Abstract
The global land surface absorbs about a third of anthropogenic emissions each year, due to the difference between two key processes: ecosystem photosynthesis and respiration. Despite the importance of these two processes, it is not possible to measure either at the ecosystem scale during daytime. Eddy-covariance measurements are widely used as the closest ‘quasi-direct’ ecosystem-scale observation from which to estimate ecosystem photosynthesis and respiration. Recent research, however, suggests that current estimates may be biased by up to 25%, due to a previously unaccounted-for process: the inhibition of leaf respiration in the light. Yet the extent of inhibition remains debated, and implications for estimates of ecosystem-scale respiration and photosynthesis remain unquantified. Here, we quantify an apparent inhibition of daytime ecosystem respiration across the global FLUXNET eddy-covariance network, and identify a pervasive influence that varies by season and ecosystem type. We develop partitioning methods that can detect an apparent ecosystem-scale inhibition of daytime respiration and find that diurnal patterns of ecosystem respiration might be markedly different than previously thought. The results call for the reevaluation of global terrestrial carbon cycle models, and also suggest that current global estimates of photosynthesis and respiration may be biased, some on the order of magnitude of anthropogenic fossil fuel emissions.
Keywords: photosynthesis, carbon cycle, terrestrial models, leaf, canopy, FLUXNET, partitioning, inhibition, respiration in the light
Introduction
The eddy-covariance technique allows for the measurement of the exchange of carbon between ecosystems and the atmosphere at a high temporal (i.e. half-hourly) frequency1. Since the 1980’s the technique has been widely deployed, and is currently used to measure land-atmosphere exchange of carbon, water, and energy at hundreds of sites around the world2.
The net measured flux of carbon (Fc) is the result of two contrasting processes: the uptake of carbon through photosynthesis, and the release of carbon through ecosystem respiration. Nighttime respiration is observed directly at the ecosystem scale using eddy-covariance, but daytime photosynthesis and respiration are mixed in the measured daytime net Fc flux. A variety of approaches have therefore been developed to estimate both the apparent photosynthesis (true photosynthesis minus photorespiration3, Fp) and ecosystem respiration (Fr) from the measured net Fc (e.g., 4,5,14–22,6–13). The partitioned estimates of Fp and Fr have been combined with machine learning to generate data-driven budgets of global photosynthesis and respiration (e.g., 23,24), allowing for new understanding of the controls of global ecosystem function and the carbon cycle (e.g., 25). They are also widely used to test and develop process-based models26 and remote-sensing based estimates of ecosystem function27.
Recent evidence, however, suggests that a key overlooked process may affect the partitioned estimates of Fp and Fr: the inhibition of leaf respiration in the light28,29. Leaf respiration is an important component of plant function30 and often accounts for 50% of whole plant respiration31. Leaf level studies have long suggested that leaf respiration is inhibited in the light32, though the responsible processes remain unclear32–34, but the lack of evidence at the ecosystem scale has historically limited research to theoretical explorations of the potential impact on estimates of apparent photosynthesis and ecosystem respiration3,6,11,22,35–39. Importantly, in the absence of ecosystem-scale evidence12,19, methods used to partition eddy-covariance have assumed that ecosystem-scale respiration is not inhibited by light. Recent isotopic evidence28,29,40 suggests that this is no longer a tenable assumption, and that considerable biases result in the two main approaches used to partition eddy-covariance observations of Fc 12,19. But evidence for an ecosystem-scale inhibition of leaf respiration in the light across a variety of ecosystems, and an assessment of implications for the two main partitioning approaches to estimate Fp and Fr, remains lacking 29,41.
There are two main approaches to partition measured eddy-covariance measurements of Fc into the component fluxes of Fp and Fr. The nighttime method (NT12) relies on the fact that fluxes measured during the night consist of purely Fr (as photosynthesis requires light). The NT method uses measured nighttime fluxes to estimate a seasonally varying reference respiration rate (Rref, at a reference temperature) and the sensitivity to temperature (e.g., 6,12,42–46). These parameters, estimated from nighttime data, are then combined to estimate Fr during the day. The difference between the observed Fc and the estimated Fr gives an estimate of Fp (Fc = Fr – Fp). In contrast, the second approach, referred to as the daytime method (DT19), uses primarily daytime data, and estimates Fp by fitting a light response curve to observations of Fc7,9,15,19,44,47. The fitted curve, informed by daytime measurements, is used to estimate Rref, and, combined with a temperature response function, to estimate nighttime Fr fluxes. Importantly, both the DT and NT methods assume that any difference between daytime and nighttime ecosystem respiration is due to temperature alone12,19.
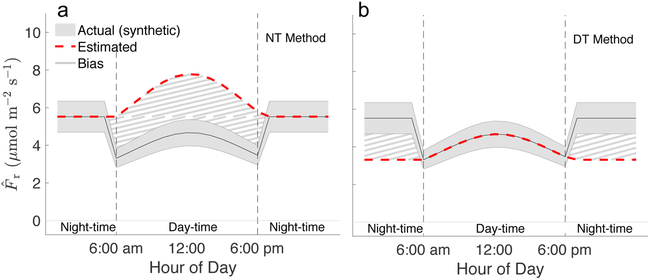
An inhibition of leaf respiration during the day would affect both the DT and NT partitioning approaches12,19, but it would do so in different ways for each. The approach focused on nighttime data12 assumes that Fr responds solely to temperature, and thus increases with temperature during the day. The NT method will thus overestimate daytime total ecosystem respiration, and consequently apparent photosynthesis (Fig. 1), if leaf respiration is inhibited during the day11. Similarly, the approach focused on daytime data19 assumes that the difference between daytime estimated Fr and Fr at night is driven solely by temperature. The DT method will thus underestimate nighttime respiration if inhibition occurs (Fig. 1). Fundamentally, both methods assume that the same Rref is applicable during daytime as at night; a questionable assumption due to the potential for the inhibition of leaf respiration in the light (e.g.,11,32).
Fig. 1 ∣. A schematic illustrating the potential bias due to a hypothetical inhibition of reference respiration (Rref) between night-time () and day-time ().
Biases are estimated for: a, ecosystem respiration (Fr) from the night-time (NT) partitioning method and, b, the day-time (DT) partitioning method, using synthetic values for ecosystem respiration (). Gray areas represent the ‘observed’ flux, and red lines the flux predicted by the NT (a) and DT (b) methods. Cross-hatched areas indicate biases. Vertical lines represent the times of hypothetical sunrise and sunset.
Here, we use globally distributed eddy-covariance observations from the FLUXNET 2015 dataset2, to develop data-driven estimates of an apparent inhibition of ecosystem scale respiration during the day. Employing multiple methods, we estimate reference respiration separately during the day () and during the night (), and use the difference between them as an estimate of the apparent inhibition of daytime ecosystem respiration. Our analysis indicates a widespread occurrence of inhibition, which follows consistent seasonal patterns within ecosystem types, with magnitudes that differ by ecosystem type, and which is in line with reports of a leaf level inhibition of non-photorespiratory mitochondrial CO2 release in the light. We assess the implications for estimates of Fp and Fr, and suggest two modified algorithms that detect and account for inhibited daytime respiration.
