Abstract
Fanconi anemia (FA) is a disorder of chromosomal fragility characterized by progression to aplastic anemia, myelodysplastic syndrome, and leukemia. FA patients are also predisposed to solid cancers. A case of FA in an adult patient who developed tongue and superficial esophageal cancers following hematopoietic stem cell transplantation is reported. This case was considered significant because it is the first reported case of superficial esophageal cancer in an FA patient that was treated successfully by endoscopic submucosal resection.
Keywords: Fanconi anemia, superficial esophageal cancer, endoscopic submucosal resection
Introduction
Fanconi anemia (FA) is an autosomal recessive disorder characterized by chromosomal fragility. Patients with FA can develop progressive pancytopenia, myelodysplastic syndrome (MDS), and acute myeloid leukemia (AML), and they may also have physical abnormalities and solid cancers. It is a very rare disease affecting approximately 5 in a million newborns in Japan (1). Patients develop bone marrow failure during early childhood, which then progresses to MDS and AML during adolescence in many cases. This increases the risk of solid cancers, such as head and neck cancer, during adulthood, with a cumulative solid cancer incidence of 28% by 40 years of age (2). Hematopoietic tumors in these patients are actively treated by hematopoietic stem cell transplantation because it is the only curative treatment. For solid cancers, surgery is selected over chemotherapy due to chromosomal fragility.
We herein report a case of FA in an adult patient who developed tongue and superficial esophageal cancers following hematopoietic stem cell transplantation. This case is presented due to its significance as the first reported case of esophageal cancer in an FA patient in whom the cancer was detected as a superficial cancer and treated successfully by endoscopic submucosal dissection (ESD).
Case Report
The patient was a Japanese man in his 30s. While his older brother had died from FA in infancy, his parents and younger sister had no notable medical history. He had been diagnosed with FA at 5 years of age when he underwent a detailed examination, and he received hematopoietic stem cell transplantation from his biological younger sister at 6 years of age. He had been regularly visiting his family physician since then.
He developed pain in his tongue approximately one month before being referred to our hospital. Upon consultation, his family physician identified a tumor with a white coating in his tongue. This was considered to be due to chronic graft-versus-host disease (GVHD), but the possibility of a malignant tumor could not be completely ruled out, so the patient was referred to our Department of Head and Neck Surgery for a detailed examination and treatment. He had no history of smoking or alcohol use and no medical history other than FA.
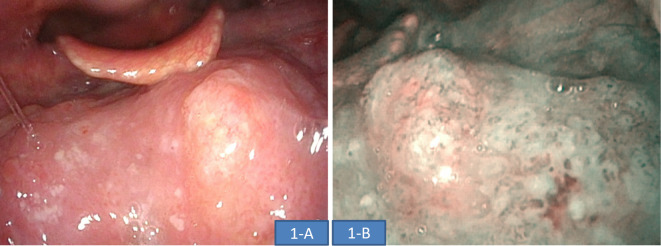
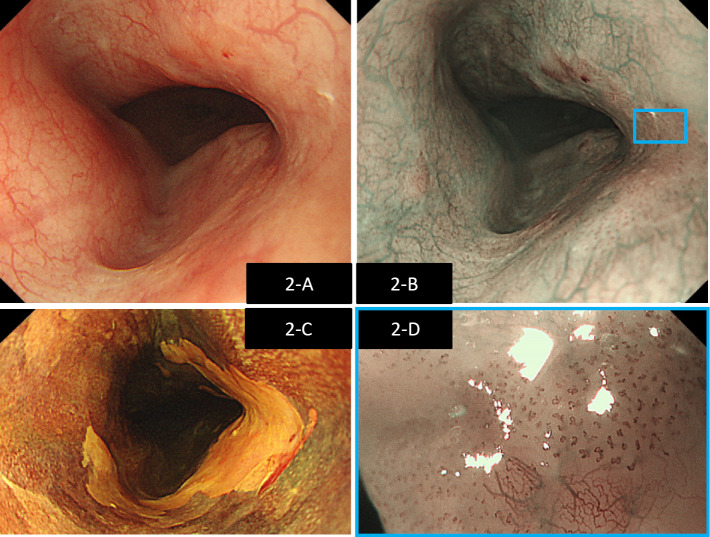
Blood tests performed during the examination did not show any abnormal findings, including tumor markers (Table). Fiberoptic laryngoscopy showed a white, elevated lesion in the left tongue root, which corresponded to a brownish area identified on narrow-band imaging (NBI) (Fig. 1). A biopsy taken from this location was determined to be squamous cell carcinoma (SCC). Upper gastrointestinal endoscopy was performed for a detailed examination of double cancers and showed a poor vascular permeability pattern in the upper thoracic esophagus, which corresponded to a brownish area identified on NBI. Magnified NBI showed that the area was composed mainly of type B1 vessels, and the depth of invasion of the lesion was determined to be the epithelium and lamina propria mucosae (EP/LPM) (Fig. 2). The area was seen as an unstained area when Lugol's solution was applied; however, multiple Lugol-voiding lesions were not observed close to the area. A biopsy taken from this location also showed SCC. Whole-body contrast-enhanced computed tomography (CT) did not identify any distant and lymph node metastases or any lesions in the esophagus. An area with slightly poorer contrast was identified in the left tongue root, suggesting that this location was the primary site of cancer in the tongue root. Given these findings, the patient was diagnosed preoperatively with cT1 N0 M0 cStageI [Union for International Cancer Control (UICC) 8th edition] oropharyngeal cancer (p16-negative), and cT1a N0 M0 cStageIA (UICC 7th edition) esophageal cancer.
