Short abstract
Objective
The present study explored how the inhibition of protease-activated receptor-2 (PAR-2) induced proliferation and apoptosis in cervical cancer in vitro and in vivo.
Methods
mRNA and protein expression of PAR2 and signal transducer and activator of transcription-3 (STAT-3) was determined by quantitative real-time PCR and western blotting. The proliferation and apoptosis of cervical cancer cells were assayed by the cell counting kit-8 kit, flow cytometry, and western blotting. The effects of PAR2 inhibition on cervical cancer were also examined in BALB/c nude mice in vivo.
Results
SLIGRL-NH2 (SL), a selective PAR-2 agonist, promoted proliferation and inhibited apoptosis of healthy cervical cancer cells and HeLa cells, while the PAR-2 antagonist FSLLRY-NH2 (FS) inhibited proliferation and led to apoptosis. SL also promoted the activation of STAT-3, while FS inhibited it and inhibited cancer growth in vivo.
Conclusion
FS inhibited cervical cancer by reducing proliferation and inducing apoptosis by interfering with STAT-3 signaling.
Keywords: Cervical cancer, protease-activated receptor-2, apoptosis, proliferation, signal transducer and activator of transcription-3, FSLLRY-NH2
Introduction
Cervical cancer originates in the uterine cervix, which is the lower end of the uterus that contacts the upper vagina. Human papillomavirus types 16 and 18 are the cause of 75% of cervical cancer cases globally.1 Cervical cancer is the fourth most common cause of cancer and the fourth most common cause of death from cancer in women worldwide. If detected early, it has a very high cure rate, and it can be treated by surgery, radiotherapy, and chemotherapy.2 Anti-cancer drugs prevent or inhibit the proliferation and metastasis of neoplasms, and have high anti-tumor efficacy and low toxicity to healthy cells. However, many anti-tumor drugs improve patient survival but are harmful to their vital organs or fertility.3,4 Therefore, the identification of novel, tumor-specific drugs with no adverse effects is an ultimate goal for anti-cancer therapy.
Protease-activated receptor-2 (PAR-2) is a member of the seven-transmembrane, G protein-coupled receptor family. It is activated by trypsin, tryptase, and coagulation factors which are the natural agonists ofPAR-2. Activated PAR-2 is involved in a series of biological behaviors including cell proliferation, invasion, and metastasis in tumors.5,6 Sánchez-Hernández and coworkers reported a strong correlation between trypsin and PAR-2 expression in all cervical cancer cell lines studied. Moreover, cervical cancer patients were shown to have high expression of PAR-2.7 A recent study by Hugo de Almeida et al.8 demonstrated crosstalk between PAR2 and epidermal growth factor receptor that contributed to chemoresistance in cervical cancer cells.
The activation of signal transducer and activator of transcription-3 (STAT-3) signaling is closely associated with cell proliferation, differentiation, and apoptosis, but continuous activation of the pathway may result in abnormal proliferation and malignant transformation of cells.9–11 STAT-3 has now been defined as an oncogene and is attracting increasing attention in the treatment of cervical cancers.
FSLLRY-NH2 (FS) is a selective PAR peptide antagonist,12 while SLIGRL-NH2 (SL) is a selective PAR-2 agonist. The aim of this study was to determine how PAR-2 affects apoptosis in cervical cancer in vitro and in vivo, and to investigate the correlation of PAR-2 with STAT-3 signaling.
Materials and methods
Cancer cell lines and cell culture
The human cervical cancer cell line HeLa was purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Beijing, China). Cells were cultured in DMEM/F12 (1:1) (GIBCO® Cell Culture, Carlsbad, CA, USA), supplemented with 10% fetal calf serum (FCS; Solarbio, Beijing, China), 100 U/mL penicillin, and 100 µg/mL streptomycin (Abcam, Cambridge, MA, USA) at 37°C with 5% CO2.
Culture of healthy primary human cervical cells
Normal primary human cervical epithelial cells were obtained from the hysterectomy specimens of four healthy women. Tissue fragments were incubated with 0.25% trypsin for 24 hours at 4°C, then cultured with DMEM/F12 (1: 1) supplemented with 10% FCS, 1 µg/mL of fungizone (Gibco BRL), 100 U/mL penicillin, and 100 µg/mL streptomycin (Gibco BRL). Subconfluent cultures were dispersed with 0.0025% trypsin and 0.02% ethylenediaminetetraacetic acid (EDTA). Sample collection was approved by the Ethical Committee of the Affiliated Hospital of Wuhan University of Science and Technology before commencing this study, and individuals gave their informed consent.
