Abstract
Background: Prostate cancer (PCa) is the second most frequent cause of cancer-related death in men worldwide. It is a heterogeneous disease at molecular and clinical levels which makes its prognosis and treatment outcome hard to predict. The epithelial-to-mesenchymal transition (EMT) marks a key step in the invasion and malignant progression of PCa. We sought to assess the co-expression of epithelial cytokeratin 8 (CK8) and mesenchymal vimentin (Vim) in locally-advanced PCa as indicators of EMT and consequently predictors of the progression status of the disease.
Methods: Co-expression of CK8 and Vim was evaluated by immunofluorescence (IF) on paraffin-embedded tissue sections of 122 patients with PCa who underwent radical prostatectomies between 1998 and 2016 at the American University of Beirut Medical Center (AUBMC). EMT score was calculated accordingly and then correlated with the patients' clinicopathological parameters and PSA failure.
Results: The co-expression of CK8/Vim (EMT score), was associated with increasing Gleason group. A highly significant linear association was detected wherein higher Gleason group was associated with higher mean EMT score. In addition, the median estimated biochemical recurrence-free survival for patients with < 25% EMT score was almost double that of patients with more than 25%. The validity of this score for prediction of prognosis was further demonstrated using cox regression model. Our data also confirmed that the EMT score can predict PSA failure irrespective of Gleason group, pathological stage, or surgical margins.
Conclusion: This study suggests that assessment of molecular markers of EMT, particularly CK8 and Vim, in radical prostatectomy specimens, in addition to conventional clinicopathological prognostic parameters, can aid in the development of a novel system for predicting the prognosis of locally-advanced PCa.
Keywords: prostate cancer, cytokeratin 8, vimentin, epithelial-to-mesenchymal transition, Gleason group, clinicopathological parameters
Introduction
Prostate Cancer (PCa) is the second most frequently diagnosed cancer and the sixth leading cause of cancer death in males worldwide (1). Screening for PCa is not routinely practiced in the Middle East, which pertains to the rising incidence rates and the high proportion of patients being diagnosed with high-risk locally-advanced and metastatic disease in this region of the world (2, 3).
Radical prostatectomy is an effective therapeutic procedure for men with organ-confined PCa. This modality, however, fails in 30–40% of patients as serum prostate-specific antigen (PSA) levels continue to rise and patients eventually develop biochemical recurrence postoperatively (4). It is of utmost importance to identify the parameters that can accurately predict the prognosis and clinical outcome following radical prostatectomy. To date, several investigators have described the usefulness of various clinicopathological factors—including PSA, Gleason scores, pathological stage, surgical margin status (SMS), perineural invasion (PNI), seminal vesicle invasion (SVI), lymphovascular invasion (LVI), and tumor volume—and their correlation with treatment failure (5–8). However, these studies have carried several limitations, such as the recent stage migration and grade inflation because of the greater aggressiveness of PCa (9), besides the differences in PCa features among diverse ethnic groups (10).
Expression of epithelial-to-mesenchymal transition (EMT) markers represents a crucial step in the malignant progression of several cancers, such as prostate, breast, ovarian, and colon cancers (11–15). This pathological process ensues the breakdown of cell-to-cell or cell-to-extracellular matrix (ECM) adhesions at the polarized epithelium lining prompting conversion into mesenchymal phenotype and enhanced cell mobility, invasion, and metastasis (14, 16). The role of EMT in PCa metastasis has been studied (16) revealing significant interplay between EMT-related genes and tissue invasion on one hand, and alterations in TGF-β (17), IL-6 (18–20), AR variants (21, 22), FGF (23), and Wnt/β-catenin signaling pathways (24–26) on the other hand.
In a previous study by our group, we have reported increased co-expression of epithelial cytokeratin 8 (CK8) and mesenchymal vimentin (Vim) markers in androgen-independent PLum-AI murine PCa cell lines, which represent advanced stages of PCa, referring to a positive EMT status in those cells, when compared to androgen-dependent PLum-AD cells which represent primary PCa (27). CK8/Vim co-expression was also reported in other murine PCa cell lines, including PLum-P and PLum-C Pten−/− TP53−/− murine prostate epithelial progenitor cells (28).
In this study, we evaluated the co-expression of two potential molecular markers of EMT, namely CK8 and Vim, in radical prostatectomy specimens of locally-advanced PCa patients using immunofluorescent (IF) staining. Accordingly, we developed a novel scoring system to quantify EMT expression (EMT score) and explored the correlation between this score and the different clinicopathological outcomes. Our results confirmed that the EMT score can predict PSA failure, and thus biochemical recurrence, irrespective of Gleason group and other conventional PCa diagnostic and prognostic parameters.
Materials and Methods
Patients Selection
Using the radical prostatectomy institutional database (1998–2016) of the American University of Beirut Medical Center (AUBMC), we identified 122 patients with locally-advanced PCa. Those patients had adverse pathological features with more than 30 months of follow-up. The study with all its experimental protocols was conducted under the Institutional Review Board (IRB) approvals of the American University of Beirut (AUB) and AUBMC. The work described herein has been carried out in accordance with relevant guidelines and regulations, and in agreement with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving human subjects.
Clinicopathological Variables
Preoperative serum PSA level, Gleason group, pathological stage, positive surgical margin (PSM), perineural invasion (PNI), seminal vesicle invasion (SVI), lymphovascular invasion (LVI), and tumor volume in the PCa specimens were recorded.
