Short abstract
A uterine tumor resembling an ovarian sex cord tumor (UTROSCT) is a rare type of neoplasm that is almost thoroughly differentiated towards ovarian sex cord elements. Because of abnormal uterine bleeding, a 64-year-old postmenopausal woman received total abdominal hysterectomy with bilateral salpingo-oophorectomy. Under a microscope, the tumor cells showed an anastomosing fascicular and trabecular pattern with a reticular architecture. Immunohistochemistry showed that the tumor cells were positive for calretinin, Wilm’s tumor-1, and vimentin. A 33-year-old woman who suffered from menorrhagia, and was treated for bilateral salpingectomy, total abdominal hysterectomy, and bilateral ovarian biopsy, was also studied. Using histology, the patient was diagnosed with UTROSCT as shown by CD99, smooth muscle actin, calretinin, vimentin, and desmin expression. As a type of rare uterine tumor, UTROSCT can be diagnosed based on morphological and immunohistochemical conditions. Generally, these tumors are benign, but can easily relapse through incomplete resection. Hysterectomy should be performed after completion of family planning.
Keywords: Uterine tumor resembling an ovarian sex cord tumor (UTROSCT), pathology, immunohistochemistry, hysterectomy, bleeding disorder, calretinin, Wilm’s tumor-1
Introduction
Uterine tumor resembling an ovarian sex cord tumor (UTROSCT) is a type of rare uterine neoplasm that was reported in 1976 by Scully and Clement.1 According to clinical and histopathological features, UTROSCTs can be divided into two types as follows: endometrial stromal tumors with a sex cord-like element (ESTSCLE) subject to recurrence and metastasis, and UTROSCT, which are defined as neoplasms resembling an ovarian sex cord tumor without an identifiable endometrial stroma.2,3 Although UTROSCTs have malignant potential, they are generally benign and sometimes relapse. Patients with UTROSCTs are typically subject to uterine mass and/or bleeding disorders. Generally, these tumors are well-bounded myometrial nodules, with infiltrating or sharp borders, and some may develop into polyps. Compared with leiomyomas, such nodules of UTROSCTs are fleshier, smoother, and are yellow-brown. Additionally, these nodules may show various histological patterns, such as glandular, trabecular, solid, diffuse, or mixed patterns. Furthermore, these nodules may lack or have abundant cytoplasms, and are usually rich in lipids. Mitoses are rare with small and inconspicuous nuclei.
UTROSCTs vary in the immunohistochemical profile. A marker panel is helpful with markers of the sex cord, including Wilm’s tumor-1 (WT-1), calretinin, and inhibin, markers of smooth muscle, including h-caldesmon, desmin, and smooth muscle actin, markers of epithelial tissues (AE1 and AE3 cytokeratin), and CD10. In 2009, Czernobilisky outlined the diagnostic criteria for UTROSCT as positivity for calretinin and positivity for at least one of the following markers: inhibin, CD99, and melan-A.4 UTROSCTs are positive for at least two sex cord markers. However, in ESTSCLEs, sex cord markers are less frequently detected.5
In this report, we describe the profiles of two cases of UTROSCTs, immunophenotypic characteristics, clinical features, therapy, and patients’ outcome.
