Short abstract
Background
The survival rate of patients undergoing hemodialysis and other renal replacement therapies has been extensively studied, but comparative studies of emergency and scheduled hemodialysis are limited.
Methods
This study included 312 patients who underwent emergency hemodialysis and 274 who received scheduled hemodialysis. We investigated the prognostic differences between these two groups of patients, including the short-term and long-term survival rates.
Results
The overall survival rate was significantly better among the patients in the scheduled hemodialysis group than emergency hemodialysis group. The mortality rate within 3 months of emergency hemodialysis was 4.8%, while that within 3 months of scheduled hemodialysis was 1.1%.
Conclusions
Significant differences were present between emergency and scheduled hemodialysis, especially the levels of serum creatinine and hemoglobin.
Keywords: Emergency hemodialysis, scheduled hemodialysis, prognosis, survival, creatinine, hemoglobin
Introduction
The incidence and prevalence of end-stage renal disease (ESRD) have increased with the development of society and changes in the disease spectrum. From 1990 to 2000, the number of patients with ESRD increased from 0.426 to 1.65 million worldwide, and it is estimated that this number will reach 3.6 million by 2020.1 Rapid population growth has led to a dramatic increase in medical costs for the treatment of ESRD.
Renal replacement therapy, including hemodialysis, peritoneal dialysis, and renal transplantation, is the primary treatment method for ESRD. Hemodialysis is the most widely used among these therapies. Although hemodialysis has saved the lives of many patients with ESRD, the overall survival rate remains low. In one study, the survival rate in patients of advanced age was 57.1%, while that in younger patients was 71.4%.2 How to improve the survival rate of patients undergoing dialysis and how to choose the most appropriate dialysis method are longstanding challenges in the medical community. Evidence suggests that early renal care can improve the prognosis of patients.3–5 Scheduled hemodialysis can provide patients with better survival and quality of life.6–8 Mendelssohn et al.9 reviewed 8 studies in Europe involving 5805 cases and found that the mortality rate was significantly higher among patients undergoing emergency hemodialysis than scheduled hemodialysis. The main causes of death were poor vascular access for dialysis, more complications before dialysis, and a worse health status at the beginning of dialysis. However, these factors were not carefully investigated and analyzed. A retrospective study in Spain also showed that patients undergoing emergency hemodialysis had a higher mortality rate than those undergoing elective hemodialysis, but other major related data such as the length of stay, clinical events, dialysis-related complications, and reasons for emergency hemodialysis were relatively limited.8
The randomized controlled trial is theoretically the most reliable study type, and some scholars have attempted such trials for comparison of dialysis methods. However, for various reasons including lack of statistical efficacy and poor reproducibility, such randomized controlled trials have not been widely carried out. Most of the currently available data regarding comparison of dialysis methods are based on large-scale observation. The data source is usually a multicenter national or even international dialysis registration system. Before randomized controlled trials are performed, large-scale observational studies are still valuable. The purpose of this study was to determine the basic features of patients undergoing emergency hemodialysis and scheduled hemodialysis, such as sex, age, primary disease, and combined diseases, to evaluate the short-term and long-term survival rates of patients undergoing emergency hemodialysis versus scheduled hemodialysis and identify the main risk factors affecting the short-term and long-term prognosis of patients undergoing hemodialysis.
Patients and methods
Patients
The study sample comprised patients aged ≥18 years who began emergency hemodialysis or scheduled hemodialysis from 1 January 2010 to 31 December 2015 and consented to long-term treatment in the Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University. Patients with acute kidney injury and acute exacerbations of chronic kidney disease were excluded.
Data collection
Demographic data, clinical data, and survival time data were collected through inpatient history-taking, outpatient history-taking, and telephone follow-up. The start date of follow-up was the beginning date of dialysis. Follow-up ended by 31 March 2016. The survival time included endpoint data and final inspection data. The endpoint data were the survival times of the patients before death during the follow-up period. The final inspection data were the survival times of the patients who were still alive and continuing hemodialysis before the end of follow-up, who underwent renal transplantation, who underwent peritoneal dialysis, who were transferred to another hospital, who discontinued treatment, or who missed any follow-up visits during the follow-up period.
