Short abstract
Objective
Intestinal permeability increases during the course of acute pancreatitis (AP). We assessed duodenal permeability alterations in patients with AP by confocal laser endomicroscopy (CLE).
Methods
Thirty patients with AP underwent CLE evaluation of the antral and duodenal mucosa. Images were graded based on the appearance of capillaries and the degree of fluorescein leakage.
Results
Patients with AP had increased duodenal mucosal permeability that could be detected by CLE. The mucosal permeability progressively increased in the gastric antrum, duodenal bulb, and descending duodenum. The CLE parameters in the antrum and duodenal bulb were not significantly different between patients with mild and severe AP. The CLE grades in the descending duodenum were higher in patients with severe than mild AP. The C-reactive protein level in AP was positively correlated with the permeability in the duodenal bulb and descending duodenum, while the computed tomography severity index score was positively correlated with the mucosal permeability in the duodenal bulb and descending duodenum.
Conclusion
CLE revealed increased duodenal permeability in patients with AP. Higher permeability in the descending duodenum was observed in severe than mild AP. Further large-scale studies are needed to confirm the relationship between altered duodenal permeability and the severity of AP.
Keywords: Confocal laser endomicroscopy, duodenal permeability, acute pancreatitis, computed tomography severity index, C-reactive protein, fluorescein
Introduction
Acute pancreatitis (AP) is a common disorder that ranges from mild disease to multiple organ failure and sepsis and that often leads to emergency hospital admission.1 Gut barrier damage plays a major role in the pathophysiology of AP, contributing to intestinal bacterial translocation and secondary infection.2–4 Gut barrier dysfunction takes place in the early phase of AP and is a result of local or systemic inflammatory responses and gut ischemia.4
Alterations in intestinal permeability have generally been assessed using the lactulose-mannitol test.3,5 However, evidence has indicated that intestinal permeability to very small sugar molecules such as lactulose or mannitol is not correlated with epithelial permeability to macromolecules.6 Observation of the intestinal ultrastructure might be necessary to identify epithelial cell shedding and loss of barrier function.
Confocal laser endomicroscopy (CLE) is a novel endoscopic technique with excellent subcellular resolution and 400-fold magnification. It has been postulated that CLE exhibits good authenticity and reproducibility for the diagnosis of AP and has potential clinical value for the diagnosis of this disorder.7 Previous studies have shown variation among confocal images in different stages of AP;8 therefore, mastering the confocal manifestations of different stages of inflammation is also very important for endoscopy practitioners to achieve an accurate diagnosis of AP using CLE. After injection of fluorescein as a contrast agent, CLE allows for in vivo examination of the cells and intracellular substance of the gastrointestinal tract mucosa during real-time endoscopy.9 In the present study, we aimed to detect duodenal permeability in patients with AP by observing epithelial cell shedding and the degree of fluorescein leakage using CLE. Alteration of the duodenal mucosa as observed by CLE might be a sensitive indicator of gut barrier dysfunction in patients with AP.
Materials and methods
Patients
Consecutive patients with AP who were admitted to the Department of Gastroenterology of The First Affiliated Hospital of Zhejiang University from March 2015 to August 2016 were included in the study. The diagnosis of AP was based on the following criteria: 1) abdominal pain, 2) a serum amylase or lipase level of at least three times the upper limit of normal, and 3) the appearance of pancreatitis on computed tomography (CT).10,11 Patients were excluded if they met the following criteria: 1) a critical condition involving multiple organ failure and inability to undergo CLE examination, 2) allergy to fluorescein sodium, 3) chronic gastrointestinal disorders at admission, and 4) unwillingness to provide written informed consent. All enrolled patients were informed about the purpose and design of the study, and written informed consent was obtained from each. All medical records were retrospectively reviewed. Ethical approval was obtained from the Ethics Committee of Ningbo No. 2 Hospital.