Table.
Blood Tests Performed during the Examination.
| Alb (g/dL) | 4.6 | 4.0-5.0 |
| BUN (mg/dL) | 19 | 8-22 |
| Cr (mg/dL) | 0.79 | 0.6-1.1 |
| AST (U/L) | 31 | 13-33 |
| ALT (U/L) | 38 | 6-30 |
| ALP (U/L) | 402 | 115-359 |
| LDH (U/L) | 201 | 119-229 |
| T-bil (mg/dL) | 0.9 | 0.3-1.2 |
| Na (mEq/L) | 139 | 138-146 |
| K (mEq/L) | 4 | 3.6-4.9 |
| Cl (mEq/L) | 102 | 99-109 |
| CRP (mg/dL) | 0.04 | 0-0.30 |
| WBC (/μL) | 7,090 | 3,300-8,600 |
| RBC (×104/μL) | 509 | 435-555 |
| Hb (g/dL) | 14.7 | 13.7-16.8 |
| Ht (%) | 45.6 | 40.7-50.1 |
| Plt (×104/μL) | 22.7 | 15.8-34.8 |
| CA19-9 (U/mL) | 18.5 | 0.0-37.0 |
| SCC (ng/mL) | 1.2 | 0.0-2.0 |
CRP: C-reactive protein, CA19-9: carbohydrate antigen 19-9, SCC: squamous cell carcinoma
Figure 1.
Findings on laryngoscopy. A white, elevated lesion was identified in the left tongue root (A). This region corresponded to a brownish area identified on NBI (B) and was classified as SCC on pathology.
Figure 2.
Findings on upper gastrointestinal endoscopy. A white region characterized by a poor vascular permeability pattern was identified in the thoracic esophagus (A). The region corresponded to a brownish area identified on NBI (B). Lugol staining was performed, showing an unstained area corresponding to approximately 2/3 of the same region (C). Magnified NBI showed that the area was mainly composed of type B1 vessels (D), and the depth of invasion was EP/LPM.
The cancer of the left tongue root was first treated by resecting the left tongue root, and then ESD was performed for the esophageal cancer. The specimen of the resected cancer in the tongue root is shown in Fig. 3. A histopathological examination showed submucosal infiltration of atypical cells arranged in alveolar and solid patterns, suggesting that it was a moderately differentiated SCC. Atypical cell infiltration was also identified in the inner sections, and the resection margin was positive. A metastatic lesion was identified in one of the dissected lymph nodes.
Figure 3.
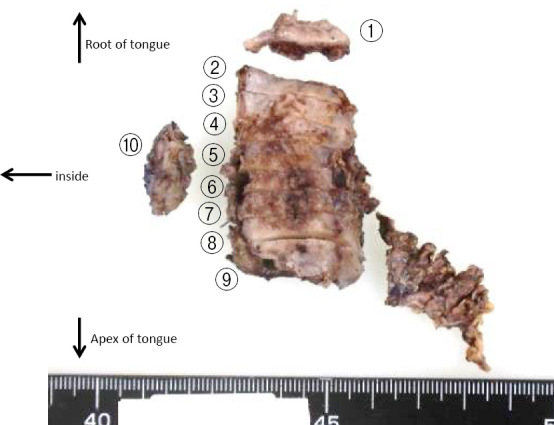
Surgical specimen of the tongue cancer. A positive margin was identified on inner section 10.
