water at a concentration of 0.1 mg/mL of hesperetin and filtered through 220 nm filters. The scattering angle was 173° and the detecting wavelength was 633 nm. Water was used as the dispersant and the temperature was set to 25 °C. The particle sizes of the micelles and the complexes are reported as the volume size distribution.
2.8. Cell culture
Human hepatocellular carcinoma (HepG2) cells and Madin-Daby canine kidney (MDCK) cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). HepG2 cells and MDCK cells were cultured in the growth medium containing Dulbecco’s modified Eagle’s medium (DMEM; Gibco, China) with 10% fetal bovine serum (FBS; Gibco, China) and antibiotics (antibiotic-antimycotic solution; Gibco, China), and maintained at 37 °C with 5% CO2.
2.9. Cytotoxicity analysis
Cytotoxicity analysis was performed by the MTT assay. HepG2 cells and MDCK cells were seeded into 96-well microplates at a density of 1×104 cells per well and incubated overnight. Hesperetin, TPGS, PC, hesperetin-TPGS micelles, or hesperetin-PC complexes were added into the culture medium at the final hesperetin concentration range of 0.01 to 10 μg/mL. After 24 h of incubation, the drug medium was removed and 200 μL of MTT solution (0.5 mg/mL) was added to each well. After 4 h of incubation at 37 °C, the MTT solution was replaced by 100 μL of dimethyl sulfoxide (DMSO) to dissolve the insoluble crystals. The absorbance of the solutions in each well was measured at 562/620 nm using a Spectramax M2 microplate reader (Molecular Devices, California, USA) after shaking for 1 min. The control group was considered to have 100% of cell viability. Triplicate experiments were performed, and five wells were set for each concentration.
2.10. Cellular antioxidant activity assay
The cellular antioxidant activity of hesperetin formulations was determined using the fluorescence assay developed by Wolfe and Liu (2007). Briefly, HepG2 cells were seeded at a density of 6×104 cells per well in a 96-well microplate. Twenty-four hours after seeding, the growth medium was removed, and the cells were washed with phosphate-buffered saline (PBS). Each group of six wells was treated for 1 h with 100 μL of growth medium containing different hesperetin samples and 100 μL of DCFH-DA (25 μmol/L) dissolved in PBS. Then the treatment medium was removed, and the cells were washed with 100 μL of PBS, after which the cells were treated with 600 μmol/L AAPH in 100 μL of HBSS and the 96-well microplate was placed into a Spectramax M2 microplate reader at 37 °C. The fluorescence intensity was measured in sextuplicate every 5 min for 1 h at the excitation and emission wavelengths of 488 and 525 nm, respectively. The blank group was treated with DCFH-DA and HBSS. The control group was treated with DCFH-DA and AAPH. The final fluorescence value curve was obtained by subtracting the blank values. The area under the curve (AUC) for fluorescence versus time was integrated to calculate the cellular antioxidant activity (CAA) value of different hesperetin samples as follows:
CAA unit=100−(∫SA/∫CA)×100,
where ∫SA is the integrated area under the sample fluorescence versus time curve, and ∫CA is the corresponding integrated area obtained from the control curve. The CAA unit was used to estimate the CAA of the samples (Wolfe and Liu, 2007).