Methods
Data regarding age, sex, duration between onset and admission, and length of stay were collected and recorded. Routine complete blood cell counts and biochemical analyses were performed at admission and before CLE examination. The serum level of C-reactive protein (CRP) was also measured. All patients underwent enhanced CT of the abdomen at admission and before CLE examination. The etiology of AP was determined by experienced gastrointestinal specialists. The severity of AP was evaluated with the Acute Physiology and Chronic Health Evaluation II (APACHE-II) score, Ranson score, and Bedside Index for Severity in AP (BISAP) score within 24 hours of hospital admission. Moreover, the CT severity index (CTSI) was calculated based on the protocol devised by Balthazar et al.12 The CT scores were based on CT scan reports provided by experienced radiologists. All patients were classified into two groups: those with mild AP (MAP) and those with severe AP (SAP). Patients with MAP recovered without local or systemic complications and without organ failure, and patients with SAP developed failure of one or more organs or a local complication.
All patients with AP were treated by fluid management, somatostatin, antibiotics, and nutrition. Initially, the patients were fasted and total parenteral nutrition was administered. When enteral nutrition was required, it was delivered by tube feeding. At this time, CLE was performed using an endomicroscope (GastroFlex UHD; Mauna Kea Technologies, Paris, France) before placement of a nasojejunal tube. All CLE procedures were performed by a single experienced endoscopist (M.Y.) (>80 previous CLE procedures) assisted by an expert nurse. First, conventional white light endoscopy was performed with the non-confocal function of the endoscope. The contrast agent (5 mL of 10% sodium fluorescence solution) was then intravenously administered, and CLE was performed. The probe was specifically focused on the gastric antrum, duodenal bulb, and descending duodenum. Biopsy specimens were taken from these sites and examined by experienced gastrointestinal pathologists.
Assessment of CLE images
Evaluation of the CLE images was based on CLE imaging criteria developed in accordance with published studies.13,14 To assess the permeability of the stomach and duodenum, the endomicroscopic changes were classified into four grades: grade 0, normal appearance of microvessels with no fluorescein leakage; grade 1, widening of capillaries with mild fluorescein leakage; grade 2, widening of capillaries with moderate fluorescein leakage; and grade 3, dilated and distorted appearance of capillaries with severely increased leakage.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean ± standard deviation (range). The unpaired Student’s t test was conducted for comparison of continuous variables. The chi-square test was applied for comparison of categorical variables. Correlations between CLE grades and clinical characteristics of AP were estimated using Spearman’s correlation. A two-tailed P value of <0.05 was considered statistically significant.
Results
Patients’ baseline characteristics
The baseline characteristics of the patients with AP are listed in Table 1. Thirty patients were included in the study, and their mean age was 49.4 ± 15.0 years. Sixteen patients were diagnosed with MAP because they had no local or systemic complications and no organ failure, while the other 14 patients were diagnosed with SAP. The length of stay among patients with MAP ranged from 3 to 34 days, while that in patients with SAP was much longer, ranging from 13 to 78 days (P = 0.013). There was no significant difference in the duration between onset and admission or in the etiology of AP between patients with MAP and SAP. As expected, the initial CRP level was significantly higher in the SAP than MAP group (P = 0.004). The APACHE-II score (P < 0.001), Ranson score (P < 0.001), BISAP score (P = 0.002), and CTSI score (P < 0.001) were significantly higher in patients with SAP than MAP.
Table 1.
Patients’ baseline characteristics.
