Short abstract
Objective
This study aimed to evaluate the clinical features, radiological findings, risk factors, and management of liver abscesses following transcatheter arterial chemoembolization (TACE) therapy in patients with primary and metastatic liver cancer.
Methods
The medical records of 163 patients who were treated with TACE in our hospital for a total of 313 times between January 2012 and January 2018 were reviewed. The incidence rate of patients who developed liver abscesses after undergoing treatment, the computed tomography findings of the abscesses, predisposing risk factors, and the method of treatment were evaluated.
Results
Formation of liver abscesses was observed after treatment in four of the 163 (2.4%) patients and in four (1.3%) of the 313 chemoembolization procedures. Additionally, bilioenteric anastomosis was found in two patients and diabetes mellitus in two patients.
Conclusion
Formation of liver abscesses after TACE is a rare, but serious, complication. Physicians should be aware of the predisposing risk factors of TACE because of the possibility of death.
Keywords: Abscess, bilioenteric anastomosis, risk factor, transcatheter arterial chemoembolization, liver cancer, diabetes mellitus
Introduction
Transcatheter arterial chemoembolization (TACE) is one of the most effective treatments for managing primary malignant liver tumors and liver metastases. Many studies have shown that TACE prolongs survival.1 Although this treatment modality is a minimally invasive procedure, it can sometimes lead to serious complications that may occasionally result in mortality, with a morbidity rate of between 13.3%and 50%.2 One of the more serious complications of TACE is formation of abscesses after TACE.3 Previous studies have shown that the incidence of abscess formation after TACE varies between 0.1% and 4.5%.4 The risk factors reported to be associated with formation of liver abscesses after TACE include biliary anastomosis, biliary anomalies, aging, diabetes mellitus (DM), a large tumor size, and portal venous occlusion.2,5 Post-TACE liver abscesses are different from primary liver abscesses because of associated conditions, such as advanced tumors, hepatic dysfunction, deterioration of arterial flow in the area, and acidity. Percutaneous drainage is recommended as the first line of treatment, and is an effective and minimally invasive treatment for liver abscesses.2,3 Taking samples is important for TACE because they can serve as a guide for selection of antibiotics. The present study aimed to determine the radiological features, incidence rate, risk factors, and percutaneous drainage of liver abscesses after TACE in patients with primary liver cancer or metastatic hepatic tumors.
Materials and methods
Patients
The study was approved by Pamukkale University Medical Faculty Non-Interventional Clinical Trials Ethics Committee, and was conducted in accordance with the Declaration of Helsinki. We retrospectively reviewed the electronic medical records of patients with primary and metastatic liver tumors who underwent TACE in the Department of Interventional Radiology at our institution between 1 January, 2012 and 31 December, 2017. Cases of liver abscess were identified after a total of 313 TACE procedures were performed in 163 patients. In this review, the relationships between abscess formation and certain risk factors (diabetes, tumor number, largest tumor size, tumor type, particulate agent size, drugs used, bilioenteric anastomosis) were examined.
TACE procedure
The patients all signed informed consent forms before the TACE procedure. Contrast-enhanced computed tomography (CT) was performed before the procedure to evaluate variations in the hepatic artery and to permit navigation to the artery feeding the tumor. All of the procedures were performed with drug-loadable particulate agents, apart from a few cases (3 procedures). The particulate agents were between 40 and 300 microns, and decisions to administer anticancer drugs were made together with the Medical Oncology Department on the basis of tumor type. Patients were discharged in the absence of any complications at 24 hours of follow-up after the procedure. Patients had a follow-up visit 1 month later to undergo contrast-enhanced CT imaging to determine the tumor response to the TACE procedure.
Certain criteria related to the patients were used in our department to perform TACE on patients with malignant liver tumors. The criteria for application of the TACE procedure are shown in Table 1.
Table 1.
Transcatheter arterial chemoembolization eligibility criteria.
| Normal coagulation parameters (INR < 1.5 or platelet count > 50,000/µL) |
| Absence of any allergy to the contrast substance |
| Presence of normal kidney function |
| Absence of jaundice (bilirubin levels > 3 mg) |
| Tumor occupying less than two thirds of the liver |
| Patients with ECOG performance scores of 0, 1, and 2 |
| Patients aged between 18 and 85 years |
INR: international normalized ratio; ECOG: European Cooperative Oncology Group.
