Abstract
Objective
The association of primary aldosteronism (PA) with thyroid disease has already been suggested. The aim of this study was to examine the presence of PA in patients with papillary thyroid carcinoma (PC) and to characterize such PC patients with PA.
Methods
We examined the presence of PA in 81 consecutive patients with PC, whose random sitting blood pressure (BP) was ≥140/90 mmHg in the office (n= 68), who had an incidental adrenal tumor or adrenal enlargement (n=9), or who showed hypokalemia (n=4). Thirty-one of these 81 patients had been treated with anti-hypertensive drugs. The plasma aldosterone concentration (PAC) and plasma renin activity (PRA) were first measured before operation in 16 patients and after operation in 65 patients. PA was diagnosed according to the guidelines of the Japan Endocrine Society.
Results
Forty patients with PC with a random PAC/PRA ratio of over 200 were subjected to a further study (12 of these patients had been treated with anti-hypertensive drugs). Ultimately, 15 patients with PC were diagnosed with PA. Adrenal venous sampling was done in 9 out of 15 patients with PC associated with PA. No patients were diagnosed as having unilateral lesions. Among the 15 patients, white-coat hypertension was observed in 5 patients, and normotension was observed in 1 patient.
Conclusion
These findings suggest that the prevalence of PA may be high among patients with PC. An active examination is needed to detect PA, as its signs and symptoms may be mild in patients with PC associated with hypertension.
Keywords: primary aldosteronism, papillary thyroid carcinoma, white-coat hypertension
Introduction
Primary aldosteronism (PA) is the most common form of endocrine hypertension (1,2). Two major subtypes of PA are aldosterone-producing adenoma (APA) and idiopathic hyperaldosteronism (IHA) (2,3). The prevalence of PA is reportedly over 5%, and may be over 10% in patients with hypertension (4).
The prevalence of ultrasonographic thyroid abnormalities is reported to be high in patients with PA (5,6). However, few cases of papillary thyroid carcinoma (PC) (5,6) or thyroid carcinoma (7) have been found in patients with PA. Furthermore, to our knowledge, the prevalence of PA in patients with PC is unclear.
We recently reported the presence of PA in nine patients with various thyroid diseases (four patients with PC, four patients with chronic thyroiditis and/or multinodular goiter, and one patient with Graves' disease) (8). The findings suggested a relationship between PC and PA.
We therefore examined the presence of PA in 81 consecutive patients with PC and characterized the patients with PC associated with PA. Four previously reported patients with PC associated with PA were also included in the present study [Patients No 4, 7, 9, and 14 in the Tables (8)]. Of note, Patient No. 7 had normotensive PA (9,10).
Materials and Methods
The plasma aldosterone concentration (PAC; pg/mL) and plasma renin activity (PRA; ng/mL/h) were measured by a radioimmunoassay using commercially available kits (SRL). The plasma cortisol concentration (μg/dL) was also measured by an enzyme-linked immunosorbent assay (ELISA) using an available kit (SRL).
Eighty-one consecutive patients with PC whose random PAC and PRA were first measured between 2013 January and August 2016 were included in the study. The diagnosis of PC was made pathologically. Of the 81 patients, 12 were men (14.8%), and the mean age was 58.1 years (range 35-79 years). Regarding the inclusion criteria, Group I had a sitting random BP ≥140/90 in the office (n=68), and 24 out of 68 patients had been treated with anti-hypertensive drugs. Group II had an incidental adrenal tumor or adrenal enlargement (n=9); 7 out of 9 adrenal lesions were detected by chest computed tomography (CT) to determine whether or not the patient had lung metastasis, and the other 2 were detected by abdominal CT conducted to assess chronic liver damage. Seven out of nine patients had been treated with anti-hypertensive drugs, and the BP of eight patients (minus one patient treated with anti-hypertensive drugs) was normal in the office. Group III had hypokalemia (3.3-3.5 mEq/L; hypokalemia <3.6 mEq/L) (n=4), and the BP of 4 patients was normal in the office without anti-hypertensive drugs.
The treatments of PC were as follows: lobectomy with neck lymph-node dissection in 19 patients and total thyroidectomy with neck lymph-node dissection in 58 patients (in 5 out of 58 patients, lobectomy followed by remaining thyroidectomy was performed because of lymph-node metastasis). In four patients, total thyroidectomy was performed without neck lymph-node dissection because the presence of PC had not been identified before the operation (three patients with multinodular goiter and one patient with Marine-Lenhart syndrome). Thyroid ablation with I-131 was done in 27 patients with PC after total thyroidectomy. The mean duration between the day when PAC and PRA were first measured and thyroidectomy was done are 2,044 days (range: -237 days to 7,570 days) (before the operation in 16 patients and after the operation in 65 patients).