| Total (n = 30) | MAP (n = 16) | SAP (n = 14) | P* | |
|---|---|---|---|---|
| Age, years | 49.4 ± 15.0 (26–80) | 47.9 ± 15.5 (26–80) | 51.1 ± 15.4 (27–77) | 0.575 |
| Male/female | 17/13 | 10/6 | 7/7 | 0.491 |
| Duration between onset and admission, days | 4.5 ± 4.7 (0–20) | 5.3 ± 5.7 (0–20) | 3.6 ± 3.5 (0.5–14) | 0.334 |
| Length of stay, days | 20.4 ± 17.0 (3–78) | 12.4 ± 7.5 (3–34) | 29.6 ± 21.0 (13–78) | 0.013 |
| Etiology | 0.975 | |||
| Biliary | 16 (53.3) | 7 (43.8) | 9 (64.3) | |
| Hyperlipidemia | 6 (20.0) | 3 (18.8) | 3 (21.4) | |
| Post-ERCP | 1 (3.3) | 1 (6.3) | 0 (0.0) | |
| Alcoholic | 1 (3.3) | 1 (6.3) | 0 (0.0) | |
| Idiopathic | 5 (16.7) | 3 (18.8) | 2 (14.3) | |
| Autoimmunity | 1 (3.3) | 1 (6.3) | 0 (0.0) | |
| Amylase (IU/L) | 505.5 ± 735.4 (19–3451) | 359.6 ± 461.0 (19–1458) | 651.4 ± 930.0 (41–3451) | 0.306 |
| Initial white cell count (109/L) | 14.3 ± 5.5 (5.0–30.8) | 12.6 ± 4.3 (8.0–25.1) | 16.2 ± 6.5 (5.0–30.8) | 0.086 |
| Follow-up white cell count (109/L) | 10.0 ± 4.3 (3.8–20.6) | 10.0 ± 4.5 (4.1–20.6) | 9.9 ± 4.2 (3.8–17.2) | 0.939 |
| Initial CRP (mg/L) | 161.2 ± 135.5 (0.8–440.1) | 97.3 ± 114.0 (0.8–440.1) | 234.1 ± 123.2 (26.3–426.7) | 0.004 |
| Follow-up CRP (mg/L) | 99.1 ± 110.2 (1.8–424.4) | 80.9 ± 122.1 (1.8–424.4) | 118.7 ± 96.4 (2.8–192.8) | 0.362 |
| APACHE-II score | 6.2 ± 5.4 (0–23) | 2.6 ± 1.8 (0–5) | 10.3 ± 5.3 (4–23) | <0.001 |
| Ranson score | 3.3 ± 1.9 (1–7) | 2.1 ± 1.1 (1–4) | 4.7 ± 1.6 (2–7) | <0.001 |
| BISAP score | 1.8 ± 1.5 (0–8) | 0.9 ± 0.7 (0–2) | 2.7 ± 1.7 (1–8) | 0.002 |
| Initial CTSI score | 5.0 ± 2.0 (0–8) | 3.9 ± 2.0 (0–4) | 6.3 ± 1.1 (4–8) | <0.001 |
| Follow-up CTSI score | 4.2 ± 2.0 (0–8) | 3.1 ± 1.9 (0–4) | 5.4 ± 1.2 (4–8) | <0.001 |
Data are presented as mean ± standard deviation (range) or n (%).
*Unpaired Student’s t test was used for comparison of continuous variables between MAP and SAP, and the chi-square test was used for comparison of categorical variables.
MAP: mild acute pancreatitis; SAP: severe acute pancreatitis; ERCP: endoscopic retrograde cholangiopancreatography; CRP: C-reactive protein; APACHE-II: Acute Physiology and Chronic Health Evaluation II; BISAP: Bedside index for Severity in Acute Pancreatitis; CTSI: computed tomography severity index.
