Abstract
Aims/hypothesis
Maternal type 2 diabetes during pregnancy and gestational diabetes is associated with childhood adiposity; however, associations of lower maternal glucose levels during pregnancy with childhood adiposity, independent of maternal BMI, remain less clear. The objective was to examine associations of maternal glucose levels during pregnancy with childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) cohort.
Methods
The HAPO Study was an observational epidemiological international multi-ethnic investigation that established strong associations of glucose levels during pregnancy with multiple adverse perinatal outcomes. The HAPO Follow-up Study (HAPO FUS) included 4832 children from ten HAPO centres whose mothers had a 75 g OGTT at ~28 weeks gestation 10–14 years earlier, with glucose values blinded to participants and clinical caregivers. The primary outcome was child adiposity, including: (1) being overweight/obese according to sex- and age-specific cut-offs based on the International Obesity Task Force (IOTF) criteria; (2) IOTF-defined obesity only; and (3) measurements >85th percentile for sum of skinfolds, waist circumference and per cent body fat. Primary predictors were maternal OGTT and HbA1c values during pregnancy.
Results
Fully adjusted models that included maternal BMI at pregnancy OGTT indicated positive associations between maternal glucose predictors and child adiposity outcomes. For one SD difference in pregnancy glucose and HbA1c measures, ORs for each child adiposity outcome were in the range of 1.05–1.16 for maternal fasting glucose, 1.11–1.19 for 1 h glucose, 1.09–1.21 for 2 h glucose and 1.12–1.21 for HbA1c. Associations were significant, except for associations of maternal fasting glucose with offspring being overweight/obese or having waist circumference >85th percentile. Linearity was confirmed in all adjusted models. Exploratory sex-specific analyses indicated generally consistent associations for boys and girls.
Conclusions/interpretation
Exposure to higher levels of glucose in utero is independently associated with childhood adiposity, including being overweight/obese, obesity, skinfold thickness, per cent body fat and waist circumference. Glucose levels less than those diagnostic of diabetes are associated with greater childhood adiposity; this may have implications for long-term metabolic health.
Keywords: Adiposity, Childhood obesity, Glucose, Pregnancy
Introduction
The intrauterine environment affects fetal development with maternal glucose and adiposity having independent associations with newborn adiposity [1-5]. Although both type 2 diabetes mellitus during pregnancy and gestational diabetes mellitus (GDM) are associated with childhood adiposity, women with these conditions are more likely to be overweight or obese. Thus, the independent relationship between maternal hyperglycaemia and childhood adiposity is less clear. Previous studies of maternal glucose levels during pregnancy and childhood obesity have generally focused on women with pre-existing diabetes (type 1 or type 2 diabetes) or GDM and were often confounded by, or did not account for, diabetes treatment or maternal BMI [6-13]. Studies of the association of maternal glucose across a spectrum of glucose values with childhood adiposity outcomes have not been reported.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study recruited a large, multinational, racially and ethnically diverse cohort of women and showed that glucose levels during pregnancy, below those diagnostic of diabetes, were associated with adverse newborn outcomes and that these associations were continuous across increasing levels of glucose [4]. The HAPO Follow-up Study (HAPO FUS) offered a unique opportunity to examine associations of maternal glucose levels during pregnancy with childhood adiposity, not confounded by treatment of maternal hyperglycaemia. We recently reported that, while GDM based on International Association of Diabetes in Pregnancy Study Groups (IADPSG) criteria [14, 15] was not associated with an increase in being overweight/obese in childhood, it was associated with a higher risk of obesity and other measures of adiposity, including sum of skinfolds, per cent body fat and waist circumference >85th percentile, in children ages 10–14 years from the HAPO FUS cohort [16]. The objective of the analyses reported herein was to assess whether in utero exposure to levels of maternal glucose across the continuous spectrum of glucose values, including values less than those diagnostic of GDM based on IADPSG criteria, is also associated with adiposity, including being overweight/obese, obesity and other anthropometric measures in children from the HAPO FUS cohort.
Methods
HAPO Study methods have been described [4, 17, 18]. Briefly, eligible women underwent a 75 g OGTT at 24–32 weeks’ gestation. Fasting, 1 h, and 2 h plasma glucose, and HbA1c were measured. Maternal height, weight and blood pressure were measured at the time of the pregnancy OGTT using standard procedures and calibrated equipment. Demographic data including race/ethnicity were collected via questionnaire and parity by medical record abstraction. OGTT results remained blinded to caregivers and participants unless fasting glucose was >5.8 mmol/l and/or 2 h glucose was >11.1 mmol/l or if either measure was <2.5 mmol/l [4, 17, 18]. Data from unblinded women were not included in HAPO analyses.
Participants
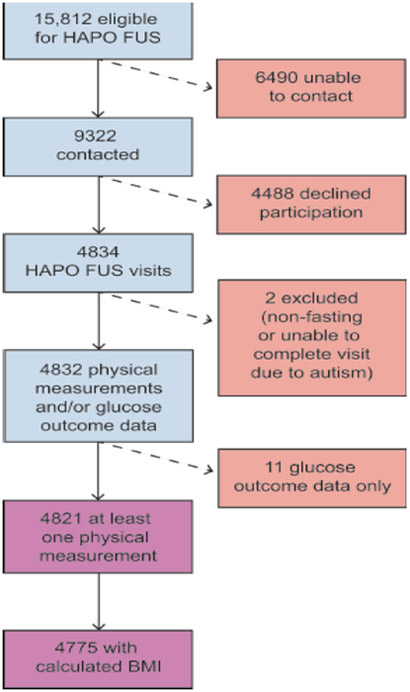
HAPO FUS participants were recruited during 2013–2016 from ten of 15 original HAPO field centres that demonstrated feasibility for this study. Eligibility criteria included the following: having remained blinded to OGTT results during HAPO; gestational age at delivery ≥37 weeks and no major neonatal malformations or fetal/neonatal death. This yielded 15,812 eligible mother–child pairs (ESM Table 1). A target sample size of 7000 was specified, with 300 mother–child pairs to be recruited at Chicago and Cleveland and 800 at each of the other eight field centres [16].
Multiple attempts were made to contact all eligible participants through various means approved by local institutional review boards. Screening questionnaires were completed by 9322 eligible mothers over the telephone to ascertain willingness to participate and confirm eligibility. From these, 4834 children completed all or part of the HAPO FUS visit, which represented 69.1% of the recruitment goal of 7000 mother–child pairs (Fig. 1). Data from two children were excluded, one due to inadequate fasting and one because of inability to complete the protocol due to autism spectrum disorder, leaving 4832 children for analyses. Of these, 4821 had at least one physical measurement, while 4775 had BMI measurements and were analysed for overweight/obesity outcomes.
Fig. 1.
Flowchart illustrating enrolment for HAPO FUS
The HAPO FUS Protocol was approved by each centre’s institutional review board. All mothers gave written informed consent for their child’s participation and children provided assent where required. The study was overseen by an external Observational Study Monitoring Board.
Study visit
During the HAPO FUS visit, height was measured twice (with the participant’s shoes removed) to the nearest 0.5 cm with a stadiometer and was measured for a third time if results differed by >1.0 cm. Weight was measured twice on a calibrated scale to the nearest 0.1 kg and was measured for a third time if results differed by >0.5 kg. Waist circumference was measured twice at the iliac crest to the nearest 0.1 cm and was measured for a third time if results differed by >1.0 cm. Skinfolds were measured (triceps, subscapular, suprailiac) twice with calibrated calipers (Harpenden, London, UK) to the nearest 0.1 mm and were measured for a third time if results differed by >1.0 mm. Means of the two measurements, or the two closest measurements if three measurements were made, were used for analysis. Per cent fat was measured by air displacement plethysmography using a BOD POD (Cosmed, Rome, Italy). Tanner staging was performed by trained individuals using breast/areolar development for girls and testicular volume (Prader orchidometer) for boys. Mothers provided girls’ menstrual status via questionnaire.