Results
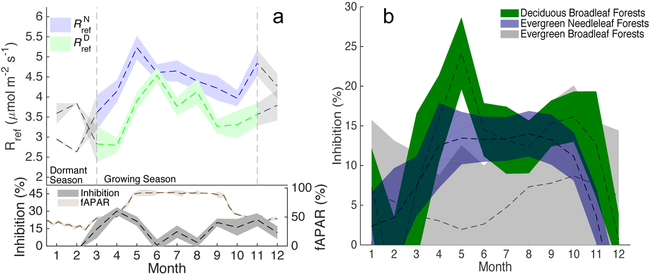
We found reference ecosystem respiration estimated using the daytime method to be consistently lower than reference respiration estimated using only nighttime observations (Fig. 2a) during the growing season. Apparent ecosystem inhibition, defined as , showed a marked ecosystem-type-specific seasonal pattern. For example, at Harvard Forest, a deciduous forest in the northeastern US, the apparent inhibition of total ecosystem respiration reached 30% during spring, dropping off to near zero shortly after peak foliage development (Fig. 2a), consistent with a previous isotope-based study at this site29, though larger than suggested by expectations based on leaf-level results (see Supplementary Methods 1). We observed a similar seasonal cycle at other deciduous broadleaved forests (Fig. 2b), with maximum apparent inhibition in early spring. The seasonal cycle in evergreen needle-leaved forests was elongated compared to deciduous forests and less pronounced in spring, and had a lower overall level of apparent ecosystem scale inhibition (Fig. 2b). Evergreen broadleaved forests showed low apparent ecosystem scale inhibition levels (Fig. 2b), potentially in contrast with reports of a consistent 30% inhibition across tropical and Mediterranean broadleaved species at the leaf level48,49. This suggests that either non-leaf respiration contributes a large proportion of ecosystem respiration in evergreen broadleaved ecosystems, or we underestimate the impact of leaf-level inhibition on an ecosystem scale for evergreen broadleaved forests. In general, the seasonal cycle of apparent inhibition generally matched the seasonal cycle of satellite-derived fraction of absorbed photosynthetically active radiation (Fig. S1), indicating a large influence of active leaf area.
Fig. 2 ∣. Seasonal cycles of Rref inferred from both day- and night-time observations.
a, Mean monthly estimates of Rref from day-time () and night-time () data (Harvard Forest, 1992-2015, top panel), and the resulting estimate of mean monthly inhibition (%) (calculated as , bottom panel), along with the satellite derived mean monthly fraction of absorbed radiation (fAPAR, 2001-2015). Vertical lines separate the growing season from the dormant season. Shaded areas represent the standard error about the mean monthly values. b, Mean monthly inhibition for three different plant functional types, deciduous broadleaf forests (n=12), evergreen needeleaf forests (n=25) and evergreen broadleaf forests (n=5) using Tier 1 sites with five years or more in the FLUXNET database. Shaded areas represent the standard error about the mean monthly values.
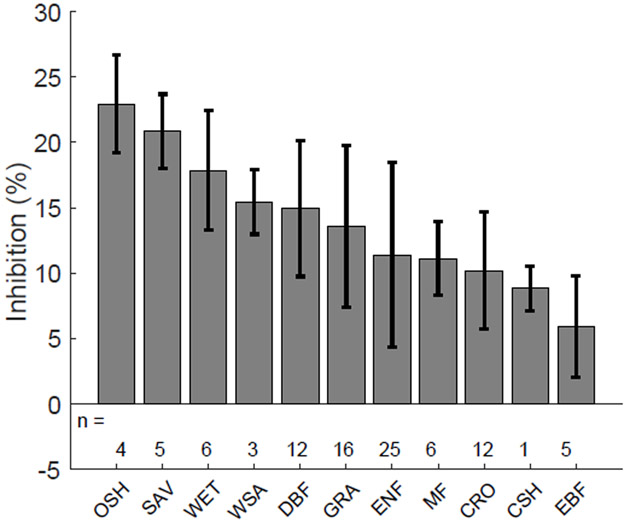
The extent of apparent inhibition differed by ecosystem plant function type (PFT, Fig. 3) with mean apparent inhibition levels during the growing season ranging from 22.9 ± 3.7% (mean, standard error) for open shrublands to a low of 5.1 ± 3.8% for evergreen broadleaved forests (Fig. 3). The plant functional types with largest apparent inhibition (open shrublands, savannahs, woody savannahs, wetlands, Fig. 3), also showed the highest bias in Fr between partitioning methods in a previous study19. Over all sites, the average apparent inhibition of ecosystem respiration during the growing season estimated by the modified DT partitioning method was 14.4 ±1.9%, which was lower than the 19.8 ±1.7% we estimated from the independent generalized additive models (GAM) approach (Fig. S2), but consistent with a hypothetical extrapolation of a range of estimates of the inhibition of leaf level respiration in the light to the ecosystem scale (Supp. Methods S1).
Fig. 3 ∣. Mean inhibition (I, %) during the growing season for each of 11 different ecosystem types.
Inhibition values are calculated as , (i.e. as the relative difference between Rref during day-time, and night-time, ) for different plant functional types (PFT) for sites with a data record of five years or more. SAV: Savannah; GRA: Grassland; DBF: Deciduous broadleaved forest; ENF: Evergreen needleleaved forest; MF: Mixed forest; OSH: Open shrubland; WSA: Woody savannah; CSH: Closed shrubland; CRO: Cropland; EBF: Evergreen broadleaved forest; WET: Wetland. Error bars represent the standard error of the mean across sites, while n represent the number of sites for each PFT.
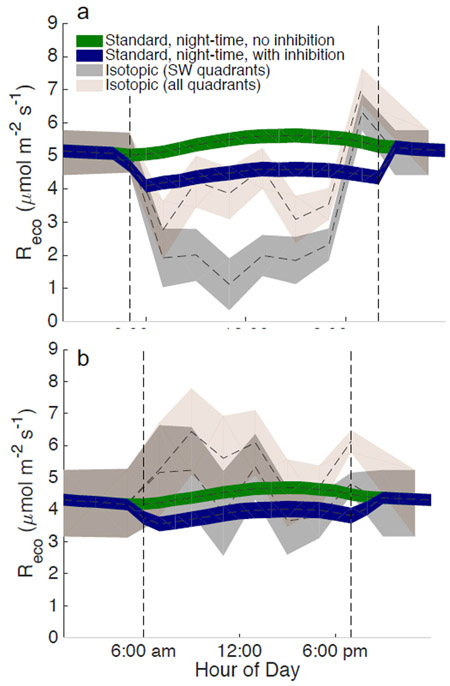
We assessed the detected apparent inhibition by comparing our estimates of Fr to independent estimates obtained from multi-year isotope records at Harvard Forest29. The apparent inhibition at Harvard Forest implied a lower rate of Fr during daytime than at night, particularly in late spring and early summer (Fig. 4a). The temporal dynamics in Fr largely matched those inferred by isotope measurements29 when using observations from all wind directions. The isotopic observations show a larger apparent inhibition when filtered for the south-western quadrant (Fig. 4a, as in29), which is a more homogenous region, dominated by deciduous trees. The lack of agreement for a particular wind direction is not surprising: the NT and DT partitioning methods are parameterized using all directions, as limiting to a specific direction limits the data available for parameterization, whereas the relative abundance of deciduous versus evergreen trees differs by wind direction29. Differences in the predominant wind direction during daytime and nighttime have also been suggested to potentially cause differences in apparent inhibition levels29, though we did not find meaningful differences in the predominant wind directions between day and night at Harvard Forest (Fig. S4). Late summer fluxes also showed evidence of apparent inhibition in the eddy-covariance flux data, in contrast to results from the isotopic data. It should be noted however, that changes in flux footprints could potentially lead to meaningful differences between the isotopic and eddy-covariance methods.