Patients’ endoscopic, CLE, and pathologic parameters
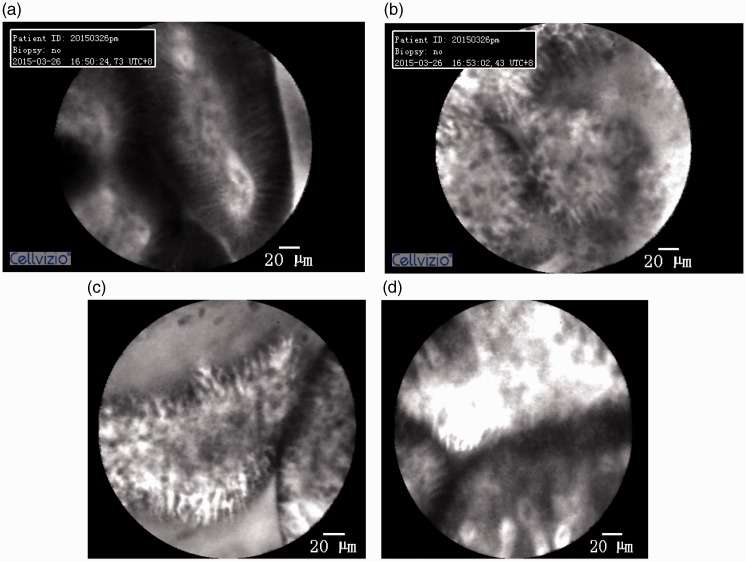
The endoscopic, CLE, and pathologic parameters of all patients with AP are shown in Table 2. In the comparisons of the gastric antrum, duodenal bulb, and descending duodenum in patients with AP, the descending duodenum had significantly fewer congested, edematous membranes or erosions under white light endoscopic examination (P = 0.006). There were no significant differences in the pathologic findings among these three parts. However, the CLE examination results showed a statistically significant difference (P < 0.001); specifically, the mucosal permeability progressively increased in the gastric antrum, duodenal bulb, and descending duodenum (Figure 1).
Table 2.
Patients’ endoscopic, CLE, and pathologic parameters.
| Site (item) | Results | Total(n = 30) | MAP(n = 16) | SAP(n = 14) | P* |
|---|---|---|---|---|---|
| Antrum (WLE) | Normal | 17 | 10 | 7 | 0.491 |
| Congested, edematous membrane or erosions | 13 | 6 | 7 | ||
| Duodenal bulb (WLE) | Normal | 17 | 7 | 10 | 0.127 |
| Congested, edematous membrane or erosions | 13 | 9 | 4 | ||
| Descending duodenum (WLE) | Normal | 27 | 14 | 13 | 0.626 |
| Congested, edematous membrane or erosions | 3 | 2 | 1 | ||
| Antrum (CLE) | Grade 0 | 5 | 3 | 2 | 0.465 |
| Grade 1 | 10 | 6 | 4 | ||
| Grade 2 | 13 | 7 | 6 | ||
| Grade 3 | 2 | 0 | 2 | ||
| Duodenal bulb (CLE) | Grade 0 | 2 | 0 | 2 | 0.281 |
| Grade 1 | 1 | 1 | 0 | ||
| Grade 2 | 20 | 12 | 8 | ||
| Grade 3 | 7 | 3 | 4 | ||
| Descending duodenum (CLE) | Grade 0 | 0 | 0 | 0 | 0.059 |
| Grade 1 | 1 | 0 | 1 | ||
| Grade 2 | 13 | 10 | 3 | ||
| Grade 3 | 16 | 6 | 10 | ||
| Antrum (Pathology) | Mild superficial gastritis | 22 | 10 | 12 | 0.334 |
| Moderate to severe superficial gastritis | 3 | 2 | 1 | ||
| Atrophic gastritis | 5 | 4 | 1 | ||
| Duodenal bulb (Pathology) | Mild superficial duodenitis | 27 | 15 | 12 | 0.464 |
| Moderate to severe superficial duodenitis | 3 | 1 | 2 | ||
| Descending duodenum (Pathology) | Mild superficial duodenitis | 25 | 15 | 10 | 0.102 |
| Moderate to severe superficial duodenitis | 5 | 1 | 4 |
*Chi-square test was used for comparison between MAP and SAP.
MAP: mild acute pancreatitis; SAP: severe acute pancreatitis; CLE: confocal laser endomicroscopy; WLE: white light endoscopy.
Figure 1.
Confocal laser endomicroscopy results. (a) Gastric antrum: mild fluorescein leakage. (b) Duodenal bulb: moderate fluorescein leakage. (c, d) Descending duodenum: severely increased fluorescein leakage.