2.11. Pharmacokinetic study in SD rats
Female Sprague Dawley (SD) rats (180–200 g, 8-week-old) were obtained from the Zhejiang Academy of Medical Sciences (Hangzhou, China) and housed under standard conditions. Before the experiment, rats were randomly divided into three groups (n=3) and fasted for 24 h with free access to water. The first group was treated with hesperetin suspension at a dose of 100 mg/kg by oral gavage. The second and third groups were treated with hesperetin-TPGS micelles and hesperetin-PC complexes, respectively, at an equivalent dose of 100 mg/kg hesperetin. After oral administration, a blood sample (0.5 mL) was collected using the orbital sinus blood sampling method at 0.25, 0.5, 0.75, 1, 1.5, 3, 5, 7, and 9 h. The blood was centrifuged at 18 800g for 5 min, and 200 μL of supernatant plasma was obtained and stored at −20 °C for further analysis. This experiment was approved by the Animal Experimental Ethical Committee of Zhejiang University, Hangzhou, China. Pharmacokinetic parameters were calculated using DRUG AND STATISTICS software (DAS, Version 2.0; Mathematical Pharmacology Professional Committee of China, Shanghai, China). The AUC of plasma concentration–time curves, the maximum concentrations of hesperetin (Cmax), the time to the peak plasma concentration (Tmax), total body clearance (Clz), mean residence time (MRT), and the apparent volume of distribution (Vz) were obtained from the non-compartmental model.
2.12. Statistical analysis
All data are presented as mean±standard deviation (SD) of ≥3 experiments or samples. A two-tailed unpaired Student’s t-test was used for statistical analysis. P<0.05 was considered statistically significant.
3. Results and discussion
3.1. Solubility study and formulation optimization
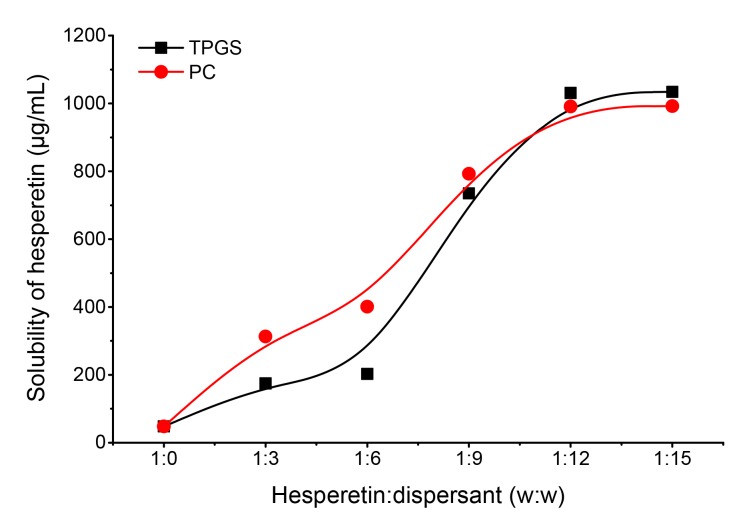
The water solubility of free hesperetin and the different hesperetin formulations was tested to find the best formulation. The amount of precipitate formed in the different formulations after 10 d was also observed. Free hesperetin was nearly insoluble in water; its solubility of 47.91 µg/mL in our test is consistent with a previous report (Liu and Chen, 2008). The solubility of hesperetin improved in hesperetin-TPGS micelles and hesperetin-PC complexes (Fig. 2). The maximum solubility of hesperetin (1031.00 µg/mL) was achieved when the weight ratio of hesperetin to TPGS was 1:12. In hesperetin-PC complexes, the highest hesperetin solubility was 991.10 µg/mL, which was also at a 1:12 weight ratio of hesperetin to PC. These two formulations with the highest water solubility and the least precipitate were chosen for further studies.
Fig. 2.
