Abstract
Objective
The standard anti-tuberculosis (TB) regimen occasionally causes acute kidney injury (AKI). The major etiology is rifampicin-induced acute interstitial nephritis. However, the standard management of AKI induced by anti-TB drugs has yet to be established.
Methods
We retrospectively reviewed patients with TB who developed AKI after starting standard anti-TB treatment between 2006 and 2016 at a single TB center. The clinical characteristics and the management are described.
Results
Among 1,430 patients with active TB, 15 (1.01%) developed AKI. The mean age (standard deviation) was 61 years (18). The median (interquartile range) time to AKI development was 45 days (21-54 days). The median serum creatinine level before anti-TB treatment was 0.7 mg/dL (0.5-1.4 mg/dL), whereas the median peak serum creatinine level after AKI onset was 4.0 mg/dL (3.08-5.12 mg/dL). Five patients (33.3%) were pathologically confirmed as having acute interstitial nephritis (AIN), and 7 patients (46.7%) had a clinical diagnosis of the disease. All anti-TB drugs were stopped, and steroids were administered to 5 (100%) patients with pathologically confirmed AIN and 3 (42.8%) patients with clinically diagnosed AIN. The renal function was normalized in 12 patients (80.0%) after restarting anti-TB treatment without rifampicin (n=12) or isoniazid (n=1). Two patients died due to severe renal failure after restarting rifampicin.
Conclusion
Rifampicin is the leading cause of AKI. Levofloxacin may be an alternative to rifampicin thanks to its safety and potency. Restarting anti-TB treatment without rifampicin and short-term steroid administration may be a feasible management for AKI.
Keywords: rifampicin, acute kidney injury, acute interstitial nephritis
Introduction
Appropriate anti-tuberculosis (TB) treatment is the most effective method of controlling the spread of the disease; however, severe adverse events occasionally interrupt treatment. The current official guidelines for anti-TB treatment contain recommendations for managing common adverse events, such as gastrointestinal upset, rash, a drug fever, hepatotoxicity, and optic neuritis (1). However, there are no recommendations for the practical management of acute kidney injury (AKI) induced by anti-TB drugs. Although AKI is a rare complication, its occurrence delays standard anti-TB treatment, potentially leading to drug-resistance due to treatment interruption.
Among the standard anti-TB drugs, rifampicin (RIF) is reportedly the most common cause of AKI (2-4). However, the exact incidence of AKI induced by standard anti-TB drugs such as RIF remains unknown (3-5). Furthermore, only a few studies have investigated acute renal dysfunction during anti-TB treatment using the diagnostic criteria for AKI (5, 6). A recent study in Taiwan focused on AKI during anti-TB treatment (5) and reported that 99 of 1,394 patients (7.1%) developed AKI after starting standard anti-TB drugs in the past 5 years, suggesting that AKI is not necessarily a rare complication. In addition, this study revealed that the presence of a fever, rash, and gastrointestinal disturbances at AKI onset may predict the recovery of the renal function. However, no studies have reported the details of any management method for AKI due to anti-TB drugs or an alternative drug treatment.
In Japan, fluoroquinolones (FQs) are empirically used as an alternative anti-TB drug when key anti-TB drugs are unable to be administered. FQs are a viable alternative owing to their high potency and safety profiles compared with older anti-TB drugs. In 2016, the Ministry of Health, Labour and Welfare of Japan approved levofloxacin as an alternative anti-TB drug based on the results of a nationwide survey of its safety and efficacy (7). Japan has a large aging population, and in 2013, the elderly population comprised approximately 25% of the total population (8). In 2015, of all patients with newly diagnosed, active TB in Japan, 58.9% were ≥70 years of age (9). Because the elderly tend to be vulnerable to AKI, we hypothesized that AKI induced by anti-TB drugs was becoming a more serious problem (10).
The current study aimed to identify the incidence of AKI and the outcomes of our management of AKI induced by anti-TB drugs at a single TB treatment center over the last 10 years. In addition, we reviewed the utility of levofloxacin as an alternative anti-TB drug following the diagnosis of AKI.
Materials and Methods
This study was conducted at Tokyo Metropolitan Tama Medical Center. We retrospectively reviewed patients with active pulmonary and extrapulmonary TB who were admitted to the TB ward between April 1, 2006, and November 30, 2016. We included patients with active TB who developed AKI within six months after starting a standard anti-TB drug, such as RIF.