Cell counting kit (CCK)-8 assay
A total of 100 µL of cell suspension containing 104 cells was added to each well of 96-well plates and cultured for 24 hours at 37°C. After serial dilution, the PAR-2 inhibitor FS or the PAR-2 agonist SL was added at a final concentration of 0, 20, 50, or 100 µM for 48 hours. FS was purchased from Sigma-Aldrich (St. Louis, MO, USA), and SL was from Abcam®. After carefully removing the supernatant, 10 µL of CCK-8 reagent was added to the cells and incubated for 3 hours. The optical density values of every well was measured at 450 nm using the SpectraMax iD5 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The experiments were repeated three times.13
Flow cytometry
HeLa cells or primary human cervical epithelial cells were treated with a final concentration of 100 µM SL or FS. Cells were then labeled with 4 µL of FITC-Annexin V and 8 µL of propidium iodide for 5 minutes at room temperature in the dark. Apoptosis was then analyzed using a flow cytometer (FACS Caliper; BD Biosciences, San Jose, CA, USA).
Quantitative real-time (qRT)-PCR
Total RNA was extracted from HeLa cells or primary human cervical epithelial cells by TRIzol (Invitrogen Corp., Carlsbad, CA, USA) and reverse transcription were performed using the QuantiTect Reverse Transcription kit (Promega, Madison, WI, USA). qPCR was performed using an ABI Prism 7000 Sequence Detection system and software (Applied Biosystems, Foster City, CA, USA) in a final volume of 20 µL containing 2 µL of cDNA as the template, 10 pmol of each primer, 10 µL of SYBR Green Master Mix (Applied Biosystems), and water to 20 µL. PCR parameters were initial denaturation at 94°C for 5 minutes, followed by 25 cycles of 94°C for 30 s and 60°C for 30 s. Primers were obtained from Takara Biotechnology Co., Ltd., Dalian, China) and were as follows: 5′-ACAGA CACGT CCTCATAACATTAAACA-3′ and 5′-TCCCTCACCTCAAAGAAA CAC TCC-3′ for PAR-2; 5′-TTTGTACCGA CCGTTCCCG-3′ and 5′-ACCACC AAG CGAGGACTGAGC-3′ for STAT-3; and 5′-GAGAAACGGCTACCACAT CCAA G-3′ and 5′-GCACCAGACTTGCCCTCC A-3′ for Homo-18SrRNA. Quantitative analysis of target gene expression data was based on the 2 ΔΔCt method.14
Western blotting
Cells were incubated with ice-cold radioimmunoprecipitation assay lysis buffer (50 mM Tris, pH 7.4, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 30 mM sodium fluoride, 5 mM EDTA, 1 mM sodium orthovanadate, and 10 mM leupeptin). The supernatant was collected after centrifugation at 10,000 × g for 15 minutes at 4°C. Protein concentrations were determined by the bicinchoninic acid assay. Approximately 80 µg of protein samples were run on 10% SDS polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membranes were blocked with western blocking buffer and incubated with the following primary antibodies (Abcam®): anti-PAR-2 (1: 250), anti-p-STAT-3 (1: 250), anti-STAT-3 (1: 250), and anti-caspase-3 or anti-GAPDH (1: 1000) at 4°C overnight. After washing three times in Tris-buffered saline and Tween 20, the membranes were incubated with goat anti-mouse IgG secondary antibody (1: 1000; Abcam) at 37°C for 2 hours. The membranes were then colored with 3,3′-diaminobenzidine for 1–2 minutes at room temperature.
In vivo tumor growth
To verify the effects of FS on cervical cancer, an in vivo model was established. Five- to six-week-old BALB/c nude mice (n = 12) were obtained from Wuhan University of Science and Technology and divided into two groups, which were inoculated with HeLa cells alone or HeLa cells + FS (20 mg/kg). All procedures involving animals and their care were approved and performed by Wuhan University of Science and Technology Institutional Animal Care and Use Committee. Each mouse was subcutaneously injected with 1 × 107 HeLa cells and half were simultaneously injected with FS (20 mg/kg). Twelve days after the injections, the tumor size was measured with a caliper; this was repeated every 3 days. The mice were killed on day 24 and the tumors underwent immunohistochemistry staining for Ki67.