Tissue Sampling and Gleason Scoring and Grouping
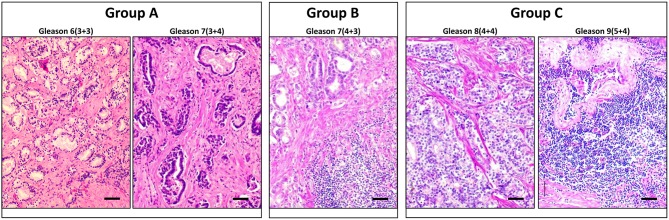
The tumor tissues were harvested and fixed in 4% formalin overnight, rinsed well in PBS and transferred to 70% ethanol before standard processing to obtain paraffin-embedded sections. Blocks of tumor tissues were identified by pathologists in terms of quality and content, and slides with unstained sections were obtained along with the H&E sections as a reference. The tumor grade and clinical stage were reviewed, and the Gleason scores were assigned by two independent pathologists according to the International Society of Urological Pathology (ISUP) criteria. This new five–grade group system has been suggested by the ISUP and accepted by the WHO in 2016, in order to address the deficiencies in the previous Gleason scoring systems (29). The sections were immunostained and analyzed for CK8/Vim co-expression, and the EMT score was then compared between three different Gleason groups that we assigned: group A (grade groups 1 and 2); group B (grade group 3); and group C (grade groups 4 and 5). Representative images of the H&E staining of PCa tissue sections that represent each of the three Gleason groups are shown in Figure 1.
Figure 1.
Representative H and E staining of PCa tissue sections that represent each of the three Gleason groups. Cross-sections of PCa tissues representing each of the three Gleason groups are stained with H and E. Left panel represents group A that includes Gleason scores 6 and 7(3+4). Middle panel represents Group B that includes Gleason score 7(4+3). Right panel represents group C that includes Gleason scores 8 and 9. Scale bars = 50 μm.
Antibodies and Reagents
Antibodies used in this study include mouse monoclonal anti-CK8 (1/200 dilution) (Covance, CA), rabbit polyclonal anti-Vim (1/50 dilution) (Santa Cruz Biotechnology, CA), Alexa 488 goat anti-rabbit, and Alexa 568 goat anti-mouse (Invitrogen, CA). All secondary Alexa Fluor antibodies were used at 1/200 dilution. Fluoro-gel II with DAPI (Electron Microscopy Sciences, PA) was used for mounting.
Immunofluorescent Staining Procedure for Tissues
Unstained formalin-fixed paraffin-embedded (FFPE) tissue sections were deparaffinized, and antigen retrieval was performed in a citrate buffer in a steamer at 100°C for 40 min. This was followed by protein blocking using the blocking buffer (3% BSA, 0.1% Triton x-100, and 10% Normal Goat Serum in PBS) for an hour at room temperature. Slides were stained using the different primary antibodies: anti-CK8 overnight, and anti-Vim for 2 h; then tissues were incubated with the corresponding secondary antibodies. Finally, slides were mounted with the anti-fade Fluoro-gel II with DAPI.
Microscope Specifications
Indirect immunofluorescence microscopic analyses were performed using Carl Zeiss Axio Observer.Z1 and LSM710 laser scanning confocal microscopes. All images were acquired and analyzed using the Carl Zeiss ZEN 2012 image software.
IF Evaluation and EMT Scoring
EMT scoring was performed manually using a 40 × objective and a Carl Zeiss Axio Observer.Z1 microscope. It was done by screening the whole tissue section in a systematic manner and counting the total number of glands, then counting the number of glands with at least one cell co-expressing CK8 and Vim. Then, the percentage was calculated by dividing the number of glands with at least one double positive cell by total number of glands, multiplied by 100. This percentage is referred to as EMT score. CK8/Vim staining was graded as double positive only when cytoplasmic staining was detectable.
Statistical Analysis
The EMT score was categorized into < 25% and more than or equal to 25%. This cutoff of 25% was assigned based on the EMT score distribution where 95.1% (116) of the total population clustered in the “less than or equal to 50% EMT score.” Chi-square test and two-tailed unpaired Student's t-test of independent variables were used to assess the association of the EMT score categorized into two groups with the sample clinicopathological characteristics such as age, PCa pathological stage, preoperative PSA, PSA failure, percentage of tumor volume involved, prostate size, perineural invasion, SVI, lympho-vascular invasion, and surgical margins. Student t-test of independent variables was used to compare the mean EMT score (as a continuous variable) between the different Gleason groups. A Mantel–Haenszel test of trend was run to determine whether a linear association existed between the EMT score categories and the different Gleason groups. In a secondary analysis, a linear regression model was built to examine the effect of the Gleason group on the EMT score while adjusting for the pathological stage and the surgical margins. EMT score in addition to the Gleason group, pathological stage, and surgical margins (the three clinicopathological variables which showed statistically significant difference between the two EMT score categories) were entered as covariates in the cox regression model. P ≤ 0.05 were considered significant. Statistical analysis was performed using the Statistical Package for the Social Sciences statistical package 21.0 software (SPSS, Inc.).