We used the diagnostic criteria for AKI established by the Acute Kidney Injury Network. An abrupt reduction in the kidney function was defined as an absolute increase in the serum creatinine level of ≥0.3 mg/dL (6). We extracted data on eligible patients from their medical records and chest X-ray, microbiological, and laboratory test findings. We clinically diagnosed acute interstitial nephritis (AIN) in patients with a diagnosis of AKI based on the findings of eosinophiluria and the uptake of 67gallium (Ga) in the bilateral kidneys. We also included patients with AKI who showed evidence of non-specific tubular damage in their urinalysis, including elevated urine N-acetyl-β-D-glucosaminidase and/or a beta-2 microglobulin concentration above the standard range at the study site after starting anti-TB treatment (11).
We excluded patients with active TB who had stage 4 or 5 chronic kidney disease (CKD) before starting anti-TB treatment. We also summarized the outcomes of patients in whom AKI developed after the start of anti-TB treatment.
Statistical analyses
We used the unpaired Student's t-test to compare two consecutive variables with a normal distribution and used the Mann-Whitney U test to compare two consecutive variables with a non-normal distribution. p<0.05 was considered to be statistically significant. The STATA software program, version 14.0 (StanCorp, College Station, USA) was used for statistical analyses.
Ethical considerations
The institutional review board of Tokyo Metropolitan Tama Medical Center approved this case series. Because all of the clinical and laboratory data of eligible patients were retrospectively collected anonymously, the institutional review board waived informed consent before enrollment.
Results
Incidence of AKI during the study period
Between April 1, 2006, and November 30, 2016, 1,430 patients with active TB were admitted to the TB ward at the Tokyo Metropolitan Tama Medical Center. AKI developed in 15 (1.04%) of these patients within 6 months after starting anti-TB treatment.
Basic characteristics of patients who developed AKI after anti-TB treatment
The mean age of the patients in whom AKI developed after starting anti-TB treatment was 61±18 years, and 13.3% of the patients were women. Five patients with AKI (26.6%) had stage 2 or 3 CKD before initiating anti-TB treatment. Their comorbidities, initial anti-TB drug regimen, co-administered drugs, symptoms, and laboratory abnormalities are summarized in Table 1. Seven patients (46.6%) had cavity lesions, whereas 5 (33.3%) had no cavity lesions, and 3 (20.0%) revealed a miliary TB pattern on their chest radiograph. The median (interquartile range) serum creatinine level of the patients before initiating anti-TB treatment was 0.7 mg/dL (range: 0.5-1.4 mg/dL).
Table 1.
Basic Characteristics of Patients who Developed AKI during Anti-TB Treatment (n=15).
| Comorbidity | n (%) |
| No comorbidity | 8 (53.3) |
| Diabetes mellitus | 3 (20.0) |
| Chronic kidney disease | 3 (20.0) |
| Malignancy | 1 (6.6) |
| Initial anti-TB drugs | n (%) |
| Isoniazid, rifampicin, pyrazinamide, and ethambutol | 10 (66.6) |
| Isoniazid, rifampicin, and ethambutol | 4 (26.6) |
| Isoniazid, rifampicin, pyrazinamide, and streptomycin | 1 (6.6) |
| Co-administered drugs | n (%) |
| None | 6 (40.0) |
| NSAIDs | 3 (20.0) |
| PPIs | 3 (20.0) |
| NSAIDs and PPIs | 3 (20.0) |
| Symptoms at AKI onset | n (%) |
| Newly developed fever | 8 (53.3) |
| Gastrointestinal discomfort | 3 (20.0) |
| No symptoms | 4 (26.7) |
| Laboratory data abnormalities at AKI onset | n (%) |
| Proteinuria | 15 (100) |
| Hyaline cast in urinalysis | 15 (100) |
| Hematuria | 8 (53.3) |
| Thrombocytopenia | 2 (20.0) |
| Liver dysfunction | 1 (6.6) |
AKI: acute kidney injury, TB: tuberculosis, PPI: proton-pump inhibitor, NSAID: non-steroidal anti-inflammatory drug
Clinical manifestations and laboratory test results of patients with AKI
At AKI onset, 8 patients (53.3%) newly developed a fever, 3 (20.0%) reported gastrointestinal discomfort, and 4 (26.7%) showed no new symptoms (Table 1). Abnormal laboratory results at the onset of AKI included proteinuria (100%), urine hyaline cast sediment (100%), microscopic hematuria (53.3%), thrombocytopenia (13.3%), and elevated liver transaminase levels (6.6%).