Enhanced water solubility of hesperetin by D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) and phosphatidylcholine (PC)
3.2. Characterization of the micelles and complexes
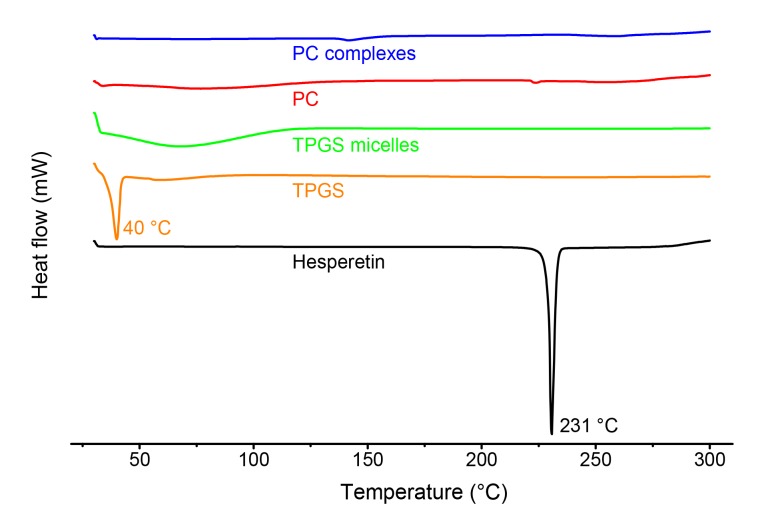
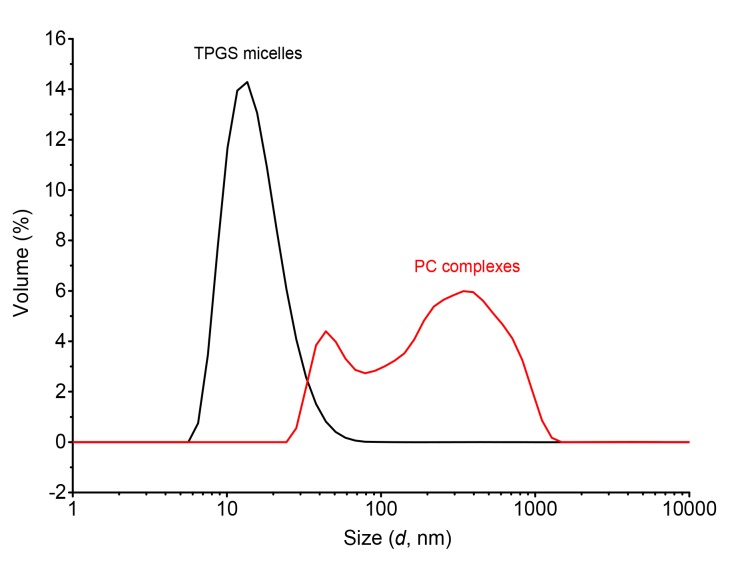
DSC is a reliable method for providing information on the melting point of compounds and the possible interactions between active ingredient and dispersants. The DSC thermograms (Fig. 3) show a sharp and narrow endothermic peak of hesperetin at 231 °C, which indicates the melting point of hesperetin. TPGS had a narrow endothermic peak at 40 °C, indicating its melting point. Once hesperetin-TPGS micelles were formed, the hesperetin peak disappeared and the peak of TPGS became broad. We infer that the state of hesperetin changed from crystalline to amorphous state by the interactions with TPGS. The peak of hesperetin at 231 °C also disappeared on DSC curve of hesperetin-PC complexes. Hydrophobic interactions and hydrogen bonding contribute to the formation of TPGS micelles and PC complexes. The aromatic rings of hesperetin can provide the hydrophobic interaction with the hydrocarbon tail of PC and the aromatic ring of TPGS, while the phenolic hydroxyl group of hesperetin contributes to the hydrogen bond with PC or TPGS (Khan et al., 2013; Shin et al., 2016). Dynamic light scattering was used to characterize the size distribution of the micelles and the complexes. The hesperetin-TPGS solution is light blue and translucent, indicating the formation of micelles with a small particle size of (26.19±0.05) nm and a polydispersity index of 0.257±0.024. In contrast, hesperetin and PC formed a milky emulsion with an average particle size of (219.15±2.05) nm and a polydispersity index of 0.264±0.004. Hesperetin-PC has a broad size distribution, which may result from the impurities in PC, such as phosphatidylethanolamine and phosphatidylserine. Other factors, such as the preparation methods, may also affect size distribution. The PC complexes prepared by super critical fluids may have larger particle sizes (Li et al., 2008). Representative volume size distributions of hesperetin-TPGS micelles and hesperetin-PC complexes are shown in Fig. 4.