Statistical methods
IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA) was used for data entry and statistical analysis. Measurement data are presented as mean ± standard deviation. The t test was used to compare the measurement data between the two groups. Count data are presented as frequency (percentage). The count data of the two groups were compared using the chi square test. For survival data, we used the Kaplan–Meier method and log-rank test. A P value of <0.05 was considered statistically significant.
Ethics statements
Ethical approval was obtained from the Ethical Community of The Northern Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University, Shanghai. All patients provided written informed consent to participate in the study.
Results
Baseline data and outcomes of enrolled patients
According to the initial dialysis method, the patients were divided into an emergency hemodialysis group and scheduled hemodialysis group. The baseline data are shown in Table 1 and Table 2. In total, 586 patients were included in the study. Of these patients, 312 (53.2%) underwent emergency hemodialysis and 274 (46.8%) underwent scheduled hemodialysis. The serum creatinine level was significantly higher in the emergency than scheduled hemodialysis group (P = 0.038), while the hemoglobin level was significantly higher in the scheduled than emergency hemodialysis group (P = 0.000). There were no significant differences in the other baseline data (age, urine volume, eGFR, immunoglobulin G level, complement C3 level, body mass index, albumin level, and all other parameters) between the two groups. Additionally, there was no significant difference in the number of patients with comorbidities at the beginning of dialysis between the two groups.
Table 1.
Baseline measurement data of patients in emergency hemodialysis group and scheduled hemodialysis group.
| Total patients(n = 586) | Emergency hemodialysis group(n = 312) | Scheduled hemodialysis group(n = 274) | P | |
|---|---|---|---|---|
| Age (years) | 55.23 ± 16.15 | 53.24 ± 15.90 | 57.54 ± 16.17 | NS |
| Height (cm) | 165.91 ± 8.32 | 167.31 ± 8.28 | 164.24 ± 8.08 | NS |
| Weight (kg) | 61.83 ± 12.01 | 63.19 ± 11.38 | 60.28 ± 12.55 | NS |
| BMI (kg/m2) | 22.34 ± 3.48 | 22.49 ± 3.43 | 22.16 ± 3.53 | NS |
| Initial urine volume (mL/day) | 1235.83 ± 561.90 | 1161.10 ± 580.15 | 1298.57 ± 532.69 | NS |
| Red blood cells (g/L) | 3.15 ± 0.75 | 3.14 ± 0.81 | 3.16 ± 0.67 | NS |
| Hemoglobin (g/L) | 92.09 ± 19.19 | 88.14 ± 18.83 | 96.58 ± 18.65 | 0.000** |
| Hematocrit (g/L) | 28.40 ± 5.50 | 28.07 ± 5.34 | 28.76 ± 5.65 | NS |
| Platelets (g/L) | 172.84 ± 72.22 | 176.49 ± 81.60 | 168.73 ± 59.83 | NS |
| White blood cells (μmol/L) | 6.53 ± 2.66 | 6.58 ± 3.09 | 6.47 ± 2.08 | NS |
| Neutrophils (%) | 68.92 ± 8.70 | 68.84 ± 8.80 | 69.01 ± 8.60 | NS |
| Total bilirubin (μmol/L) | 5.82 ± 5.07 | 5.81 ± 5.80 | 5.82 ± 4.09 | NS |
| Conjugated bilirubin (μmol/L) | 2.32 ± 4.01 | 2.45 ± 5.26 | 2.16 ± 1.78 | NS |
| Albumin (g/L) | 40.29 ± 5.10 | 39.94 ± 5.40 | 40.70 ± 4.71 | NS |
| Globulin (g/L) | 27.92 ± 6.11 | 28.37 ± 5.89 | 27.41 ± 6.33 | NS |