Serum creatinine level trends
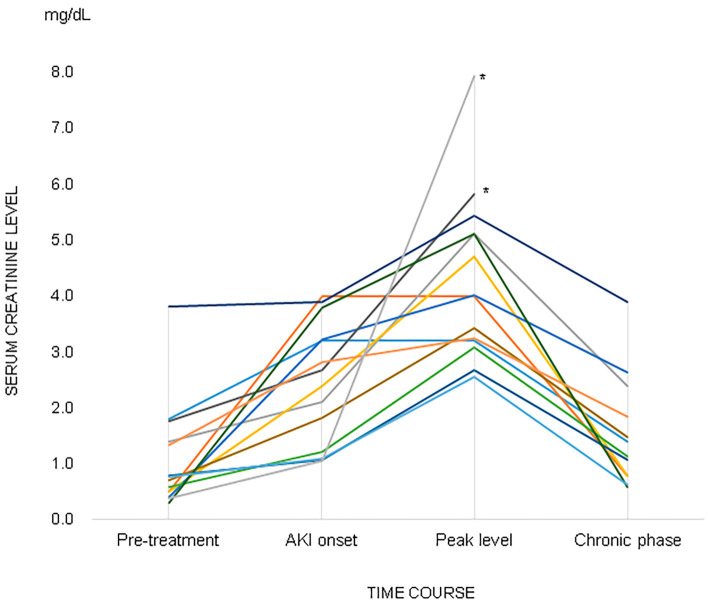
The median serum creatinine level before initiating anti-TB treatment was 0.7 mg/dL (range: 0.5-1.4 mg/dL), and the mean serum creatinine level at AKI onset was 2.4 mg/dL (range: 1.15-3.22 mg/dL) (Fig. 1). Even after interrupting anti-TB treatment, the serum creatinine level tended to increase, and the mean of the highest serum creatinine levels was 4.0 mg/dL (range: 3.08-5.12 mg/dL). Among the 13 surviving patients, the median serum creatinine level was significantly higher in the recovery phase [1.14 mg/dL (range: 0.80-1.85 mg/dL)] than in the pretreatment phase [0.70 mg/dL (range: 0.5-1.33 mg/dL); p=0.03; Mann-Whitney U test].
Figure 1.
Serum creatinine levels in the pretreatment phase, AKI onset phase, and poorest renal function phase (n=15). The chronic phase comprised data from 13 patients who survived after developing AKI. *Patients who died.
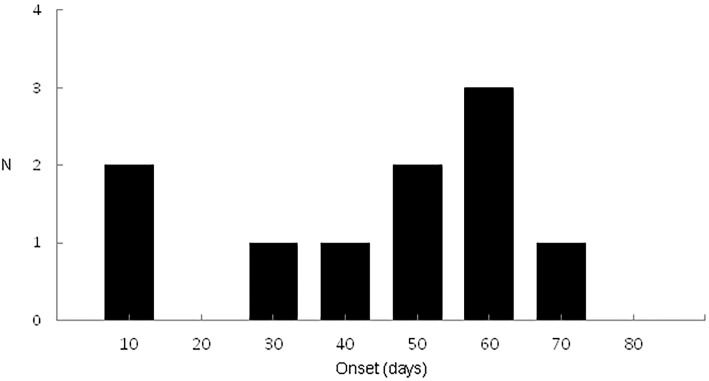
Time to AKI development after starting anti-TB drugs and the method of diagnosing AKI
The overall median time to AKI development was 45 days (range: 21-54 days) (Fig. 2). The median time to AKI development was 45 days (range:6-60 days) in patients with CKD (n=3) before initiating anti-TB treatment. The median time to AKI development was also 45 days (range: 23-52) in patients without CKD (n=12) before initiating anti-TB treatment. There was no significant difference in the median time to AKI onset between patients with or without CKD before initiating anti-TB treatment (p=0.243; Mann-Whitney U test). Five patients (33.3%) had a pathological diagnosis of AIN based on an ultrasound-guided percutaneous kidney biopsy. It was not possible to obtain a kidney biopsy from 10 patients (66.6%); therefore, we clinically diagnosed AIN induced by RIF or other anti-TB drugs based on a compatible clinical course, symptoms, and laboratory test results. The laboratory test results revealed that 4 patients (40.0%) had eosinophiluria, 2 (13.3%) had the accumulation of 67Ga in the bilateral kidneys, and 1 (6.6%) had both eosinophiluria and the accumulation of 67Ga in the bilateral kidneys (Table 2).