Results
Clinicopathological Characteristics of PCa Patients and Their Correlation With the EMT Score
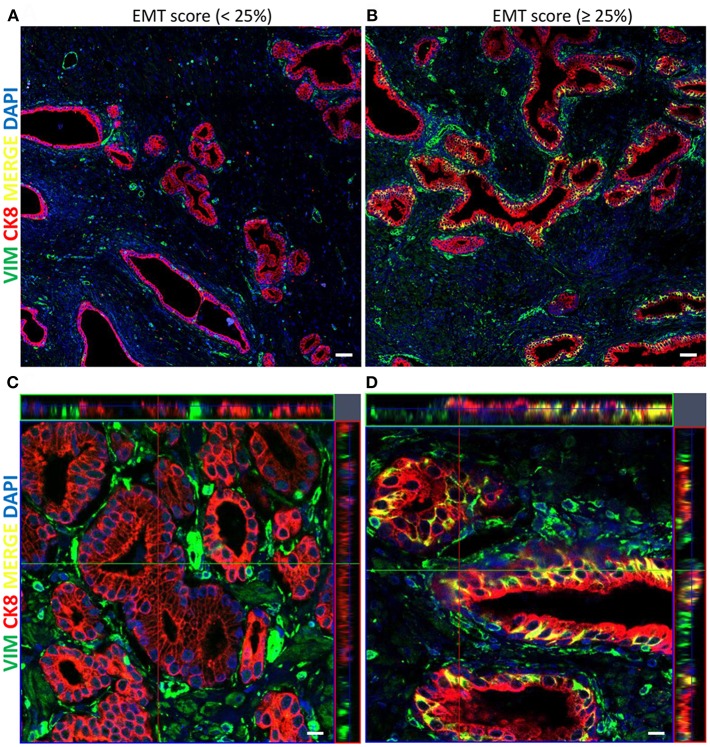
A total of 122 radical prostatectomy specimens were analyzed. Table 1 summarizes the clinicopathological characteristics of the 122 patients. Association of several clinicopathological variables and EMT score is shown in Table 2. The specimens were analyzed by IF and examined for CK8/Vim co-expression (EMT score) (Figure 2).
Table 1.
Clinicopathological characteristics of the 122 patients with PCa included in our study.
| Clinicopathological variable | Total N | Categories | n (%) |
|---|---|---|---|
| Age (in years) | 122 | Mean (±SD) | 62.1(±6.5) |
| < 70 | 109 (89.3) | ||
| ≥70 | 13 (10.7) | ||
| Gleason groups | 122 | A: Gleason scores 6 and 7(3 + 4) | 63 (51.6) |
| B: Gleason score 7(4 + 3) | 30 (24.6) | ||
| C: Gleason scores 8 and 9 | 29 (23.8) | ||
| Lympho-vascular invasion | 61 | Absent | 55 (90.2) |
| Present | 6 (9.8) | ||
| Perineural invasion | 94 | Absent | 28 (29.8) |
| Present | 66 (70.2) | ||
| Seminal vesicle invasion | 118 | Absent | 96 (81.4) |
| Present | 22 (18.6) | ||
| Lymph node invasion | 28 | Absent | 25 (89.3) |
| Present | 3 (10.7) | ||
| Pathological stage | 120 | pT2 | 35 (29.2) |
| ≥pT3 | 85 (70.8) | ||
| Preoperative PSA (in ng/mL) | 116 | Mean (±SD) | 10.7(±9.8) |
| < 10 | 76 (65.5) | ||
| ≥10 | 40 (34.5) | ||
| Prostate size (in g) | 120 | Mean (±SD) | 58.3 (±59.5) |
| < 50 | 65 (54.2) | ||
| ≥50 | 55 (45.8) | ||
| Tumor volume (in cc) | 113 | Mean (±SD) | 14.9 (±21.9) |
| < 5 | 26 (23) | ||
| ≥5 | 87 (77) | ||
| PSA failure | 87 | No | 45 (51.7) |
| Yes | 42 (48.3) |
Table 2.
Correlation of EMT score with the patients' clinicopathological variables.
| Clinicopathological variable | EMT score | P-value | |||
|---|---|---|---|---|---|
| <25 | ≥25 | Total | |||
| N (%) | N (%) | N (%) | |||
| Age (in years) | Mean (±SD) | 61.6 (±6.2) | 63.6 (±7.2) | 62 (±6.5) | 0.167 |
| <70 | 85 (93.4) | 22 (81.5) | 107 (90.7) | 0.061 | |
| ≥70 | 6 (6.6) | 5 (18.5) | 11 (9.3) | ||
| Total | 91 (100) | 27 (100) | 118 (100) | ||
| Gleason groups | A: Gleason scores 6 and 7(3 + 4) | 51 (56) | 9 (33.3) | 60 (50.8) | 0.014 |
| B: Gleason score 7(4 + 3) | 24 (26.4) | 6 (22.2) | 30 (25.4) | ||
| C: Gleason scores 8 and 9 | 16 (17.6) | 12 (44.4) | 28 (23.7) | ||
| Total | 91 (100) | 27 (100) | 118 (100) | ||
| Lympho-vascular invasion | Absent | 32 (88.9) | 22 (91.7) | 54 (90) | 0.725 |
| Present | 4 (11.1) | 2 (8.3) | 6 (10) | ||
| Total | 36 (100) | 24 (100) | 60 (100) | ||
| Perineural invasion | Absent | 15 (23.1) | 10 (38.5) | 25 (27.5) | 0.137 |
| Present | 50 (76.9) | 16 (61.5) | 66 (72.5) | ||
| Total | 65 (100) | 26 (100) | 91 (100) | ||
| Seminal vesicle invasion | Absent | 74 (83.1) | 19 (73.1) | 93 (80.9) | 0.251 |
| Present | 15 (16.9) | 7 (26.9) | 22 (19.1) | ||
| Total | 89 (100) | 26 (100) | 115 (100) | ||
| Lymph node invasion | Absent | 11 (91.7) | 13 (86.7) | 24 (88.9) | 0.681 |
| Present | 1 (8.3) | 2 (13.3) | 3 (11.1) | ||
| Total | 12 (100) | 15 (100) | 27 (100) | ||
| Pathological stage | pT2 | 21 (23.6) | 13 (48.1) | 34 (29.3) | 0.014 |
| ≥pT3 | 68 (76.4) | 14 (51.9) | 82 (70.7) | ||
| Total | 89 (100) | 27 (100) | 116 (100) | ||
| PSA failure | No | 32 (49.2) | 11 (55) | 43 (50.6) | 0.625 |
| Yes | 33 (50.8) | 9 (45) | 42 (49.4) | ||
| Total | 65 (100) | 20 (100) | 85 (100) | ||
| Surgical margins | Negative | 17 (18.7) | 12 (44.4) | 29 (24.6) | 0.006 |
| Positive | 74 (81.3) | 15 (55.6) | 89 (75.4) | ||
| Total | 91 (100) | 27 (100) | 118 (100) | ||
| Preoperative PSA (in ng/mL) | Mean (±SD) | 10 (±7.1) | 13.7 (±16.4) | 10.9 (±9.9) | 0.289 |
| < 10 | 55 (63.2) | 17 (68) | 72 (64.3) | 0.66 | |
| ≥10 | 32 (36.8) | 8 (32) | 40 (35.7) | ||
| Total | 87 (100) | 25 (100) | 112 (100) | ||
| Prostate size (in g) | Mean (±SD) | 58.5 (±67.7) | 60.1 (±24.8) | 58.9 (±60.4) | 0.903 |