| ALT (U/L) | 19.84 ± 19.74 | 19.04 ± 17.87 | 20.75 ± 21.67 | NS |
| AST (U/L) | 21.18 ± 16.08 | 20.84 ± 15.78 | 21.57 ± 16.43 | NS |
| Pre-albumin (g/L) | 0.33 ± 0.08 | 0.33 ± 0.08 | 0.33 ± 0.08 | NS |
| Blood urea nitrogen (mmol/L) | 26.23 ± 8.27 | 26.16 ± 9.00 | 26.31 ± 7.36 | NS |
| Serum creatinine (μmol/L) | 944.76 ± 659.80 | 994.89 ± 873.30 | 887.68 ± 240.69 | 0.038* |
| Serum uric acid (μmol/L) | 452.20 ± 137.97 | 440.15 ± 140.00 | 465.92 ± 134.57 | NS |
| Sodium (mmol/L) | 139.62 ± 3.46 | 139.61 ± 3.91 | 139.65 ± 2.86 | NS |
| Potassium (mmol/L) | 4.83 ± 0.89 | 4.88 ± 0.84 | 4.79 ± 0.94 | NS |
| Chloride (mmol/L) | 100.93 ± 4.25 | 100.77 ± 4.43 | 101.10 ± 4.04 | NS |
| CO2-CP (mmol/L) | 23.28 ± 4.80 | 23.57 ± 5.44 | 22.95 ± 3.92 | NS |
| Calcium (mmol/L) | 2.52 ± 1.65 | 2.58 ± 1.91 | 2.45 ± 1.30 | NS |
| Phosphorus (mmol/L) | 2.21 ± 0.66 | 2.20 ± 0.66 | 2.22 ± 0.67 | NS |
| Magnesium (mmol/L) | 1.22 ± 0.26 | 1.22 ± 0.28 | 1.21 ± 0.23 | NS |
| IgG (g/L) | 9.61 ± 3.74 | 9.47 ± 3.26 | 9.84 ± 3.91 | NS |
| C3 (g/L) | 1.04 ± 0.23 | 0.91 ± 0.21 | 1.07 ± 0.39 | NS |
| Serum iron (g/L) | 13.33 ± 5.52 | 13.30 ± 6.02 | 13.36 ± 4.87 | NS |
| Transferrin (%) | 2.92 ± 14.46 | 2.09 ± 2.86 | 3.91 ± 21.17 | NS |
| Serum ferritin (μg/L) | 267.35 ± 251.71 | 265.66 ± 243.20 | 269.30 ± 261.68 | NS |
| iPTH (ng/L) | 233.60 ± 347.20 | 256.56 ± 412.04 | 206.35 ± 247.37 | NS |
| eGFR (mL/min/1.73 m2) | 7.87 ± 3.18 | 7.82 ± 3.51 | 7.91 ± 2.77 | NS |
Data are presented as mean ± standard deviation.
BMI, body mass index; ALT, alanine transaminase; AST, aspartate transaminase; CO2-CP, carbon dioxide combining power; IgG, immunoglobulin G; C3, complement C3; iPTH, intact parathyroid hormone; eGFR, estimated glomerular filtration rate (Cockcroft–Gault formula); NS, no significant difference.
*P < 0.05, **P < 0.01 (t test).
Table 2.
Baseline enumeration data of patients in emergency hemodialysis group and scheduled hemodialysis group.
| Total patients | Emergency hemodialysis group | Scheduled hemodialysis group | P* | |
|---|---|---|---|---|
| Combined diseases | ||||
| Hypertension | 558 (95.2) | 300 (96.2) | 258 (94.2) | NS |
| DM | 129 (22.0) | 73 (23.4) | 56 (20.4) | NS |
| Cerebrovascular accident | 50 (8.5) | 30 (9.6) | 20 (7.3) | NS |
| Ischemic heart disease | 43 (7.3) | 20 (6.4) | 23 (8.4) | NS |
| CHF | 98 (16.7) | 57 (18.3) | 41 (15.0) | NS |
| Left ventricular hypertrophy | 166 (28.3) | 88 (28.2) | 78 (28.5) | NS |
| Arrhythmia | 174 (29.7) | 101 (32.4) | 73 (26.6) | NS |
| Chronic liver disease | 12 (2.0) | 7 (2.2) | 5 (1.8) | NS |
| COPD | 14 (2.4) | 8 (2.6) | 6 (2.2) | NS |
| Tumor | 26 (4.4) | 9 (2.9) | 17 (6.2) | NS |
Data are presented as n (%).
DM, diabetes mellitus; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; NS, no significant difference.
*Chi square test.
A total of 124 (21.2%) patients died, and 319 (54.4%) patients were still alive and continuing hemodialysis at the end of follow-up. Sixty-six (11.3%) patients consented to renal transplantation during follow-up, and 12 (2.0%) were transferred to peritoneal dialysis. Other outcomes were observed in the remaining 65 (11.1%) patients.