Fig. 3.
DSC thermograms of hesperetin, TPGS, PC, hesperetin-TPGS micelles, and hesperetin-PC complexes
Fig. 4.
Volume size distributions of hesperetin-TPGS micelles and hesperetin-PC complexes in water
The average particle sizes of hesperetin-TPGS micelles and hesperetin-PC complexes are (26.19±0.05) nm with a polydispersity index of 0.257±0.024 and (219.15±2.05) nm with polydispersity index of 0.264±0.004, respectively
3.3. Cytotoxicity analyzed by the MTT assay
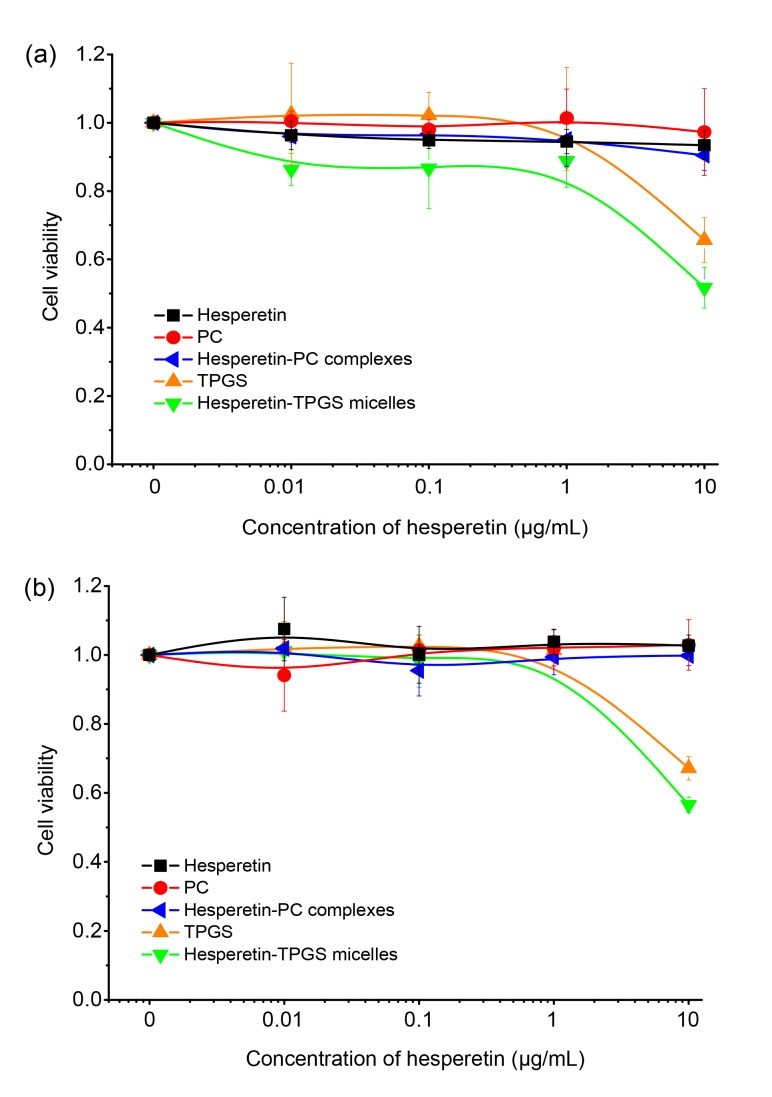
The MTT assay was used to investigate the cytotoxicity of the formulations and the dispersants on both cancer and normal cell lines (Fig. 5). PC and hesperetin-PC complexes did not show significant cytotoxicity compared with hesperetin, which agrees with other studies (Khan et al., 2013). TPGS and hesperetin-TPGS micelles exhibited a low degree of toxicity at the equivalent hesperetin concentration of 10 μg/mL (or 120 μg/mL of TPGS, according to the weight ratio of 1:12). Previous studies have found no obvious side effects when TPGS was orally administered at 1000 mg/kg body weight per day (Krasavage and Terhaar, 1977; Choudhury et al., 2017).