Diagnosis and treatment of abscesses
The patients were discharged within 24 hours of the procedure after being informed of potential complications, and asked to report to the hospital in the event of symptoms, such as abdominal pain and fever. Contrast CT scans of patients who were clinically suspected of having abscesses were obtained. CT scans were also evaluated in terms of peripheral rim contrast enhancement, with or without an air–fluid level abscess, and with hypoattenuation in the treated segment. Ultrasound (US)-guided percutaneous catheter drainage therapy was carried out in patients who had abscesses.
After skin sterilization, a local anesthetic agent (2% prilocaine hydrochloride) was administered into the skin, subcutaneous tissue, and liver capsule under US guidance to provide local anesthesia. An 18-gauge Cook needle was inserted into the abscess and approximately 5 cc of aspirate was obtained for bacterial culture and antibiogram tests from culture. A 0.038-inch (1 inch = 2.54 cm) guide wire (Amplatz Super Stiff; Boston Scientific, Heredia, Costa Rica) was then inserted through the needle and the needle was removed. A 10 French (F) or 12 F drainage catheter was inserted into the abscess along the guide wire, and then left for free drainage. Irrigation with 0.9% saline was recommended twice daily to prevent the catheter from becoming occluded. Additionally, broad-spectrum antibiotics were initiated until the results of the antibiogram were collected. The size of the abscess was assessed with US when free drainage from the catheter fell below 10 cc per day. If the size of the abscess had shrunk (<2 cm) and the clinical and laboratory findings had improved, the catheter was withdrawn, assuming that the abscess had recorded improvement.
Statistical analysis
The data were analyzed using the SPSS program package version 21.0 (IBM SPSS, Armonk, NY, USA). The patients’ age, sex, presence of diabetes mellitus, tumor size, tumor number, tumor type, anticancer drugs used, particle size used, bacteriological results of the patients, and the presence of bilioenteric anastomosis and biliary stents were examined. Numerical data are expressed as mean ± standard deviation or percentage.
Results
Of the 163 patients who underwent TACE procedures, 110 (67.5%) were men and 53 (32.5%) were women. The patients’ mean age was 61.96 years (34–84 years). Liver abscesses developed in four (2.4%) of the 163 patients and in four (1.3%) of the 313 TACE procedures. Liver abscesses developed in one (0.91%) of 110 men and in three (5.66%) of 53 women (Table 2). All of the patients reported development of an abscess when the first TACE operation was performed, and no second TACE procedure was performed in these patients.
Table 2.
Characteristics of patients who developed liver abscesses after transcatheter arterial chemoembolization.
| Patients | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Sex | Male | Female | Female | Female |
| Age (years) | 34 | 69 | 78 | 71 |
| Tumor type | Pancreas CA | Ovarian CA | Colon CA | Hepatocellular CA |
| Bilioenteric anastomosis | + | − | + | − |
| Diabetes mellitus | + | − | − | + |
| Treated lesion size (mm) | 32 × 26 | 103 × 92 | 35 × 24 | 64 × 43 |
| Tumor number | 1 | 13 | 9 | 1 |
| Anticancer drugs | 50 mg doxorubicin | 100 mg doxorubicin | 200 mg irinotecan | 100 mg doxorubicin |
| Particle size | 50 µ | 75 µ | 40 µ | 100–300 µ |
| Bacteriological results | Escherichia coli | Escherichia coli | Escherichia coli and Enterobacter | Escherichia coli |
CA: carcinoma.
Bilioenteric anastomosis was present in two of the patients and two of the patients were receiving medication for DM. Of the patients with abscesses, three underwent TACE for metastatic disease because of the presence of hepatocellular cancer. The treated tumor size in all of the patients was greater than 3 cm. None of the patients had leukopenia, while leukocytosis was present in one patient (10800/µL). Three of the patients who developed abscesses were treated with 50 to 100 mg doxorubicin and one of the patients was treated with 200 mg irinotecan. The particle sizes used ranged between 40 and 300 microns. Postembolization syndrome developed in all patients with abscesses immediately after undergoing the TACE procedure.