Figure 2.
Time to the onset of acute kidney injury (n=15).
Table 2.
Method of Diagnosing left, Pathological Findings of the Renal Biopsy Cases, and Laboratory Abnormalities of Clinically Diagnosed Cases.
| Laboratory and image findings of clinically diagnosed acute interstitial nephritis (n=7) | n (%) |
| Eosinophiluria | 4 (57.1) |
| 67Ga accumulation in the kidneys | 2 (28.6) |
| Eosinophiluria and 67Ga accumulation | 1 (14.3) |
| Pathological findings of biopsy confirmed acute interstitial nephritis (n=5) | n (%) |
| Acute interstitial nephritis | 4 (80.0) |
| Acute interstitial nephritis and mesangial proliferative glomerular nephritis | 1 (20.0) |
AIN: acute interstitial nephritis
AKI management and its prognosis
Anti-TB drug treatment was discontinued as soon as AKI development was confirmed. RIF was suspected of inducing AKI in 14 patients (93.3%), and isoniazid (INH) was suspected in 1 patient (6.6%). After stopping all anti-TB drugs, 6 patients (40.0%) were observed until their serum creatinine levels decreased without steroid administration; steroid was immediately administered to 9 patients (60.0%), with the median initial steroid dose at 50 mg (range: 40-50 mg). Steroid use for AKI treatment was mainly decided by each pulmonologist. The median duration of steroid administration was 52 days (range: 25-150 days) in 7 patients who survived and for whom data were available. The remaining two patients were transferred to another hospital to continue anti-TB treatment while receiving steroid treatment. Therefore, their data were not available for the duration of the steroid administration. One patient was excluded from the analysis due to death within a few weeks of stopping the anti-TB drugs despite receiving steroid treatment for 11 days. In 1 patient (6.6%), INH was suspected of inducing AKI due to a relapse of AKI after RIF was switched with INH. A renal biopsy confirmed the presence of AIN in this patient. When INH was stopped and RIF was re-administered to this patient, the AKI resolved.
The remaining 14 patients received a diagnosis of RIF-induced AKI based on pathological or clinical findings and stopped receiving anti-TB drugs (Table 3). Of these 14 patients, 12 (85.7%) who recovered their renal function did not resume RIF treatment but continued treatment with levofloxacin. The remaining 2 patients (14.3%) had been desensitized to RIF by the referring physician, who failed to consider the cause of the AKI clinically or pathologically, and consequently developed rapid progressive renal failure and died within several weeks of admission to our hospital despite continuous renal replacement therapy. Of the 12 patients who survived after AKI development, the renal function finally returned to the pre-anti-TB treatment levels in 8 patients (66.6%). Four patients (33.3%) partially recovered and achieved a sufficient renal function to receive a modified anti-TB treatment regimen (Table 3). No significant difference was observed in the baseline renal function recovery rate between the steroid administration group and non-administration group (p=0.558) among the surviving patients (Table 3).
Table 3.
Management of Anti-TB Drug-induced AKI and Renal Prognosis (n=15).
| AKI cause | Modification of anti-TB drugs | Steroid | Renal function outcome and prognosis | AIN cases | Anti-TB treatment outcome | Outcome | |
|---|---|---|---|---|---|---|---|
| RIF | Substitute RIF with LVFX n=12 |
Steroid (+) n=7 |
Baseline recovery | 5 (71.4%) | p-AIN 3 (42.8%) |
Accomplish | Survive |
| c-AIN 2 (28.5%) |
|||||||
| Partial recovery | 2 (28.6%) | p-AIN 1 (14.2%) |
|||||
| c-AIN 1 (14.2%) |
|||||||
| Steroid (−) n=5 |
Baseline recovery | 3 (60%) | c-AIN 2 (40%) |
Accomplish | Survive | ||
| Partial recovery | 2 (40%) | c-AIN 2 (40%) |
|||||
| RIF desensitization n=2 |
Steroid (+) n=1 |
End-stage renal failure | 2 (100%) | Fail | Die | ||
| Steroid (−) n=1 |
|||||||
| INH | Avoid INH and restarting RIF n=1 |
Steroid (+) n=1 |
Baseline recovery | 1 (100%) | p-AIN 1 (100%) |
Accomplish | Survive |
AIN: acute interstitial nephritis, INH: isoniazid, LVFX: levofloxacin, RIF: rifampicin, c-AIN: clinically diagnosed AIN, p-AIN: pathologically confirmed AIN
In addition, the underlying factors and comorbidities between the baseline renal function recovery group (n=8) and partial renal function recovery group (n=4) were compared. There was no significant difference between the groups in the proportion of patients using non-steroidal anti-inflammatory drugs (NSAIDs) or proton-pump inhibitors (PPIs) or who had CKD or diabetes mellitus. The serum creatinine level before starting anti-TB treatment and at AKI onset were similar between the baseline recovery and partial recovery groups. For all 12 surviving patients, RIF was switched to levofloxacin in the anti-TB treatment regimen after the AKI resolved (Table 3). These 12 patients eventually resumed the modified anti-TB treatment regimen without RIF and completed their anti-TB treatment.