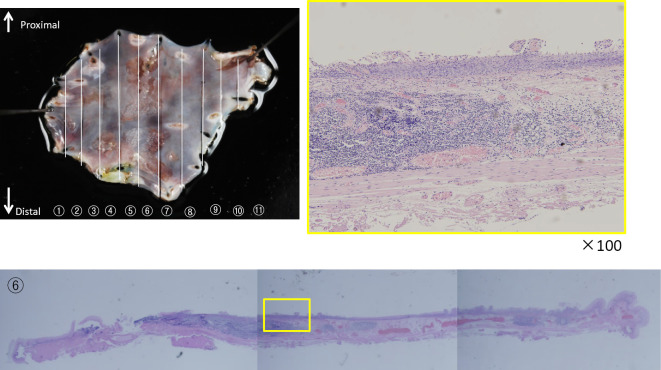
After confirming that the general condition of the patient was stable following the surgery, ESD was performed to completely resect the esophageal cancer. A pathological examination showed some tumor cell infiltration into the lamina propria; however, the horizontal and vertical margins were negative with no invasion into the lymphatic or vascular systems (Fig. 4). Based on these findings, the patient was diagnosed postoperatively with T1N1M0 pStageIII oropharyngeal cancer (p16-negative) and LPM ly-v-HM-VM-esophageal cancer, and curative resection of the esophageal cancer was achieved by ESD.
Figure 4.
Surgical specimen of the esophageal cancer. Growth of atypical squamous cells was identified on sections 2-7. Cells were found to have infiltrated into the lamina propria, and there was no invasion into the lymphatic or vascular systems.
The tongue cancer had a positive margin; however, there was a high risk of serious complications if the patient chose to undergo additional chemotherapy or radiation therapy, given his FA background. Thus, following discussions with the patient, no additional treatments were given, and the patient has instead been carefully monitored on follow-up visits. Currently, six months after the surgery, the patient has been monitored as an outpatient and has not developed any recurrent disease.
Discussion
FA is characterized by chromosomal instability due to defects in the DNA repair mechanisms, and 21 DNA repair genes involved in FA have been identified so far (3). A study demonstrated that 16% and 5% of FA patients developed hematological malignancies and solid cancers in their lifetime, respectively (2). Among the 21 genes associated with FA, BRCA1 and BRCA2 have been shown to be involved in the development of solid cancers (4); however, multiple mechanisms are likely involved in the development of solid cancers, since a single mutation is not sufficient to promote cancer development (5). Solid cancers in patients who did not undergo transplantation are often found to be SCC in the head and neck, as well as hepatic tumors, esophageal cancers, vulvar cancers, and brain tumors. SCC in the head and neck is the most common type of solid cancer found in patients who have undergone transplantation (4), and the cancer risk is 4.4 times higher in those who undergo transplantation than in those who do not (4,6,7). In general, cancer occurrence following hematopoietic stem cell transplantation is associated with chronic GVHD and is thought to be caused by radiation therapy and immunosuppression prior to and after hematopoietic stem cell transplantation, tumor-associated viruses, and mutations in p35 gene (8). Among SCCs, the incidence of esophageal cancer following hematopoietic stem cell transplantation is very rare, with only three cases reported in Japan (9-11). Outside of Japan, Alter et al. reported that none of 12 FA patients who received transplantation developed esophageal cancer (12). Hosoya et al. demonstrated that the esophageal mucosa of patients does not exhibit the pathological characteristics of chronic GVHD, which is found in the head and neck region (11). This may explain the low incidence of esophageal cancer following transplantation.
Both head and neck and esophageal cancers are commonly treated by surgical resection, chemotherapy, and radiation therapy, depending on the clinical stage of the disease. However, FA patients have congenital defects in the pathways to repair DNA crosslinks. Thus, DNA crosslinking anti-cancer agents, such as cisplatin, are difficult to prescribe to these patients, as they can significantly increase the risk of chromosomal breakage (5,13), leading to serious side effects, even when administered at a low dosage. Targeted radiation therapy is an alternative option. However, Alter et al. (14) demonstrated that even targeted radiation induced serious unexpected side effects, including severe hematotoxicity, mucositis, stomatitis, and ulceration, leading to a treatment-associated mortality rate of 80% (15). Surgery is therefore recommended as the first treatment of choice for solid cancers in FA patients (16,17). In terms of surgical resection, Kataoka et al. (16) suggested that surgical resections in FA patients should be minimally invasive, and treatment options should be considered for possible postoperative complications. A recent study demonstrated that photodynamic therapy (PDT) was effective as a minimally invasive strategy in these patients (18). Endoscopic resection is another effective technique that is less invasive than surgery. Ishihara et al. (19) reported that ESD had a significantly higher curability rate than endoscopic mucosal resection (EMR). In the present study, ESD was selected because the esophageal cancer was superficial, with a size of 35 mm, and had not metastasized to the lymph nodes. The patient was successfully treated with ESD and did not develop any intraoperative serious complications, such as bleeding or perforation, or postoperative complications, such as aspiration pneumonitis. This case was considered significant because it is the first reported case of superficial esophageal cancer in an FA patient that was treated successfully by ESD.