Fig. 5.
Cell viability of HepG2 cells (a) and MDCK cells (b) after treatments of hesperetin, TPGS, PC, hesperetin-TPGS micelles, and hesperetin-PC complexes for 24 h
The data are from MTT assays and normalized against untreated cells. Data are expressed as mean±SD (n=3)
3.4. Cellular antioxidant activity of the formulations
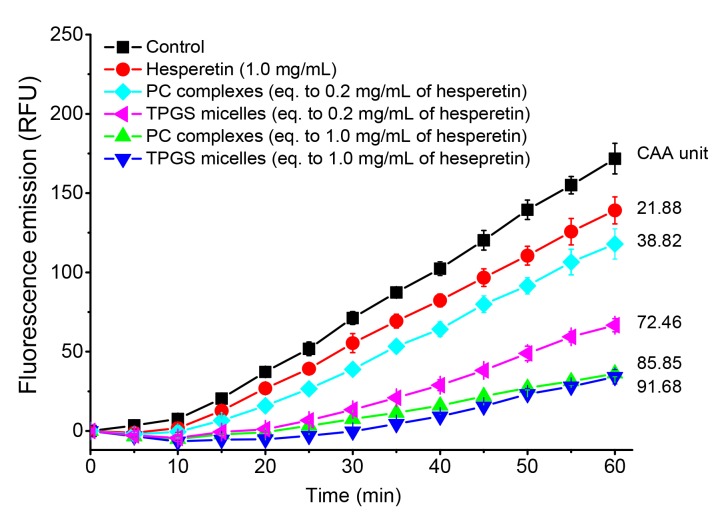
The CAA assay was performed to compare the antioxidant activity of different formulations (Fig. 6). Hesperetin (1.0 mg/mL) had inherent antioxidant activity with the CAA unit of 21.88. Either encapsulated into TPGS micelles or forming PC complexes, even 0.2 mg/mL of hesperetin increased the CAA level compared to a free hesperetin suspension (1.0 mg/mL). In addition, higher antioxidant activity was observed for hesperetin-TPGS micelles (CAA=91.68) and hesperetin-PC complexes (CAA=85.85) when the equivalent concentration of hesperetin was increased to 1.0 mg/mL. Increased solubility, improved cell membrane permeability and cellular uptake of hesperetin may contribute to the enhanced antioxidant activity (Varma and Panchagnula, 2005). Interestingly, TPGS has inherent antioxidant activity because of the vitamin E structure in the molecule (Singh et al., 2017), which may further improve the antioxidant activity of hesperetin-TPGS micelles.
Fig. 6.