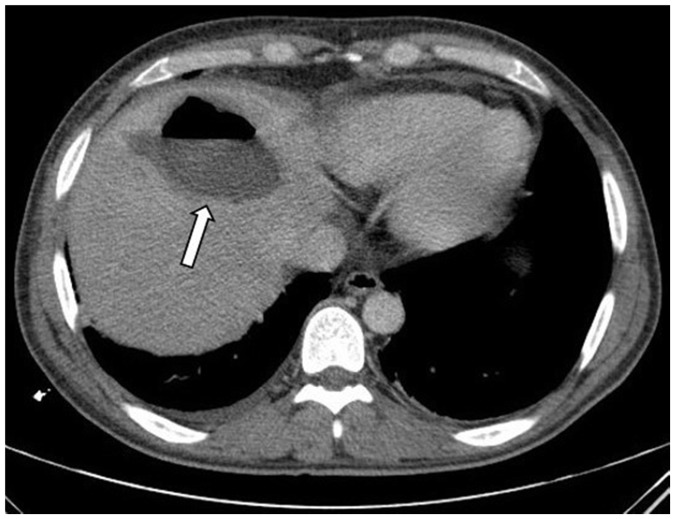
Abscesses were diagnosed at an average of 13 days (10–19 days) after the procedure by CT. The patients’ complaints at the time of presentation were right upper quadrant pain (100%), fever (75%), and malaise (50%). Diagnoses of abscesses were made by contrast-enhanced CT in all patients (Figure 1). All of the patients had hypoattenuating lesions, with an average size of 77 mm (42–113 mm). Some lesions showed peripheral rim enhancement and contained gas. There was only one abscess in one patient, while there were multiple abscesses in the other three patients. US-guided percutaneous abscess drainage therapy was performed in all patients. Escherichia coli growth in pouch culture was reported in three patients, while Escherichia coli and Enterobacter was found in one patient. One patient died 6 days after insertion of a drainage catheter as a result of liver abscess progressing to sepsis. Drainage catheters were removed after an average of 28 days (12–54 days).
Figure 1.
Axial computed tomographic scan image shows a hypoattenuating abscess (arrow) with an air–fluid level in the left lobe of the liver.
Liver abscesses were expected to develop after TACE in patients with a bilioenteric fistula and associated DM. However, we were unable to obtain significant results associated with abscess formation after TACE because of the small number of patients (4 patients) in the sample. Information on patients in whom abscesses formed is shown in Table 2.
Discussion
The incidence of abscesses following TACE has been reported in a wide range of studies (0.19%–5%). Kim et al.6 performed a retrospective analysis of 397 procedures in 157 patients who had TACE. They found that 4.5% of the patients and 2% of the procedures experienced liver abscesses. Song et al.1 examined the follow-up results of TACE in 2459 patients and found that the liver abscess development rate was 0.2% per patient. A study with a large sample size reported the lowest incidence of abscess per procedure.3 This previous study showed that, among 3613 (0.58%) patients who underwent 11,054 TACE procedures (0.19%), liver abscess formation was reported in 21 of the cases. Previous studies showed that there was an abscess rate of 1.2% per procedure, and the incidence of abscess formation was more common in liver metastases than in primary tumors.6,7 We consider that the differences in incidence rates among studies may be due to the different diagnostic distribution of patients, leading to different rates of bilioenteric anastomosis in patients. Lv et al.3 did not report the effect of tumor type on abscess formation, although most (78%) of their patients had hepatocellular carcinoma. The low incidence (0.19%) of abscess formation after TACE may explain this finding. However, Woo et al.2 and Song et al.4 reported that tumor type was not an important factor in development of abscesses. Lv et al.3 examined liver abscesses in 21 cases after TACE and found that the risk factors that affected abscess formation were bilioenteric anastomosis, DM, biliary stents, hypoalbuminemia, and portal venous tumor thrombus. In their study, 57.1% of patients with abscesses had a history of bilioenteric anastomosis or biliary stent implantation, and hypoalbuminemia, portal venous thrombosis, and DM were also found at a rate of 47.6%, 28.6%, and 23.8%, respectively. Woo et al.4 examined 65 TACE procedures and reported that in 25 patients with bilioenteric anastomosis, 12 (48%) developed 17 (26.2%) abscesses. In their study, risk factors for abscess formation after TACE in patients with bilioenteric anastomosis were leukopenia, first-time TACE treatment, and chemoembolization using a high-risk technique.4
All patients who developed abscess had undergone a TACE procedure for the first time, and no second TACE procedure was performed in these patients. Patients and physicians avoid repeated TACE after abscesses due to the procedure because abscess is an important cause of morbidity and mortality. If an abscess does not develop in the first TACE, a repeated TACE procedure can be performed with greater safety.