CLE grade in relation to AP severity
The CLE parameters in the antrum and duodenal bulb were not significantly different between patients with MAP and SAP. Interestingly, the CLE grades in the descending duodenum were higher in patients with SAP than MAP (P = 0.059, borderline significance) (Table 2). Spearman’s correlation tests indicated that the permeability of the duodenal mucosa as measured by CLE was positively correlated with the CRP level at a mean follow-up of 4 months (duodenal bulb: R = 0.293, P = 0.012; descending duodenum: R = 0.290, P = 0.013). In addition, the CLE grade of the duodenum was significantly positively correlated with the follow-up CTSI score (duodenal bulb: R = 0.395, P < 0.001; descending duodenum: R = 0.297, P = 0.010). Nevertheless, the CLE grades in the gastric antrum, duodenal bulb, and descending duodenum were not correlated with the other clinical characteristics (age, length of stay, initial CRP level, APACHE-II score, Ranson score, BISAP score, and initial CTSI score) (Table 3).
Table 3.
Correlation between confocal laser endomicroscopy grades and clinical characteristics.
| Clinical characteristics |
Antrum |
Duodenal bulb |
Descending duodenum |
|||
|---|---|---|---|---|---|---|
| R | P | R | P | R | P | |
| Age | 0.263 | 0.161 | 0.127 | 0.276 | 0.012 | 0.919 |
| Length of stay | −0.103 | 0.594 | −0.045 | 0.707 | 0.083 | 0.490 |
| Initial CRP (mg/L) | −0.070 | 0.712 | 0.042 | 0.721 | 0.013 | 0.912 |
| Follow-up CRP (mg/L) | 0.281 | 0.140 | 0.293 | 0.012 | 0.290 | 0.013 |
| APACHE-II score | 0.081 | 0.670 | 0.042 | 0.720 | 0.083 | 0.481 |
| Ranson score | −0.030 | 0.874 | 0.002 | 0.988 | 0.016 | 0.895 |
| BISAP score | 0.220 | 0.242 | 0.004 | 0.972 | 0.064 | 0.587 |
| Initial CTSI score | 0.005 | 0.981 | 0.129 | 0.268 | 0.049 | 0.678 |
| Follow-up CTSI score | 0.125 | 0.510 | 0.395 | <0.001 | 0.297 | 0.010 |
All tests were performed using the Spearman’s correlation.
R: correlation coefficient; CRP: C-reactive protein; APACHE-II: Acute Physiology and Chronic Health Evaluation II; BISAP: Bedside Index for Severity in Acute Pancreatitis; CTSI: computed tomography severity index.
Discussion
In this study, we found that CLE could be used to identify increased duodenal permeability in patients with AP. Patients with SAP had higher permeability in the descending duodenum than those with MAP. The CRP level in patients with AP was positively correlated with the permeability in the duodenal bulb and descending duodenum as well as with the CTSI score.
CLE is a recently developed endoscopic technique that can provide in vivo histological evaluation. Previous studies have shown that CLE identifies loss of the mucosal barrier in patients with gastric cancer,15–17 colorectal cancer,18 and inflammatory bowel disease.13,14 Lim et al.14 indicated that CLE revealed epithelial damage and barrier loss in the duodenum of patients with inflammatory bowel disease that was not apparent on conventional endoscopy or histology. According to previous studies, intestinal barrier dysfunction under CLE was characterized by an altered appearance of the capillaries and fluorescein leakage. In the present study, CLE was used to effectively confirm these alterations in the duodenal mucosa in patients with AP.