| < 50 | 48 (53.9) | 13 (48.1) | 61 (52.6) | 0.598 | |
| ≥50 | 41 (46.1) | 14 (51.9) | 55 (47.4) | ||
| Total | 89 (100) | 27 (100) | 116 (100) | ||
| Tumor volume (in cc) | Mean (±SD) | 16.1 (±24.7) | 12.3 (±9.4) | 15.2 (±22.2) | 0.461 |
| < 5 | 18 (21.4) | 7 (28) | 25 (22.9) | 0.493 | |
| ≥5 | 66 (78.6) | 18 (72) | 84 (77.1) | ||
| Total | 84 (100) | 25 (100) | 109 (100) | ||
Bold values represent statistically significant data.
Figure 2.
Representative immunofluorescent images of the co-expression of CK8/Vim molecular markers in PCa tissue specimens stained with CK8 (red), Vimentin (green), and DAPI (blue). (A) Tile scan image (5 x 5) of PCa tissue showing low EMT score <25% (scale bar = 50μm). (B) Tile scan image (5 x 5) of PCa tissue showing high EMT score ≥25% (scale bar = 50 μm). (C) Z-stack with maximal and orthogonal projection of PCa tissue showing low EMT score <25% (scale bar = 10 μm). (D) Z-stack with maximal and orthogonal projection of PCa tissue showing high EMT score ≥25% (scale bar = 10μm).
In studying the sample distribution statistics between the two categories of the EMT score, a significant statistical difference was detected between the two categories in terms of Gleason group (p = 0.014), pathological stage (p = 0.014), and surgical margins (p = 0.006). No significant differences in the patient's age, pre-operative PSA, PSA failure (defined by an increase in blood PSA level at or above 0.2 ng/mL following surgery), and tumor volume were observed (Table 2).
High Mean EMT Score Is Significantly Associated With Higher Gleason Group
To investigate the difference in the mean EMT score between the assigned Gleason groups, an independent t-test was run. There were 60 patients in group A, 30 patients in group B, and 28 patients in group C. There was no statistical difference in the mean EMT score between group A and B. Nonetheless, the mean EMT score was higher in group B (M = 15.3%, SD = 21.3%) than group A (M = 10.7 %, SD = 11.6%), with a mean difference (M = −4.62, 95% CI [−13.04;3.8], p = 0.274). When comparing the mean EMT score of the 60 patients in the Gleason group A (M = 10.7 %, SD = 11.6%) to the 28 patients in group C (M = 26.8%, SD = 29.1%), a significant difference with quite high mean difference was recorded (M = −16.09, 95% CI [−27.71; −4.47], p = 0.008). The mean EMT score comparison between groups B and C revealed no significant difference, although a higher mean was recorded in the higher Gleason group (M = −11.47, 95% CI [−24.99; −2.06], p = 0.091) (Table 3).
Table 3.
Comparison of the mean EMT scores between Gleason groups.
| Gleason group | N | Mean (±SD) | Mean difference (95% CI) | P-value | |
|---|---|---|---|---|---|
| Mean EMT score | A: Gleason scores 6 and 7(3 + 4) | 60 | 10.7 (±11.6) | −4.62 [−13.04; 3.8] | 0.274 |
| B: Gleason score 7(4 + 3) | 30 | 15.3 (±21.3) | |||
| B: Gleason score 7(4 + 3) | 30 | 15.3 (±21.3) | −11.47 [−24.99; 2.06] | 0.091 | |
| C: Gleason scores 8 and 9 | 28 | 26.8 (±29.1) | |||
| A: Gleason scores 6 and 7(3 + 4) | 60 | 10.7 (±11.6) | −16.09 [−27.71; −4.47] | 0.008 | |
| C: Gleason scores 8 and 9 | 28 | 26.8 (±29.1) |
Bold values represent statistically significant data.
A mean plot of the EMT score vs. the three Gleason groups is shown in Supplementary Figure 1. A Mantel–Haenszel test of trend was run to determine whether a linear association existed between EMT score categorized into two groups (< 25% and more than or equal to 25%) and the assigned Gleason groups. The Mantel–Haenszel test of trend showed a statistically significant linear association between them [ = 7.547, p < 0.007, r = 0.254], where higher Gleason group was associated with a higher EMT score (Supplementary Table 1). A scatterplot simplifying the linear association between EMT score and the Gleason groups is presented in Supplementary Figure 2.