Fig. 4. A comparison of the standard night-time partitioning, with and without inhibition, to the partitioning inferred from carbon isotope measurements at Harvard Forest.
Ecosystem respiration (Fr) for the June-July (a) and August-September (b) periods. The carbon isotope inferred fluxes are those presented in Wehr et al. (2016). Shaded areas represent one standard error about the mean.
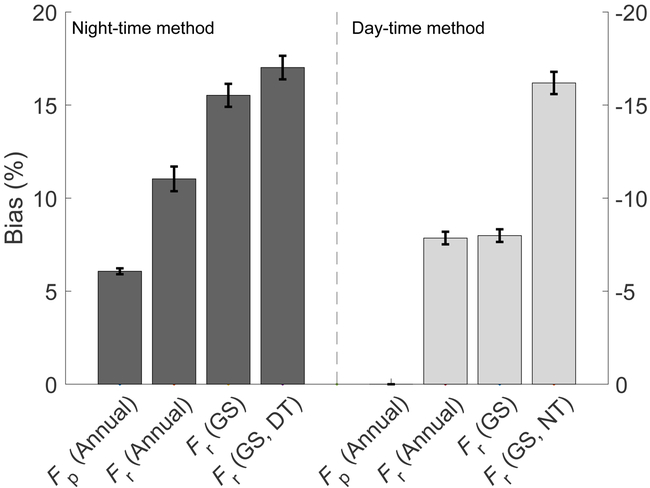
The prevalence of apparent inhibition suggests that previous approaches to partition Fc into Fr and Fp are likely biased. We compared estimates of Fr and Fp from both the DT and NT partitioning methods, with and without the modifications that allow for an apparent inhibition of ecosystem respiration (see methods). As expected, the DT method showed no bias in Fp on any timescale (Fig. 5), as any bias introduced by light inhibition of leaf respiration in the daytime method would primarily affect the DT method estimates of respiration at night (Fig. 1). Indeed, not taking apparent inhibition into account in the DT method led to an underestimation of total annual Fr by 7.9 ± 0.4% (Fig. 5). This bias was prevalent during the growing season only, and was due to a 16.2 ± 0.6% underestimation of growing season nighttime Fr (Fig. 5, Fig. S3). In contrast, for the NT partitioning method, apparent inhibition led to positive biases, i.e. an overestimation in both Fp and Fr. Biases in Fp, which by definition occur during growing season daytime conditions, led to an overestimation of total annual Fp by 7.0 ± 0.2%. Total annual biases in Fr of 11.4 ± 0.7% were primarily due to an overestimation of 17.4 ± 0.6% during growing season daytime conditions (Fig. 5).
Fig. 5 ∣. Relative biases in estimates of photosynthesis (Fp) and respiration (Fr).
Biases are calculated on both an annual and growing season (GS) basis, and for the growing season during the day for the night-time method (GS, DT), and during the night for the day-time method (GS, NT). Biases (positive, left; negative, right) are defined for each method as the difference between the version of the method that does not allow for an inhibition of ecosystem respiration during the day and the version that does. Positive biases (left) indicate that the method version that does not allow for inhibition overestimates net flux components compared to the version that does allow for inhibition. Values are calculated using all data from the FLUXNET Tier 1 dataset. Error bars represent the standard error of the mean bias across all sites.
Discussion
The lack of evidence of the influence of the inhibition of leaf respiration in the light on canopy scale processes has led to much debate and allowed ecosystem models and eddy covariance partitioning methods to omit the process altogether11,32,38. Recent results using isotopic flux observations29,41, however, confirmed that ecosystem scale respiration was often lower during the day at two sites, and attributed the response to the inhibition of leaf respiration in the light. In a study at a deciduous temperate forest29, Fr was more than 2 times lower during the day than at night in the early growing season. This difference was not captured by the non-isotopic partitioning approaches tested, leading to an overestimation of ~25% of apparent photosynthesis in spring at that forest. Similarly, a short campaign of isotopic flux observations in an alfalfa field41 found lower Fr during the day, and subsequently a bias in the partitioning methods tested. Our results suggest that inhibition is indeed a pervasive phenomenon, but one that varies in magnitude by season and plant functional type. The resulting biases are smaller than previously reported29, particularly at annual scales (Fig. 5), but have important implications for diel cycles, partitioning methods, and ecosystem models.
The seasonal cycle of apparent inhibition we report is in line with previous results showing that apparent inhibition is stronger in the early growing season at Harvard Forest29. One explanation for such a dynamic is found in the relative contribution of aboveground and belowground respiration to the total respiratory flux. At Harvard Forest, for example, the early season respiratory flux is ~50% aboveground respiration, driven by leaf growth and development, compared to 10% later in the growing season, when soil respiration plays a larger role50. This is consistent with reports that leaf respiration is highest in late spring and decreases during the course of the summer51,52, due to higher metabolic activity associated with development of new leaves and shoots52. Seasonality of apparent inhibition at the ecosystem scale is likely influenced by multiple factors, in particular seasonal changes in the ratio of leaf to branch, stem and soil respiration11,38,53,54, seasonal changes in the components of foliar respiration (i.e. the construction costs of new leaves is higher in spring55,56), increases in the proportional soil respiration due to priming by root exudates, and increases in the shaded leaf fraction with canopy development57. Consistent with the latter, an influence of total leaf area has also been proposed11,38 and is supported here by comparisons to seasonal cycles of fAPAR (Fig. S1), with higher leaf area potentially leading to higher leaf respiration and thus a higher influence on apparent inhibition. That said, higher leaf area can be associated with denser forests with high soil and woody biomass and respiration rates58,59, and we did not observe a relationship between maximum fAPAR and apparent inhibition across sites. This suggests that the distribution of apparent inhibition across PFTs is more related to the ratio of leaf to non-leaf respiration than to total leaf area. Measurements of seasonal cycles of leaf-level inhibition of leaf respiration in the light across a variety of plant types, along with measurements of non-leaf (soil, roots, bole and branch) respiration rates, would help elucidate the seasonality and between-site inhibition differences reported here.
Other factors, unrelated to actual leaf-scale process, could also affect the apparent difference between daytime and nighttime respiration reported here. Nighttime observations are often associated with low and sporadic turbulence, and although the observations are processed to minimize the effect of low turbulence, other forms of transport (e.g. advection) may bias the observed fluxes17. Advective losses of CO2 would result in an underestimation of nighttime fluxes (and thus ), however, and consequently an underestimation of inhibition. Advective losses are highly site dependent, but intercomparison experiments using eddy covariance fluxes and upscaled chamber estimates suggest an underestimation of nighttime respiration up to 30%60–62. Similarly, the boundary layer can become stratified at night due to radiative cooling of the canopy, with an associated increase in storage of respired CO2 within the canopy63. Increases in turbulence in the early morning can cause vertical advection64, as is commonly observed in sites with more complex canopy structure (e.g., 65), which could lead to an overestimation of apparent , and thus an underestimation of apparent inhibition. These potential biases, along with results of the independent GAM method and synthetic analyses (Fig. S2), suggest that the levels of apparent inhibition reported here represent a conservative estimate. Other potential biases, such as the choice of temperatures for partitioning (e.g., air, leaf, wood and soil temperatures;53,54), also deserve further attention. The single source models used here have the potential to be over-parameterized12,19, however, so an approach that adds more parameters for ecosystem components at different temperatures and sensitivities is unlikely to be widely applicable53.