Although the overall mortality rate of AP has decreased in the past 10 years, SAP is closely associated with high mortality, especially for those with severe infection. However, many patients with SAP do not show organ failure or pancreatic necrosis at the initial diagnosis, leading to a delay in clinical treatment measures. Therefore, it is very important to accurately determine the patient’s condition within the first 48 hours after admission. The gut appears to play a major role in the pathophysiology of AP.19 Gut barrier dysfunction plays an important role in the occurrence, development, and prognosis of SAP. It is widely accepted that the gut barrier becomes compromised and intestinal permeability increases during the course of AP. This is mainly due to hypovolemia, splanchnic hypoperfusion, and intestinal ischemia.20 Moreover, ischemia–reperfusion injury in the intestine causes the relapse of oxygen radicals and a variety of inflammatory cytokines, which further destroy the gut barrier function.19,21 As a consequence, intestinal bacterial overgrowth and translocation lead to endotoxemia, systemic inflammatory response syndrome, and even multiorgan failure.20
This is the first study in which increased intestinal permeability in patients with AP was detected by CLE. The grading criteria for CLE were established according to the appearance of capillaries and the degree of fluorescein leakage in the duodenal mucosa. The breaking of the epithelial line in the mucosa might lead to increased epithelial gaps and cell shedding with resultant fluorescein leakage, which could be quantified by fluorescein-aided CLE.14 As the initial part of the intestine, barrier loss should first occur in the duodenum in patients with AP. Because the duodenum is a convenient site for performing CLE, CLE might be an effective way to detect altered duodenal permeability in patients with AP.
Given the relationship between intestinal barrier dysfunction and SAP, it is reasonable to assume that early gut barrier function assessment contributes to the disease prediction of AP. Some evidence has demonstrated that infections occur in the early phase of AP.5,23 Moreover, increased intestinal permeability is more relevant and persistent in severe cases.22 In a study by Sharma et al.,3 patients with complications of SAP showed increased intestinal permeability, and higher endotoxemia predicted poorer outcomes. Increased duodenal permeability in patients with SAP was also observed by CLE in the present study. Duodenal permeability was positively correlated with the CRP level at a mean follow-up of 4 months as well as with the CTSI score, but it was not correlated with the length of stay, initial CRP level, APACHE-II score, Ranson score, BISAP score, or initial CTSI score. This might be related to the timing of the CLE examination; CLE was performed at the time of bowel function recovery when enteral nutrition was required in our study. At this stage of AP, the duodenal permeability might also improve in many cases.
Our study had some limitations that warrant mention. First, the number of patients was small. Second, the patients did not undergo CLE at the same stage of the disease course. A better understanding of the association between intestinal permeability and severity of AP would have been obtained if CLE had been performed in the early phase of AP and at a later follow-up visit. However, because CLE is an invasive examination, it was performed before gastroscopic placement of a nasojejunal tube for enteral nutrition. This study design minimized the patients’ pain. Third, although the CRP level and white blood cell counts were evaluated and the APACHE-II, Ranson, BISAP, and CTSI scores were estimated, no parameters reflecting endotoxemia were measured. Finally, it would have been better to examine the validity of CLE for estimation of intestinal permeability if the lactulose-mannitol test had been performed in both patients and controls followed by comparison of the CLE and lactulose-mannitol test results.