Gleason Groups Can Predict EMT Score Irrespective of the Pathological Stage and Surgical Margins
A multiple regression model was built to study if Gleason group can predict EMT score while adjusting for the pathological stage and surgical margins, the variables which showed statistically significant difference between the two EMT score categories (Table 2). The multiple regression model significantly predicted EMT score, F(3, 112) = 7.037, p < 0.001. R2 for the overall model was 15.9 % with an adjusted R2 of 13.6%. Only Gleason group added statistical significance to the prediction, p = 0.001. Regression coefficients and their P-values can be found in Supplementary Table 2.
EMT Score Can Predict PSA Failure Irrespective of Gleason Group, Pathological Stage, or Surgical Margins
To study the correlation between EMT score and PSA failure, a Cox regression model was built. Time to PSA failure was considered time to event, and EMT score, Gleason group, pathological stage, and surgical margins were added as covariates to the model using forward method. EMT score was found to be an independent predictor of PSA failure. Biochemical recurrence was higher in patients with EMT score ≥25% (OR: 2.23, 95% CI [1.018; 4.895], p = 0.045). The overall model has a χ2 of 4.221, with a P-value of 0.04. Biochemical recurrence-free survival curve estimating PSA failure based on the patients' EMT score is shown in Figure 3.
Figure 3.
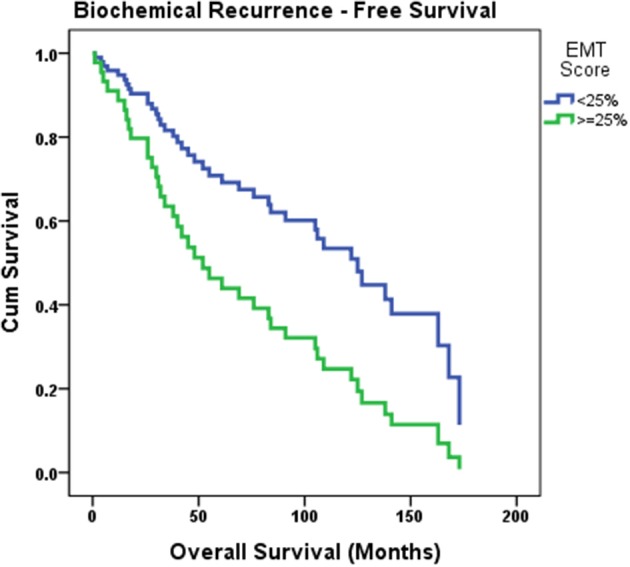
Biochemical recurrence-free survival curve estimating PSA failure based on the patients' EMT score. Cox regression model was built where time to PSA failure was considered time to event, and EMT score, Gleason group, pathological stage and surgical margins were added as covariates to the model. Biochemical recurrence was found to be higher in patients with EMT score ≥25% (p = 0.045).
Discussion
Despite the advances in the treatment of metastatic PCa, most patients eventually die from their disease. This is due to the rapid and poorly understood progression of PCa from a primary stage to an advanced and metastatic castration-resistant PCa (mCRPC) stage which involves several mechanisms, including epithelial-to-mesenchymal transition (EMT). The latter is recognized in endorsing the invasiveness of PCa cells due to increased mobility and migration of mesenchymal cells (16). In addition to the role of EMT in PCa progression, it has been identified as playing a substantial role in PCa therapeutic resistance to anti-androgens and radiotherapy (30). Therefore, it has been postulated that targeting EMT may improve the overall survival of patients with PCa (16). The main cause of PCa mortality is the progression to metastatic castration-resistant PCa (mCRPC); therefore, identifying the onset of metastatic dissemination through assessment of molecular markers of EMT can aid in the development of a novel system for predicting the prognosis of PCa. Nonetheless, the translation of EMT into clinical applicability presents substantial challenges (31). This can be attributed to tumor heterogeneity and diverse metastatic behavior, which is underrepresented in currently used homogenous cell lines and preclinical models (32). Yet, several studies have addressed the changes in the expression levels of genes and/or proteins associated with EMT in human tumor samples to establish an association with clinical significance.
In carcinoma, invasion and metastasis are associated with transition of cancer cells form an epithelial keratins-expressing phenotype to a mesenchymal vimentin (Vim)-expressing phenotype (33, 34). The importance of assessing the EMT status through investigating Vim overexpression was highlighted in different solid malignancies. For instance, Vim expression was associated with adverse prognosis in ductal breast carcinoma (35). Besides, in triple-negative breast cancer, Vim expression was significantly higher compared to other subtypes, and was shown to be associated with a worse prognosis and a more aggressive phenotype, thereby assisting as a biomarker for the prognosis of this aggressive subtype of breast cancer (36). In a study by Bukhari et al., Vim was also suggested to aid in predicting the risk of developing colon cancer and its use was proposed to serve as an antigen for tumor vaccination for colon cancers (37). Additionally, a significant increase in Vim expression coupled with a decrease in cytokeratin expression were observed in advanced grades of transitional cell carcinoma of the bladder, suggesting the potential use of these biomarkers for early diagnosis of bladder carcinoma (38). In PCa, Gravdal et al. focused on the independent relationship between an E-cadherin to N-cadherin switch and patient prognosis by unraveling the importance of EMT in PCa progression (39).