An additional source of uncertainty lies in the fact that the temperature sensitivity of non-photorespiratory mitochondrial CO2 release has been reported to be lower during the day than at night66,67. We assessed the implications of a lower leaf E0 for our results by rerunning the partitioning and analysis with a lower E0 imposed for daytime respiration, setting a conservative66 ratio of nighttime to daytime leaf E0d_leaf = 0.5.E0n. In order to scale to ecosystem respiration, we assumed that leaf respiration is 50% of total ecosystem respiration. There is considerable variation in this scaling ratio between sites, but 50% represents a conservative estimate for Harvard Forest50 and temperate forests more broadly. The results show that applying a lower E0d_leaf leads to only small changes in the magnitude of the detected response. At Harvard Forest, for example, the apparent inhibition is reduced in the August-September period, but not in June-July (Supplementary Figure 6), and the reduction does not affect the general magnitude of inhibition or its seasonal cycle at this site (Supplementary Figure 7). Across all sites globally, using the lower E0 for leaf daytime respiration leads to a small reduction in the bias between methods (Supplementary Figure 8). E0d_leaf could also vary seasonally due to acclimation, though there is little consensus regarding whether and how E0d_leaf acclimates. For example, McLaughlin et al.68 report long-term acclimation of the temperature response of E0d_leaf in one species but not in another. Other studies also report seasonal acclimation69–71, but many studies report no acclimation between seasons72,73. Most recently, Heskel et al.74 found no seasonal variation in the temperature sensitivity of daytime leaf respiration for the dominant species (Red Oak) at Harvard Forest. Crous et al.75 conclude that it is not known whether or how much E0d_leaf varies seasonally under field conditions, and hypothesize that the difference between study results may reflect a species-specific ability to acclimate, and may be restricted to fast growing species.
Ultimately, independent measurements of each ecosystem respiration and temperature component, and photosynthesis proxies, are needed in order to reduce uncertainty in current estimates of apparent photosynthesis and respiration at eddy-covariance sites. A full characterization of the uncertainties involved will require the incorporation of multiple alternative partitioning approaches and assumptions.
Neither the nighttime or daytime based partitioning algorithms most commonly used account for the inhibition of respiration during the day. Previous results suggest that this omission would lead to a 10 to 25% overestimation of daily apparent photosynthesis at specific sites11,29,41. Here we show that the implications are more nuanced, at times in the opposite direction to that previously suggested, and depend on the partitioning method used. The DT method showed no effect of inhibition on estimates of either apparent photosynthesis or daytime respiration, but did underestimate respiration at night (Fig. 5). In contrast, both apparent photosynthesis and respiration estimates from the NT method were biased by the apparent inhibition, leading to an overestimation of both. The mean growing season bias in respiration during day or night in the NT and DT methods (respectively 17.4 ± 0.6%, −16.2 ± 0.6%, mean, standard err., Fig. 5) is in line with published estimates of inhibition at the leaf scale38 (Supp. Methods S1). The annual biases we report are comparable to previous analyses of methodological bias. For example, Falge et al.44, using different methods and a limited number of sites, reported an annual respiration bias of ~6% between different daytime and nighttime partitioning approaches, whereas both Suyker and Verma7 and Xu and Baldocchi76 report a bias of up to 20%, compared to our reported average bias of 9.7% (Fig. 5). Lasslop et al.19, however, reported a small median bias in annual ecosystem respiration of 13 g C m−2 yr−1 between the DT and NT methods, compared to our median biases of −43.4 ± 0.08 and 77.7 ± 0.2 g C m−2 yr−1 for the DT and NT methods respectively.
As both the NT and DT methods are commonly used by upscaling approaches to estimate global budgets of photosynthesis and respiration (e.g. 23,24), our results suggest a bias in previous global estimates based on eddy-covariance data. That said, although biases were relatively high at certain times of the year (e.g., during the day in the growing season in the NT method; during the night in the DT method), annual totals were less affected. Our estimates suggest that annual apparent photosynthesis was overestimated by the NT method by an average of 7.0 ± 0.2% at the studied sites, and annual respiration overestimated by 11.4 ± 0.7%. For the DT method, the only biases were for respiration, ranging from 16.2 ± 0.6% for nighttime respiration during the growing season to 7.9 ± 0.3% on an annual scale.
Although the most commonly used NT and DT methods do not account for a lower basal respiration during the day, both can be modified to allow them to do so. In the case of the DT method19, the modification is relatively straightforward (see methods). Our results suggest that future partitioning efforts should include a modified DT method, where is used to estimate respiration during the night, not . In the case of the NT method12, accounting for inhibition requires an independent estimate of . Here we use a fitted light response curve to estimate the applied in the modified nighttime method. Note that this approach, to an extent, preserves the original distinction between the NT and DT methods. The original NT method uses only nighttime observations, while the original DT method uses primarily daytime observations but also uses nighttime observations to estimate the temperature sensitivity of ecosystem respiration (E0, Eq.119). Here, the modified DT method additionally uses nighttime observations to estimate , and the modified NT method uses daytime observations to estimate . As with the original NT method, the modified NT method estimates Fp as the residual between observed Fc and modeled Fr. The modified DT method preserves the approach of the original DT method by estimating Fp as a function of light, temperature and vapor pressure deficit. It is worth noting however that the modified methods proposed here, as with the original DT method, do not preserve full independence between nighttime and daytime data, which could lead to self-correlation (cf. 77).
In order to assess the robustness of our results, we developed an independent machine learning approach (see methods) to estimate and using generalized additive models (GAMs78). The strength of such an inductive approach is that it does not require the functional form of the response to be specified a-priori, thus reducing the influence of model structural error, which is known to lead to biases in estimates of 9,79. Estimates of apparent inhibition from the GAM method were larger than those from the modified DT and NT methods, suggesting that the results presented herein may be conservative estimates of ecosystem scale inhibition. Being unconstrained, however, the GAM approach can lead to implausible responses (e.g., a negative quantum yield of photosynthesis) if such responses are supported by the observations for specific windows. Although the GAM method used here is therefore not readily applicable for partitioning eddy-covariance flux observations, advanced applications of machine learning methods to flux partitioning (e.g., 13,16,41) may prove effective.
Our results have potentially important implications for models of the terrestrial carbon cycle. Few such models include an inhibition of leaf respiration in the light, and those that do lack the information necessary for adequate parameterization32,38,80, though previous studies have tested the potential bias implicated11. Eddy-covariance observations are commonly used to develop and test all other estimates of ecosystem scale photosynthesis and respiration (e.g., land surface models, remote sensing). We show that the fluxes of respiration and apparent photosynthesis previously used were incorrect, with biases that vary on both diel and seasonal cycles. The biases uncovered here thus likely apply to land surface models and remote sensing based estimates of photosynthesis and respiration.