Immunofluorescence and immunohistochemistry
HeLa cells and primary human cervical epithelial cells treated with or without SL or FS were subjected to p-STAT-3 immunofluorescence. Subcutaneous tumor tissues from the in vivo model were isolated and fixed in formaldehyde for at least 24 h, after which they were dehydrated, embedded, sectioned, and subjected to Ki67 immunohistochemistry staining. The integral optical density values of Ki67 protein in the cervical cancer sections were measured by the CMIAS-8 color pathological image analysis system. All antibodies were from Abcam®.
Statistical analysis
Statistical analysis was performed with SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). The Student’s t-test (two-tailed distribution with a two sample equal variance) was used for analysis. All results were presented as the mean ± SD. P < 0.05 was considered to be statistically significant.
Results
PAR-2 promoted the proliferation of HeLa cells and primary human cervical epithelial cells
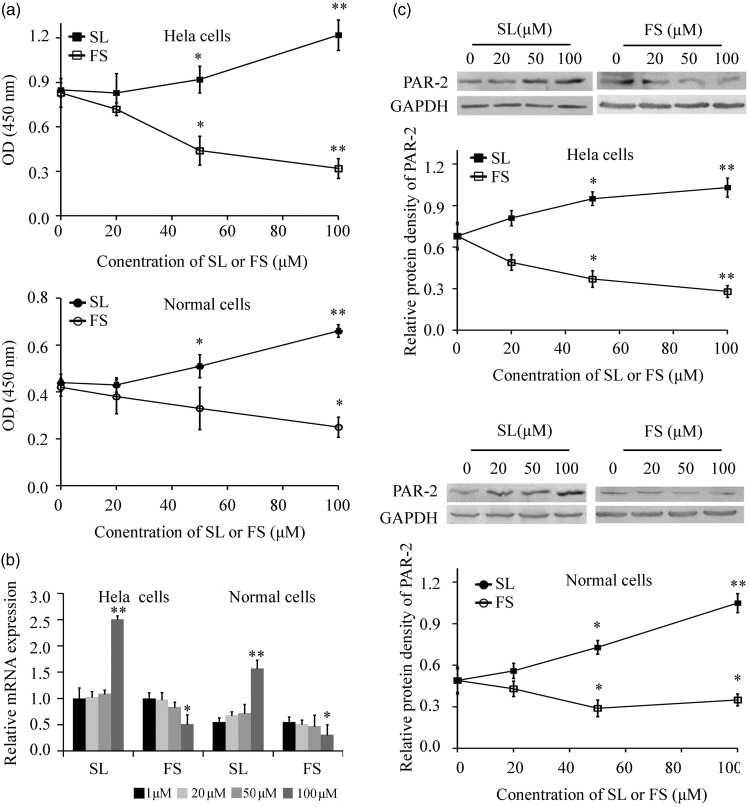
The effects of PAR-2, FS, and SL were tested on HeLa cells and primary human cervical epithelial cells. As shown in Figure 1a, FS displayed strong inhibitory effects on cell growth, while SL strongly promoted the growth of both primary cervical cells and HeLa cells. Next, we examined the mRNA and protein expression of PAR-2 when treated with different concentrations of FS or SL for 48 hours. Real-time PCR showed that 100 µM FS inhibited the expression of PAR-2, and that 100 µM SL promoted the expression of PAR-2 in both primary cervical cells and HeLa cells, (Figure 1b). Similarly, western blotting showed that 50 and 100 µM FS inhibited the expression of PAR-2 and 50 and 100 µM SL promoted the expression of PAR-2 (Figure 1c).
Figure 1.
PAR-2 promoted the proliferation of HeLa cells and primary cervical cells (n = 6). A: Proliferation was assayed by CCK-8 after treatment with different concentrations of SL or FS for 48 hours. B: PAR-2 mRNA expression after treatment with different concentrations of FS or SL for 48 hours was assayed by real-time PCR (n = 5). C: PAR-2 protein expression after treatment with different concentrations of FS or SL for 48 hours was assayed by western blotting. *P < 0.05 and ** P < 0.01 vs. 0 hours.