In the present study, we investigated the correlation between EMT score on one hand, designated by estimating the percentage of glands co-expressing epithelial CK8 and mesenchymal Vim markers out of the total glands counted within a PCa radical prostatectomy tissue section, and the various clinicopathological parameters among locally-advanced PCa patients on the other hand. The value of Vim expression as a predictor of recurrence was established in a previous study where Zhang et al. performed an immunohistochemical study and reported that risk of biochemical recurrence is associated with high levels of Vim which was described to be independent of Gleason score (40). In our patients, representing a cohort of high-risk locally-advanced PCa from the Middle East region, looking at co-expression patterns of CK8 and Vim revealed that the mean EMT score increases significantly as disease becomes more poorly differentiated reflected by higher Gleason group (Table 3). Our results show that there is a highly significant difference in the mean EMT score between Gleason groups A and C (10.7 ± 11.6% in Gleason group A vs. 26.8 ± 29.1% in Gleason group C, p = 0.008). Furthermore, there is a highly significant linear association based on Mantel–Haenszel test (p = 0.007) whereby higher Gleason groups were associated with higher EMT scores (Supplementary Figure 2). The added value of this EMT scoring system is the fact that it can predict PSA failure irrespective of Gleason group, pathological stage, and surgical margins (41). As PSA recurrence is a powerful predictor of distant metastasis, cancer-specific survival, and overall survival, these results suggest that the EMT score can be used to estimate the biochemical recurrence-free survival of a patient irrespective of other clinicopathological parameters.
A possible explanation of the link between EMT status and disease progression is the fact that cells with hybrid epithelial/mesenchymal phenotypes possess a large repertoire of survival strategies under many stress conditions (42). EMT has been linked to circulating tumor cells (CTCs) generation and subsequently metastasis. In colorectal cancer, for instance, the presence of biophenotypic and mesenchymal CTCs, rather than epithelial CTCs, is indicative of a more advanced disease stage and metastasis (43).
Conclusions
In conclusion, this study underscores the importance of EMT markers (increased Vim and decreased CK8 expression) for predicting the prognosis of PCa. Whereas, previous studies have indicated reduced expression of epithelial markers and increasing expression of mesenchymal markers, an EMT phenotype and the co-expression of such markers specifically CK8 and Vim and their association with outcome data have not been described. Since these markers could have a significant effect on the management of PCa patients, including projections of targeted therapy, we suggest the extrapolation of this study to larger cohorts of patients from different ethnicities to further validate our findings. Besides, since androgen receptor (AR) expression and EMT have been recently reported to be mutually exclusive (44), future studies are indeed warranted to evaluate expression levels of AR and PSA in the PCa tissue samples and their correlation with EMT score.
Study Limitations
We recognize that our study has some limitations. First, as a clinical study the sample size is relatively small, therefore the results obtained require further investigation on a larger cohort. Second, samples were collected retrospectively over the period of 18 years with around 75% of the samples having a positive margin and around 70% with a pathological stage greater than pT3. The latter identified the study sample as a high-risk cohort thus restricting the results obtained to such sample characteristics. Third, the retrospective collection of data led to missing information regarding the SVI, PNI, and LNM status of the patients; this might explain the lack of significant correlation between the EMT score and the metastatic status.
Author Contributions
KC, HB, OH, ES, CD, MH, MS, ZM, SN, AT, MB, RK, WW, RN, AS, ST, and ME-S contributed to the project design and execution of experiments. KC, HB, OH, and ES contributed to the analysis of results and writing of manuscript. AE-H, DM, and WA-K contributed to overlooking and following up with experiments, result analysis, and manuscript proofreading. AE-H, DM, and WA-K contributed to project design, result analysis, manuscript writing, and proofreading. All authors critically revised and edited the manuscript and approved the final draft.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all members of the Abou-Kheir's Laboratory for their support. In addition, we would like to thank all members of the core facilities in the DTS Building for their help and support. We would like to dedicate this work to the late Dr. Mark Jabbour (May his soul rest in peace).
Footnotes
Funding. This research was supported by funding from the Medical Practice Plan (MPP) at the American University of Beirut Faculty of Medicine (AUB-FM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00131/full#supplementary-material
Gleason group distribution among the EMT score categories.
Regression coefficients of the multiple regression model.
Mean plot of mean EMT score in percentage vs. different Gleason groups showing a linear association. Linear association exists between mean EMT score and the assigned Gleason groups A; Gleason scores 6 and 7(3 + 4), group B; Gleason score 7(4 + 3), and group C; Gleason scores 8 and 9, where the mean percentage EMT score increases drastically when the gleason group increases.