Case report
Case 1
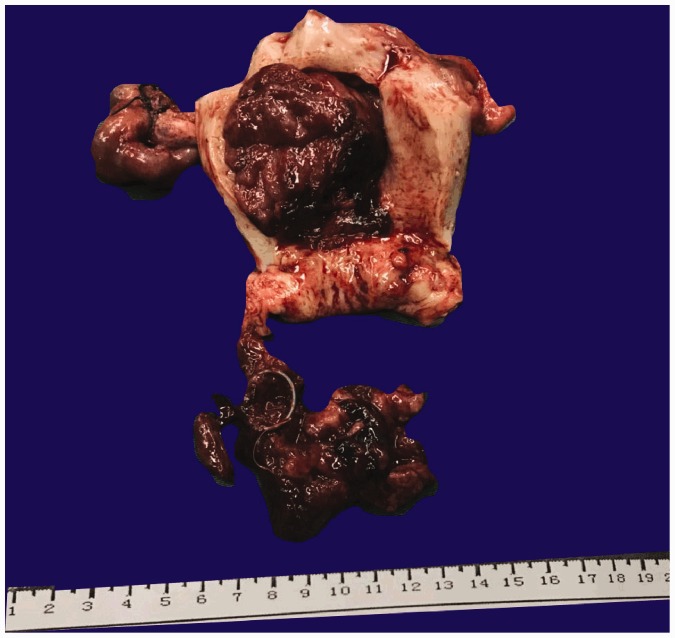
The first patient was a postmenopausal woman (64 years old) who experienced 15 days of abnormal uterine bleeding. B-ultrasound showed that she had uterine fibroids and an intrauterine device. Computed tomography (CT) showed that she had an intrauterine mass with hemorrhage, indicating the presence of endometrial cancer. In the retroperitoneal, pelvic cavity and bilateral groins, enlarged lymph nodes were found, and were considered as inflammatory swelling. The laboratory examination results were as follows: hemoglobin, 98 g/L; carcinoma antigen-125 (CA125), 68.8 U/mL; squamous cell antigen, 1.6 ng/mL; and CA72-4, 19.51 U/mL. CA19-9, α-fetoprotein, and carcinoembryonic antigen values were normal. Obtaining effective preoperative histological verification by biopsy was difficult with a large number of blood clots occluding the cervix because this can easily cause false negatives. On the basis of these findings, the patient underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy. Tumor samples were collected and sent to the histopathology laboratory for analysis. Through gross examination, a mass (10 × 5 × 4 cm) with a pedicle (3 × 4 cm) was found to be connected to the uterus (Figure 1). The tumor had a red cut surface, and the samples appeared similar to fish flesh with local necrosis. Under a microscope, the tumor cells showed an anastomosing fascicular and trabecular pattern with a reticular architecture (Figure 2). The overlying endometrium showed that the pattern of the tumor was atrophic with a compact stroma and inactive glands. A histological examination showed that both ovaries were normal. Additionally, immunohistochemical stained was performed. The tumor cells were positive for vimentin, calretinin, WT-1, cytokeratin (CK), and progesterone receptor (PR). Cells were also positive for Ki-67 and inhibin (Figure 3). Additionally, a small amount of cells were positive for CD10, CA125, and p16. Negative stains included human melanoma black 45, CD99, PAX-8, melan-A, Myo-D1, chromogranin A, synaptophysin, S-100, smooth muscle actin (SMA), CK7, desmin, caldesmon, P53, and estrogen receptor. To further guarantee the accuracy of the diagnosis, senior pathologists from other organizations were consulted and they confirmed the diagnosis of UTROSCT. CA125 and CA199 values were normal at 6 months after the operation. CT did not show any enlarged lymph nodes at this time. Furthermore, there was no discomfort during a 1-year telephone follow-up.
Figure 1.
Gross photograph of the uterus. A mass (10 × 5 × 4 cm) with a pedicle (3 × 4 cm) and a cut surface appears similar to fish meat with local necrosis (case 1).
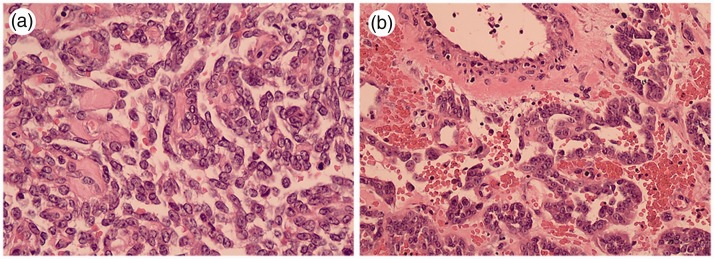
Figure 2.