Outcomes
Child adiposity was the primary outcome and included the following: (1) children who were overweight/obese according to age- and sex-specific cut-offs based on International Obesity Task Force (IOTF) criteria (Asian-specific cut-offs were used for self-reported Asian children and international cut-offs were used for all other children [19]); (2) IOTF-defined obesity only; and (3) measurements of per cent body fat (BOD POD), waist circumference and sum of skinfolds >85th percentile according to quantile regression adjusted for sex, age and field centre. BMI, BMI z score [19], per cent body fat, waist circumference and sum of skinfolds were also assessed as continuous outcomes.
Predictors
The primary predictors were continuous values of fasting, 1 h and 2 h glucose and HbA1c from the original HAPO OGTT during pregnancy. Continuous predictors were scaled by their SDs as estimated in original HAPO data.
Additionally, to confirm graded associations, these continuous variables were divided into discrete categorical variables with five categories for each measure. The fifth and highest category for each measure corresponded to IADPSG diagnostic thresholds for GDM. The lowest three categories for fasting, 1 h and 2 h glucose were the same as those used for analyses of glucose levels during the original HAPO Study. The fourth category included values between the third and fifth categories. For the HbA1c values, the lowest three categories were used during the original HAPO Study and the fourth and fifth categories combined smaller categories used for original analyses of HbA1c in HAPO. Categories were defined as follows: fasting glucose ≤4.2, 4.2–4.4, 4.5–4.7, 4.8–5.0, ≥5.1 mmol/l; 1 h glucose ≤5.8, 5.9–7.3, 7.4–8.6, 8.7–9.9, ≥10.0 mmol/l; 2 h glucose ≤5.0, 5.1–6.0, 6.1–6.9, 7.0–8.4, ≥8.5 mmol/l; HbA1c ≤25, 26–28, 29–31, 32–36, ≥37 mmol/mol (≤4.4, 4.5–4.7, 4.8–5.0, 5.1–5.4, ≥5.5%).
Statistical analyses
HAPO FUS data were summarised using frequencies and counts for categorical variables and means and SDs for continuous variables. Summary statistics from pregnancy were also compared for eligible children who did and did not participate in HAPO FUS, including field-centre summaries weighted by the proportion of the total eligible at each field centre. Histograms and boxplots were examined to determine the shape of distributions and to identify potential outlying observations. Multiple logistic regression was used for dichotomous outcomes; results are reported as ORs with 95% CIs. Multiple linear regression was used for continuous outcomes; results are reported as regression coefficients (β estimates) with 95% CIs. Multiple models were considered for all outcomes, with variables identified according to study design, known potential confounders and adjustments used in HAPO analyses [4, 16]. Covariate adjustments were examined as follows: Model 1: field centre, sex, child’s age at follow-up. Since IOTF definitions are sex- and age-specific, Model 1 for IOTF outcomes included field centre adjustment only. For per cent body fat, waist circumference and sum of skinfolds >85th percentiles, logistic regression Model 1 was unadjusted since 85th percentiles included age, sex and field centre in their definition. Model 2 was as for Model 1 plus adjustment for child’s pubertal status (Tanner stage 1, 2/3, 4/5) with sex-by-Tanner stage interaction. Model 3 was as for Model 2 plus adjustment for maternal variables at pregnancy OGTT (age, height, family history of diabetes in first-degree relatives, mean arterial pressure, parity [0, 1+], smoking (yes/no), alcohol (yes/no), gestational age). Model 4 was as for Model 3 plus adjustment for maternal BMI at pregnancy OGTT. Exploratory analyses were also conducted to evaluate differences in associations according to sex and race/ethnicity, using field centre as a proxy for race/ethnicity. Statistical interaction terms between continuous maternal glucose and HbA1c with sex and field centre were reviewed, and group-specific model variable estimates were compared. Multiple imputation under a ‘missing at random’ assumption [20, 21] using the mice R package[22] was used for imputation of all missing data, with Tanner stage being the primary source of missingness, and incorporated sex steroid measurements (ESM Methods). Logistic regression model fit was measured by C statistics and confirmed by Hosmer–Lemeshow goodness-of-fit tests [23]. Linear regression model fit was assessed by scatterplots of residuals vs fitted values, histograms and qqplots of residuals, and DFbeta statistics. Adjusted R2 values were used to gauge variability explained in linear models. Quadratic terms and restricted cubic splines estimated with the rms R package [24] were used to assess linearity between the continuous predictor and the log odds of the outcome for logistic regression models and continuous outcomes for linear regression models. All analyses were conducted in R (3.4.1) [25].
Results
Participants
Characteristics of participating children during HAPO FUS and their mothers during HAPO are shown in Table 1. The mean age of the children at follow-up was 11.4 years. The mean age at the time of the HAPO OGTT and frequency of GDM according to IADPSG criteria [14] among mothers of offspring who did not participate (unable to contact or declined) was 29.1 years and 16.9%, respectively, compared with 30.0 years and 14.9% among mothers of offspring who participated (ESM Table 1). Mean maternal BMI, HbA1c and fasting, 1 h and 2 h glucose during the HAPO OGTT and race/ethnicity were similar between groups who did and did not participate (summaries weighted to reflect full eligible population, ESM Table 1).
Table 1.
Characteristics of mothers during HAPO pregnancy OGTT and their children at follow-up
| Characteristic | N=4832 Mean (SD) |
|---|---|
| Mothers during HAPO pregnancy | |
| Age at OGTT, years | 29.9 (5.7) |
| Gestational age at OGTT, weeks | 27.7 (1.7) |
| Height, cm | 161.8 (6.8) |
| Weight, kg | 72.0 (14.2) |
| BMI, kg/m2 | 27.5 (4.9) |
| Mean arterial pressure, mmHg | 80.5 (8.0) |
| Fasting glucose, mmol/l | 4.5 (0.4) |
| 1 h glucose, mmol/l | 7.4 (1.7) |
| 2 h glucose, mmol/l | 6.1 (1.3) |
| HbA1c, mmol/l | 29.0 (4.5) |
| HbA1c, % | 4.8 (0.4) |
| Race/Ethnicity | |
| White, non-Hispanic, n (%) | 2287 (47.3) |
| Hispanic, n (%) | 507(10.5) |
| Black, non-Hispanic, n (%) | 775 (16.0) |
| Asian, n (%) | 1176 (24.3) |
| Other, n (%) | 87(1.8) |
| Any prenatal smoking, n (%) | 245 (5.1) |
| Any prenatal alcohol use, n (%) | 406 (8.4) |
| Parity (any prior delivery ≥20 weeks), n (%) | 2485 (51.4) |
| Family history of diabetes, n (%) | 1077 (22.3) |
| Children at follow-up | |
| Age, years | 11.4 (1.2) |
| Height, cm | 148.6 (10.2) |
| Weight, kg | 43.2 (13.3) |
| Sex, female, n (%) | 2367 (49.0) |
| Tanner stage, girls, n (%) | |
| 1 | 381 (19.0) |
| 2/3 | 853 (42.5) |
| 4/5 | 774 (38.5) |
| Tanner stage, boys, n (%) | |
| 1 | 565 (36.0) |
| 2/3 | 726 (46.2) |
| 4/5 | 279 (17.8) |
Data are shown as means (SD) or n (%)
Model diagnostics
Hosmer–Lemeshow p values for logistic regression models with continuous maternal glucose and HbA1c predictors were in the range 0.06–0.99 for all outcomes, indicating reasonable model fit. C statistics for logistic regression models with continuous maternal glucose and HbA1c predictors were in the range 0.54–0.77 and R2 values were in the range 0.04–0.35 for continuous maternal glucose and HbA1c predictors with continuous outcomes. Both C statistics and R2 values increased across Models 1–4, suggesting improved fit for additional covariate adjustments (Tables 2, 3). Co-linearity was not a concern with pairwise correlations in the range 0–0.20 for model covariates. Visual inspection of residual plots confirmed linear modelling assumptions and DFbeta statistics indicated no observations of undue influence. Quadratic terms and restricted cubic spline modelling indicated no significant departure from linearity for all analyses of continuous maternal glucose and HbA1c predictors with p values in the range 0.29–0.98.