A positive margin was detected on pathology in the resected tongue cancer, suggesting that tumor cells may have remained around the lesion. However, no postoperative treatments were given in this case; instead, given the possible lethal complications that might be caused by chemotherapy and radiation therapy in FA patients, this patient has been monitored on follow-up visits. Kluter et al. (20) reported that FA patients who underwent surgery for head and neck cancer had recurrence within a mean of 16 months after the index surgery. Thus, the risk of recurrence is very high in the present patient, including in the tongue, where tumor cells likely remained. Therefore, the patient has been carefully monitored on his monthly visits to the Department of Head and Neck Surgery by clinical and endoscopic examinations for the early detection of recurrence. In addition, while curative resection of the esophageal cancer was achieved, the patient is being further monitored by upper gastrointestinal endoscopy every six months and CT every four months for the early detection of double cancers or recurrence in a different location.
Minimally invasive surgical resection is the first treatment of choice in FA patients who have undergone hematopoietic stem cell transplantation. Early detection is critical in these patients. Patients should be screened regularly for head and neck cancer, to which these patients are known to be predisposed. In addition, regular screening for double esophageal cancers by upper gastrointestinal endoscopy is needed, even in young patients. Whole-body cancer screening should be performed in FA patients because they are at risk of developing solid cancers across different organs of the body, and coordination with experts is needed once cancer is suspected.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Akira O. Japan National registry of aplastic anemia in children 1988-2000: clinical features and prognosis of idiopathic aplastic anemia. Nihon Shouni Ketsueki Gakkai Zasshi 22: 53-62, 2008(in Japanese). [Google Scholar]
- 2.Kulter DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry. Blood 101: 1249-1256, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi T, D'Andrea AD. Molecular pathogenesis of Fanconi anemia:recent progress. Blood 107: 4223-4233, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood 101: 822-826, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Spanier G, Pohl F, Giese T, Meier JK, Koelbl O, Reichert TE. Fatal course of tonsillar squamous cell carcinoma associated with Fanconi anaemia: a mini review. J Craniomaxillofac Surg 40: 510-515, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Alter BP. Cancer in Fanconi anemia, 1927-2001. Cancer 97: 425-440, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood 105: 67-73, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Socie G, Scieux C, Gluckman E, et al. Squamous cell carcinoma after allogeneic bone marrow transplantation for aplastic anemia; futher evidence of a multistep process. Transplantation 66: 667-670, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Nara N, Miyamoto T, Kurisu A. Two siblings with Fanconi's anemia developing squamous cell carcinoma. Rihsho Ketsueki 21: 1944-1950, 1980(in Japanese, Abstract in English). [Google Scholar]
- 10.Aho S. Clinical conferences. Case of Fanconi' anemia. Kyobu Geka (Jpn J Thorac Surg) 33: 397-399, 1980(in Japanese). [PubMed] [Google Scholar]
- 11.Hosoya Y, Lefor A, Hirashima Y, et al. Successful treatment of esophageal squamous cell carcinoma in a patient with Fanconi anemia. Jpn J Clin Oncol 40: 805-810, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Alter BP. Fanconi's anemia, transplantation, and cancer. Pediatr Transplantation 9(Suppl): 81-86, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Spanier G, Pohl F, Giese T, et al. Fatal course of tonsillar squamous cell carcinoma associated with Fanconi anaemia: a mini review. J Craniomaxillofac Surg 40: 510-515, 2012(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 14.Alter BP. Radiosensitivity in Fanconi's anemia patients. Radiother Oncol 62: 345-347, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Marcou Y, D'Andrea A, Jeggo PA, Plowman PN. Normal cellular radiosensitivity in an adult Fanconi anaemia patient with marked clinical radiosensitivity. Radiother Oncol 60: 75-79, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka Y, Honda K, Asato R. A case of advanced carcinoma of the gingiva in a patient with Fanconi anemia. Kouku Intou Ka Gakkai Zasshi 27: 173-177, 2014(in Japanese). [Google Scholar]
- 17.Jerjes W, Upile T, Hamdoon Z, Alexander Mosse C, Morcos M, Hopper C. Photodynamic therapy outcome for T1/T2 No oral squamous cell carcinoma. Lasers Surg Med 43: 463-469, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Schethenbach K, Wagenmann M, Freund M, Schipper J, Hanenberg H. Squamous cell carcinomas of the head and neck in fanconi anemia: risk, prevention, theraphy, and the need for guidelines. Klin Padiatr 224: 132-138, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancer in Japan. Gastrointest Endosc 68: 1066-1072, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Kulter DI, Auerbach AD, Satagopan J, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg 129: 106-112, 2003. [DOI] [PubMed] [Google Scholar]