PAR-2 inhibited the apoptosis of HeLa cells and primary human cervical epithelial cells
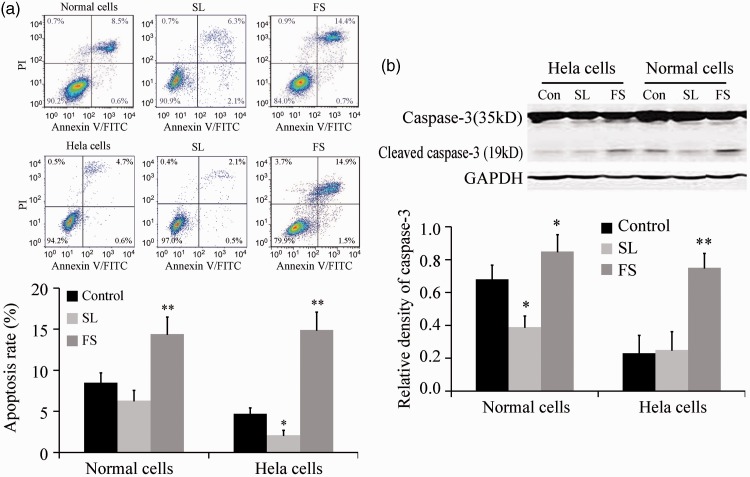
Apoptosis in cervical cells was then examined after treatment with 100 µM of FS or SL for 48 hours. FS promoted the apoptosis of primary cervical cells and HeLa cells, while SL inhibited the apoptosis of HeLa cells (Figure 2a). Western blotting showed that SL inhibited the expression of the apoptosis marker cleaved caspase-3 in normal primary cervical cells, while FS promoted its expression in primary cervical cells and HeLa cells (Figure 2b). These data suggest that PAR-2 inhibited the apoptosis of HeLa cells and primary cervical cells.
Figure 2.
PAR-2 inhibited the apoptosis of HeLa cells and primary cervical cells (n = 5). A: Apoptosis was assayed by flow cytometry after treatment with 100 µM of FS or SL for 48 hours. B: Caspase-3 expression was assayed by western blotting after treatment with 100 µM of FS or SL for 48 hours. *P < 0.05 and **P < 0.01 vs. control.
PAR-2 promoted the expression of STAT-3 in HeLa cells and primary human cervical epithelial cells
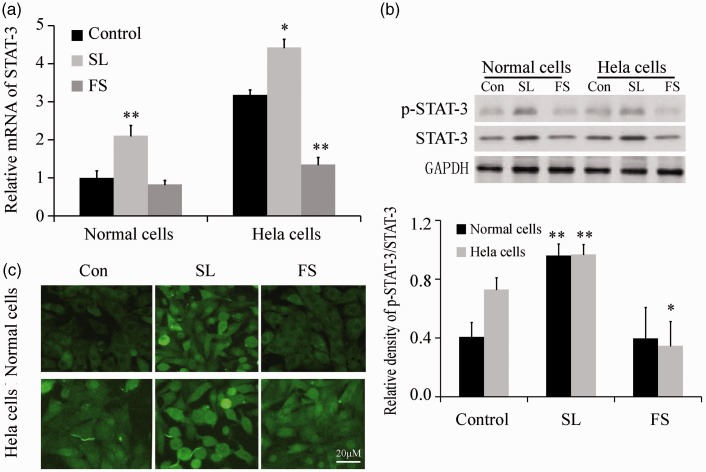
Real-time PCR showed that the expression of STAT-3 mRNA increased after pretreatment with 100 µM SL for 48 hours in primary cervical cells, while it increased after pretreatment with 100 µM SL and decreased after pretreatment with 100 µM FS for 48 hours in HeLa cells (Figure 3a). Similarly, western blotting showed that activation of the STAT-3 protein increased after SL pretreatment in primary cervical normal cells, and increased after SL pretreatment but decreased after FS pretreatment in HeLa cells (Figure 3b). p-STAT-3 immunofluorescence staining analysis revealed similar findings (Figure 3c).
Figure 3.