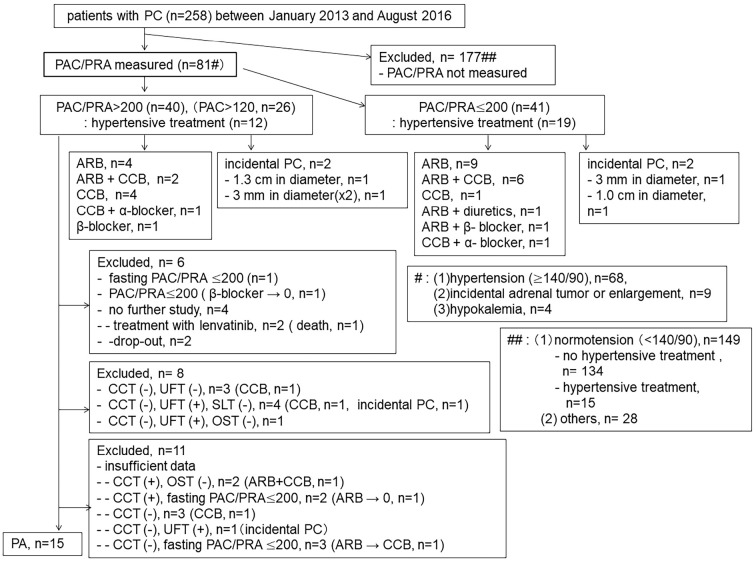
In the present study, we excluded 177 patients with PC (27 men) in whom the PAC and PRA values had not been measured during the investigation period (Figure). One-hundred and forty-nine of 177 patients with PC showed a normal BP in the office with (n=15) or without (n=134) anti-hypertensive drugs. In addition, 28 patients with PC treated with anti-hypertensive drugs were also excluded from the study because their medical records were insufficient for the analysis (e.g., BP was not recorded during the investigation period), because their random sitting BP was ≥140/90 mmHg in the office but their PAC and PRA were not measured, because of a poor general condition, including an advanced age, the need for hemodialysis, or because the patient had a history of cerebral bleeding.
Figure.
Flow chart of the study. PC: papillary thyroid carcinoma, PAC: plasma aldosterone concentration, PRA: plasma renin activity, ARB: angiotensin II receptor blocker, CCB: calcium channel blocker, CCT: captopril-challenge test, UFT: upright furosemide-loading test, SLT: saline-loading test, OST: oral salt-loading test, (+): positive result, (-): negative result
Written informed consent was not obtained for the study from the patients because we retrospectively reviewed the patients and the data were anonymous in nature. This study was approved by the institutional review board of our hospital (I7121302).
The diagnosis of PA (according to Japan Endocrine Society guidelines) (11)
1) Screening for PA
Patients with a random sitting PAC (pg/mL)/PRA(ng/mL/h) ratio exceeding 200 were subjected to further study, regardless of the treatment with anti-hypertensive drugs.
2) The definitive diagnosis of PA (Table 1)
Table 1.
PA Confirmatory Test.
| Test | Procedure | Criteria for positve results |
|---|---|---|
| Captopril-challenge test | oral administraion of captopril (50 mg) | PAC/PRA (90 min)>200 |
| Upright furosemide-loading test | intravenous injection of furosemide (40 mg) followed by upright posture (2 h) | PRA (2 h)<2.0 ng/mL/h |
| Saline-loading test | intravenous drip infusion of saline (2 L) over 4 h | PAC (4 h)>60 pg/mL |
| Oral salt-loading test | 24-h urine collection | urinary aldosterone>8 μg/day (urinary Na>170 mEq/day) |
PA: primary aldosteronism, PAC: plasma aldosterone concentration (pg/mL), PRA: plasma renin activity (ng/mL/h)
PA was diagnosed when at least two of the following three tests were positive: 1) captopril-challenge test, 2) upright furosemide-loading test, and 3) saline-loading test (or oral salt-loading test). Angiotensin II receptor blockers (ARBs) were changed to calcium channel blockers (CCBs) (n=1) or stopped (n=4) in 5 out of 6 patients before the PA confirmatory tests in Figure and Tables 2 and 3, as indicated by the guidelines (11).
Table 2.