Mortality within 3 months in scheduled versus emergency hemodialysis groups
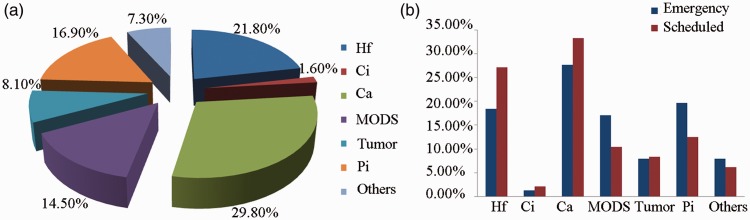
Among all 124 patients with heart failure who died, the causes of death were as follows: heart failure in 27 (21.8%) patients, myocardial infarction in 2 (1.6%), cerebral vascular accident in 37 (29.8%), multiple organ failure in 18 (14.5%), tumors in 10 (8.1%), pulmonary infection in 21 (16.9%), and other causes in 9 (7.3%) (Table 3-1, Figure 3).
Table 3-1.
Death and other endpoint events in emergency and scheduled hemodialysis groups.
| Total patients | Emergency hemodialysis group | Scheduled hemodialysis group | P | |
|---|---|---|---|---|
| Total death | 124 (21.2) | 76 (24.4) | 48 (17.5) | 0.043* |
| Endpoint events | NS | |||
| CVD | 29 (23.4) | 15 (19.7) | 14 (29.2) | NS |
| Heart failure | 27 (21.8) | 14 (18.4) | 13 (27.1) | NS |
| Cardiac infarction | 2 (1.6) | 1 (1.3) | 1 (2.1) | NS |
| Cerebrovascular accident | 37 (29.8) | 21 (27.6) | 16 (33.3) | NS |
| MODS | 18 (14.5) | 13 (17.1) | 5 (10.4) | NS |
| Tumor | 10 (8.1) | 6 (7.9) | 4 (8.3) | NS |
| Pulmonary infection | 21 (16.9) | 15 (19.7) | 6 (12.5) | NS |
| Others | 9 (7.3) | 6 (7.9) | 3 (6.2) | NS |
| Renal transplant | 66 (11.3) | 36 (11.5) | 30 (10.9) | NS |
| Transfer to peritoneal dialysis | 12 (2.0) | 6 (1.9) | 6 (2.2) | NS |
| Other outcomes | 65 (11.1) | 36 (11.5) | 29 (10.6) | NS |
Data are presented as n (%).
CVD, cardiovascular disease; MODS, multiple organ dysfunction syndrome; NS, no significant difference.
*P < 0.05.
Figure 3.
Causes of death. (a) Causes of death in all patients. (b) Causes of death in emergency and scheduled hemodialysis groups.
Forty-six patients died within 1 year after hemodialysis, accounting for 7.8% of the total number of deaths. Among them, 34 patients were in the emergency hemodialysis group, accounting for 10.9% of all emergency hemodialysis deaths, and 12 were in the scheduled hemodialysis group, accounting for 4.4% of all hemodialysis deaths. The difference between the two groups was significant (P = 0.003). Eighteen patients died within 3 months after dialysis, accounting for 3.1% of the total deaths. Among them, 15 patients were in the emergency hemodialysis group, accounting for 4.8% of all emergency hemodialysis deaths, and 3 were in the scheduled hemodialysis group, accounting for 1.1% (P = 0.009) of all hemodialysis deaths. Nine patients died during the first 3 to 6 months, accounting for 1.5% of the total number of deaths. Among them, five patients were in the emergency hemodialysis group, accounting for 1.6% of all emergency hemodialysis deaths, and four were in the scheduled hemodialysis group, accounting for 1.4% of all scheduled hemodialysis deaths. Nineteen patients died during the first 7 to 12 months, accounting for 3.2% of the total number of deaths. Among them, 14 patients were in the emergency hemodialysis group, accounting for 4.5% of all emergency hemodialysis deaths, and 5 were in the scheduled hemodialysis group, accounting for 1.8% of all hemodialysis deaths (Table 3-2).