The inhibition of leaf respiration in the light has long been acknowledged32, and is supported by various lines of evidence38, and estimation techniques32, though different interpretations exist regarding the actual mechanisms involved32–34,81,82. Tcherkez et al.32 summarize various explanations for the inhibition of leaf respiration in the light, and conclude that it is likely due to a combination of different processes. Previous studies have suggested the inhibition may also affect ecosystem scale fluxes22,29,41. Here, we demonstrate that ecosystem basal respiration is systematically lower during the day than at night in a wide variety of ecosystem types. The observed apparent inhibition is consistent with previous reports of leaf-level inhibition of respiration in the light, though we do not identify the underlying cause. The results suggest that previous eddy-covariance based estimates of global photosynthesis and respiration are likely biased high, and call for a reevaluation of terrestrial ecosystem models.
Methods
Eddy-covariance observations
We used eddy-covariance observations of carbon fluxes between ecosystems and the atmosphere from the FLUXNET 2015 openly available (Tier 1) database. The database contains observations from 166 sites around the world (Table S1, www.fluxnet.org), incorporating data collected at sites from multiple regional flux networks. The data used includes half-hourly or hourly observations of net carbon fluxes (Fc) and meteorological observations (incoming radiation [SW_IN_FILL], air temperature [TA_F], and vapor pressure deficit (VPD_F)). All analysis was performed on data that was pre-filtered by the FLUXNET network to exclude conditions of low turbulence or conditions that do not meet the requirement of the eddy covariance technique. The Fc estimate used was NEE_VUT_USTAR50, which applied a variable threshold of friction velocity (USTAR) for each year from the 50th percentile of USTAR thresholds identified. The associated uncertainty estimate used is NEE_VUT_USTAR50_RANDUNC. All data used are freely available for download, along with detailed descriptions, at http://fluxnet.fluxdata.org/.
Partitioning methods
We applied the two most commonly used partitioning methods, one focused on the use of nighttime data12 and the other primarily focused on the use of daytime data19. Here we describe both methods as applied, and then describe the modifications made to each to allow the detection and incorporation of an apparent inhibition of respiration in the light.
Nighttime partitioning method
The nighttime (NT) partitioning method relies on the fact that photosynthesis is zero at night, so any nighttime measurements purely contain the respiratory flux. The NT method uses nighttime measurements to estimate a reference respiration rate, which is then projected into the day using a temperature response function that is directly parameterized by nighttime observations12. The difference between this estimate of daytime respiration (Fr) and the observed net carbon flux (Fc) is then attributed to apparent photosynthesis (Fp). Formally, the model is constructed using an Arrhenius-type model after Lloyd & Taylor83 to describe the temperature dependence of Fr as:
| (1) |
where Rref (μmol C m−2 s−1) is the reference respiration rate at the reference temperature (Tref = 15 ºC), and E0 (ºC) is the temperature sensitivity. Tair is the air temperature, and the parameter T0 (ºC) is set to a constant −46.02 ºC following Lloyd & Taylor83. A constant value is estimated for E0 for the whole year, while Rref is estimated every 5 days using a 15-day window (following 12). Here, . It should be noted that the true driving temperature is likely a combination of air, leaf, wood and soil temperatures53,54, the approach applied here follows convention in using air temperature observations, as those are most commonly available across a wide range of sites. The nighttime method is thus applied to partition the observed flux data from the FLUXNET 2015 Tier 1 data release (Table S1), and the R code implementation is available to download from https://github.com/bgctw/REddyProc84.
Daytime partitioning method
The daytime (DT) partitioning method differs from the nighttime partitioning method in that it uses observations during the daytime to parameterize a light response curve, from which it estimates both the reference respiration Rref and the photosynthetic carbon flux (Fp). Nighttime data are also used in the DT method, but only to estimate the temperature sensitivity parameter E0. Formally, the net carbon flux (Fc) is modeled following Lasslop et al.19 using a combination of the rectangular hyperbolic light-response curve8and an ecosystem respiration term9, as:
| (2) |
where α (μmol C J−1) is the canopy-scale quantum yield (i.e. the initial slope of the light response curve), β (μmol C m−2 s−1) is the maximum rate of CO2 uptake of the canopy at light saturation, Rg is the global radiation (W m−2) and γ (μmol C m−2 s−1) is the modeled ecosystem respiration (described below). Parameter β is estimated as an exponentially decreasing function of atmospheric vapor pressure deficit of air (VPD), in order to account for the effect of VPD on apparent photosynthesis, as:
| (3) |
where β0, k and VPD0 are fit parameters.
The modeled respiration term, γ, is estimated using the same function as in Eq. 1 (i.e. γ = Fr). Here, E0 is first estimated as in the NT method, by fitting Eq. 1 to nighttime observations. With the fixed E0, the remaining parameters (, α, β0, k and VPD0) are estimated by fitting the entire model (Eq. 2) to the daytime data. Nighttime fluxes of Fr are then estimated by using the fit model (with , α, β0, k and VPD0 from daytime, and E0 from nighttime data) along with the observed nighttime air temperatures. The daytime method is thus applied to partition the observed flux data from the FLUXNET 2015 Tier 1 data release, and the R code implementation84 is available to download from https://github.com/bgctw/REddyProc. In both the daytime and nighttime methods, day and night were determined based on the corresponding flags in the FLUXNET data archive (i.e. variable NIGHT).
Modified partitioning methods that allow for inhibition
Both of the approaches described above are built on the assumption that the reference respiration rate (Rref) does not change between night and day (i.e. ). The nighttime approach applies an Rref that is estimated using nighttime data to the daytime, and the daytime approach applies an Rref that is estimated using primarily daytime data to the nighttime. Clearly, if the reference respiration rate is lower during the day than during the night, as has been suggested by recent studies29,41, then the nighttime method will overestimate daytime respiration (and thus by definition apparent photosynthesis), and the daytime method will underestimate nighttime respiration.
We modified both the standard DT and NT partitioning methods12,19 described above to account for an apparent inhibition by estimating and applying and separately. Here, we describe the modifications performed and their motivation.
For the modified DT method, we changed the implementation to allow for a difference between the reference respiration that is applied to estimate nighttime and daytime fluxes. The standard DT method estimates and uses is it as a prior to estimate . It then uses to estimate both night- and daytime respiratory fluxes. In our modified daytime method we applied to estimate daytime fluxes only, and applied to estimate nighttime fluxes. Otherwise, the modified DT method preserves the structure of the original DT method, with both Fr and Fp estimated by equations 1 and 2, with parameters E0 and estimated from nighttime data, and with parameters , α, β0, k and VPD0 estimated from daytime data. To test the efficacy of the modified DT methods, we compared the estimates of Fr from both the original and modified DT method to observed nighttime Fr (Fig. S3).
For the modified NT method, we similarly changed the implementation to allow for a difference between the reference respiration that is applied to estimate nighttime and daytime fluxes. The standard NT method estimates from nighttime data and applies this to calculate daytime Fr. In our modified method, we used the nighttime method derived to estimate nighttime fluxes, as in the original method, but used an independently derived to estimate daytime fluxes. The used in the modified NT method is calculated following the same procedure as in the DT method, based on the intercept of a light response curve fit to daytime observations. Otherwise, the modified NT method preserves the structure of the original, with Fr estimated by equation 1, and Fp taken as the residual between the observed Fc and the modeled Fr, with parameters E0 and estimated from nighttime data, and parameters estimated from daytime data. These modifications largely preserve the original differences between the NT and DT methods but allow for an independent reference respiration to be used during the night and day in both the nighttime and the daytime methods. It should be noted however that the modified NT method is not solely based on nighttime data, as daytime observations are used to estimate the daytime reference respiration based on the fit of a light response curve.