Table 2.
Associations of continuous measures of maternal glucose and HbA1c during pregnancy with dichotomous adiposity outcomes among children at follow-up
| Predictor | Model 1a | Model 2b | Model 3c | Model 4d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
p value | C statistic | OR (95% CI) | p value | C statistic | OR (95% CI) | p value |
C
statistic |
OR (95% CI) | p value | C statistic | |
| IOTF overweight/obese | ||||||||||||
| Fasting glucose | 1.27 (1.19, 1.36) | <0.001*** | 0.64 | 1.26 (1.18, 1.35) | <0.001*** | 0.67 | 1.22 (1.13, 1.31) | <0.001*** | 0.70 | 1.05 (0.98, 1.14) | 0.19 | 0.74 |
| 1 h glucose | 1.22 (1.14, 1.30) | <0.001*** | 0.64 | 1.21 (1.13, 1.29) | <0.001*** | 0.67 | 1.18 (1.10, 1.27) | <0.001*** | 0.69 | 1.11 (1.03, 1.19) | 0.006** | 0.74 |
| 2 h glucose | 1.17 (1.10, 1.25) | <0.001*** | 0.64 | 1.18 (1.10, 1.26) | <0.001*** | 0.67 | 1.15 (1.07, 1.23) | <0.001*** | 0.69 | 1.09 (1.01, 1.17) | 0.019* | 0.74 |
| HbA1c | 1.23 (1.15, 1.33) | <0.001*** | 0.64 | 1.25 (1.16, 1.35) | <0.001*** | 0.67 | 1.24 (1.15, 1.34) | <0.001*** | 0.69 | 1.12 (1.04, 1.22) | 0.004** | 0.74 |
| IOTF obesity | ||||||||||||
| Fasting glucose | 1.40 (1.28, 1.54) | <0.001*** | 0.69 | 1.40 (1.27, 1.53) | <0.001*** | 0.71 | 1.34 (1.21, 1.48) | <0.001*** | 0.74 | 1.16 (1.05, 1.29) | 0.005** | 0.77 |
| 1 h glucose | 1.24 (1.13, 1.36) | <0.001*** | 0.68 | 1.23 (1.12, 1.35) | <0.001** | 0.70 | 1.20 (1.08, 1.32) | <0.001*** | 0.73 | 1.13 (1.01, 1.25) | 0.025* | 0.77 |
| 2 h glucose | 1.28 (1.17, 1.40) | <0.001*** | 0.68 | 1.29 (1.18, 1.41) | <0.001*** | 0.71 | 1.25 (1.14, 1.38) | <0.001*** | 0.71 | 1.21 (1.09, 1.34) | <0.001*** | 0.78 |
| HbA1c | 1.37 (1.23, 1.52) | <0.001*** | 0.69 | 1.37 (1.23, 1.52) | <0.001*** | 0.71 | 1.36 (1.22, 1.52) | <0.001*** | 0.74 | 1.21 (1.08, 1.35) | <0.001*** | 0.77 |
| BOD POD % fat >85th percentilee | ||||||||||||
| Fasting glucose | 1.35 (1.25, 1.47) | <0.001*** | 0.54 | 1.34 (1.24, 1.46) | <0.001*** | 0.62 | 1.29 (1.18, 1.41) | <0.001*** | 0.65 | 1.15 (1.05, 1.26) | 0.002** | 0.68 |
| 1 h glucose | 1.27 (1.17, 1.38) | <0.001*** | 0.55 | 1.25 (1.15, 1.36) | <0.001*** | 0.62 | 1.23 (1.13, 1.34) | <0.001*** | 0.65 | 1.18 (1.08, 1.29) | <0.001*** | 0.69 |
| 2 h glucose | 1.20 (1.10, 1.29) | <0.001*** | 0.54 | 1.20 (1.11, 1.30) | <0.001*** | 0.61 | 1.19 (1.09, 1.29) | <0.001*** | 0.64 | 1.15 (1.06, 1.26) | 0.001** | 0.69 |
| HbA1c | 1.25 (1.14, 1.36) | <0.001*** | 0.52 | 1.25 (1.14, 1.37) | <0.001*** | 0.61 | 1.23 (1.12, 1.35) | <0.001*** | 0.64 | 1.14 (1.03, 1.25) | 0.009** | 0.68 |
| Waist circumference > 85th percentilee | ||||||||||||
| Fasting glucose | 1.34 (1.23, 1.45) | <0.001*** | 0.54 | 1.31 (1.21, 1.42) | <0.001*** | 0.61 | 1.25 (1.14, 1.36) | <0.001*** | 0.65 | 1.09 (0.99, 1.19) | 0.067 | 0.69 |
| 1 h glucose | 1.22 (1.13, 1.32) | <0.001*** | 0.55 | 1.22 (1.12, 1.32) | <0.001*** | 0.60 | 1.21 (1.11, 1.32) | <0.001*** | 0.64 | 1.15 (1.05, 1.26) | 0.002** | 0.69 |
| 2 h glucose | 1.20 (1.10, 1.29) | <0.001*** | 0.53 | 1.21 (1.12, 1.31) | <0.001*** | 0.60 | 1.20 (1.11, 1.31) | <0.001*** | 0.64 | 1.17 (1.07, 1.27) | 0.003** | 0.69 |
| HbA1c | 1.23 (1.13, 1.34) | <0.001*** | 0.52 | 1.27 (1.16, 1.38) | <0.001*** | 0.60 | 1.26 (1.15, 1.38) | <0.001*** | 0.65 | 1.15 (1.05, 1.27) | 0.004** | 0.69 |
| Sum of skinfolds > 85th percentilee | ||||||||||||
| Fasting glucose | 1.36 (1.25, 1.47) | <0.001*** | 0.54 | 1.34 (1.23, 1.45) | <0.001*** | 0.61 | 1.30 (1.19, 1.41) | <0.001*** | 0.64 | 1.16 (1.06, 1.27) | 0.001** | 0.67 |
| 1 h glucose | 1.25 (1.16, 1.36) | <0.001*** | 0.55 | 1.26 (1.16, 1.37) | <0.001*** | 0.60 | 1.24 (1.14, 1.35) | <0.001*** | 0.64 | 1.19 (1.09, 1.30) | <0.001*** | 0.68 |
| 2 h glucose | 1.23 (1.14, 1.33) | <0.001*** | 0.54 | 1.25 (1.15, 1.36) | <0.001*** | 0.60 | 1.24 (1.14, 1.35) | <0.001*** | 0.63 | 1.21 (1.11, 1.31) | <0.001*** | 0.68 |
| HbA1c | 1.25 (1.15, 1.37) | <0.001*** | 0.52 | 1.26 (1.15, 1.38) | <0.001*** | 0.59 | 1.24 (1.13, 1.36) | <0.001*** | 0.63 | 1.15 (1.05, 1.26) | 0.004** | 0.67 |
ORs for the dichotomous outcomes are for the continuous predictors higher by one SD estimated in the original HAPO data: fasting glucose 0.4 mmol/l; 1 h glucose 1.7 mmol/l; 2 h glucose 1.3 mmol/l; HbA1c 5.0 mmol/mol (0.45%).