Scatterplot of the EMT score vs. different Gleason groups showing a linear association. A Mantel–Haenszel test of trend was run to determine whether a linear association existed between EMT score categorized into two groups (<25 and ≥25%) and the assigned Gleason groups revealing a statistically significant linear association between them (Supplementary Table 1, p < 0.007), where higher Gleason group was associated with a higher EMT score.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. (2011) 61:69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Shamseddine A, Saleh A, Charafeddine M, Seoud M, Mukherji D, Temraz S, et al. Cancer trends in Lebanon: a review of incidence rates for the period of 2003-2008 and projections until 2018. Popul Health Metr. (2014) 12:4. 10.1186/1478-7954-12-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukherji D, Massih SAE, Daher M, Chediak A, Charafeddine M, Shahait M, et al. Prostate cancer stage at diagnosis: first data from a Middle-Eastern cohort. J Clin Oncol. (2017) 35:e552 10.1200/JCO.2017.35.6_suppl.e552 [DOI] [Google Scholar]
- 4.Amling CL. Biochemical recurrence after localized treatment. Urol Clin North Am. (2006) 33:147–59. 10.1016/j.ucl.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 5.Quinn DI, Henshall SM, Haynes AM, Brenner PC, Kooner R, Golovsky D, et al. Prognostic significance of pathologic features in localized prostate cancer treated with radical prostatectomy: implications for staging systems and predictive models. J Clin Oncol. (2001) 19:3692–705. 10.1200/JCO.2001.19.16.3692 [DOI] [PubMed] [Google Scholar]
- 6.Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. (2005) 66:1245–50. 10.1016/j.urology.2005.06.108 [DOI] [PubMed] [Google Scholar]
- 7.Carvalhal GF, Daudi SN, Kan D, Mondo D, Roehl KA, Loeb S, et al. Correlation between serum prostate-specific antigen and cancer volume in prostate glands of different sizes. Urology. (2010) 76:1072–6. 10.1016/j.urology.2009.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright JL, Dalkin BL, True LD, Ellis WJ, Stanford JL, Lange PH, et al. Positive surgical margins at radical prostatectomy predict prostate cancer specific mortality. J Urol. (2010) 183:2213–8. 10.1016/j.juro.2010.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson IM, Canby-Hagino E, Lucia MS. Stage migration and grade inflation in prostate cancer: will Rogers meets Garrison Keillor. J Natl Cancer Inst. (2005) 97:1236–7. 10.1093/jnci/dji286 [DOI] [PubMed] [Google Scholar]
- 10.Byun SS, Lee S, Lee SE, Lee E, Seo SI, Lee HM, et al. Recent changes in the clinicopathologic features of Korean men with prostate cancer: a comparison with Western populations. Yonsei Med J. (2012) 53:543–9. 10.3349/ymj.2012.53.3.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs IB, Lichtenegger W, Buehler H, Henrich W, Stein H, Kleine-Tebbe A, et al. The prognostic significance of epithelial-mesenchymal transition in breast cancer. Anticancer Res. (2002) 22:3415–9. [PubMed] [Google Scholar]
- 12.Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M. Vimentin and epithelial-mesenchymal transition in human breast cancer–observations in vitro and in vivo. Cells Tissues Org. (2007) 185:191–203. 10.1159/000101320 [DOI] [PubMed] [Google Scholar]
- 13.Loboda A, Nebozhyn MV, Watters JW, Buser CA, Shaw PM, Huang PS, et al. EMT is the dominant program in human colon cancer. BMC Med Genomics. (2011) 4:9. 10.1186/1755-8794-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant CM, Kyprianou N. Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl Androl Urol. (2013) 2:202–11. 10.3978/j.issn.2223-4683.2013.09.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takai M, Terai Y, Kawaguchi H, Ashihara K, Fujiwara S, Tanaka T, et al. The EMT (epithelial-mesenchymal-transition)-related protein expression indicates the metastatic status and prognosis in patients with ovarian cancer. J Ovarian Res. (2014) 7:76. 10.1186/1757-2215-7-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo UG, Lee CF, Lee MS, Hsieh JT. The role and mechanism of epithelial-to-mesenchymal transition in prostate cancer progression. Int J Mol Sci. (2017) 18:E2079. 10.3390/ijms18102079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CL, Mahalingam D, Osmulski P, Jadhav RR, Wang CM, Leach RJ, et al. Single-cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT-related genes in metastatic prostate cancer. Prostate. (2013) 73:813–26. 10.1002/pros.22625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojas A, Liu G, Coleman I, Nelson PS, Zhang M, Dash R, et al. IL-6 promotes prostate tumorigenesis and progression through autocrine cross-activation of IGF-IR. Oncogene. (2011) 30:2345–55. 10.1038/onc.2010.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu CT, Hsieh CC, Lin CC, Chen WC, Hong JH, Chen MF. Significance of IL-6 in the transition of hormone-resistant prostate cancer and the induction of myeloid-derived suppressor cells. J Mol Med. (2012) 90:1343–55. 10.1007/s00109-012-0916-x [DOI] [PubMed] [Google Scholar]
- 20.Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: the role of interleukin 6 (IL-6). BJU Int. (2014) 113:986–92. 10.1111/bju.12452 [DOI] [PubMed] [Google Scholar]
- 21.Sun F, Chen HG, Li W, Yang X, Wang X, Jiang R, et al. Androgen receptor splice variant AR3 promotes prostate cancer via modulating expression of autocrine/paracrine factors. J Biol Chem. (2014) 289:1529–39. 10.1074/jbc.M113.492140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Qiu Y. Role of androgen receptor splice variants in prostate cancer metastasis. Asian J Urol. (2016) 3:177–84. 10.1016/j.ajur.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Jin C, Hamana T, Liu J, Wang C, An L, et al. Overexpression of FGF9 in prostate epithelial cells augments reactive stroma formation and promotes prostate cancer progression. Int J Biol Sci. (2015) 11:948–60. 10.7150/ijbs.12468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Jiao L, Hou J, Xu C, Wang L, Yu Y, et al. Dishevelled-2 silencing reduces androgen-dependent prostate tumor cell proliferation and migration and expression of Wnt-3a and matrix metalloproteinases. Mol Biol Rep. (2013) 40:4241–50. 10.1007/s11033-013-2506-6 [DOI] [PubMed] [Google Scholar]