Overall survival rate in scheduled versus emergency hemodialysis group
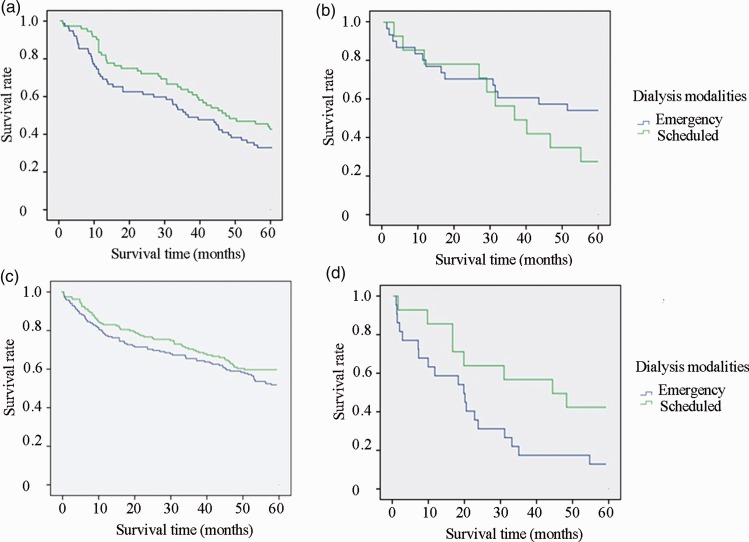
We further divided the patients in the emergency and scheduled hemodialysis groups into subgroups according to age (≥65 or <65 years) and primary disease (diabetic nephropathy or other). Kaplan–Meier survival analysis showed that among patients with diabetes aged <65 years, the survival rate was significantly higher in the scheduled than emergency hemodialysis group (P = 0.044, log-rank test) (Figure 2).
Figure 2.
Survival rate curves. (a) Survival rate curve of patients aged ≥65 years with nondiabetic nephropathy in emergency and scheduled hemodialysis groups (P > 0.05). (b) Survival rate curve of patients aged ≥65 years with diabetic nephropathy in emergency and scheduled hemodialysis groups (P > 0.05). (c) Survival rate curve of patients aged <65 years with nondiabetic nephropathy in emergency and scheduled hemodialysis groups (P > 0.05). (d) Survival rate curve of patients aged <65 years with diabetic nephropathy in emergency and scheduled hemodialysis groups (P = 0.044).
The survival rates in the emergency and scheduled hemodialysis groups in each period were as follows. Within the first 3, 6, and 12 months, the survival rates of patients in the emergency and scheduled hemodialysis groups were 92.3% and 96.4%, 87.2% and 93.4%, and 76.3% and 83.6%, respectively (Table 4-1, Figure 1).
Table 3-2.
Mortality in emergency hemodialysis group and scheduled hemodialysis group within 1 year after hemodialysis.
| Total patients | Emergency hemodialysis group | Scheduled hemodialysis group | P | |
|---|---|---|---|---|
| Total deaths | 46 (7.8) | 34 (10.9) | 12 (4.4) | 0.003** |
| Deaths in 1–3 months | 18 (3.1) | 15 (4.8) | 3 (1.1) | 0.009** |
| Deaths in 3–6 months | 9 (1.5) | 5 (1.6) | 4 (1.4) | NS |
| Deaths in 7–12 months | 19 (3.2) | 14 (4.5) | 5 (1.8) | NS |
Data are presented as n (%).
NS, no significant difference.
**P < 0.01.
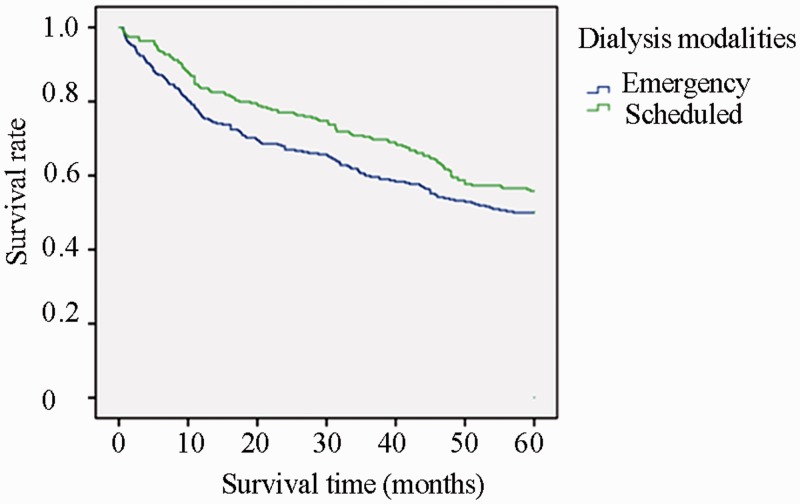
Figure 1.