Cellular antioxidant activity (CAA) levels of hesperetin, hesperetin-TPGS micelles, and hesperetin-PC complexes examined by the CAA assay
The CAA unit was calculated by the difference between the areas under the curve (AUCs) of sample groups and that of the control group. RFU: relative fluorescence unit. Data are expressed as mean±SD (n=6)
3.5. Pharmacokinetic study in SD rats
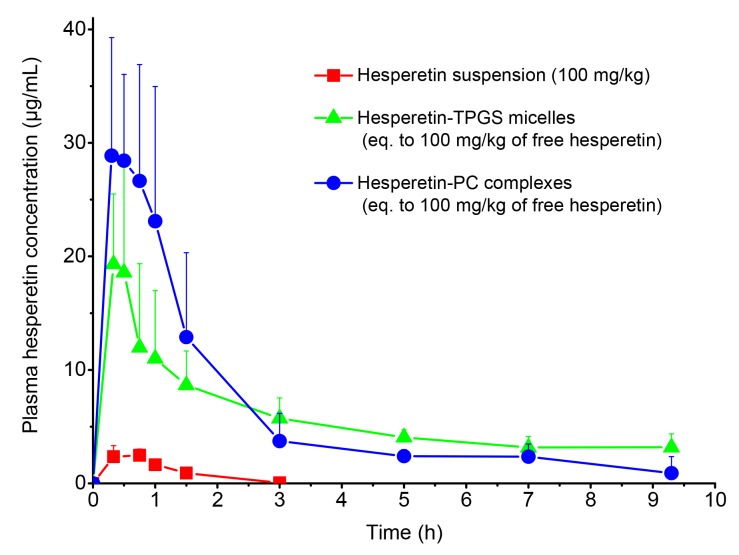
Plasma concentration–time profiles of the hesperetin suspension, hesperetin-TPGS micelles, and hesperetin-PC complexes after oral administration are shown in Fig. 7 and the main pharmacokinetic parameters are listed in Table 1. The micelles and complexes significantly increased the Cmax of hesperetin from 2.64 μg/mL to 20.67 and 33.09 μg/mL, respectively. Importantly, these two formulations increased the AUC to 16.2-fold (53.01 h∙μg/mL vs. 3.28 h∙μg/mL) and 18.0-fold (59.13 h∙μg/mL vs. 3.28 h∙μg/mL), indicating that TPGS micelles and PC complexes remarkably improved the oral bioavailability of hesperetin. Although there were no significant statistical differences in the pharmacokinetic parameters between the micelles and the complexes, the mechanisms of the enhanced solubility and oral absorption may be distinct. Absorbed hesperetin is reported to be pumped out by P-glycoprotein (P-gp), which is extensively expressed in the intestinal epithelium and pumps xenobiotics back into the intestinal lumen (Brand et al., 2008). TPGS functions as an inhibitor of P-gp (Dintaman and Silverman, 1999). In addition, increased solubility of hesperetin could accelerate the absorption and reduce the exposure time to bacteria, resulting in decreased hesperetin degradation by intestinal bacteria. Therefore, TPGS can not only increase the water solubility and the oral absorption of hesperetin through micelle formation, but it can also reduce the degradation and prevent the excretion of absorbed hesperetin by P-gp inhibition. As a component of the cell membrane, PC is easily absorbed into gastrointestinal tract, facilitating the membrane transport of the dispersed and encapsulated hesperetin. In addition, lipid-based nanoparticles, especially those containing phospholipids, are absorbed by the lymphatic system, through which the hepatic first-pass effect can be avoided (Attili-Qadri et al., 2013; Zhao et al., 2018).
Fig. 7.
Concentration–time profiles of total hesperetin in plasma after oral administration of hesperetin suspension, hesperetin-TPGS micelles, or hesperetin-PC complexes in rats
Data are expressed as mean±SD (n=3)
Table 1.
Main pharmacokinetic parameters of hesperetin in rats after oral administration of hesperetin suspension, hesperetin-TPGS micelles, or hesperetin-PC complexes
| Hesperetin formulation | Cmax (μg/mL) | Tmax (h) | AUC0–t (h∙μg/mL) | MRT (h) | t1/2z (h) | Clz (L/h) | Vz (L) |
| Hesperetin suspension | 2.64±0.76 | 0.61±0.24 | 3.28±0.68 | 0.93±0.06 | 0.39±0.00 | 31.11±6.26 | 17.38±3.64 |
| Hesperetin-TPGS micelles | 20.67±8.27** | 0.39±0.10 | 53.01±4.39** | 3.31±0.69** | 18.45±17.86 | 1.00±0.75** | 14.66±5.97 |
| Hesperetin-PC complexes | 33.09±10.70** | 0.45±0.26 | 59.13±12.50** | 2.17±0.79* | 2.48±2.67 | 1.56±0.32** | 5.21±5.16** |
Cmax: peak plasma concentration; Tmax: the time to Cmax; AUC0–t: area under the concentration–time curve; MRT: mean residence time; t1/2z: half-life of drug; Clz: clearance; Vz: volume of distribution. Oral administration of hesperetin suspension: 100 mg/kg, per oral (p.o.); Oral administration of hesperetin-TPGS micelles or hesperetin-PC complexes: equivalent to 100 mg/kg of free hesperetin, p.o. Data are expressed as mean±SD (n=3).