P values are from logistic regression model
p<0.05
p<0.01
p<0.001
Model 1: adjusted for field centre for being overweight/obese and obesity outcomes; unadjusted for BOD POD % fat, waist circumference and sum of skinfolds >85th percentile outcomes
Model 2: Model 1 + child pubertal status (Tanner stage 1, 2/3, 4/5, sex × Tanner stage interaction)
Model 3: Model 2 + maternal variables during pregnancy OGTT (age, height, any family history of diabetes, mean arterial pressure, parity [0, 1+], smoking [yes/no], alcohol [yes/no], gestational age)
Model 4: Model 3 + maternal BMI during pregnancy
85th percentiles were determined using quantile regression adjusted for age, sex and field centre
Table 3.
Associations of continuous measures of maternal glucose and HbA1c during pregnancy with continuous adiposity outcomes among children at follow-up
| Predictor | Model 1a | Model 2b | Model 3c | Model 4d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p value | Adjusted
R2 |
β (95% CI) | p value | Adjusted R2 | β (95% CI) |
p value | Adjusted
R2 |
β (95% CI) | p value | Adjusted
R2 |
|
| BMI z score | ||||||||||||
| Fasting glucose | 0.14 (0.10, 0.18) | <0.001*** | 0.07 | 0.13 (0.09, 0.16) | <0.001*** | 0.13 | 0.10 (0.06, 0.14) | <0.001*** | 0.15 | −0.001 (−0.04, 0.04) | 0.96 | 0.22 |
| 1 h glucose | 0.10 (0.06,0.14) | <0.001*** | 0.07 | 0.09 (0.06, 0.12) | <0.001*** | 0.14 | 0.07 (0.03, 0.10) | <0.001*** | 0.14 | 0.02 (−0.01, 0.05) | 0.26 | 0.22 |
| 2 h glucose | 0.08(0.04,0.11) | <0.001*** | 0.07 | 0.08 (0.04, 0.11) | <0.001*** | 0.12 | 0.05 (0.02, 0.09) | 0.003** | 0.14 | 0.02 (−0.02, 0.05) | 0.38 | 0.22 |
| HbA1c | 0.13 (0.09, 0.16) | <0.001*** | 0.07 | 0.14(0.10, 0.18) | <0.001*** | 0.21) | 0.13 (0.09, 0.16) | <0.001*** | 0.22 | 0.06 (0.02,0.10) | 0.001** | 0.29 |
| BOD POD % fat | ||||||||||||
| Fasting glucose | 1.35 (1.04, 1.66) | <0.001*** | 0.08 | 1.29 (0.99, 1.59) | <0.001*** | 0.13 | 1.09 (0.78, 1.40) | <0.001*** | 0.14 | 0.46 (0.14,0.77) | 0.004** | 0.18 |
| 1 h glucose | 1.11 (0.81, 1.41) | <0.001*** | 0.08 | 1.05 (0.75, 1.34) | <0.001*** | 0.12 | 0.93 (0.63, 1.24) | <0.001*** | 0.14 | 0.62 (0.32, 0.92) | <0.001*** | 0.18 |
| 2 h glucose | 0.87(0.57, 1.17) | <0.001*** | 0.08 | 0.86 (0.57, 1.16) | <0.001*** | 0.12 | 0.75 (0.45, 1.05) | <0.001*** | 0.14 | 0.50 (0.20, 0.80) | <0.001*** | 0.18 |
| HbA1c | 0.66 (0.34, 0.99) | <0.001*** | 0.08 | 0.81 (0.49, 1.13) | <0.001*** | 0.19 | 0.68 (0.36, 1.01) | <0.001*** | 0.21 | 0.25 (−0.07, 0.58) | 0.13 | 0.25 |
| Waist circumference, cm | ||||||||||||
| Fasting glucose | 1.50 (1.15, 1.85) | <0.001*** | 0.10 | 1.35 (1.02, 1.67) | <0.001*** | 0.21 | 1.09 (0.75, 1.43) | <0.001*** | 0.22 | 0.24 (−0.09, 0.28) | 0.16 | 0.28 |
| 1 h glucose | 1.07(0.73, 1.41) | <0.001*** | 0.09 | 0.99 (0.68, 1.31) | <0.001*** | 0.20 | 0.86 (0.53, 1.19) | <0.001*** | 0.22 | 0.45 (0.13,0.76) | 0.006** | 0.28 |
| 2 h glucose | 0.98 (0.64, 1.32) | <0.001*** | 0.09 | 0.91 (0.59, 1.22) | <0.001*** | 0.20 | 0.78 (0.46, 1.11) | <0.001*** | 0.22 | 0.46 (0.14,0.77) | 0.005** | 0.28 |
| HbA1c | 0.51 (0.15, 0.88) | 0.005** | 0.09 | 1.07 (0.73, 1.41) | <0.001*** | 0.28 | 0.96 (0.61, 1.31) | <0.001*** | 0.30 | 0.39(0.05,0.74) | 0.025* | 0.35 |
| Sum of skinfolds, mm | ||||||||||||
| Fasting glucose | 2.71 (2.05, 3.35) | <0.001*** | 0.05 | 2.70 (2.06, 3.34) | <0.001*** | 0.09 | 2.32(1.67, 2.99) | <0.001*** | 0.10 | 0.98 (0.31, 1.65) | 0.004** | 0.14 |
| 1 h glucose | 1.89(1.26, 2.52) | <0.001*** | 0.04 | 1.91 (1.29, 2.53) | <0.001*** | 0.08 | 1.62 (0.97, 2.26) | <0.001*** | 0.10 | 0.95 (0.31, 1.58) | 0.003** | 0.15 |
| 2 h glucose | 1.66(1.03,2.29) | <0.001*** | 0.04 | 1.76(1.14, 2.38) | <0.001*** | 0.08 | 1.48(0.84, 2.13) | <0.001*** | 0.10 | 0.95 (0.32, 1.58) | 0.003** | 0.15 |
| HbA1c | 1.61 (0.94, 2.29) | <0.001*** | 0.04 | 1.96(1.29, 2.64) | <0.001*** | 0.17 | 1.74(1.04, 2.43) | <0.001*** | 0.18 | 0.83 (0.14, 1.51) | 0.018* | 0.22 |
β estimates represent change in each continuous outcome for the continuous predictors higher by one SD estimated in the original HAPO data: fasting glucose 0.4 mmol/l; 1 h glucose 1.7 mmol/l; 2 h glucose 1.3 mmol/l; HbA1c 5.0 mmol/mol (0.45%)
p values are from linear regression model;
p<0.05
p<0.01 and
p<0.001
Model 1: adjusted for field centre for being overweight/obese and obesity outcomes; unadjusted for BOD POD % fat, waist circumference and sum of skinfolds >85th percentile outcomes
Model 2: Model 1 + child pubertal status (Tanner stage 1, 2/3, 4/5, sex × Tanner stage interaction)
Model 3: Model 2 + maternal variables during pregnancy OGTT (age, height, any family history of diabetes, mean arterial pressure, parity [0, 1+], smoking [yes/no], alcohol [yes/no], gestational age)
Model 4: Model 3 + maternal BMI during pregnancy
Continuous measures of maternal glucose level and childhood adiposity
Initial analyses examined the association of maternal glucose across the continuum with dichotomous child adiposity outcomes. ORs for each dichotomous child adiposity outcome were estimated for one SD difference in maternal fasting, 1 h and 2 h glucose, and HbA1c during pregnancy (Table 2). In Models 1–3, positive associations for each child adiposity outcome were observed for higher levels of each glucose measure and HbA1c. ORs were attenuated after adjusting for maternal BMI during pregnancy (Model 4). However, for one SD difference in pregnancy glucose ad HbA1c measures, ORs for each child adiposity outcome were in the range of 1.05–1.16 for maternal fasting glucose, 1.11–1.19 for 1 h glucose, 1.09–1.21 for 2 h glucose and 1.12–1.21 for HbA1c (Table 2). All of these results were significant except for the associations of maternal fasting glucose with offspring being overweight/obese or having waist circumference >85th percentile. Linearity for the continuous maternal glucose and HbA1c predictors was confirmed as described above.