Kaplan–Meier survival curve of emergency and scheduled hemodialysis groups according to initial dialysis method. The log-rank test showed a statistically significant difference between the two groups (P = 0.034).
After exclusion of patients who died within the first 3 months, there was no significant difference in the survival rate between the two groups. The survival rates in the emergency and scheduled hemodialysis groups in each period were as follows. Within the first 6 months, the survival rates in the emergency and scheduled hemodialysis groups were 94.4% and 97.3%, respectively. Within the first 12 months, these survival rates were 82.6% and 86.7%, respectively (Table 4-2). Our results indicate that the highest incidence of death in the emergency dialysis group was in the first 3 months after hemodialysis.
Table 4-2.
Survival rate of patients in emergency and scheduled hemodialysis groups (except for death in first 3 months).
| Emergency hemodialysis | Scheduled hemodialysis | P | |
|---|---|---|---|
| 6 months | 94.4% | 97.3% | NS |
| 1 year | 82.6% | 86.7% | NS |
| 2 years | 73.6% | 79.2% | NS |
| 3 years | 64.9% | 71.6% | NS |
| 4 years | 58.0% | 61.0% | NS |
| 5 years | 51.4% | 57.2% | NS |
NS, no significant difference.
Cox regression analysis
Univariate Cox regression analysis showed that one factor affecting the survival rate was whether the starting hemodialysis was on an emergency or scheduled basis. The survival rate was better in the scheduled than emergency hemodialysis group (P < 0.05). Other factors were as follows: diabetes mellitus (P < 0.05), history of pre-ischemic heart disease (P < 0.05), history of pre-penetrating congestive heart failure (P < 0.05), history of pre-penetrating arrhythmia (P < 0.05), platelet count (P < 0.05), and serum albumin level (P < 0.05).
When all of the above factors were included in the Cox regression model, forward stepwise regression (conditional logistic regression) analysis showed that after adjusting for confounding factors, the survival rates in the emergency and scheduled hemodialysis groups were not significantly different. The presence of concurrent or combined diabetes (hazard ratio (HR), 1.253; 95% confidence interval (CI), 1.010–1.556; P = 0.041) and the pre-albumin level (HR, 0.972; 95% CI, 0.956–0.988; P = 0.000) were the primary factors affecting the survival rate of patients treated with hemodialysis. Patients with concurrent or combined diabetes and low pre-albumin levels had lower survival rates (Table 5-1). In the univariate Cox regression analysis, significant risk factors (pre-ischemic heart disease, congestive heart failure, arrhythmia, and the serum albumin level) were not included in the final screening results. These factors may have interacted with other factors.
Table 4-1.
Survival rate of patients in emergency and scheduled hemodialysis groups.
| Emergency hemodialysis | Scheduled hemodialysis | P | |
|---|---|---|---|
| 3 months | 92.3% | 96.4% | 0.037* |
| 6 months | 87.2% | 93.4% | 0.012* |
| 1 year | 76.3% | 83.6% | 0.029* |
| 2 years | 67.0% | 76.3% | 0.013* |
| 3 years | 59.9% | 69.0% | 0.023* |
| 4 years | 53.5% | 58.8% | NS |
| 5 years | 47.4% | 55.1% | NS |
NS, no significant difference.
*P < 0.05.
Table 5-1.
Factors affecting survival of patients undergoing hemodialysis (Cox regression analysis).
| Β | Relative risk | 95% CI | P | |
|---|---|---|---|---|
| Pre-albumin | −0.029 | 0.972 | 0.956–0.988 | 0.000** |
| Co-occurring or associated diabetes | 0.226 | 1.253 | 1.010–1.556 | 0.041* |
CI, confidence interval.
*P < 0.05, **P < 0.01.