Clinical Characteristics of 15 Patients with PC with PA.
| Patient No. | Gender | Age #(years) | Age at thyroidectomy (years) | Random sitting BP (mmHg) | Random sitting PAC/PRA | K## (mEq/L) |
|---|---|---|---|---|---|---|
| 1 | F | 41 | 42□ | 154/80 | 136/0.5 | 3.7-4.5 |
| 2a | F | 42 | 35○ | 145/95 | 66.2/0.2(ARB) | 3.3-4.0 |
| 3 | F | 47 | 43○ | 146/80 | 181/0.3(ARB+CCB) | 3.7-4.0 |
| 4 | M | 49 | 48○A | 156/100 | 98.7/0.3 | 4.0-4.5 |
| 5 | F | 51 | 47○A | 156/104 | 237/0.5 | 3.5-4.2 |
| 6 | F | 53 | 37□ | 160/90 | 138/≤0.1 | 3.8-5.1 |
| 7 | F | 53 | 46○ | 121/68 | 202/0.3 | 3.5-4.3 |
| 8 | F | 54 | 54○A | 160/86 | 273/1.2(CCB+α-blocker) | 3.9-5.1 |
| 9 | F | 55 | 48□ | 152/80 | 127/0.6(ARB) | 3.8-4.4 |
| 10 | F | 55 | 51○A | 159/100 | 148/≤0.1 | 3.5-4.3 |
| 11 | F | 56 | 51○ | 140/90 | 121/0.3 | 3.5-4.0 |
| 12 | F | 57 | 51□ | 134/90 | 262/0.2 | 3.7-4.8 |
| 13b | F | 60 | 53○ | 155/95 | 177/0.7 | 3.8-5.1 |
| 14 | F | 63 | 48○ | 154/84 | 74.3/0.2 | 3.7-4.2 |
| 15 | F | 75 | 65□ | 123/72 | 236/0.2(CCB) | 3.9-4.2 |
PC: papillary thyroid carcinoma, PA: primary aldosteronism, PAC: plasma aldosterone concentration (pg/mL), PRA: plasma renin activity (ng/mL/h), ARB: angiotensin II receptor blocker, CCB: calcium channel blocker
a: CKD (chronic kidney disease), eGFR (estimated glomerular filtration rate) 71.8 mL/min/1.73m2
b: CKD, eGFR 73.4 mL/min/1.73m2
#: when PAC/PRA were first measured.
##: during the follow-up period in our hospital
□: lobectomy
○: total thyroidectomy
A: I-131 ablation
Table 3.
Rusults of PA Confirmatory Test.
| Patient No. | Captopril-challenge test | Upright furosemide- loading test | Saline-loading test | Oral salt-loading test | |||
|---|---|---|---|---|---|---|---|
| before | 90 min | before | 2 h | before | 4 h | urinary aldosterone (μg/day)/ urinary Na (mEq/day) |
|
| PAC/PRA | PAC/PRA | PAC/PRA | |||||
| 1 | 78.8/0.3 | 81.1/0.3 | 79.1/0.3 | 308/1.3(1 h)# | |||
| 2 | 96.1/0.2 | 67.5/0.2 | 121/0.2 | 388/0.5 | |||
| 3a | 149/≤0.1 | 114/0/2 | 182/≤0.1 | 276/0.2 | |||
| 4 | 80.5/0.2 | 65.5/0.4 | 76.9/0.2 | 154/0.4 | 102/0.2 | 60.2/0.2 | |
| 5 | 206/0.5 | 108/0.6 | 176/0.4 | 586/0.8 | 252/0.4 | 104/0.3 | |
| 6 | 88.9/0.2 | 80.9/0.2 | 101/0.2 | 364/0.4 | |||
| 7 | 123/0.2 | 84.8/0.2 | 126/0.3 | 636/2.3 | 142/≤0.1 | 104/≤0.1 | |
| 8b | 78.7/≤0.1 | 69.3/≤0.1 | 105/0.4 | 392/1.5 | |||
| 9 | 80.8/≤0.1 | 70.9/0.2 | 81.4/0.2 | 334/1.0 | |||
| 10 | 84.3/0.2 | 63.7/0.2 | 109/0.2 | 357/0.4 | |||
| 11 | 59.3/≤0.1 | 50.2/0.2 | 87.7/0.2 | 215/0.9 | |||
| 12 | 107/0.2 | 91.5/0.2 | 96.7/≤0.1 | 216/0.3 | |||
| 13 | 105/0.4 | 67.0/0.4 | 134/0.5 | 457/1.5 | 14/179 | ||
| 14 | 77.6/≤0.1 | 67.4/≤0.1 | 63.4≤0.1 | 143/0.2 | |||
| 15a | 208/≤0.1 | 257/≤0.1 | 22/247 | ||||
PA: primary aldosteronism, PAC: plasma aldosterone concentration (pg/mL), PRA: plasma renin activity (ng/mL/h)
#: The test was discontinued 1h later because she could not keep standing.