Linear associations of continuous child BMI z score, per cent fat, waist circumference and sum of skinfolds with maternal fasting, 1 h and 2 h glucose and HbA1c during pregnancy were also evaluated (Table 3). In Models 1–3, positive associations for each continuous child adiposity outcome were observed for one SD differences in each maternal glucose measure and HbA1c. Associations were again attenuated after adjusting for maternal BMI during pregnancy (Model 4). For child BMI z score, associations with maternal fasting, 1 h and 2 h glucose were not significant after adjustment for maternal BMI during pregnancy. Associations between child per cent fat and maternal HbA1c and child waist circumference and maternal fasting glucose were also not significant in the fully adjusted Model 4. However, all other associations between continuous maternal glucose levels and HbA1c and continuous child adiposity outcomes were positive and statistically significant. As described above, linearity of all observed associations was confirmed.
Maternal glucose categories and childhood adiposity
To complement the above analyses, analyses of childhood adiposity across categories of maternal glucose levels during the HAPO OGTT and HbA1c at the time of the OGTT were also conducted. Frequencies of childhood adiposity outcomes, including being overweight/obese and obesity, and per cent fat, waist circumference and sum of skinfolds >85th percentiles, were higher across increasing categories of maternal glucose levels and HbA1c (ESM Fig. 1 and ESM Table 2). Adjusted associations of childhood adiposity outcomes across maternal glucose and HbA1c categories were examined next. Although adjustment for maternal BMI during pregnancy (Model 4) attenuated the associations, ORs for being overweight/obese and obesity, and per cent body fat, waist circumference and sum of skinfolds >85th percentiles, increased across higher categories of maternal glucose levels and HbA1c in all models (ESM Fig. 2 and ESM Tables 3–7). This confirmed higher risk of each outcome with increasing levels of maternal glucose and HbA1c (ESM Fig. 2 and ESM Tables 3–7).
To confirm a gradation of outcome means across maternal glucose categories, means of continuous childhood adiposity measures, including BMI z score, per cent fat, waist circumference and sum of skinfolds, by categories of maternal fasting, 1 h and 2 h glucose and HbA1c during pregnancy were also determined (ESM Fig. 3 and ESM Table 8). In general, means were greater for higher levels of maternal glucose. Adjusted mean differences for each child adiposity outcome across increasing categories of maternal glucose relative to the lowest category of maternal glucose were also determined (ESM Tables 9–12). In Models 1–3, adjusted mean differences for child BMI z score, per cent body fat, waist circumference and sum of skinfolds across categories of maternal fasting, 1 h and 2 h glucose and HbA1c, relative to the lowest category for each measure, confirmed higher means for each continuous child outcome across higher categories of maternal glucose and HbA1c. Adjustment for maternal BMI during pregnancy (Model 4) attenuated the adjusted mean differences of per cent fat, waist circumference and sum of skinfolds but the differences were still generally higher across categories of maternal glucose and HbA1c (ESM Tables 9–12). For child BMI z score, differences were no longer evident across categories of fasting, 1 h and 2 h glucose but were still present for categories of HbA1c.
Analyses to explore sex- and field centre-specific associations
HAPO FUS was not intentionally powered to evaluate differences in associations according to subgroups, so exploratory analyses were performed to evaluate sex- and race/ethnicity-specific associations using field centre as a proxy for race/ethnicity. Statistical interactions between sex and 2 h glucose yielded p<0.05 for per cent fat >85th percentile and per cent fat as a continuous outcome, which reflected stronger associations for these outcomes in boys than in girls (Tables 4, 5). The statistical interaction between fasting glucose and sex was also significant for obesity, reflecting a higher OR estimate for girls than boys (Table 4). All other sex-specific analyses and statistical interaction terms confirmed generally consistent associations for boys and girls. No significant differences in associations across field centres were observed.
Table 4.
Sex-specific fully adjusted associations of continuous measures of maternal glucose and HbA1c during pregnancy with dichotomous adiposity outcomes among children at follow-up
| Predictor | Boys | Girls | Interaction term p valuec | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p valuea | C statistic | OR (95% CI) | p valueb | C statistic | ||
| IOTF overweight/obese | |||||||
| Fasting glucose | 1.05 (0.96, 1.17) | 0.33 | 0.73 | 1.04 (0.93, 1.16) | 0.48 | 0.76 | 0.82 |
| 1 h glucose | 1.10 (1.00, 1.22) | 0.06 | 0.73 | 1.10 (0.99, 1.23) | 0.08 | 0.76 | 0.98 |
| 2 h glucose | 1.14 (1.03, 1.26) | 0.01* | 0.73 | 1.03 (0.93, 1.15) | 0.55 | 0.76 | 0.12 |
| HbA1c | 1.10 (0.99, 1.23) | 0.07 | 0.73 | 1.14 (1.02, 1.27) | 0.03* | 0.76 | 0.74 |
| IOTF obesity | |||||||
| Fasting glucose | 1.07 (0.93, 1.24) | 0.34 | 0.76 | 1.26 (1.08, 1.47) | 0.003** | 0.81 | 0.03* |
| 1 h glucose | 1.14 (0.99, 1.32) | 0.06 | 0.76 | 1.12 (0.96, 1.30) | 0.16 | 0.80 | 0.66 |
| 2 h glucose | 1.31 (1.14, 1.50) | <0.001*** | 0.77 | 1.14 (0.98, 1.32) | 0.10 | 0.80 | 0.08 |
| HbA1c | 1.21 (1.04, 1.42) | 0.02* | 0.77 | 1.18 (0.99, 1.40) | 0.62 | 0.80 | 0.98 |
| BOD % fat >85th percentiled | |||||||
| Fasting glucose | 1.12 (0.99, 1.28) | 0.07 | 0.66 | 1.18 (1.03, 1.34) | 0.01* | 0.72 | 0.36 |
| 1 h glucose | 1.18 (1.05, 1.33) | 0.01* | 0.67 | 1.19 (1.05, 1.36) | 0.01* | 0.72 | 0.77 |
| 2 h glucose | 1.23 (1.09-1.39) | <0.001*** | 0.67 | 1.08 (0.95, 1.22) | 0.24 | 0.72 | 0.04* |
| HbA1c | 1.22 (1.07, 1.39) | 0.003** | 0.67 | 1.03 (0.90, 1.19) | 0.63 | 0.71 | 0.12 |
| Waist circumference >85th percentiled | |||||||
| Fasting glucose | 1.08 (0.95, 1.22) | 0.25 | 0.65 | 1.11 (0.97, 1.27) | 0.13 | 0.73 | 0.39 |
| 1 h glucose | 1.12 (0.99, 1.27) | 0.06 | 0.66 | 1.20 (1.05, 1.36) | 0.01* | 0.74 | 0.79 |
| 2 h glucose | 1.20 (1.06, 1.35) | 0.003** | 0.66 | 1.14 (1.00, 1.29) | 0.04* | 0.73 | 0.29 |
| HbA1c | 1.21 (1.06, 1.37) | 0.005** | 0.66 | 1.08 (0.94, 1.25) | 0.27 | 0.73 | 0.24 |
| Sum of skinfolds >85th percentiled | |||||||
| Fasting glucose | 1.07 (0.95, 1.22) | 0.27 | 0.65 | 1.26 (1.11, 1.43) | <0.001*** | 0.70 | 0.07 |
| 1 h glucose | 1.14 (1.01, 1.29) | 0.03* | 0.65 | 1.26 (1.11, 1.43) | <0.001*** | 0.70 | 0.41 |
| 2 h glucose | 1.19 (1.06, 1.35) | 0.004** | 0.66 | 1.23 (1.09, 1.39) | 0.001** | 0.70 | 0.96 |
| HbA1c | 1.19 (1.04, 1.35) | 0.009** | 0.65 | 1.10 (0.96, 1.27) | 0.17 | 0.69 | 0.63 |
ORs for the dichotomous outcomes are for the continuous predictors higher by one SD estimated in the original HAPO data: fasting glucose 0.4 mmol/l; 1 h glucose 1.7 mmol/l; 2 h glucose 1.3 mmol/l; HbA1c 5.0 mmol/mol (0.45%). Models are adjusted for field centre for being overweight/obesity and IOTF obesity outcomes, child pubertal status (Tanner Stage 1, 2/3, 4/5), maternal variables during pregnancy OGTT (age, height, any family history of diabetes, mean arterial pressure, parity [0, 1+], smoking [yes/no], alcohol [yes/no], gestational age, maternal BMI during pregnancy
p value from regression model for boys only
p<0.05
p<0.01, and
p<0.001
p value from regression model for girls only
p<0.05 and
p<0.01
p value for sex × maternal predictor interaction term from regression model including boys and girls
p<0.05 and
p<0.01
85th percentiles were determined using quantile regression adjusted for age, sex, and field centre
Table 5.