The emergency and scheduled hemodialysis groups were assessed by Cox regression analysis. The above-mentioned factors were also included in the Cox regression model. Using forward stepwise regression (conditional logistic regression) analysis, we found that after adjusting for confounding factors, the key factor affecting the survival rate in the emergency hemodialysis group was the pre-albumin level (HR, 0.964; 95% CI, 0.944–0.984; P = 0.000). Patients with a lower pre-albumin level in the emergency hemodialysis group had a lower survival rate. Diabetes mellitus was not the key factor affecting the survival rate of patients in the emergency hemodialysis group (Table 5-2). In the scheduled hemodialysis group, the primary factor affecting the survival rate was concurrent or combined diabetes mellitus (HR, 1.497; 95% CI, 1.089–2.057; P = 0.013). The survival rate of patients with concurrent or combined diabetes was low. The pre-albumin level was not the main factor influencing the survival rate in the emergency hemodialysis group (Table 5-3).
Table 5-2.
Factors affecting survival of patients undergoing emergency hemodialysis (Cox regression analysis).
| Β | Relative risk | 95% CI | P | |
|---|---|---|---|---|
| Pre-albumin | −0.037 | 0.964 | 0.944–0.984 | 0.000** |
CI, confidence interval.
**P < 0.01.
Table 5-3.
Factors affecting survival of patients undergoing scheduled hemodialysis (Cox regression analysis).
| Β | Relative risk | 95% CI | P | |
|---|---|---|---|---|
| Co-occurring or associated diabetes | 0.403 | 1.497 | 1.089–2.057 | 0.013* |
CI, confidence interval.
*P < 0.05.
Discussion
In the present observational study of the demographic and clinical data of patients who underwent either emergency or scheduled hemodialysis at the Department of Nephrology in the Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University, we evaluated and compared the short- and long-term survival rates between the two groups of patients. We also explored the primary factors affecting the short- and long-term prognosis of patients who underwent emergency and scheduled hemodialysis.
Our results showed that the serum creatinine level in the emergency hemodialysis group was significantly higher than that in the scheduled hemodialysis group, while the hemoglobin level was significantly higher in the scheduled hemodialysis group (P < 0.05). These findings are basically the same as previously reported findings.6,10–13 However, there was no significant difference in age, body mass index, albumin level, electrolyte levels, cerebral vascular events, cardiovascular events, or congestive heart failure between the two groups before the first hemodialysis treatment. The renal function, nutritional status, and general condition were worse among patients in the emergency than scheduled hemodialysis group; these factors are also poor prognostic factors.
Consistent with the results reported by Couchoud et al.,11 Górriz et al.,6 Metcalfe et al.,14 and other researchers, our results showed that the mortality rate was significantly higher in the emergency than scheduled hemodialysis group.6,11–13 The Kaplan–Meier survival curve also showed that the overall survival rate was better among patients undergoing scheduled than emergency hemodialysis. In each period, the survival rate was lower in the emergency than scheduled hemodialysis group, but the extent of the difference in the survival rate between the two groups gradually decreased as time progressed. The 3-year survival rate significantly differed between the two groups, but the long-term survival rate was not significantly different. We also found that the survival rate was lower in the emergency than scheduled hemodialysis group in each period. However, when death within the first 3 months was excluded, the survival rates in the two groups were not significantly different. This result indicates that the difference in the survival rate between the two groups occurred mainly within the first 3 months after hemodialysis, while the long-term survival rate was not significantly different between the two groups. In addition, we found that the primary cause of death was non-renal disease. Therefore, close clinical observation of the indications for hemodialysis and the performance of early preventive hemodialysis for patients with indications before the development of severe complications can reduce patients’ mortality and improve their quality of life. We also found significant differences in the survival rates between the two hemodialysis groups among patients who were <65 years of age and whose primary disease was diabetic nephropathy; this finding has not been mentioned in most of the relevant literature. It is necessary to strengthen the follow-up diagnosis and treatment of patients with diabetic nephropathy.