In conclusion, this study has demonstrated that CLE can be used to identify alterations in duodenal permeability in patients with AP. Patients with SAP had higher permeability in the descending duodenum than did patients with MAP. Duodenal permeability was correlated with the CRP level and CTSI score. Further robust studies are needed to confirm the relationship between altered duodenal permeability and the severity of AP.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med 1994; 330: 1198–1210. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Li W, Wang X, et al. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas 2008; 36: 192–196. [DOI] [PubMed] [Google Scholar]
- 3.Sharma M, Sachdev V, Singh N, et al. Alterations in intestinal permeability and endotoxemia in severe acute pancreatitis. Trop Gastroenterol 2012; 33: 45–50. [DOI] [PubMed] [Google Scholar]
- 4.Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg 1996; 20: 411–417. [DOI] [PubMed] [Google Scholar]
- 5.Nagpal K, Minocha VR, Agrawal V, et al. Evaluation of intestinal mucosal permeability function in patients with acute pancreatitis. Am J Surg 2006; 192: 24–28. [DOI] [PubMed] [Google Scholar]
- 6.Vojdani A. For the assessment of intestinal permeability, size matters. Altern Ther Health Med 2013; 19: 12–24. [PubMed] [Google Scholar]
- 7.Telek G, Scoazec JY, Chariot J, Ducroc R, Feldmann G, Roz C. Cerium-based histochemical demonstration of oxidative stress in taurocholate-induced acute pancreatitis in rats. A confocal laser scanning microscopic study. J Histochem Cytochem 1999; 47(9): 1201–1212. [DOI] [PubMed] [Google Scholar]
- 8.Bhutani MS, Koduru P, Joshi V, Karstensen JG, Saftoiu A, Vilmann P, Giovannini M. EUS-Guided Needle-Based Confocal Laser Endomicroscopy: A Novel Technique With Emerging Applications. Gastroenterol Hepatol (N Y) 2015; 11(4): 235–240. [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann H, Kiesslich R, Wallace MB, et al. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology 2010; 139: 388–392, 392.e1–2. [DOI] [PubMed] [Google Scholar]
- 10.Johnson CD, Besselink MG, Carter R. Acute pancreatitis. BMJ 2014; 349: g4859. [DOI] [PubMed] [Google Scholar]
- 11.Working Group IAPAPAAPG. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013; 13: e1–e15. [DOI] [PubMed] [Google Scholar]
- 12.Balthazar EJ, Robinson DL, Megibow AJ, et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990; 174: 331–336. [DOI] [PubMed] [Google Scholar]
- 13.Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 2012; 61: 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim LG, Neumann J, Hansen T, et al. Confocal endomicroscopy identifies loss of local barrier function in the duodenum of patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 2014; 20: 892–900. [DOI] [PubMed] [Google Scholar]
- 15.Kitabatake S, Niwa Y, Miyahara R, et al. Confocal endomicroscopy for the diagnosis of gastric cancer in vivo. Endoscopy 2006; 38: 1110–1114. [DOI] [PubMed] [Google Scholar]
- 16.Li WB, Zuo XL, Li CQ, et al. Diagnostic value of confocal laser endomicroscopy for gastric superficial cancerous lesions. Gut 2011; 60: 299–306. [DOI] [PubMed] [Google Scholar]
- 17.Jeon SR, Cho WY, Jin SY, et al. Optical biopsies by confocal endomicroscopy prevent additive endoscopic biopsies before endoscopic submucosal dissection in gastric epithelial neoplasias: a prospective, comparative study. Gastrointest Endosc 2011; 74: 772–780. [DOI] [PubMed] [Google Scholar]
- 18.Xie XJ, Li CQ, Zuo XL, et al. Differentiation of colonic polyps by confocal laser endomicroscopy. Endoscopy 2011; 43: 87–93. [DOI] [PubMed] [Google Scholar]
- 19.Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas 2003; 26: 122–129. [DOI] [PubMed] [Google Scholar]
- 20.Rahman SH, Ammori BJ, Holmfield J, et al. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg 2003; 7: 26–35; discussion 35–36. [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Li L, Yan J, et al. The loss of alphaSNAP downregulates the expression of occludin in the intestinal epithelial cell of acute pancreatitis model. Pancreatology 2014; 14: 347–355. [DOI] [PubMed] [Google Scholar]
- 22.Dervenis C, Smailis D, Hatzitheoklitos E. Bacterial translocation and its prevention in acute pancreatitis. J Hepatobiliary Pancreat Surg 2003; 10: 415–418. [DOI] [PubMed] [Google Scholar]
- 23.Besselink MG, van Santvoort HC, Boermeester MA, et al. Timing and impact of infections in acute pancreatitis. Br J Surg 2009; 96: 267–273. [DOI] [PubMed] [Google Scholar]
- 24.Penalva JC, Martinez J, Laveda R, et al. A study of intestinal permeability in relation to the inflammatory response and plasma endocab IgM levels in patients with acute pancreatitis. J Clin Gastroenterol 2004; 38: 512–517. [DOI] [PubMed] [Google Scholar]