Sex-specific fully adjusted associations of continuous measures of maternal glucose and HbA1c during pregnancy with continuous adiposity outcomes among children at follow-up
| Predictor | Boys | Girls | Interaction term p valuec | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p valuea | Adjusted R2 | β (95% CI) | p valueb | Adjusted R2 | |||
| BMI z score | ||||||||
| Fasting glucose | −0.005 (−0.06, 0.05) | 0.84 | 0.17 | 0.007 (−0.04, 0.06) | 0.77 | 0.31 | 0.92 | |
| 1 h glucose | 0.02 (−0.03 0.07) | 0.46 | 0.17 | 0.01 (−0.03, 0.06) | 0.56 | 0.31 | 0.46 | |
| 2 h glucose | 0.02 (−0.03, 0.07) | 0.45 | 0.17 | 0.02 (−0.03, 0.06) | 0.44 | 0.31 | 0.35 | |
| HbA1c | 0.03 (−0.02, 0.09) | 0.22 | 0.23 | 0.04 (−0.009, 0.09) | 0.10 | 0.37 | 0.61 | |
| BOD POD % fat | ||||||||
| Fasting glucose | 0.37 (−0.10, 0.83) | 0.12 | 0.16 | 0.53 (0.11, 0.94) | 0.01* | 0.23 | 0.51 | |
| 1 h glucose | 0.73 (0.29, 1.17) | 0.001** | 0.16 | 0.49 (0.09, 0.89) | 0.02* | 0.23 | 0.08 | |
| 2 h glucose | 0.69 (0.25, 1.14) | 0.002** | 0.16 | 0.31 (−0.09, 0.70) | 0.13 | 0.23 | 0.005** | |
| HbA1c | 0.33 (−0.15, 0.80) | 0.18 | 0.23 | 0.15 (−0.28, 0.59) | 0.49 | 0.29 | 0.52 | |
| Waist circumference, cm | ||||||||
| Fasting glucose | 0.12 (−0.37, 0.60) | 0.63 | 0.22 | 0.35 (−0.10, 0.81) | 0.13 | 0.35 | 0.85 | |
| 1 h glucose | 0.43 (−0.03 0.89) | 0.07 | 0.23 | 0.44 (0.0002, 0.87) | 0.05 | 0.35 | 0.28 | |
| 2 h glucose | 0.51 (0.04, 0.97) | 0.03* | 0.23 | 0.39 (−0.04, 0.82) | 0.07 | 0.35 | 0.11 | |
| HbA1c | 0.41 (−0.08, 0.90) | 0.10 | 0.30 | 0.37 (−0.10, 0.85) | 0.13 | 0.41 | 0.79 | |
| Sum of skinfolds, mm | ||||||||
| Fasting glucose | 0.60 (−0.39, 1.59) | 0.24 | 0.12 | 1.36 (0.48, 2.24) | 0.002** | 0.18 | 0.53 | |
| 1 h glucose | 0.89 (−0.05, 1.83) | 0.06 | 0.12 | 0.97 (0.13, 1.81) | 0.02* | 0.18 | 0.34 | |
| 2 h glucose | 0.98 (0.03, 1.93) | 0.04* | 0.12 | 0.92 (0.09, 1.76) | 0.03* | 0.18 | 0.14 | |
| HbA1c | 1.09 (0.09, 2.10) | 0.03* | 0.20 | 0.51 (−0.42, 1.43) | 0.28 | 0.25 | 0.42 | |
β estimates represent change in each continuous outcome for the continuous predictors higher by one SD estimated in the original HAPO data: fasting glucose 0.4 mmol/l; 1 h glucose 1.7 mmol/l; 2 h glucose 1.3 mmol/l; HbA1c 5.0 mmol/mol (0.4%). Models are adjusted for field centre, child pubertal status (Tanner stage 1, 2/3, 4/5), maternal variables during pregnancy OGTT (age, height, any family history of diabetes, mean arterial pressure, parity [0, 1+], smoking [yes/no], alcohol [yes/no], gestational age, maternal BMI during pregnancy)
p value from regression model for boys only
p<0.05 and
p<0.01
p value from regression model for girls only
p<0.05 and
p<0.01
p value for sex × maternal predictor interaction term from regression model including boys and girls
p<0.01
Discussion
We recently reported that, while GDM based on IADPSG criteria was not associated with an increase in being overweight/obese, it was associated with a higher risk of obesity and measures of adiposity >85th percentile in children from the HAPO FUS cohort [16]. The present study now shows that the positive association between maternal glucose levels during pregnancy and measures of child adiposity in HAPO FUS children (aged 10–14 years) is present across the spectrum of maternal glucose levels during pregnancy. This was demonstrated in a number of ways. First, except for the association of being overweight/obese and waist circumference >85th percentile with fasting glucose, the risk for each of the child adiposity outcomes was significantly higher for each one SD difference in maternal fasting, 1 h or 2 h glucose levels as well as HbA1c. Second, other than the absence of an association between maternal glucose and child BMI z score, the continuous childhood adiposity outcomes were in general significantly higher for each one SD difference in continuous maternal glucose and HbA1c predictors, even after adjustment for maternal BMI during pregnancy. In addition, the linearity of the observed associations was confirmed. Finally, complementary studies examining the prevalence of child adiposity outcomes (being overweight/obese, obesity and sum of skinfolds, waist circumference and per cent body fat >85th percentiles) and means of continuous measures of child adiposity (BMI z score, per cent fat, sum of skinfolds and waist circumference) across increasing categories of maternal glucose generally confirmed the relationships described above. Thus, the present study demonstrates that the link between maternal glucose levels and child adiposity outcomes extends across the spectrum of maternal glucose levels, including glucose levels below the diagnostic threshold for GDM defined by IADPSG.
The original HAPO Study demonstrated continuous and graded associations between maternal glucose levels during pregnancy and newborn adiposity outcomes [4]. Recent smaller studies of children ages 5–10 years reported positive associations between maternal glucose levels during pregnancy and measures of adiposity [26, 27]. The HAPO FUS now demonstrates the novel finding that the continuous and graded association between maternal glucose levels and offspring adiposity is also observed in a multi-ethnic cohort during adolescence. Whether these relationships will continue into adulthood is yet to be determined; ongoing surveillance of this well-phenotyped at-risk population is required. However, previous studies have demonstrated associations of childhood obesity with higher risk for obesity as well as other cardiometabolic diseases as an adult [28-33], suggesting that children with greater childhood adiposity are at risk for poor metabolic outcomes as adults.