PAR-2 promoted the expression of STAT-3 in HeLa cells and primary cervical cells (n = 5). A: STAT-3 mRNA expression was assayed by real-time PCR after treatment with 100 µM of FS or SL for 48 hours. B: p-STAT-3 and STAT-3 were assayed by western blotting after treatment with 100 µM of FS or SL for 48 hours. c: p-STAT-3 immunofluorescence staining. *P<0.05 and **P < 0.01 vs. control.
FS suppressed cervical cancer growth in vivo
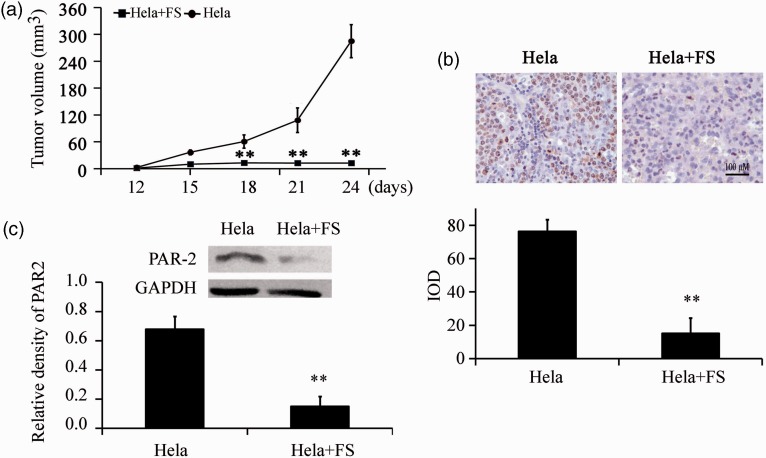
To explore the effect of PAR-2 on cervical cancer cell growth in vivo, HeLa cells were injected subcutaneously into nude mice. Tumor growth rate of the HeLa group was significantly higher than in the HeLa+FS group after serial observation for 24 days (P = 0.0153, Figure 4a). Ki67 staining of tumor tissues confirmed that tumors in the HeLa group were stained more strongly than in the HeLa+FS group (Figure 4b). Moreover, PAR-2 protein expression was also significantly decreased in the HeLa+FS group compared with the HeLa group (P < 0.01, Figure 4c). These results showed that the inhibition of PAR-2 repressed cancer growth in vivo.
Figure 4.
FS inhibited cervical cancer growth in vivo (n=12). Nude mice were subcutaneously injected with 1 × 107 HeLa cells, and half were simultaneously injected with 20 mg/kg FS. A: Tumor growth (**P = 0.0153). B: Ki67 immunohistochemical staining (magnification ×400). C: PAR-2 protein expression was assayed by western blotting.
Discussion
Cervical cancer is a malignant tumor deriving from the cells of the cervix. Uncontrolled cell growth and reduced apoptosis are important characteristics of malignancies, and treatment with anti-cancer drugs is used to achieve the inhibition of tumor cell proliferation.
In the present study, we found that PAR-2 was highly expressed in HeLa cells. We used a selective PAR-2 antagonist, FS, and a selective PAR-2 agonist, SL, to investigate the effects of PAR-2 on cervical cell growth and apoptosis, with the aim of evaluating its therapeutic potential in cervical cancer. SL promoted the expression of PAR-2 and FS inhibited it in cervical cells in a concentration-dependent manner. The increased or decreased expression of PAR-2 was related to receptor activation or degradation at agonist or antagonist concentrations of 50 µM, and to receptor regulation and mRNA transcription at concentrations of 100 µM. FS induced the apoptosis of both primary cervical cells and HeLa cells. Our data suggested that PAR-2 inhibition blocked the proliferation and promoted the apoptosis of cervical cancer cells.
PAR-2 is a G protein-coupled receptor that regulates the biological characteristics of tumor cells after activation by multiple signal pathways. These pathways that affect the proliferation, metastasis, and invasion of malignant tumors include the mitogen-activated protein kinase (MAPK) signaling pathway and its downstream cascade reactions.15 MAPK signaling activates c-Jun N-terminal kinase phosphorylation and STAT-3, which enhanced the occurrence of epithelial–mesenchymal transition and extracellular matrix degradation, and promoted the invasion and metastasis of tumors.16 However, the correlation between PAR-2 activation and STAT-3 phosphorylation had not been studied. We report, for the first time, that PAR-2 promoted the activation of STAT-3 in both primary cervical cells and HeLa cells, which was associated with increased mRNA expression.