Estimating apparent inhibition
We estimated apparent inhibition (I) as the difference between Rref calculated separately from nighttime () and daytime () observations. To ensure internal consistency, both and were estimated using the daytime method, as the prior (nighttime based) and posterior (daytime based) estimates of Rref. This implies that the same temperature sensitivity (E0), and data window lengths, are applied to both and for estimating I. We then estimated the percent apparent inhibition from the estimated parameters on a monthly basis as . Note that we implicitly assume that I is independent of light level as I is typically observed to start at very low light levels32, though a dependence on light level has been reported85.
Independent test based on Generalized Additive Models.
We developed an approach based on generalized additive models to derive independent estimates of , , and thus apparent inhibition, to compare to the inhibition estimates derived from the partitioning approach described above. Generalized additive models (GAMs) are a form of generalized linear model in which the predicted variable depends on smooth functions of predictor variables, thus allowing for unprescribed non-linear responses78. We derived estimates of by fitting a GAM every second day to 12-day moving windows of nighttime observations, using air temperature as a predictor. The GAM for estimating utilized penalized regression smoothing splines with a basis dimension of n knots (i.e. fit <- gam(y ~ s(x, k = n))). We estimated as the GAM prediction at given a reference temperature of the mean hourly temperature of each window. Similarly, for , we fit a GAM every second day to 12-day moving windows of daytime observations, using air temperature, light and VPD as predictors. Here, the GAM utilized penalized regression smoothing splines with a basis dimension of 3, 5, and 3 knots for air temperature, light and VPD respectively. The higher number of knots for the light response allowed the GAM to capture the non-linear form of the light response curve. Only windows with 10 or more observations were used. We then estimated as the GAM prediction at a given reference temperature of the mean hourly air temperature for each window, with zero light and window-mean VPD. The resulting apparent inhibition estimates were calculated as . The GAM analysis was implemented in R (version 3.3.3) using the Mixed GAM Computational Vehicle with Automatic Smoothness Estimation package (MGCV, version 1.8-23), with all parameters set to package defaults other than those specified here.
Satellite estimates of vegetation
As the inhibition of ecosystem respiration in the light is hypothesized to be driven by a suppression of leaf respiration38, the presence of active leaf area can be useful to determine periods during which apparent inhibition might be expected. We used satellite estimates of the fraction of absorbed photosynthetically active radiation (fAPAR) from the Moderate Resolution Imaging Spectroradiometer (MODIS) as a proxy for the extent of active leaf area. fAPAR estimates were obtained from the MOD15A2 fAPAR product at a 1km resolution for a 3×3 pixel area around each site, on an 8-day temporal resolution for the period March 1st 2000 to December 31st 2015. These data were quality controlled and aggregated to monthly averages for comparison to the seasonal cycles of apparent inhibition across sites.
Supplementary Material
Acknowledgements
TFK was supported by the NASA Terrestrial Ecology Program IDS Award NNH17AE86I. DP thanks the RINGO project funded by the European Union's Horizon 2020 Research and Innovation Programme under grant agreement 730944. We also acknowledge support from the Director, Office of Science, Office of Biological and Environmental Research of the US Department of Energy under the AmeriFlux Management Project. This work used eddy covariance data acquired and shared by the FLUXNET community, including these networks: AmeriFlux, AfriFlux, AsiaFlux, CarboAfrica, CarboEuropeIP, CarboItaly, CarboMont, ChinaFlux, Fluxnet-Canada, GreenGrass, ICOS, KoFlux, LBA, NECC, OzFlux-TERN, TCOS-Siberia, and USCCC. The ERA-Interim reanalysis data are provided by ECMWF and processed by LSCE. The FLUXNET eddy covariance data processing and harmonization was carried out by the European Fluxes Database Cluster, AmeriFlux Management Project, and Fluxdata project of FLUXNET, with the support of CDIAC and ICOS Ecosystem Thematic Center, and the OzFlux, ChinaFlux and AsiaFlux offices. We especially acknowledge all the PIs who contributed data to the FLUXNET Tier 1 dataset.
Footnotes
Data availability statement
This work used openly available FLUXNET 2015 v3 Tier 1 eddy covariance data acquired and shared by the FLUXNET community. All related data is publicly available for download at http://fluxnet.fluxdata.org
Code availability statement
Code used in the analysis presented in this paper is available online in two repositories. The first contains the modified REddyProc partitioning algorithms and can be accessed at https://github.com/trevorkeenan/REddyProc. The second contains the post-partitioning data processing pipeline code, and can be accessed at https://github.com/trevorkeenan/inhibitionPaperCode.
Declaration of competing interests
The authors declare no competing interests.
References
- 1.Baldocchi D TURNER REVIEW No. 15. ‘Breathing’ of the terrestrial biosphere: lessons learned from a global network of carbon dioxide flux measurement systems. Aust. J. Bot. 56, 1 (2008). [Google Scholar]
- 2.Pastorello G et al. A New Data Set to Keep a Sharper Eye on Land-Air Exchanges. Eos (Washington. DC). 1–6 (2017). doi: 10.1029/2017EO071597 [DOI] [Google Scholar]
- 3.Wohlfahrt G & Gu L The many meanings of gross photosynthesis and their implication for photosynthesis research from leaf to globe. Plant Cell Environ. 38, 2500–2507 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granier A et al. The carbon balance of a young Beech forest. Funct. Ecol. 14, 312–325 (2000). [Google Scholar]
- 5.Barford CC et al. Factors controlling long- and short-term sequestration of atmospheric CO2 in a mid-latitude forest. Science (80-. ). 294, 1688–91 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Janssens IA et al. Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob. Chang. Biol. 7, 269–278 (2001). [Google Scholar]
- 7.Suyker AE & Verma SB Year-round observations of the net ecosystem exchange of carbon dioxide in a native tallgrass prairie. Glob. Chang. Biol. 7, 279–289 (2001). [Google Scholar]
- 8.Falge E et al. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric. For. Meteorol. 107, 43–69 (2001). [Google Scholar]
- 9.Gilmanov TG et al. Gross primary production and light response parameters of four Southern Plains ecosystems estimated using long-term CO 2 -flux tower measurements. Global Biogeochem. Cycles 17, 1071 (2003). [Google Scholar]
- 10.Yi C et al. A nonparametric method for separating photosynthesis and respiration components in CO2 flux measurements. Geophys. Res. Lett. 31, 1–5 (2004). [Google Scholar]
- 11.Wohlfahrt G, Bahn M, Haslwanter A, Newesely C & Cernusca A Estimation of daytime ecosystem respiration to determine gross primary production of a mountain meadow. Agric. For. Meteorol. 130, 13–25 (2005). [Google Scholar]