Previous studies that examined the association of maternal glucose levels during pregnancy with adiposity in childhood typically compared offspring of mothers without vs with GDM [9, 10, 34-37]. In many cases, the associations were confounded by treatment for GDM. In contrast, HAPO mothers were not treated and remained blinded to their glucose levels during the pregnancy. Women with glucose levels during the HAPO pregnancy OGTT that exceeded levels for unblinding were treated according to local practice and were excluded from HAPO Study analyses. Thus, the present study was not confounded by treatment. Moreover, the relationships found in the present study, between glucose levels less than those diagnostic of GDM and childhood adiposity outcomes, demonstrated that the relationships between maternal glucose and measures of child adiposity were largely continuous without evidence for a threshold effect. These findings may have implications for target glucose levels in women with GDM, including in clinical trials designed to examine the effect of GDM treatment on childhood adiposity outcomes, and may also inform approaches to the monitoring of women with other independent risk factors for higher childhood adiposity (e.g. obesity).
In previous studies, associations between maternal glucose levels during pregnancy, typically in GDM, and measures of child adiposity were attenuated after adjustment for maternal BMI prior to or during pregnancy [9, 10, 35, 36]. In the present study, the associations of maternal glucose levels with risk for the five different child adiposity outcomes were also attenuated by adjustment for maternal BMI during pregnancy but remained significant, with the exception of the associations between maternal fasting glucose and risk for being overweight/obese and waist circumference >85th percentile. These data are consistent with an independent association of maternal glucose and measures of child adiposity. Whether this relationship is due solely to maternal glucose or to other metabolites related to maternal glucose has not been determined. While transplacental transfer of glucose undoubtedly contributes to fetal growth, it has been suggested that lipids, including triacylglycerol and non-esterified fatty acids, are important contributors to excess fetal growth and fat accretion [38]. Indeed, in previous metabolomics studies performed in the HAPO cohort, we demonstrated associations of maternal 1 h glucose levels with maternal serum levels of triacylglycerol, non-esterified fatty acids, β-hydroxybutyrate and several amino acids [39]. This is consistent with the hypothesis proposed by Freinkel and colleagues that the association of GDM with newborn and childhood outcomes is mediated through the transplacental transfer of mixed nutrients [40].
Differences in adiposity in girls vs boys have been previously reported. At birth, per cent body fat is higher in girls than in boys [41], and sex-specific associations between maternal glucose levels and childhood adiposity have been described. In the present study, associations of maternal blood glucose levels with measures of child adiposity were generally consistent for boys and girls, although stronger associations of maternal 2 h glucose with per cent fat was observed in boys and the OR estimate for obesity with higher levels of maternal fasting glucose was higher in girls. Other studies have also reported inconsistent associations. In one, GDM was associated with higher fat mass in boys but not girls at age ~8 years, while lesser degrees of maternal hyperglycaemia were associated with higher fat mass in girls only [42]. In an Indian cohort, at age ~9.5 years, higher adiposity was observed in girl but not boy offspring of mothers with GDM [43]. Similarly, among HAPO participants from Hong Kong, maternal glucose levels during the HAPO OGTT were associated with being overweight/obese and adiposity in girls but not boys [26]. Thus, consistent sex-specific effects of GDM and maternal blood glucose levels on measures of child adiposity have not been observed.
This study has several strengths. First, HAPO was a blinded observational study in which pregnant women and their clinical caregivers were not made aware of their glucose status. Thus, treatment is not a factor in this study of childhood outcomes. Second, the large number and racial/ethnic diversity of participants included in the HAPO FUS make the findings broadly applicable. Third, in addition to determining pubertal status of the children, this prospective study also used standardised procedures to characterise childhood adiposity via multiple methods, with generally consistent results across different measures. Finally, the HAPO FUS addressed limitations of previous studies that examined the association of GDM with childhood adiposity, including lack of complete data on glucose values in pregnancy, maternal BMI and child measures of age-adjusted BMI [6-13].
There are some limitations. The proportion of participants who met IADPSG GDM criteria and participated in the HAPO FUS (weighted estimate 14.9%) was lower than the frequency in all eligible participants (16.2%). In addition, we used maternal BMI at the pregnancy OGTT and not pre-gestational BMI, since objectively measured pre-pregnancy weight was not available in HAPO. The HAPO FUS also did not have detailed paternal data or data related to postnatal lifestyle variables which may affect childhood adiposity. By design, participants with fasting and/or 2 h OGTT glucose values during pregnancy that were above predefined thresholds were unblinded during the HAPO Study and were excluded from the HAPO FUS. This subgroup (1.8% of the HAPO cohort) is likely to have included children at highest risk of being overweight/obese and therefore the reported associations may be underestimates. Finally, the number of mother–child pairs recruited was 69.1% of the target, although the maternal characteristics during pregnancy for children who did and did not participate were similar. In summary, the HAPO FUS provides new evidence for a continuous relationship between maternal glucose levels during pregnancy and childhood adiposity outcomes that is independent of maternal BMI. Since obese children are at high risk of obesity and related metabolic disorders as adults [28, 29], this has important public health implications and may have implications for treatment targets in mothers with pre-existing or gestational diabetes.
Supplementary Material
Acknowledgements
The HAPO FUS investigators are grateful to all mothers and children who participated in the HAPO and HAPO Follow-up studies. Study data were collected and managed using REDCap electronic data capture tools hosted at Northwestern University Feinberg School of Medicine (FSM). REDCap is supported at FSM by the Northwestern University Clinical and Translational Science (NUCATS) Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
The HAPO Follow-up Study is funded by grant 1U01DK094830 from the National Institute of Diabetes and Digestive and Kidney Diseases and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR001422. BL (NIH) was a participating member of the study Steering Committee and the Writing Group for this manuscript because of the cooperative funding agreement. She was involved in the design of the study but not the conduct of the study; she was not involved in the collection, management and analysis of the data and she was involved in the preparation, review and approval of the manuscript and the decision to submit the manuscript for publication.
Abbreviations
- GDM
Gestational diabetes mellitus
- HAPO
Hyperglycemia and Adverse Pregnancy Outcome Study
- HAPO FUS
HAPO Follow-up Study
- IADPSG
International Association of Diabetes in Pregnancy Study Groups
- IOTF
International Obesity Task Force
Appendix
HAPO Follow-up Study Cooperative Research Group, field centre principal investigators and study staff
Bangkok C. Deerochanawong, T. Tanaphonpoonsuk (Rajavithi Hospital), S. Binratkaew U. Chotigeat, W. Manyam, (Queen Sirikit National Institute of Child Health)
Barbados M. Forde, A. Greenidge, K. Neblett, P. M. Lashley, D. Walcott (Queen Elizabeth Hospital/School of Clinical Medicine and Research, University of the West Indies, Barbados)
Belfast K Corry, L. Francis, J. Irwin, A. Langan, D. R. McCance, M. Mousavi, (Belfast Health and Social Care Trust), I. S. Young (Queen’s University Belfast)
Bellflower J. Gutierrez, J. Jimenez, J. M. Lawrence, D. A. Sacks, H. S. Takhar, E. Tanton (Kaiser Permanente Southern California)
Chicago W. J. Brickman, J. Howard, J. L. Josefson, L. Miller, R. Steuer (Ann and Robert H. Lurie Children’s Hospital/Northwestern University Feinberg School of Medicine)
Cleveland J. Bjaloncik, P. M. Catalano, A. Davis, K. Koontz, L. Presley, S. Smith, A. Tyhulski (MetroHealth Medical Center/Case Western Reserve University)