P<0.05,
P<0.01, vs. hesperetin suspension
4. Conclusions
In conclusion, we developed two oral formulations of hesperetin (hesperetin-TPGS micelles and hesperetin-PC complexes) by a simple preparation process. The micelles and complexes increased the solubility and improved the antioxidant activity of hesperetin dramatically. Importantly, these two formulations remarkably increased the oral absorption of hesperetin in vivo and the excipients used in these formulations are US FDA-approved. Thus, these two formulations may have potential applications in drugs and healthcare products.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 51773176, 51522304, and U1501243) and the Natural Science Foundation of Zhejiang Province (No. LY17H300002), China
Contributors: Su-fang GU and Xiang-rui LIU designed the research. Su-fang GU and Li-ying WANG performed the research. Ying-jie TIAN and You-qing SHEN contributed reagents/cells. Su-fang GU, Li-ying WANG, Ying-jie TIAN, Zhu-xian ZHOU, Jian-bin TANG, Xiang-rui LIU, Hai-ping JIANG, and You-qing SHEN analyzed data. Su-fang GU, Xiang-rui LIU, and Hai-ping JIANG wrote the paper.
Compliance with ethics guidelines: Su-fang GU, Li-ying WANG, Ying-jie TIAN, Zhu-xian ZHOU, Jian-bin TANG, Xiang-rui LIU, Hai-ping JIANG, and You-qing SHEN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Attili-Qadri S, Karra N, Nemirovski A, et al. Oral delivery system prolongs blood circulation of docetaxel nanocapsules via lymphatic absorption. Proc Natl Acad Sci USA. 2013;110(43):17498–17503. doi: 10.1073/pnas.1313839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand W, van der Wel PAI, Rein MJ, et al. Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab Dispos. 2008;36(9):1794–1802. doi: 10.1124/dmd.107.019943. [DOI] [PubMed] [Google Scholar]
- 3.Choudhury H, Gorain B, Pandey M, et al. Recent advances in TPGS-based nanoparticles of docetaxel for improved chemotherapy. Int J Pharm. 2017;529(1-2):506–522. doi: 10.1016/j.ijpharm.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 4.de Souza VT, de Franco ÉPD, de Araújo MEMB, et al. Characterization of the antioxidant activity of aglycone and glycosylated derivatives of hesperetin: an in vitro and in vivo study. J Mol Recognit. 2016;29(2):80–87. doi: 10.1002/jmr.2509. [DOI] [PubMed] [Google Scholar]
- 5.Dintaman JM, Silverman JA. Inhibition of P-glycoprotein by D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) Pharm Res. 1999;16(10):1550–1556. doi: 10.1023/A:1015000503629. [DOI] [PubMed] [Google Scholar]
- 6.Kanaze FI, Bounartzi MI, Georgarakis M, et al. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr. 2007;61(4):472–477. doi: 10.1038/sj.ejcn.1602543. [DOI] [PubMed] [Google Scholar]
- 7.Khan J, Alexander A, Ajazuddin , et al. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J Control Release. 2013;168(1):50–60. doi: 10.1016/j.jconrel.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Jeon SM, Lee MK, et al. Comparison of hesperetin and its metabolites for cholesterol-lowering and antioxidative efficacy in hypercholesterolemic hamsters. J Med Food. 2010;13(4):808–814. doi: 10.1089/jmf.2009.1320. [DOI] [PubMed] [Google Scholar]