Low-power view showing classic oncocytoma with reticular (a) and trabecular (b) architecture (case 1).
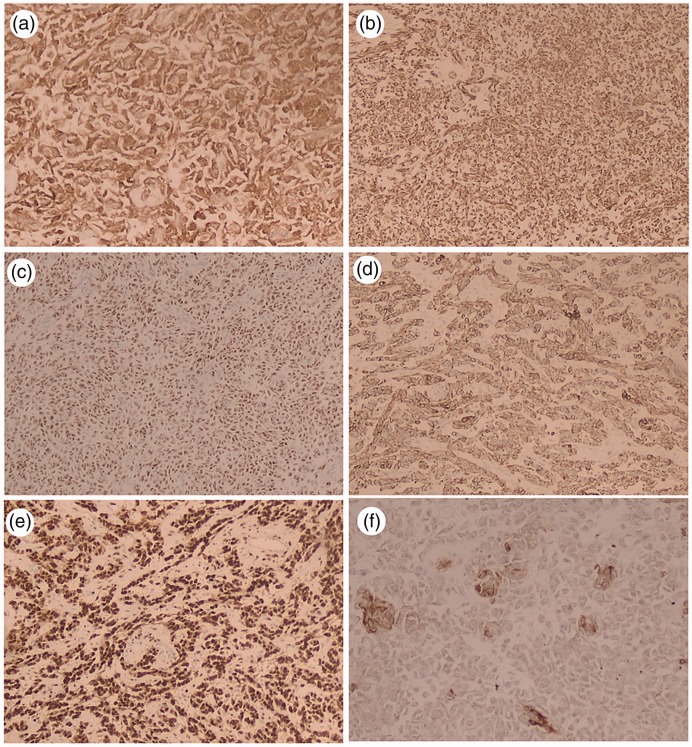
Figure 3.
Neoplastic cells positive for calretinin (a), vimentin (b), Wilm’s tumor-1 (c), cytokeratin (d), and progesterone receptor (e). Occasional cells indicate inhibin positivity (f) (case 1).
Case 2
The second patient was a 33-year-old woman with menorrhagia, who was gravida 2, para 1. B-ultrasound showed that she had an enlarged uterus with uterine leiomyoma. A uterus-preserving surgery was discussed with this patient. However, the patient had no fertility requirement. Therefore, total abdominal hysterectomy and bilateral ovarian biopsy were performed. Pathology showed no tumors in both ovaries, which were preserved. A round, gray-red mass with a size of 3.5 × 2.5 cm was found on the right lateral wall of the uterus. The tumor boundary was unclear and invaded the gland on the surface of the endometrium, which was intact. After a retrospective review of the microscopic and macroscopic features of the specimens by pathologists, the patient was histologically diagnosed with UTROSCT as shown by CD99, SMA, calretinin, vimentin, and desmin expression. However, CK or Ki-67 was not expressed. Gross and pathological sections were not clear when these were collected during the time of the diagnosis. At a postoperative telephone follow-up after 12 years, the patient was still alive without evidence of recurrence.
Ethics
Verbal consent was obtained from both patients for the publication of their information and images. Additionally, the present study was approved (L-2018-16) by the Research Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University.