Hong Kong A. Li, R. C. Ma, R. Ozaki, W. H. Tam, M. Wong, C. Yuen (The Chinese University of Hong Kong/Prince of Wales Hospital)
Manchester P. E. Clayton, A. Khan, A. Vyas (Royal Manchester Children’s Hospital, Manchester University Hospitals NHS Foundation Trust, Manchester Academic Healthy Sciences Centre/School of Medical Sciences, Faculty of Biology, Medicine & Health, University of Manchester), M. Maresh (St Mary’s Hospital, Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre)
Petah-Tiqva H. Benzaquen, N. Glickman, A. Hamou, O. Hermon, O. Horesh, Y. Keren, S. Shalitin (Schneider Children’s Medical Center of Israel), Y. Lebenthal (Jesse Z and Sara Lea Shafer Institute for Endocrinology and Diabetes, National Center for Childhood Diabetes, Schneider Children’s Medical Center of Israel, Sackler Faculty of Medicine, Tel Aviv University)
Toronto K. Cordeiro, J. Hamilton, H.Y. Nguyen, S. Steele (The Hospital for Sick Children, University of Toronto)
Coordinating Centres F. Chen, A. R. Dyer, W. Huang, A. Kuang, M. Jimenez, L. P. Lowe, W.L. Lowe, Jr, B. E. Metzger, M. Nodzenski, A. Reisetter, D. Scholtens, P. Yim (Northwestern University Feinberg School of Medicine)
Consultants D. Dunger, A. Thomas
NIDDK M. Horlick, B. Linder, A. Unalp-Arida
NICHD G. Grave
Footnotes
Data availability
Data will be made available by the authors upon request.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
References
- [1].Metzger BE (2007) Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol 50: 972–979 [DOI] [PubMed] [Google Scholar]
- [2].Pettitt DJ, Baird HR, Aleck KA, Bennett PH, Knowler WC (1983) Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N Engl J Med 308: 242–245 [DOI] [PubMed] [Google Scholar]
- [3].Silverman BL, Rizzo TA, Cho NH, Metzger BE (1998) Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 21 Suppl 2: B142–149 [PubMed] [Google Scholar]
- [4].Metzger BE, Lowe LP, Dyer AR, et al. (2008) Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358: 1991–2002 [DOI] [PubMed] [Google Scholar]
- [5].(2010) Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG 117: 575–584 [DOI] [PubMed] [Google Scholar]
- [6].Tam WH, Ma RC, Yang X, et al. (2008) Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero. Pediatrics 122: 1229–1234 [DOI] [PubMed] [Google Scholar]
- [7].Catalano PM, Farrell K, Thomas A, et al. (2009) Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 90: 1303–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pirkola J, Pouta A, Bloigu A, et al. (2010) Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care 33: 1115–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Philipps LH, Santhakumaran S, Gale C, et al. (2011) The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia 54: 1957–1966 [DOI] [PubMed] [Google Scholar]
- [10].Kim SY, England JL, Sharma JA, Njoroge T (2011) Gestational diabetes mellitus and risk of childhood overweight and obesity in offspring: a systematic review. Exp Diabetes Res 2011: 541308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim SY, Sharma AJ, Callaghan WM (2012) Gestational diabetes and childhood obesity: what is the link? Curr Opin Obstet Gynecol 24: 376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Donovan LE, Cundy T (2015) Does exposure to hyperglycaemia in utero increase the risk of obesity and diabetes in the offspring? A critical reappraisal. Diabet Med 32: 295–304 [DOI] [PubMed] [Google Scholar]
- [13].Grunnet LG, Hansen S, Hjort L, et al. (2017) Adiposity, Dysmetabolic Traits, and Earlier Onset of Female Puberty in Adolescent Offspring of Women With Gestational Diabetes Mellitus: A Clinical Study Within the Danish National Birth Cohort. Diabetes Care 40: 1746–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].(2010) International association of diabetes and pregnancy groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].(2014) Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract 103: 341–363 [DOI] [PubMed] [Google Scholar]
- [16].Lowe WL Jr., Scholtens DM, Lowe LP, et al. (2018) Association of Gestational Diabetes With Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 320: 1005–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].(2002) The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int J Gynaecol Obstet 78: 69–77 [DOI] [PubMed] [Google Scholar]
- [18].Nesbitt GS, Smye M, Sheridan B, Lappin TR, Trimble ER (2006) Integration of local and central laboratory functions in a worldwide multicentre study: Experience from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Clin Trials 3: 397–407 [DOI] [PubMed] [Google Scholar]
- [19].Cole TJ, Lobstein T (2012) Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatric obesity 7: 284–294 [DOI] [PubMed] [Google Scholar]
- [20].White IR, Daniel R, Royston P (2010) Avoiding bias due to perfect prediction in multiple imputation of incomplete categorical variables. Comput Stat Data Anal 54: 2267–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rubin DB (1987) Multiple imputation for nonresponse in surveys. Wiley, New York [Google Scholar]
- [22].Van Buuren S, Groothuis-Oudshoorn K (2011) Multivariate imputation by chained equations in R. Journal of Statistical Software 45: 1–67 [Google Scholar]
- [23].Hosmer DW, Lemeshow S (2013) Applied Logistic Regression. Wiley, New York [Google Scholar]
- [24].Harrell FE Jr. (2018) rms: Regression Modeling Strategies. R package version 51-2 (accessed June 2018) [Google Scholar]
- [25].Team RC (2016) A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- [26].Tam WH, Ma RCW, Ozaki R, et al. (2017) In Utero Exposure to Maternal Hyperglycemia Increases Childhood Cardiometabolic Risk in Offspring. Diabetes Care 40: 679–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Landon MB, Mele L, Varner MW, et al. (2018) The relationship of maternal glycemia to childhood obesity and metabolic dysfunction(double dagger). J Matern Fetal Neonatal Med: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Simmonds M, Llewellyn A, Owen CG, Woolacott N (2016) Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev 17: 95–107 [DOI] [PubMed] [Google Scholar]
- [29].Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH (1997) Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 337: 869–873 [DOI] [PubMed] [Google Scholar]
- [30].Kelsey MM, Zaepfel A, Bjornstad P, Nadeau KJ (2014) Age-related consequences of childhood obesity. Gerontology 60: 222–228 [DOI] [PubMed] [Google Scholar]
- [31].Liang Y, Hou D, Zhao X, et al. (2015) Childhood obesity affects adult metabolic syndrome and diabetes. Endocrine 50: 87–92 [DOI] [PubMed] [Google Scholar]
- [32].Maffeis C, Tato L (2001) Long-term effects of childhood obesity on morbidity and mortality. Horm Res 55 Suppl 1: 42–45 [DOI] [PubMed] [Google Scholar]
- [33].Reilly JJ, Kelly J (2011) Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond) 35: 891–898 [DOI] [PubMed] [Google Scholar]
- [34].Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ (2007) Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care 30: 2287–2292 [DOI] [PubMed] [Google Scholar]
- [35].Kubo A, Ferrara A, Windham GC, et al. (2014) Maternal hyperglycemia during pregnancy predicts adiposity of the offspring. Diabetes Care 37: 2996–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhao P, Liu E, Qiao Y, et al. (2016) Maternal gestational diabetes and childhood obesity at age 9–11: results of a multinational study. Diabetologia 59: 2339–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Patel S, Fraser A, Davey Smith G, et al. (2012) Associations of gestational diabetes, existing diabetes, and glycosuria with offspring obesity and cardiometabolic outcomes. Diabetes Care 35: 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Barbour LA, Hernandez TL (2018) Maternal Non-glycemic Contributors to Fetal Growth in Obesity and Gestational Diabetes: Spotlight on Lipids. Curr Diab Rep 18: 37. [DOI] [PubMed] [Google Scholar]
- [39].Jacob S, Nodzenski M, Reisetter AC, et al. (2017) Targeted Metabolomics Demonstrates Distinct and Overlapping Maternal Metabolites Associated With BMI, Glucose, and Insulin Sensitivity During Pregnancy Across Four Ancestry Groups. Diabetes Care 40: 911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Freinkel N (1980) Banting Lecture 1980. Of pregnancy and progeny. Diabetes 29: 1023–1035 [DOI] [PubMed] [Google Scholar]
- [41].Hawkes CP, Hourihane JO, Kenny LC, Irvine AD, Kiely M, Murray DM (2011) Gender- and gestational age-specific body fat percentage at birth. Pediatrics 128: e645–651 [DOI] [PubMed] [Google Scholar]
- [42].Regnault N, Gillman MW, Rifas-Shiman SL, Eggleston E, Oken E (2013) Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care 36: 3045–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Krishnaveni GV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH (2010) Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care 33: 402–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.