- 12.Reichstein M et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 11, 1424–1439 (2005). [Google Scholar]
- 13.Hagen SC et al. Statistical uncertainty of eddy flux - Based estimates of gross ecosystem carbon exchange at Howland Forest, Maine. J. Geophys. Res. Atmos. 111, 1–12 (2006). [Google Scholar]
- 14.Stoy PC et al. An evaluation of models for partitioning eddy covariance-measured net ecosystem exchange into photosynthesis and respiration. Agric. For. Meteorol. 141, 2–18 (2006). [Google Scholar]
- 15.Gilmanov TG et al. Partitioning European grassland net ecosystem CO2 exchange into gross primary productivity and ecosystem respiration using light response function analysis. Agric. Ecosyst. {&} Environ. 121, 93–120 (2007). [Google Scholar]
- 16.Desai AR et al. Cross-site evaluation of eddy covariance GPP and RE decomposition techniques. Agric. For. Meteorol. 148, 821–838 (2008). [Google Scholar]
- 17.van Gorsel E et al. Application of an alternative method to derive reliable estimates of nighttime respiration from eddy covariance measurements in moderately complex topography. Agric. For. Meteorol. 148, 1174–1180 (2008). [Google Scholar]
- 18.Scanlon TM & Sahu P On the correlation structure of water vapor and carbon dioxide in the atmospheric surface layer: A basis for flux partitioning. Water Resour. Res. 44, 1–15 (2008). [Google Scholar]
- 19.Lasslop G et al. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: Critical issues and global evaluation. Glob. Chang. Biol. 16, 187–208 (2010). [Google Scholar]
- 20.Scanlon TM & Kustas WP Partitioning carbon dioxide and water vapor fluxes using correlation analysis. Agric. For. Meteorol. 150, 89–99 (2010). [Google Scholar]
- 21.Sulman BN, Roman DT, Scanlon TM, Wang L & Novick KA Comparing methods for partitioning a decade of carbon dioxide and water vapor fluxes in a temperate forest. Agric. For. Meteorol. 226–227, 229–245 (2016). [Google Scholar]
- 22.Bruhn D et al. Estimating daytime ecosystem respiration from eddy-flux data. BioSystems 103, 309–313 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Beer C et al. Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. Science 329, 834–8 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Jung M et al. Global patterns of land-atmosphere fluxes of carbon dioxide, latent heat, and sensible heat derived from eddy covariance, satellite, and meteorological observations. J. Geophys. Res. 116, 1–16 (2011). [Google Scholar]
- 25.Jung M et al. Compensatory water effects link yearly global land CO2 sink changes to temperature. Nature 541, 516–520 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Williams M et al. Improving land surface models with FLUXNET data. Biogeosciences 6, 1341–1359 (2009). [Google Scholar]
- 27.Running SW et al. A Global Terrestrial Monitoring Network Integrating Tower Fluxes , Flask Sampling , Ecosystem Modeling and EOS Satellite Data. System 127, 108–127 (1999). [Google Scholar]
- 28.Kok B On the interrelation of respiration and photosynthesis in green plants. Biochim. Biophys. Acta 3, 625–631 (1949). [Google Scholar]
- 29.Wehr R et al. Seasonality of temperate forest photosynthesis and daytime respiration. Nature 534, 680–683 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Atkin OK et al. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 206, 614–636 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Amthor J The McCree–de Wit–Penning de Vries–Thornley Respiration Paradigms: 30 Years Later. Ann. Bot. 86, 1–20 (2000). [Google Scholar]
- 32.Tcherkez G et al. Leaf day respiration: low CO2 flux but high significance for metabolism and carbon balance. New Phytol. 216, 986–1001 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Farquhar GD & Busch FA Changes in the chloroplastic CO2 concentration explain much of the observed Kok effect: a model. New Phytol. 214, 570–584 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Buckley TN, Vice H & Adams MA The Kok effect in Vicia faba cannot be explained solely by changes in chloroplastic CO 2 concentration. New Phytol. (2017). doi: 10.1111/nph.14775 [DOI] [PubMed] [Google Scholar]
- 35.Amthor JS & Baldocchi DD Terrestrial Higher Plant Respiration and Net Primary Production. Terr. Glob. Product. 33–59 (2001). doi: 10.1016/B978-012505290-0/50004-1 [DOI] [Google Scholar]
- 36.Morgenstern K et al. Sensitivity and uncertainty of the carbon balance of a Pacific Northwest Douglas-fir forest during an El Niño/La Niña cycle. Agric. For. Meteorol. 123, 201–219 (2004). [Google Scholar]
- 37.Chambers JQ et al. Respiration from a tropical forest ecosystem: Partitioning of sources and low carbon use efficiency. Ecol. Appl. 14, S72–S88 (2004). [Google Scholar]
- 38.Heskel MA, Atkin OK, Turnbull MH & Griffin KL Bringing the Kok effect to light: A review on the integration of daytime respiration and net ecosystem exchange. Ecosphere 4, 1–14 (2013). [Google Scholar]
- 39.Baldocchi DD & Harley PC Scaling carbon dioxide and water vapour exchange from leaf to canopy in a deciduous forest. I. Leaf model parametrization. Plant, Cell Environ. 18, 1157–1173 (1995). [Google Scholar]
- 40.Gong XY, Schäufele R, Lehmeier CA, Tcherkez G & Schnyder H Atmospheric CO 2 mole fraction affects stand-scale carbon use efficiency of sunflower by stimulating respiration in light. Plant. Cell Environ. 40, 401–412 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Oikawa PY et al. Revisiting the partitioning of net ecosystem exchange of CO2 into photosynthesis and respiration with simultaneous flux measurements of 13CO2 and CO2, soil respiration and a biophysical model, CANVEG. Agric. For. Meteorol. 234–235, 149–163 (2017). [Google Scholar]
- 42.Hollinger DY et al. Forest-atmosphere carbon dioxide exchange in eastern Siberia. Agric. For. Meteorol. 90, 291–306 (1998). [Google Scholar]
- 43.MIGLIETTA F et al. Severe drought effects on ecosystem CO2 and H2O fluxes in three Mediterranean evergreen ecosystems: revision of current hypotheses? Glob. Chang. Biol. 8, 999–1017 (2002). [Google Scholar]
- 44.Falge E et al. Seasonality of ecosystem respiration and gross primary production as derived from FLUXNET measurements. Agric. For. Meteorol. 113, 53–74 (2002). [Google Scholar]
- 45.Law BE, Hall R, Forestry C & State O Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric. For. Meteorol. 113, 97–120 (2002). [Google Scholar]
- 46.Rambal S, Joffre R, Ourcival JM, Cavender-Bares J & Rocheteau A The growth respiration component in eddy {CO}2 flux from a Quercus ilex mediterranean forest. Glob. Chang. Biol. 10, 1460–1469 (2004). [Google Scholar]
- 47.Gilmanov TG, Johnson D. a & Saliendra NZ Growing season CO2 fluxes in a sagebrush-steppe ecosystem in Idaho: bowen ratio/energy balance measurements and modeling. Basic Appl. Ecol. 4, 167–183 (2003). [Google Scholar]
- 48.Weerasinghe LK et al. Canopy position affects the relationships between leaf respiration and associated traits in a tropical rainforest in Far North Queensland. Tree Physiol. 34, 564–584 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Turnbull MH et al. Light inhibition of foliar respiration in response to soil water availability and seasonal changes in temperature in Mediterranean holm oak (Quercus ilex) forest. Funct. Plant Biol. 44, 1178–1193 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Giasson M-A et al. Soil respiration in a northeastern US temperate forest: a 22-year synthesis. Ecosphere 4, art140 (2013). [Google Scholar]