Discussion
Since 1976, when Clenment and Scully first studied UTROSCTs, researchers have made great efforts to further determine the features of these uterine tumors.1,6–18 To date, no more than 80 cases of UTROSCTs have been reported. However, endometrial stromal tumors (possessing sex cord-like elements) are only deemed as essential endometrial stromal tumors, making the essence of “pure” UTROSCTs enigmatic. Histologically, UTROSCTs consist of ribbons, small nests, and trabeculae or tubules, which resemble Sertoli cell tumors or granulosa of the ovary. Hemorrhage and necrosis are unusual in UTROSCTs.19 Many investigations have provided evidence that UTROSCTs have stromal differentiation,1,8 true sex cord differentiation,6,8,13,14 epithelial differentiation,20 or smooth muscle differentiation.21 In a recent immunohistochemical study on the polyphenotypic condition of such tumors, these tumors were hypothesized to have been created by pluripotent mesenchymal cells.11 In 1976, five successful pregnancies with uterus-sparing treatment of UTROSCTs3,22–24 were discussed in the medical literature. In an ultrastructural study (13 cases), UTROSCTs were determined as polyphenotypic neoplasms with sex cord-like and focal epithelial differentiation.8 In endometrial stromal tumors, divergent differentiation may cause such tumors, which could represent a special group of uterine tumors with sex cord-like differentiation that are closer in histogenesis to ovarian sex cord stromal tumors.
Morphologically, UTROSCTs completely consist of elements that simulate ovarian sex cord tumors in a number of architectural patterns, including anastomosing trabeculae, tubules, plexiform cords, microfollicles, retiform islands, and glomeruloid structures.10,25 Therefore, distinguishing UTROSCTs from plexiform tumorlets, epithelial tumors, metastatic stromal ovarian sex cord tumors, and plexiform leiomyoma may be challenging. Additionally, focal sex cord elements could also be misdiagnosed as endometrial stromal sarcoma. Therefore, multiple studies need to be conducted for thorough sampling to guarantee the correct diagnosis.
UTROSCTS have a diverse immunohistochemical profile, as indicated by co-expression of sex cord, epithelial, and smooth muscle markers in one third of cases.26 In UTROSCTS, the most frequently expressed proteins are calretinin and WT-1. Calretinin is a protein bound by calcium, and is found in hilar, theca interna, stromal, and mesothelial cells, as well as ovarian epithelia.27,28 Although the specificity of calretinin is less than inhibin, calretinin has been widely used in diagnosing tumors as a marker for steroid cell and stromal sex cord tumors because of its sensitivity.29–32 Calretinin is positive in most UTROSCTs7,10–12,16,26,33,34 that involve stromal, endometrial, and non-neoplastic cells of the layer of the superficial functionalis, and in ESTSCLEs11,32 in the secretory and proliferative phases.35 WT-1 is expressed in the gonads, and in the mesothelium, metanephros, and mesonephros in fetuses. With regard to the frequency of WT-1, Hurrel and McCluggage10 observed diffuse nuclear staining of moderate intensity for WT-1 in all four cases of UTROSCT. In contrast to the series reported by de Leval et al.,26 only four of 12 UTROSCTs were positive for WT-1. A previous study reported that 11 of 12 UTROSCTs were positive for at least one smooth muscle marker, and SMA and desmin were the two most commonly expressed markers.26 UTROSCT cells are usually immunoreactive for CK and WT-1, frequently reactive for SMA and desmin, and commonly reactive for at least two markers of sex cord differentiation (e.g., inhibin, calretinin, CD99 and melan-A).11 Immunohistochemical expression of epithelial membrane antigen, CK7, and CA125 are helpful for differentiating UTROSCT from primary ovarian epithelial carcinoma. Chromogranin A is a water-soluble acidic glyco-protein contained in the secretory vesicles of neurons and neuroendocrine cells. Chromogranin A is also a neurosecretion along with synaptophysin, and can be used as a specific marker to distinguish neuroendocrine tumors from UTROSCT.36,37 CD56 is a neural cell adhesion molecule. CD56 is strongly expressed in ovarian stromal cells, but not in endometrial stromal cells.38 Antigens that are frequently expressed by other types of spindle cell tumors of fibroblastic/myofibroblastic origin,39 such as S-100, are usually negative in UTROSCTs. These previous findings suggest that caution is advised for diverse immunohistochemistry when the diagnosis cannot be established according to histological morphology.