Discussion
This study showed that RIF was the most common causative drug in AKI during standard anti-TB treatment, although we identified one case of INH-induced AKI. In line with previous studies, we found that AIN was the most common etiology of AKI during anti-TB treatment (3, 4). All patients with AKI who underwent a renal biopsy had AIN, and those who did not undergo a renal biopsy were found to have AIN based on clinical findings. We consider it important to specify the etiology of AKI, as management of the condition may differ depending on its etiology. In cases where a renal biopsy cannot be used to specify the etiology, clinical symptoms, eosinophiluria, and the accumulation of 67Ga in the bilateral kidneys may be used to corroborate the diagnosis of AIN; eosinophiluria or the accumulation of 67Ga alone is not sensitive or specific enough (12). Patients with AIN exhibit systemic symptoms, such as nausea, a fever, and rash; however, these are not frequently observed in patients with AKI with other etiologies. In this study, gastrointestinal discomfort was present among 20% of the patients, which is similar to the findings of a recent study of anti-TB drug-induced AKI in Taiwan (5). Therefore, we speculated that gastrointestinal discomfort may be an important sign of AIN.
In the present study, AKI mostly occurred during the intensive anti-TB treatment phase. We observed no marked difference in the time of AKI onset after initiating anti-TB treatment between patients with and without CKD. However, this finding was not conclusive, as the number of patients was small. Proteinuria and hematuria were observed in our patients after AKI developed, indicating that a urinalysis may be used as an early screening test for AKI if sequentially performed during the intensive phase of anti-TB treatment.
A recent, large-scale case series of AIN diagnosed by a biopsy suggested that PPIs NSAIDs, and antibiotics were common causes of drug-induced AIN (13). In this study, we found that NSAIDs and PPIs were frequently administered to patients with active TB.
Interrupting anti-TB treatment should be avoided, even in patients with AKI, as it is important to complete anti-TB treatment in order to maximize the reduction of Mycobacterium tuberculosis (MTB) bacilli during the intensive phase of treatment. Therefore, we administered steroids to patients who had a pathological or clinical diagnosis of AIN in order to promote the rapid recovery of their renal function. Although the use of steroids for AIN remains controversial (12, 13), one study clearly showed that the early administration of steroids resulted in a higher rate of successful renal function recovery after the development of AIN (14). Our data suggested that some patients only showed partial renal function recovery and developed CKD even when anti-TB treatment was immediately discontinued and steroids were administered. However, the number of patients in this study was too small to assess the efficacy of steroid therapy.
We believe that restarting RIF, even at low doses, should be avoided in patients with RIF-induced AIN, as patients who become desensitized to RIF therapy are at an increased risk of mortality. One case report showed that restarting RIF caused more severe AKI even after exposure to much smaller doses than those used at the initial administration (15). Several studies have suggested that the complete discontinuation of the causative drug is essential for managing drug-induced AIN (12, 13). Based on these suggestions, we recommend avoiding restarting RIF, including desensitization therapy, if the cause of AKI is suspected to be AIN.