a : CCB (calcium channel blocker), b : CCB+α-blocker
3) The subtype diagnosis of PA [adrenal venous sampling (AVS)] (11)
AVS was performed in patients who wished to undergo surgical treatment when the site of aldosterone hypersecretion was unilateral. Blood was sampled after stimulation with bolus adrenocorticotropic hormone (ACTH) (0.25 mg CortrosinⓇ ). A unilateral lesion was considered to be present when 1) the adrenal vein aldosterone concentration (A) was ≥14,000 pg/mL on 1 side; 2) the lateralized ratio (LR)=[adrenal vein (A)/cortisol (C) ratio on the high-value side]/(adrenal vein A/C ratio on the low-value side) was ≥2.6; and 3) the contralateral ratio (CR)=(adrenal vein A/C ratio on the low-value side)/[A/C ratio in the inferior vena cava (IVC)] was <1.0.
Hypertension
BP was divided into 3 categories based on the JSH 2014 (1): 1) normotension (home BP <135/85 mmHg and office BP <140/90 mmHg), 2) white-coat hypertension (home BP <135/85 mmHg and office BP ≥140/90 mmHg), or 3) (sustained) hypertension (home BP ≥135/85 mmHg and office BP ≥140/90 mmHg). In the present study, the patients who had been treated with anti-hypertensive drugs were categorized as having hypertension. A diagnosis of white-coat hypertension was made after the BP was measured at home for at least two weeks.
Results
In screening, the random PAC/PRA was over 200 in 40 of 81 patients. Twelve of these 40 patients had been treated with anti-hypertensive drugs, as shown in Figure. The PAC was over 120 pg/mL in 25 of the 40 patients. These 40 patients were further investigated. We excluded 25 patients for various reasons (shown in Figure). Ultimately, 15 patients with PC were diagnosed as having PA (Tables 2 and 3). Thirteen and 2 patients (Patient No. 7 and 15) with PA were included in groups I and II, respectively, and no patients were included in group III. In addition, PA was not detected in four patients with incidental PC. AVS was performed in 9 of the 15 patients, as shown in Table 4. No patients were diagnosed with unilateral lesions.
Table 4.
Type of Hypertension and Results of AVS.
| Patient No. | Presence of adrenal tumor | Type of hypertension | ACTH-AVS (PAC, pg/mL/cortisol, μg/dL) | LR | CR | Type | ||
|---|---|---|---|---|---|---|---|---|
| right adrenal vein | left adrenal vein | inferior vena cava | ||||||
| 1 | no | WCH | 15,000/653 | 10,300/643 | 250/31.1 | 1.43 | 1.99 | IHA |
| 2 | no | sustained | ||||||
| 3 | not denied | sustained | ||||||
| 4 | no | sustained | 18,500/2,100 | 11,400/1,680 | 110/19.9 | 1.3 | 1.23 | IHA |
| 5 | no | sustained | 45,300/1,070 | 43,000/804 | 445/28.2 | 1.26 | 2.68 | IHA |
| 6 | no | sustained | ||||||
| 7 | yes | NT | 17,300/1,520 | 30,900/1,100 | 716/30.7 | 2.47 | 0.49 | IHA |
| 8 | no | sustained | ||||||
| 9 | no | sustained | 21,900/1,410 | 13,300/1,000 | 146/24.0 | 1.17 | 2.19 | IHA |
| 10 | no | WCH | 13,300/538 | 9,200/583# | 259/28.4 | 1.57 | 1.73 | IHA |
| 11 | no | WCH | 17,700/814 | 9,500/497 | 189/25.0 | 1.14 | 2.53 | IHA |
| 12 | yes | WCH | 14,000/1,420 | 10,800/520 | 141/22.9 | 2.11 | 1.6 | IHA |
| 13 | no | WCH | ||||||
| 14 | no | sustained | 9,250/992 | 10,400/899## | 118/21.6 | 1.24 | 1.71 | IHA |
| 15 | yes | sustained | ||||||
AVS: adrenal venous sampling, PAC: plasma aldosterone concentration, LR: lateralized ratio, CR: contralateral ratio, WCH: white-coat hypertension, NT: normotension, IHA: idiopathic hyperaldosteronism
# 20,400/651 (superior tributary)
## 18,100/1,020 (lateral tributary)
Clinical characteristics of patients with PC with PA (n=15)
Continuous hypokalemia (K <3.6 mEq/L) was not observed in any patients, as shown in Table 2. The mean duration between the day when PAC and PRA were first measured and thyroidectomy was 2,266 days in patients with PC associated with PA (range: -237 days to 6,177 days). White-coat hypertension was observed in five patients, normotension in one patient, and hypertension in the remaining nine patients. The nine patients subjected to AVS all had IHA. Treatment with eplerenone was started in all patients.