- 51.Falge E, Graber W, Siegwolf R & Tenhunen JD A model of the gas exchange response ofPicea abies to habitat conditions. Trees 10, 277–287 (1996). [Google Scholar]
- 52.Brooks JR, Hinckley TM, Ford DE & Sprugel DG Foliage dark respiration in Abies amabilis (Dougl.) Forbes: variation within the canopy. Tree Physiol. 9, 325–338 (1991). [DOI] [PubMed] [Google Scholar]
- 53.Wohlfahrt G & Galvagno M Revisiting the choice of the driving temperature for eddy covariance CO2 flux partitioning. Agric. For. Meteorol. 237–238, 135–142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lasslop G et al. On the choice of the driving temperature for eddy-covariance carbon dioxide flux partitioning. Biogeosciences 9, 5243–5259 (2012). [Google Scholar]
- 55.Landhäusser SM, Desrochers A & Lieffers VJ A comparison of growth and physiology in Picea glauca and Populus tremuloides at different soil temperatures. Sci. York 1929, 1922–1929 (2001). [Google Scholar]
- 56.Migliavacca M et al. Influence of physiological phenology on the seasonal pattern of ecosystem respiration in deciduous forests. Glob. Chang. Biol. 21, 363–376 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Law BE, Cescatti A & Baldocchi DD Leaf area distribution and radiative transfer in open-canopy forests: implications for mass and energy exchange. Tree Physiol. 21, 777–787 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Moyano FE, Kutsch WL & Rebmann C Soil respiration fluxes in relation to photosynthetic activity in broad-leaf and needle-leaf forest stands. Agric. For. Meteorol. 148, 135–143 (2008). [Google Scholar]
- 59.Migliavacca M et al. Semiempirical modeling of abiotic and biotic factors controlling ecosystem respiration across eddy covariance sites. Glob. Chang. Biol. 17, 390–409 (2011). [Google Scholar]
- 60.Goulden ML, Munger JW, Fan SM, Daube BC & Wofsy SC Measurements of carbon sequestration by long-term eddy covariance: Methods and a critical evaluation of accuracy. Glob. Chang. Biol. 2, 169–182 (1996). [Google Scholar]
- 61.Lavigne MB et al. Comparing nocturnal eddy covariance measurements to estimates of ecosystem respiration made by scaling chamber measurements at six coniferous boreal sites. J. Geophys. Res. 102, 977–985 (1997). [Google Scholar]
- 62.Law BE, Baldocchi DD & Anthoni PM Below-canopy and soil CO2 fluxes in a ponderosa pine forest. Agric. For. Meteorol. 94, 171–188 (1999). [Google Scholar]
- 63.van Gorsel E et al. Estimating nocturnal ecosystem respiration from the vertical turbulent flux and change in storage of CO2. Agric. For. Meteorol. 149, 1919–1930 (2009). [Google Scholar]
- 64.Leuning R, Zegelin SJ, Jones K, Keith H & Hughes D Measurement of horizontal and vertical advection of CO2 within a forest canopy. Agric. For. Meteorol. 148, 1777–1797 (2008). [Google Scholar]
- 65.De Araújo AC et al. Nocturnal accumulation of CO2 underneath a tropical forest canopy along a topographical gradient. Ecol. Appl. 18, 1406–1419 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Atkin OK, Evans JR, Ball MC, Lambers H & Pons TL Leaf Respiration of Snow Gum in the Light and Dark. Interactions between Temperature and Irradiance. Plant Physiol. 122, 915–924 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ayub G, Smith RA, Tissue DT & Atkin OK Impacts of drought on leaf respiration in darkness and light in Eucalyptus saligna exposed to industrial-age atmospheric CO2 and growth temperature. New Phytol. 190, 1003–1018 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Mclaughlin BC, Xu CY, Rastetter EB & Griffin KL Predicting ecosystem carbon balance in a warming Arctic: The importance of long-term thermal acclimation potential and inhibitory effects of light on respiration. Glob. Chang. Biol. 20, 1901–1912 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Atkin OK, Scheurwater I & Pons T High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Glob. Chang. Biol. 12, 500–515 (2006). [Google Scholar]
- 70.Crous KY et al. Light inhibition of leaf respiration in field-grown Eucalyptus saligna in whole-tree chambers under elevated atmospheric CO2 and summer drought. Plant, Cell Environ. 35, 966–981 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Zaragoza-Castells J, Sánchez-Gómez D, Valladares F, Hurry V & Atkin OK Does growth irradiance affect temperature dependence and thermal acclimation of leaf respiration? Insights from a Mediterranean tree with long-lived leaves. Plant, Cell Environ. 30, 820–833 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Heskel MA et al. Thermal acclimation of shoot respiration in an Arctic woody plant species subjected to 22 years of warming and altered nutrient supply. Glob. Chang. Biol. 20, 2618–2630 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Way DA, Holly C, Bruhn D, Ball MC & Atkin OK Diurnal and seasonal variation in light and dark respiration in field-grown Eucalyptus pauciflora. Tree Physiol. 35, 840–849 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Heskel MA, Tang J & Way D Environmental controls on light inhibition of respiration and leaf and canopy daytime carbon exchange in a temperate deciduous forest. Tree Physiol. 1–17 (2018). doi: 10.1093/treephys/tpy103 [DOI] [PubMed] [Google Scholar]
- 75.Crous KY, Wallin G, Atkin OK, Uddling J & Ekenstam AA Acclimation of light and dark respiration to experimental and seasonal warming are mediated by changes in leaf nitrogen in Eucalyptus globulus. Tree Physiol. 37, 1069–1083 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Xu L & Baldocchi DD Seasonal variation in carbon dioxide exchange over a Mediterranean annual grassland in California. Agric. For. Meteorol. 123, 79–96 (2004). [Google Scholar]
- 77.Lasslop G, Reichstein M, Detto M, Richardson AD & Baldocchi DD Comment on Vickers et al. : Self-correlation between assimilation and respiration resulting from flux partitioning of eddy-covariance CO2 fluxes. Agric. For. Meteorol. 150, 312–314 (2010). [Google Scholar]
- 78.Efron B & Hastie T Computer age statistical inference. (Cambridge University Press, 2016). [Google Scholar]
- 79.Moffat AM A new methodology to interpret high resolution measurements of net carbon fluxes between terrestrial ecosystems and the atmosphere. 113 (2012). [Google Scholar]
- 80.Huntingford C et al. Implications of improved representations of plant respiration in a changing climate. Nat. Commun. 8, 1602 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loreto F, Velikova V & Di Marco G Respiration in the light measured by 12CO2 emission in 13CO2 atmosphere in maize leaves. Aust. J. Plant Physiol. 28, 1103–1108 (2001). [Google Scholar]
- 82.Tcherkez G et al. Tracking the origins of the Kok effect, 70 years after its discovery. New Phytol. 214, 506–510 (2017). [DOI] [PubMed] [Google Scholar]
- 83.Lloyd J & Taylor JA On the temperature dependence of soil respiration. Funct. Ecol. 8, 315–323 (1994). [Google Scholar]
- 84.Wutzler T et al. Basic and extensible post-processing of eddy covariance flux data with REddyProc. Biogeosciences 15, 5015–5030 (2018). [Google Scholar]
- 85.Peisker M & Apel H Inhibition by light of CO2 evolution from dark respiration: Comparison of two gas exchange methods. Photosynth. Res. 70, 291–298 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.