- 25.Baruah MM, Khandwekar AP, Sharma N. Quercetin modulates Wnt signaling components in prostate cancer cell line by inhibiting cell viability, migration, and metastases. Tumour Biol. (2016) 37:14025–34. 10.1007/s13277-016-5277-6 [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Ye L, Zhang X, Wang M, Lin C, Huang S, et al. FZD8, a target of p53, promotes bone metastasis in prostate cancer by activating canonical Wnt/beta-catenin signaling. Cancer Lett. (2017) 402:166–76. 10.1016/j.canlet.2017.05.029 [DOI] [PubMed] [Google Scholar]
- 27.Daoud G, Monzer A, Bahmad H, Chamaa F, Hamdar L, Mouhieddine TH, et al. Primary versus castration-resistant prostate cancer: modeling through novel murine prostate cancer cell lines. Oncotarget. (2016) 7:28961–75. 10.18632/oncotarget.8436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abou-Kheir W, Hynes PG, Martin P, Yin JJ, Liu YN, Seng V, et al. Self-renewing Pten-/- TP53-/- protospheres produce metastatic adenocarcinoma cell lines with multipotent progenitor activity. PLoS ONE. (2011) 6:e26112 10.1371/journal.pone.0026112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, et al. A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol. (2016) 69:428–35. 10.1016/j.eururo.2015.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark TW, Hensley PJ, Spear A, Pu H, Strup SS, Kyprianou N. Predictive value of epithelial-mesenchymal-transition (EMT) signature and PARP-1 in prostate cancer radioresistance. Prostate. (2017) 77:1583–91. 10.1002/pros.23435 [DOI] [PubMed] [Google Scholar]
- 31.Tarin D. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. (2005) 65:5996–6001. 10.1158/0008-5472.CAN-05-0699 [DOI] [PubMed] [Google Scholar]
- 32.Santamaria PG, Moreno-Bueno G, Portillo F, Cano A. EMT: present and future in clinical oncology. Mol Oncol. (2017) 11:718–38. 10.1002/1878-0261.12091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyer B, Tucker GC, Valles AM, Franke WW, Thiery JP. Rearrangements of desmosomal and cytoskeletal proteins during the transition from epithelial to fibroblastoid organization in cultured rat bladder carcinoma cells. J Cell Biol. (1989) 109:1495–509. 10.1083/jcb.109.4.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valles AM, Boyer B, Badet J, Tucker GC, Barritault D, Thiery JP. Acidic fibroblast growth factor is a modulator of epithelial plasticity in a rat bladder carcinoma cell line. Proc Natl Acad Sci USA. (1990) 87:1124–8. 10.1073/pnas.87.3.1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemalatha A, Suresh TN, Kumar ML. Expression of vimentin in breast carcinoma, its correlation with Ki67 and other histopathological parameters. Indian J Cancer. (2013) 50:189–94. 10.4103/0019-509X.118724 [DOI] [PubMed] [Google Scholar]
- 36.Yamashita N, Tokunaga E, Kitao H, Hisamatsu Y, Taketani K, Akiyoshi S, et al. Vimentin as a poor prognostic factor for triple-negative breast cancer. J Cancer Res Clin Oncol. (2013) 139:739–46. 10.1007/s00432-013-1376-6 [DOI] [PubMed] [Google Scholar]
- 37.Bukhari S, Mokhdomi TA, Chikan NA, Amin A, Qazi H, Wani SH, et al. Affinity proteomics led identification of vimentin as a potential biomarker in colon cancers: insights from serological screening and computational modelling. Mol Biosyst. (2015) 11:159–69. 10.1039/C4MB00506F [DOI] [PubMed] [Google Scholar]
- 38.Rahmani AH, Babiker AY, Alwanian WM, Elsiddig SA, Faragalla HE, Aly SM. Association of cytokeratin and vimentin protein in the genesis of transitional cell carcinoma of urinary bladder patients. Dis Markers. (2015) 2015:204759. 10.1155/2015/204759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. (2007) 13:7003–11. 10.1158/1078-0432.CCR-07-1263 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q, Helfand BT, Jang TL, Zhu LJ, Chen L, Yang XJ, et al. Nuclear factor-kappaB-mediated transforming growth factor-beta-induced expression of vimentin is an independent predictor of biochemical recurrence after radical prostatectomy. Clin Cancer Res. (2009) 15:3557–67. 10.1158/1078-0432.CCR-08-1656 [DOI] [PubMed] [Google Scholar]
- 41.Lee DK, Park JH, Kim JH, Lee SJ, Jo MK, Gil MC, et al. Progression of prostate cancer despite an extremely low serum level of prostate-specific antigen. Korean J Urol. (2010) 51:358–61. 10.4111/kju.2010.51.5.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jolly MK, Boareto M, Huang B, Jia D, Lu M, Ben-Jacob E, et al. Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front Oncol. (2015) 5:155. 10.3389/fonc.2015.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao R, Cai Z, Li S, Cheng Y, Gao H, Liu F, et al. Expression and clinical relevance of epithelial and mesenchymal markers in circulating tumor cells from colorectal cancer. Oncotarget. (2017) 8:9293–302. 10.18632/oncotarget.14065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin CY, Jan YJ, Kuo LK, Wang BJ, Huo C, Jiang SS, et al. Elevation of androgen receptor promotes prostate cancer metastasis by induction of epithelial-mesenchymal transition and reduction of KAT5. Cancer Sci. (2018) 109:3564. 10.1111/cas.13776 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gleason group distribution among the EMT score categories.
Regression coefficients of the multiple regression model.
Mean plot of mean EMT score in percentage vs. different Gleason groups showing a linear association. Linear association exists between mean EMT score and the assigned Gleason groups A; Gleason scores 6 and 7(3 + 4), group B; Gleason score 7(4 + 3), and group C; Gleason scores 8 and 9, where the mean percentage EMT score increases drastically when the gleason group increases.
Scatterplot of the EMT score vs. different Gleason groups showing a linear association. A Mantel–Haenszel test of trend was run to determine whether a linear association existed between EMT score categorized into two groups (<25 and ≥25%) and the assigned Gleason groups revealing a statistically significant linear association between them (Supplementary Table 1, p < 0.007), where higher Gleason group was associated with a higher EMT score.