- 9.Krasavage WJ, Terhaar CJ. d-α-Tocopheryl poly(ethylene glycol) 1000 succinate. Acute toxicity, subchronic feeding, reproduction, and teratologic studies in the rat. J Agric Food Chem. 1977;25(2):273–278. doi: 10.1021/jf60210a002. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Yang DJ, Chen SL, et al. Process parameters and morphology in puerarin, phospholipids and their complex microparticles generation by supercritical antisolvent precipitation. Int J Pharm. 2008;359(1-2):35–45. doi: 10.1016/j.ijpharm.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Liu LX, Chen J. Solubility of hesperetin in various solvents from (288.2 to 323.2) K. J Chem Eng Data. 2008;53(7):1649–1650. doi: 10.1021/je800078j. [DOI] [Google Scholar]
- 12.Maiti K, Mukherjee K, Murugan V, et al. Exploring the effect of hesperetin-HSPC complex–a novel drug delivery system on the in vitro release, therapeutic efficacy and pharmacokinetics. AAPS PharmSciTech. 2009;10(3):943–950. doi: 10.1208/s12249-009-9282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Németh K, Plumb GW, Berrin JG, et al. Deglycosylation by small intestinal epithelial cell β-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr. 2003;42(1):29–42. doi: 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- 14.Parhiz H, Roohbakhsh A, Soltani F, et al. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29(3):323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 15.Qin LH, Niu YW, Wang YM, et al. Combination of phospholipid complex and submicron emulsion techniques for improving oral bioavailability and therapeutic efficacy of water-insoluble drug. Mol Pharm. 2018;15(3):1238–1247. doi: 10.1021/acs.molpharmaceut.7b01061. [DOI] [PubMed] [Google Scholar]
- 16.Roohbakhsh A, Parhiz H, Soltani F, et al. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin–a mini-review. Life Sci. 2014;113(1-2):1–6. doi: 10.1016/j.lfs.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Shete G, Pawar YB, Thanki K, et al. Oral bioavailability and pharmacodynamic activity of hesperetin nanocrystals generated using a novel bottom-up technology. Mol Pharm. 2015;12(4):1158–1170. doi: 10.1021/mp5008647. [DOI] [PubMed] [Google Scholar]
- 18.Shin GH, Li JL, Cho JH, et al. Enhancement of curcumin solubility by phase change from crystalline to amorphous in cur-TPGS nanosuspension. J Food Sci. 2016;81(2):N494–N501. doi: 10.1111/1750-3841.13208. [DOI] [PubMed] [Google Scholar]
- 19.Singh H, Narang JK, Singla YP, et al. TPGS stabilized sublingual films of frovatriptan for the management of menstrual migraine: formulation, design and antioxidant activity. J Drug Deliv Sci Technol. 2017;41:144–156. doi: 10.1016/j.jddst.2017.07.008. [DOI] [Google Scholar]
- 20.Testai L, Calderone V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients. 2017;9(5):502. doi: 10.3390/nu9050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varma MVS, Panchagnula R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo. Eur J Pharm Sci. 2005;25(4-5):445–453. doi: 10.1016/j.ejps.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Wang JN, Wang LL, Zhang L, et al. Studies on the curcumin phospholipid complex solidified with Soluplus®. J Pharm Pharmacol. 2018;70(2):242–249. doi: 10.1111/jphp.12857. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe KL, Liu RH. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem. 2007;55(22):8896–8907. doi: 10.1021/jf0715166. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZP, Tan SW, Feng SS. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–4906. doi: 10.1016/j.biomaterials.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 25.Zhao BX, Gu SF, Du Y, et al. Solid lipid nanoparticles as carriers for oral delivery of hydroxysafflor yellow A. Int J Pharm. 2018;535(1-2):164–171. doi: 10.1016/j.ijpharm.2017.10.040.. [DOI] [PubMed] [Google Scholar]