Among second-line anti-TB drugs, levofloxacin is considered to be a viable substitute for RIF, and switching RIF to levofloxacin in this study resulted in no serious adverse events or AKI relapse. Alternative anti-TB treatment using levofloxacin successfully achieved culture-negative conversion and was able to be completed by extending the treatment duration from 6 months to at least 18 months after confirming culture-negative conversion if the MTB was drug-susceptible. Levofloxacin is categorized as a group 3 anti-TB drug and is recommended for use against multidrug-resistant TB if the strain is susceptible to levofloxacin, according to the treatment guidelines of the World Health Organization (16). In Japan, levofloxacin was approved as a third-line anti-TB drug by the Ministry of Health, Labor and Welfare in 2016 based on a nationwide surveillance of its use in anti-TB treatment (7). Although several studies have failed to show that FQs are not inferior to first-line anti-TB drugs, such as INH and RIF (17, 18), we consider FQs to be relatively safe and viable alternatives if the first-line drugs cause serious adverse events, such as AKI, severe skin rash, or severe liver dysfunction.
Several limitations associated with the present study warrant mention. First, the study design was retrospective and descriptive. Furthermore, it was only conducted at a single TB center in Japan. Because the number of patients with AKI during anti-TB treatment was small, identifying the risk factors for AKI development was difficult. However, this study also has a strength in that we performed baseline laboratory tests for all patients before initiating anti-TB treatment and routinely monitored laboratory parameters, such as the renal function weekly during the intensive anti-TB treatment phase. Therefore, the AKI onset and changes in the renal function were accurately described. In addition, patient adherence to anti-TB drug treatment was guaranteed because we performed directly observed therapy for all admitted patients during this study period.
Conclusion
In conclusion, our study showed that RIF is the leading causative drug in AKI during anti-TB treatment. Restarting RIF after diagnosing AIN may be lethal and should be avoided. As an alternative to RIF, levofloxacin currently appears to be viable owing to its safety profile and potency. Because there is no organized system for reporting adverse events associated with anti-TB treatment in Japan, further nationwide studies are warranted to establish an appropriate clinical management protocol for AKI during anti-TB treatment.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Drs. Kazuaki Enatsu and Takahiro Kiryu for their support in interpreting the renal pathological findings.
References
- 1.Nahid P, Dorman SE, Alipanah N, et al. Executive summary: Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of drug-susceptible tuberculosis. Clin Infect Dis 63: 853-867, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poole G, Stradling P, Worlledge S. Potentially serious side effects of high-dose twice-weekly rifampicin. Br Med J 3: 343-347, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covic A, Goldsmith DJ, Segall L, et al. Rifampicin-induced acute renal failure: a series of 60 patients. Nephrol Dial Transplant 13: 924-929, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Muthukumar T, Jayakumar M, Fernando EM, Muthusethupathi MA. Acute renal failure due to rifampicin: a study of 25 patients. Am J Kidney Dis 40: 690-696, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Chang C-H, Chen Y-F, Wu V-C, et al. Acute kidney injury due to anti-tuberculosis drugs: a five-year experience in an aging population. BMC Infect Dis 14: 23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Japanese Society for Tuberculosis TC. [The outcome of a survey on the use of levofloxacin for the treatment of tuberculosis]. Kekkaku 87: 599-608, 2012(in Japanese). [PubMed] [Google Scholar]
- 8.Arai H, Ouchi Y, Toba K, et al. Japan as the front-runner of super-aged societies: perspectives from medicine and medical care in Japan. Geriatr Gerontol Int 15: 673-687, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Tuberculosis RI of. Japan Anti-tuberculosis Association. Statistics of TB 2016 (in Japanese) [Internet]. [cited 2017 May 6]. Available from: http://www.jata.or.jp/rit/ekigaku/toukei/nenpou/ [Google Scholar]
- 10.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193-207, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Trof RJ, Di Maggio F, Leemreis J, Groeneveld ABJ. Biomarkers of acute renal injury and renal failure. Shock 26: 245-253, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol 6: 461-470, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis 64: 558-566, 2014. [DOI] [PubMed] [Google Scholar]
- 14.González E, Gutiérrez E, Galeano C, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 73: 940-946, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Feinfeld DA, Ansari N, Nuovo M, Hussain A, Mir R. Tubulointerstitial nephritis associated with minimal self reexposure to rifampin. Am J Kidney Dis 33: e3, 1999. [DOI] [PubMed] [Google Scholar]
- 16.In: WHO, The World Health Organization. Treatment of tuberculosis: guidelines. 4th ed 2010: 160. [PubMed] [Google Scholar]
- 17.Gninafon M, Lo MB, Mthiyane T, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med 371: 1588-1598, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Gillespie SH, Crook AM, McHugh TD, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med 371: 1577-1587, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]