In the present study, all four patients with abscesses had right upper quadrant pain, fever, malaise, and nausea-vomiting. There was leukocytosis in one patient. The CT findings of the patients were also consistent with classical liver abscesses. We believe that the mortality rate is high in these patients because arterial nourishment is disrupted after TACE, the patients have tumors, some patients have low albumin levels, and because of possible hepatic dysfunction. The increased incidence of mortality after TACE may be attributed to an inability of oxygen and antibiotics to reach the vicinity of the necrotic tissue via the arterial pathway. In addition to the direct antibacterial effects of oxygen, TACE might hinder the wound healing effects of oxygen. Furthermore, the biliary origin of liver abscesses and the high bilirubin levels in these patients may result in an increase in mortality rate.8 No previous studies have compared the mortality rate of liver abscesses after TACE that was not related to TACE. Previous studies have shown that the presence of thrombus in the portal vein increases the incidence of abscess formation.4 A deterioration in arterial circulation with TACE in patients with impaired portal nourishment of the liver not only increases the incidence of abscesses, but also makes healing difficult. As a result, TACE is contraindicated in patients with portal venous thrombosis.
A positive pouch culture was obtained in all of the patients in the present study. Escherichia coli was isolated from the cultures of all four patients with abscesses. Escherichia coli is the most commonly isolated agent from cultures of all liver abscesses9 and in liver abscesses3 that develop after TACE. Broad spectrum antibiotic therapy should be started until microbiology culture and antibiotic susceptibility test results are obtained.10 Our patients were started on third-generation cephalosporin and vancomycin by a specialist in infectious diseases after being diagnosed with an abscess. There is still no consensus on pre-TACE prophylactic antibiotic therapy. Plentz et al.11 identified no significant differences between patients who received ciprofloxacin and metronidazole before the procedure and those who did not. Woo et al.4 showed that use of prophylactic antibiotics in patients with bilioenteric anastomosis did not reduce the incidence of abscess formation. However, Khan et al.12 reported that no abscess formation occurred after TACE when moxifloxacin was provided to patients who had been previously subjected to biliary interventions. Geschwind et al.13 reported that use of prophylactic tazobactam-piperacillin and pre-treatment bowel cleansing could prevent development of abscesses after TACE in patients with bilioenteric anastomosis. The patients in the present study were not administered prophylactic antibiotics before the TACE procedure.
Percutaneous drainage is recommended as the first-line drainage method in cases of liver abscess because of its minimally invasive nature, and also because of the high technical success rate when carried out with guidance of imaging techniques. Needle aspiration alone is recommended if the abscess size is less than 5 cm, whereas catheter drainage is recommended for abscesses larger than 5 cm. Percutaneous needle aspiration and percutaneous catheter drainage are recommended as the first-line treatment method, except in cases requiring emergency surgery for peritonitis.14 All of the patients in the present study underwent US-guided percutaneous drainage with a 12 French pigtail catheter, and the mean duration of catheter use was 16 days (6–34). The patients were asked to undergo a follow-up US after fluid from the catheter fell below 10 cc per day. The catheter was removed if the abscess size decreased to less than 2 cm, or if there was no remaining fluid in the abscess. Pressure irrigation with 0.9% saline was performed if no considerable reduction was noted in the size of the abscess. If the catheter holes were considered to be occluded, the catheter was replaced over a new wire (only 1 patient).
A limitation of the present study is the small number of patients who developed abscesses after TACE. This small number is due to the rarity of abscess occurrence, and this prevented any statistical analysis from being performed. The retrospective nature of the study is another limitation.