In the present study, 2 of 15 patients with PA had been treated with anti-hypertensive drugs before thyroidectomy. The other 13 patients did not receive anti-hypertensive drugs before thyroidectomy. The office BP had been ≥140/90 mmHg on at least 1 occasion in six of the 13 patients before thyroidectomy. Treatment with anti-hypertensive drug was started later in one of the six patients. The BP was only measured one or two times before thyroidectomy in the remaining seven patients because it was normal at the first visit. Two of the seven patients were later treated with anti-hypertensive drugs.
Discussion
The present findings indicated that patients with PA associated with PC have the following characteristics: [1] white-coat hypertension (observed in 33% of the patients); [2] a not-very-high PAC (<200; noted in 3 out of 15 patients in random sampling and detected in many patients 30 minutes. after resting in the supine position in the morning while fasting), although the Japan Endocrine Society has recently recommended that random PAC >120 pg/mL be added to the screening criteria (12), as already indicated by The Japanese Society of Hypertension (1); and [3] no APA (not found in any patients studied). The PAC/PRA was first measured in many patients with PC several years after thyroidectomy, as the strong association of PC and PA had not been previously suggested and BP was not constantly high in many patients. We now actively measure the PAC/PRA in patients with various thyroid diseases, including PC, whose random BP is ≥140/90 mmHg when measured during a visit to our clinic.
Whether or not eplerenone should be given to patients whose BP is normal or who have white-coat hypertension is unclear at present. However, we administered eplerenone to these patients because high aldosterone levels are reported to damage DNA via oxidative stress (13,14) and to induce the development and growth of renal cell carcinoma (7,15). Furthermore, eplerenone was reported to inhibit hepatocellular carcinoma (HCC) growth and angiogenesis via the suppression of vascular endothelial growth factor (VEGF) in mice (16). In addition, PAC in PA patients with malignancy is reportedly higher than in patients without malignancy (7). It is also suggested that high aldosterone levels influence the thyroid immune system (17) and thyroid function (17,18). If the course of PC is indeed affected by high aldosterone levels, the administration of eplerenone may help prevent the recurrence of PC. Greenman et al. (19) reported the expression of aldosterone synthase in a papillary thyroid carcinoma specimen. Further studies are needed to clarify the effect of the blockade of aldosterone action on the PC course.
We selected patients with PC based on the following: random sitting blood pressure of ≥140/90 mmHg with or without anti-hypertensive drugs, incidental adrenal tumor or adrenal enlargement, or hypokalemia. We then focused on those patients whose PAC/PRA was >200. Ultimately, 15 patients with PC were considered to have PA. Tanabe et al. reported that the PAC and PAC/PRA values were sometimes normal in patients with PA (20). However, we did not measure the PAC/PRA multiple times in the same patient. Furthermore, patients with a normal pressure with or without anti-hypertensive drugs were not included in the study despite the presence of PA having been reported in some pre-hypertensive subjects (BP, 120-139/80-89 mmHg) (21) and in normal-pressure subjects (9,10). In addition, for the patient selection, we did not measure the PAC and PRA after stopping anti-hypertensive drugs, although some drugs, such as ARBs and diuretics, can cause false-negative results (1). These are some of the limitations associated with the present study.
Armanini et al. (5) analyzed 80 patients with PA (40 with APA and 40 with IHA) and 80 normotensive healthy controls. They found that three patients with IHA had PC, but no PC was detected in patients with APA or in controls. Turchi et al. (6) also analyzed 92 patients with PA (33 with APA and 59 with IHA) and 96 patients with EH as controls. They found that 3 patients (3% in their paper) with PA had PC. However, the incidence of PA in controls was not described. In 2016, Lang et al. (7) examined the prevalence of malignancy in 335 patients with PA (about 50% with APA according to their figures) and 335 age- and sex-matched hypertensive controls. They found that three patients with APA had thyroid carcinoma, compared with one control patient. These previous findings suggest that the incidence of PC (or thyroid carcinoma) in PA is about 1-4%. However, the relationship between PC and the PA subtype (APA or IHA) remains unclear.