In vivo, we found that FS inhibited cancer growth and Ki67 staining. Ki67 expression reflects cancer cell proliferation, with higher Ki67 expression seen in more active cancer cells. FS also significantly decreased the protein expression of PAR-2, indicating that PAR-2 inhibition repressed cancer growth in vivo. However, a limitation of this study was that the effect of PAR-2 was not studied in several different cervical cancer cell lines.
In conclusion, we showed that PAR-2 inhibition blocked cervical cancer by specifically reducing proliferation and inducing apoptosis through the inhibition of STAT-3 signaling. Therefore, FS is a promising candidate for an anti-tumor drug.
Author contribution statement
Design: YL; Collection of data: HS, XL, LX, WH; Data analysis: ZM; Manuscript writing: YL; Final approval of manuscript: All authors.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Molijn A, Jenkins D, Chen W, et al. The complex relationship between human papillomavirus and cervical adenocarcinoma. Int J Cancer 2016; 138: 409–416. [DOI] [PubMed] [Google Scholar]
- 2.Lecavalier-Barsoum M, Chaudary N, Han K, et al. Targeting the CXCL12/CXCR4 pathway and myeloid cells to improve radiation treatment of locally advanced cervical cancer. Int J Cancer 2018; 143: 1017–1028. [DOI] [PubMed] [Google Scholar]
- 3.Dueñas-González A, Cetina L, Coronel J, et al. The safety of drug treatments for cervical cancer. Expert Opin Drug Saf 2016; 15: 169–180. [DOI] [PubMed] [Google Scholar]
- 4.Dueñas-Gonzalez A, Cetina L, Coronel J, et al. New pharmacotherapy options for cervical cancer. Expert Opin Pharmacother 2014; 15: 51–60. [DOI] [PubMed] [Google Scholar]
- 5.Jahan I, Fujimoto J, Alam SM, et al. Role of protease activated receptor-2 in tumor advancement of ovarian cancers. Ann Oncol 2007; 18: 1506–1512. [DOI] [PubMed] [Google Scholar]
- 6.Jahan I, Fujimoto J, Alam SM, et al. Role of protease activated receptor-2 in lymph node metastasis of uterine cervical cancers. BMC Cancer 2008; 8: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sánchez-Hernández PE, Ramirez-Dueñas MG, Albarran-Somoza B, et al. Protease-activated receptor-2 (PAR-2) in cervical cancer proliferation. Gynecol Oncol 2008; 108: 19–26. [DOI] [PubMed] [Google Scholar]
- 8.Hugo de Almeida V, Guimarães IDS, Almendra LR, et al. Positive crosstalk between EGFR and the TF-PAR2 pathway mediates resistance to cisplatin and poor survival in cervical cancer. Oncotarget 2018; 9: 30594–30609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadducci A, Guerrieri ME, Greco C. Tissue biomarkers as prognostic variables of cervical cancer. Crit Rev Oncol Hematol 2013; 86: 104–129. [DOI] [PubMed] [Google Scholar]
- 10.Miranda C, Fumagalli T, Anania MC, et al. Role of STAT3 in in vitro transformation triggered by TRK oncogenes. PLoS One 2010; 5: e9446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poli V, Camporeale A. STAT3-mediated metabolic reprograming in cellular transformation and implications for drug resistance. Front Oncol 2015; 5: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLarty JL, Meléndez GC, Brower GL, et al. Tryptase/protease-activated receptor 2 interactions induce selective mitogen-activated proteinkinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension 2011; 58: 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Liu M, Kang L, et al. HHEX: a crosstalker between HCMV infection and proliferation of VSMCs. Front Cell Infect Microbiol 2016; 6: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Wu G, Qin X, et al. Expression of nodal on bronchial epithelial cells influenced by lung microbes through DNA methylation modulates the differentiation of T-helper cells. Cell Physiol Biochem 2015; 37: 2012–2022. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie E, Saka M, Mackenzie C, et al. Cytokine upregulation of proteinase-activated-receptors 2 and 4 expression mediated by p38 MAP kinase and inhibitory kappa B kinase beta in human endothelial cells. Br J Pharmacol 2007; 150: 1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colomiere M, Ward AC, Riley C, et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer 2009; 100: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]