A fertility-preserving option for younger women with UTROSCT has only recently been suggested by some authors.3,19,23,24,40–43 In 2007, Schraag et al. reported two cases of recurrence of UTROSCT, which were most likely caused by an incomplete resection during the first operation.22 However, five successful pregnancies following the uterus-sparing treatment of UTROSCT have been described in the medical literature since 1945.3,22–24 A fertility-sparing approach should always be considered in women with UTROSCT who wish to preserve their fertility. Nonetheless, because of the possibility of late local recurrence and the lack of experience with UTROSCT, hysterectomy should be performed after completion of family planning.
Although UTROSCTs generally behave in a benign manner, they may undergo malignant transformation and metastasize in rare cases. Tumor metastasis can occur to the ovary,13 omentum,13 lymph nodes,44 and epiploic appendix.44 In the literature, we found three cases of UTROSCT with recurrence after hysterectomy. Biermann et al.45 first described the 4-year follow-up of a 68-year-old patient who developed intestinal obstruction with a 10-cm nodular tumor in the small bowel, which also showed microscopic features of UTROSCT. A 48-year-old woman developed pelvic recurrence and galactorrhea 1.5 years after hysterectomy for UTROSCT.46 In 2016, Endo et al. reported UTROSCT pelvic lymph node recurrence in a 62-year-old woman 23 years after hysterectomy.47 Although the recurrence rate of this tumor is low, the feasibility of hysterectomy alone is questionable, with requirement of long-term follow-up and setting a standard.
Conclusion
In conclusion, UTROSCTs are uterine tumors of unknown histogenesis with various architectural patterns, and they can extensively express epithelial, stromal, and sex cord markers. This finding suggests that caution is required for diverse immunohistochemistry when the diagnosis cannot be established according to histological morphology. Generally, these tumors are usually benign, but can easily relapse through incomplete resection. A fertility-sparing approach should always be considered in women with UTROSCT who wish to preserve their fertility. Hysterectomy should be performed after completion of family planning. Because these tumors are unusual, long-term follow-up is recommended.
Acknowledgments
We are grateful for the support provided by Dr Liang-liang Mao for selection and preparation of the pathological figures. We also thank staff at the Center for the Uterine Cancer Diagnosis and Therapy Research of Zhejiang Province.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Clement PB, Scully RE. Uterine tumors resembling ovarian sex-cord tumors. A clinicopathologic analysis of fourteen cases. Am J Clin Pathol 1976; 66: 512–525. [DOI] [PubMed] [Google Scholar]
- 2.Tavassoli FA, Devilee P. Sex cord-like, neuroectodermal and neuroendocrine tumours, lyphomas and leukaemias. Pathology and genetics: tumours of the breast and female genital organs. In: WHO Classification of tumours series Lyon: IARC Press, 2003.
- 3.Jeong KH, Lee HN, Kim MK, et al. Successful delivery after conservative resectoscopic surgery in a patient with a uterine tumor resembling ovarian sex cord tumor with myometrial invasion. Obstet Gynecol Sci 2015; 58: 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czernobilsky B. Uterine tumors resembling ovarian sex cord tumors: an update. Int J Gynecol Pathol 2008; 27: 229–235. [DOI] [PubMed] [Google Scholar]
- 5.Liu CY, Shen Y, Zhao JG, et al. Clinical experience of uterine tumors resembling ovarian sex cord tumors: a clinicopathological analysis of 6 cases. Int J Clin Exp Pathol 2015; 8: 4158–4164. [PMC free article] [PubMed] [Google Scholar]