While several assumptions have been made, the concurrent presence of these two diseases might merely be a chance occurrence.
The circadian clock plays an important role in the regulation of the physiological functions. An altered circadian clock has been reported in pathological conditions, such as sleep disorder (22), hypertension (23,24), metabolic syndrome (24-26), and cancer (27,28). It was reported that mice lacking Cryptochrome (Cry) 1 and Cry 2, which are core clock genes, showed salt-sensitive hypertension (high PAC and low PRA) with bilateral hyperplasia of the zona glomerulosa cells, a condition similar to that of IHA in humans (23,29,30). In addition, the expression of Cry 2 is reportedly reduced, while that of brain and muscle Arnt-like protein-1 (BMAL1) is increased in human PC tissue (28). Thus, disruption in the clock gene expression may cause both IHA and PC in predisposed subjects. Sabbadin et al. (31) reported that the prevalence of anti-thyroid antibodies in PA patients was significantly higher than that in normotensive controls. They proposed the hypothesis that the course of autoimmune thyroiditis may be worsened by aldosterone, via the local invasion of T cells (32-34). Furthermore, ultrasonography showed that autoimmune thyroiditis was more frequent in patients with IHA (20.8%) than in patients with APA (9.3%). A significant association between thyroid carcinoma and thyroid autoimmunity (autoimmune thyroiditis) has been pointed out (35-37). Thus, immunological factors may play a role in the development of PC in predisposed subjects. As reported in patients with PA who showed ultrasonographic thyroid abnormalities (5,6,17), an imbalance between growth factors or cytokines (38-40) may contribute to the development of PC. The thyroid volume and thyroid nodule formation were reportedly increased in subjects with insulin resistance and/or metabolic syndrome (41,42). The associations between the serum aldosterone concentration and insulin resistance (43) and metabolic syndrome (43,44) have been shown in the general population. Insulin resistance was also reported in patients with PA (45,46). Turchi et al (6) demonstrated that insulin resistance in PA patients whose thyroid had an abnormal ultrasonographic appearance was higher than in PA patients whose thyroid had a normal ultrasonographic appearance. In addition, the prevalence of metabolic syndrome was reportedly high in patients with PA (47). Rezzónico et al. (48) indicated that insulin resistance may play a role in the development of differentiated thyroid carcinoma (DTC) because the incidence of insulin resistance was high in patients with DTC. In fact, the overexpression of insulin receptors and insulin-like growth factor (IGF)-I receptors was reported in PC tissue (49).
A high aldosterone concentration alone is believed to be incapable of leading to the development of PC, as [1] the presence of PC was not found in patients with Bartter's syndrome, Gitelman's syndrome, or renovascular hypertension, who had a high aldosterone concentration; and [2] the prevalence of PC was not reported to be high in patients with PA (5-7). Thus, a patient's predisposition is thought to play an important role in the development of PC.
Whether or not PA antedates the occurrence of PC is important, but it is difficult to determine when these two diseases occur because they are usually recognized by chance without signs or symptoms. In the present study, the BP was not measured frequently in most of the patients because the randomly measured BP values were not very high. In addition, the BP was not recorded in some patients who had lived with PC for a long time. We were therefore unable to determine the exact time at which PA developed.
The prevalence of PA in PC has not been studied, although there are a few case reports in which PA was found to be associated with PC (50,51). In the present study, the prevalence of PA in PC was at least 18.5% (15/81), since not all confirmatory tests were performed in the 25 patients with PA whose random PAC/PRA was over 200. In addition, only 1 assay result (PAC/PRA >200) was used for the first screening. We cannot completely rule out that patients with PC with PA may have been among patients with a PAC/PRA ≤200 in the first screening, especially among patients treated with ARBs and diuretics. Based on these points, the prevalence of PA in PC may be much higher than was suggested in the present study.
In conclusion, the present study indicates that [1] there may be some relationship between high aldosterone levels and the occurrence of PC; [2] an active examination is needed to determine the presence of PA in patients with PC, since signs and symptoms of PA are mild; [3] one cause of white-coat hypertension is PA (in patients with PC); and [4] further investigations are needed to examine the presence of PA in patients with PC whose blood pressure is normal with anti-hypertensive drugs.