Conclusion
In conclusion, formation of liver abscesses is a rare, but serious, complication. Awareness of predisposing risk factors is important because arterial nourishment can have a fatal course in the presence of a damaged liver. CT imaging of patients complaining of right upper quadrant pain and fever within 2 weeks of a TACE procedure is necessary for early diagnosis of an abscess, and percutaneous drainage is an effective and minimally invasive method of treatment. Obtaining samples for bacteriology and antibiotic susceptibility tests during the procedure can be advantageous in determining antibiotics for later use. Bilioenteric anastomosis, DM, biliary stents, hypoalbuminemia, and portal venous tumor thrombosis are important predisposing factors in development of abscesses. Especially patients with bilioenteric anastomosis should be informed about the increased risk of abscess formation, and they should be advised to visit the hospital when symptoms of an abscess develop. If an abscess does not develop in the first TACE, this process can be performed more safely with repeated TACE. Antibiotic prophylaxis and bowel cleansing can be beneficial. However, there is a need for prospective, controlled studies to confirm these recommendations.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37: 429–442. [DOI] [PubMed] [Google Scholar]
- 2.Song SY, Chung JW, Han JK, et al. Liver abscess after transcatheter oily chemoembolization for hepatic tumors: incidence, predisposing factors, and clinical outcome. J Vasc Interv Radiol 2001; 12: 313–320. [DOI] [PubMed] [Google Scholar]
- 3.Lv WF, Lu D, He YS, et al. Liver abscess formation following transarterial chemoembolization: clinical features, risk factors, bacteria spectrum, and percutaneous catheter drainage. Medicine 2016; 95: e3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woo S, Chung JW, Hur S, et al. Liver abscess after transarterial chemoembolization in patients with bilioenteric anastomosis: frequency and risk factors. AJR 2013; 200: 1370–1377. [DOI] [PubMed] [Google Scholar]
- 5.Sun Z, Li G, Ai X, et al. Hepatic and biliary damage after transarterial chemoembolization for malignant hepatic tumors: incidence, diagnosis, treatment, outcome and mechanism. Crit Rev Oncol Hematol 2011; 79: 164–174. [DOI] [PubMed] [Google Scholar]
- 6.Kim W, Clark TW, Baum RA, et al. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol 2001; 12: 965–968. [DOI] [PubMed] [Google Scholar]
- 7.Korkmaz M, Bozkaya H, Cınar C, et al. Liver abscess following radioembolization with yttrium-90 microspheres. Wien Klin Wochenschr 2014; 126: 785–788. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz-Hernandez JJ, Leon-Mazorra M, Conde-Martel A, et al. Pyogenic liver abscesses: mortality-related factors. Eur J Gastroenterol Hepatol 2007; 19: 853–858. [DOI] [PubMed] [Google Scholar]
- 9.Yang CC, Yen CH, Ho MW, et al. Comparison of pyogenic liver abscess caused by non‐Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect 2004; 37: 176–184. [PubMed] [Google Scholar]
- 10.Ryan JM, Ryan BM, Smith TP. Antibiotic prophylaxis in interventional radiology. J Vasc Interv Radiol 2004; 15: 547–556. [DOI] [PubMed] [Google Scholar]
- 11.Plentz RR, Lankisch TO, Basturk M, et al. Prospective analysis of German patients with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization with or without prophylactic antibiotic therapy. J Gastroenterol Hepatol 2005; 20: 1134–1136. [DOI] [PubMed] [Google Scholar]
- 12.Khan W, Sullivan KL, McCann JW, et al. Moxifloxacin prophylaxis for chemoembolization or embolization in patients with previous biliary interventions: a pilot study. AJR 2011; 197: 343–345. [DOI] [PubMed] [Google Scholar]
- 13.Geschwind JF, Kaushik S, Ramsey DE, et al. Influence of a new prophylactic antibiotic therapy on the incidence of liver abscesses after chemoembolization treatment of liver tumors. J Vasc Interv Radiol 2002; 13: 1163–1166. [DOI] [PubMed] [Google Scholar]
- 14.Patel PB, Shah J, Baria S. A profile study of 50 cases of liver abcess treated by percutaneous catheter drainage. Int J Surg 2017; 4: 2490–2494. [Google Scholar]