The principal risk factors affecting the survival rate of patients undergoing hemodialysis were screened using a Cox regression model. The key risk factors were diabetes mellitus and the pre-albumin level. The survival rate was low among patients with diabetes mellitus and a low pre-albumin level. This further proved that the presence of associated disease (especially diabetes mellitus with multiple organ damage) and the nutritional status upon beginning hemodialysis can affect the survival rate. The influence of hemodialysis methods on the survival rate is partly due to the interaction of these factors. Many previous reports have provided similar descriptions of the above-mentioned risk factors. For example, a study by Couchoud et al.11 showed that diabetes is the primary risk factor for a low survival rate among patients undergoing hemodialysis. Mendelssohn et al.9 and other researchers11,15 also confirmed that patients with low pre-albumin levels have lower survival rates. Some factors in the univariate analysis were proven to be significant risk factors, including ischemic heart disease, congestive heart failure, arrhythmia, and chronic obstructive pulmonary disease. However, these factors were not entered into the final screening results. This may have been because of the small sample size and the interaction between the factors, which needs further research.
Authors’ contributions
Shijian Zhu participated in the study design, manuscript drafting, data interpretation. Zhixiang Bian was involved in the data analysis and interpretation. Huiyi Gu and Peihua Chen both took part in collecting the patients’ information.
Availability of data and materials
Data are available from the authors upon reasonable request and with permission of The Northern Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University, Shanghai.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Tan Yipu WH. Welcome “World Kidney Day”: pay attention to the early diagnosis and prevention of chronic kidney disease. Chinese Journal of Medicine 2006; 86: 649–651. [Google Scholar]
- 2.Yang DC, Luo CG, Zhou WM, et al. Clinical analysis of 30 cases of senile acute renal failure . Chinese Community Physician 2011; 24: 186–187. [Google Scholar]
- 3.Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Int Med 2002; 137: 479. [DOI] [PubMed] [Google Scholar]
- 4.Chan MR, Dall AT, Fletcher KE, et al. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med 2007; 120: 1063–1070. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura S, Nakata H, Yoshihara F, et al. Effect of early nephrology referral on the initiation of hemodialysis and survival in patients with chronic kidney disease and cardiovascular diseases. Circ J 2007; 71: 511–516. [DOI] [PubMed] [Google Scholar]
- 6.Górriz JL, Sancho A, Pallardó LM, et al. Prognostic significance of programmed dialysis in patients who initiate renal substitutive treatment. Multicenter study in Spain. Nefrología 2002; 22: 49–59 [in Spanish, English Abstract]. [PubMed] [Google Scholar]
- 7.Buck J, Baker R, Cannaby AM, et al. Why do patients known to renal services still undergo urgent dialysis initiation? A cross-sectional survey. Nephrol Dial Transplant 2007; 22: 3240–3245. [DOI] [PubMed] [Google Scholar]
- 8.Watson D. Post-dialysis “pre-dialysis” care: the cart before the horse–advanced practice nurse intervention and impact on modality selection. CANNT J 2008; 18: 30–33. [PubMed] [Google Scholar]
- 9.Mendelssohn DC, Malmberg C, Hamandi B. An integrated review of “unplanned” dialysis initiation: reframing the terminology to “suboptimal” initiation. BMC Nephrol 2009; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellano I, Gallego S, Labrador PJ, et al. The start of renal replacement therapy in a Spanish department. Nefrologia 2006; 26: 445–451. [PubMed] [Google Scholar]
- 11.Couchoud C, Moranne O, Frimat L, et al. Associations between comorbidities, treatment choice and outcome in the elderly with end-stage renal disease. Nephrol Dial Transplant 2007; 22: 3246–3254. [DOI] [PubMed] [Google Scholar]
- 12.Information CIfH: The cost of hospital stays: why costs vary 2008.
- 13.Ifudu O, Dawood M, Homel P, et al. Excess morbidity in patients starting uremia therapy without prior care by a nephrologist. Am J Kidney Dis 1996; 28: 841–845. [DOI] [PubMed] [Google Scholar]
- 14.Metcalfe W, Khan IH, Prescott GJ, et al. Can we improve early mortality in patients receiving renal replacement therapy? Kidney Int 2000; 57: 2539–2545. [DOI] [PubMed] [Google Scholar]
- 15.Caskey FJ, Wordsworth S, Ben T, et al. Early referral and planned initiation of dialysis: what impact on quality of life? Nephrol Dial Transplant 2003; 18: 1330–1338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the authors upon reasonable request and with permission of The Northern Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University, Shanghai.