Patient No. 15 was later diagnosed with IHA based on the findings of AVS performed at another hospital.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Shimamoto K, Ando K, Fujita T, et al. The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res 37: 253-392, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res 27: 193-202, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Miyake Y, Tanaka K, Nishikawa T, et al. Prognosis of primary aldosteronism in Japan: results from a nationwide epidemiological study. Endocr J 61: 35-40, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 101: 1889-1916, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Armanini D, Nacamulli D, Scaroni C, et al. High prevalence of thyroid ultrasonographic abnormalities in primary aldosteronism. Endocrine 22: 155-159, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Turchi F, Ronconi V, di Tizio V, Boscaro M, Giacchetti G. Blood pressure, thyroid-stimulating hormone, and thyroid disease prevalence in primary aldosteronism and essential hypertension. Am J Hypertens 24: 1274-1279, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Lang K, Weber K, Quinkler M, et al. Prevalence of malignancies in patients with primary aldosteronism. J Clin Endocrinol Metab 101: 1656-1663, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura S. Primary aldosteronism in patients with thyroid diseases. Medical Journal of Japanese Red Cross Gifu Hospital 26: 3-8, 2014(in Japanese). [Google Scholar]
- 9.Médeau V, Moreau F, Trinquart L, et al. Clinical and biochemical characteristics of normotensive patients with primary aldosteronism: a comparison with hypertensive cases. Clin Endocrinol (Oxf) 69: 20-28, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y, Takeda R, Takeda Y. Subclinical primary aldosteronism. Best Pract Res Clin Endocrinol Metab 26: 485-495, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa T, Omura M, Satoh F, et al. Guidelines for the diagnosis and treatment of primary aldosteronism - The Japan Endocrine Society 2009 - . Endocr J 58: 711-721, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Consensus Statement on the Clinical Practice of Primary Aldosteronism in Japan-Jaoan Endocrine Society. Folia Endocrinol Jap 92(Suppl): 1-49, 2016(in Japanese). [Google Scholar]
- 13.Queisser N, Oteiza PI, Stopper H, Oli RG, Schupp N. Aldosterone induces oxidative stress, oxidative DNA damage and NF-κB-activation in kidney tubule cells. Mol Carcinog 50: 123-135, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Queisser N, Happ K, Link S, et al. Aldosterone induces fibrosis, oxidative stress and DNA damage in livers of male rats independent of blood pressure changes. Toxicol Appl Pharmacol 280: 399-407, 2014. [DOI] [PubMed] [Google Scholar]
- 15.King S, Bray S, Galbraith S, Christie L, Fleming S. Evidence for aldosterone-dependent growth of renal cell carcinoma. Int J Exp Pathol 95: 244-250, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaji K, Yoshiji H, Kitade M, et al. Selective aldosterone blocker, eplerenone, attenuates hepatocellular carcinoma growth and angiogenesis in mice. Hepatol Res 40: 540-549, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Krysiak R, Okopien B. Coexistence of primary aldosteronism and Hashimoto's thyroiditis. Rheumatol Int 32: 2561-2563, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santori C, Di Veroli C, Di Lazzaro F, et al. High prevalence of thyroid disfunction in primary hyperaldosteronism. Recenti Prog Med 96: 352-356, 2005(in Italian, Abstract in English). [PubMed] [Google Scholar]
- 19.Greenman Y, Trostanetsky Y, Ben-Shemen S, et al. Thyroid cysts: a new extra-adrenal site of aldosterone synthase expression and increased aldosterone content. Clin Endocrinol (Oxf) 66: 886-889, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Tanabe A, Naruse M, Takagi S, et al. Variability in the renin/aldosterone profile under random and standardized sampling conditions in primary aldosteronism. J Clin Endocrinol Metab 88: 2489-2494, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Ito Y, Takeda R, Karashima S, Yamamoto Y, Yoneda T, Takeda Y. Prevalence of primary aldosteronism among prehypertensive and stage 1 hypertensive subjects. Hypertens Res 34: 98-102, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Khan S, Duan P, Yao L, Hou H. Shiftwork-mediated disruptions of circadian rhythms and sleep homeostasis cause serious health problems. Int J Genomics 2018: 8576890, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doi M, Takahasi Y, Komatsu R, et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med 16: 67-74, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Rahman A, Hasan AU, Nishiyama A, Kobori H. Altered circadian timing system-mediated non-dipping pattern of blood pressure and associated cardiovascular disorders in metabolic and kidney diseases. Int J Mol Sci 19: E400, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 308: 1043-1045, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinemia and diabetes. Nature 466: 627-631, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111: 41-50, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Mannic T, Meyer P, Triponez F, et al. Circadian clock characteristics are altered in human thyroid malignant nodules. J Clin Endocrinol Metab 98: 4446-4456, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Doi M. Circadian clock-deficient mice as a tool for exploring disease etiology. Biol Pharm Bull 35: 1385-1391, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Okamura H, Doi M, Goto K, Kojima R. Clock genes and salt-sensitive hypertension: a new type of aldosterone-synthesizing enzyme controlled by the circadian clock and angiotensin II. Hypertens Res 39: 681-687, 2016. [DOI] [PubMed] [Google Scholar]
- 31.Sabbadin C, Mian C, Nacamulli D, et al. Association of primary aldosteronism with chronic thyroiditis. Endocrine 55: 303-306, 2017. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y, Wang H, Su Z, et al. Differentiation imbalance of Th1/Th17 in peripheral blood mononuclear cells might contribute to pathogenesis of Hashimoto's thyroiditis. Scand J Immunol 72: 250-255, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Herrada AA, Contreras FJ, Marini NP, et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol 184: 191-201, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Herrada AA, Campino C, Amador CA, Michea LF, Fardella CE, Kalergis AM. Aldostetrone as a modulater of immunity: implications in the organ damage. J Hypertens 29: 1684-1692, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Boi F, Lai ML, Marziani B, Minerba L, Faa G, Mariotti S. High prevalence of suspicious cytology in thyroid nodules associated with positive thyroid autoantibodies. Eur J Endocrinol 153: 637-642, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Spencer CA. Clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab 96: 3615-3627, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Paparodis R, Imam S, Todorova-Koteva K, Staii A, Jaume JC. Hashimoto's thyroiditis pathology and risk for thyroid cancer. Thyroid 24: 1107-1114, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuki K, Hathaway CK, Chang AS, Smithies O, Kakoki M. Transforming growth factor beta1 and aldosterone. Curr Opin Nephrol Hypertens 24: 139-144, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Šomlóová Z, Petrák O, Rosa J, et al. Inflammatory markers in primary aldosteronism. Physiol Res 65: 229-237, 2016. [DOI] [PubMed] [Google Scholar]
- 40.Petramala L, Iacobellis G, Carnevale R, et al. Enhanced soluble serum CD40L and serum P-selectin levels in primary aldosteronism. Horm Metab Res 48: 440-445, 2016. [DOI] [PubMed] [Google Scholar]
- 41.Rezzonico J, Rezzonico M, Pusiol E, Pitoia F, Niepomniszcze H. Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid 18: 461-464, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Ayturk S, Gursoy A, Kut A, Anil C, Nar A, Tutuncu NB. Metabolic syndrome and its components are associated with increased thyroid volume and nodule prevalence in a mild-to-moderate iodine-deficient area. Eur J Endocrinol 161: 599-605, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Min SH, Kim SH, Jeong IK, et al. Independent association of serum aldosterone level with metabolic syndrome and insulin resistance in Korean adults. Korean Circ J 48: 198-208, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hannemann A, Meisinger C, Bidlingmaier M, et al. Association of plasma aldosterone with the metabolic syndrome in two German populations. Eur J Endocrinol 164: 751-758, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Catena C, Lapenna R, Baroselli S, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab 91: 3457-3463, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Fallo F, Della Mea P, Sonino N, et al. Adiponectin and insulin sensitivity in primary aldosteronism. Am J Hypertens 20: 855-861, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Fallo F, Veglio F, Bertello C, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab 91: 454-459, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Rezzónico JN, Rezzónico M, Pusiol E, Pitoia F, Niepomniszcze H. Increased prevalence of insulin resistance in patients with differentiated thyroid carcinoma. Metab Syndr Relat Disord 7: 375-380, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Vella V, Sciacca L, Pandini G, et al. The IGF system in thyroid cancer: new concepts. Mol Pathol 54: 121-124, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishikawa M, Inada M, Kurata S, et al. Primary aldosteronism associated with a primary double cancer of the thyroid and rectum: report of a case. Endocrinol Jpn 28: 735-739, 1981. [DOI] [PubMed] [Google Scholar]
- 51.Wanta SM, Basina M, Chang SD, et al. A rare case of an aldosterone secreting metastatic adrenocortical carcinoma and papillary thyroid carcinoma in a 31-year-old male. Rare Tumors 3: e45, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]