- 6.Baker RJ, Hildebrandt RH, Rouse RV, et al. Inhibin and CD99 (MIC2) expression in uterine stromal neoplasms with sex-cord-like elements. Hum Pathol 1999; 30: 671–679. [DOI] [PubMed] [Google Scholar]
- 7.Czernobilsky B, Mamet Y, David MB, et al. Uterine retiform sertoli-leydig cell tumor: report of a case providing additional evidence that uterine tumors resembling ovarian sex cord tumors have a histologic and immunohistochemical phenotype of genuine sex cord tumors. Int J Gynecol Pathol 2005; 24: 335–340. [DOI] [PubMed] [Google Scholar]
- 8.Gupta M, de Leval L, Selig M, et al. Uterine tumors resembling ovarian sex cord tumors: an ultrastructural analysis of 13 cases. Ultrastruct Pathol 2010; 34: 16–24. [DOI] [PubMed] [Google Scholar]
- 9.Hauptmann S, Nadjari B, Kraus J, et al. Uterine tumor resembling ovarian sex-cord tumor–a case report and review of the literature. Virchows Arch 2001; 439: 97–101. [DOI] [PubMed] [Google Scholar]
- 10.Hurrell DP, McCluggage WG. Uterine tumour resembling ovarian sex cord tumour is an immunohistochemically polyphenotypic neoplasm which exhibits coexpression of epithelial, myoid and sex cord markers. J Clin Pathol 2007; 60: 1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irving JA, Carinelli S, Prat J. Uterine tumors resembling ovarian sex cord tumors are polyphenotypic neoplasms with true sex cord differentiation. Mod Pathol 2006; 19: 17–24. [DOI] [PubMed] [Google Scholar]
- 12.Kabbani W, Deavers MT, Malpica A, et al. Uterine tumor resembling ovarian sex-cord tumor: report of a case mimicking cervical adenocarcinoma. Int J Gynecol Pathol 2003; 22: 297–302. [DOI] [PubMed] [Google Scholar]
- 13.Kantelip B, Cloup N, Dechelotte P. Uterine tumor resembling ovarian sex cord tumors: report of a case with ultrastructural study. Hum Pathol 1986; 17: 91–94. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamurthy S, Jungbluth AA, Busam KJ, et al. Uterine tumors resembling ovarian sex-cord tumors have an immunophenotype consistent with true sex-cord differentiation. Am J Surg Pathol 1998; 22: 1078–1082. [DOI] [PubMed] [Google Scholar]
- 15.McCluggage WG. Uterine tumours resembling ovarian sex cord tumours: immunohistochemical evidence for true sex cord differentiation. Histopathology 1999; 34: 375–376. [DOI] [PubMed] [Google Scholar]
- 16.Nogales FF, Isaac MA. Functioning uterine sex cord tumour. Histopathology 2002; 41: 277–279. [DOI] [PubMed] [Google Scholar]
- 17.Oliva E, Young RH, Amin MB, et al. An immunohistochemical analysis of endometrial stromal and smooth muscle tumors of the uterus: a study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am J Surg Pathol 2002; 26: 403–412. [DOI] [PubMed] [Google Scholar]
- 18.McCluggage WG, Burton J, Maxwell P, et al. Immunohistochemical staining of normal, hyperplastic, and neoplastic adrenal cortex with a monoclonal antibody against alpha inhibin. J Clin Pathol 1998; 51: 114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano G, Lombardi M, Brigati F, et al. Clinicopathologic features of 2 new cases of uterine tumors resembling ovarian sex cord tumors. Int J Gynecol Pathol 2010; 29: 459–467. [DOI] [PubMed] [Google Scholar]
- 20.Fekete PS, Vellios F, Patterson BD. Uterine tumor resembling an ovarian sex-cord tumor: report of a case of an endometrial stromal tumor with foam cells and ultrastructural evidence of epithelial differentiation. Int J Gynecol Pathol 1985; 4: 378–387. [PubMed] [Google Scholar]
- 21.McCluggage WG, Shah V, Walsh MY, et al. Uterine tumour resembling ovarian sex cord tumour: evidence for smooth muscle differentiation. Histopathology 1993; 23: 83–85. [DOI] [PubMed] [Google Scholar]
- 22.Schraag SM, Caduff R, Dedes KJ, et al. Uterine tumors resembling ovarian sex cord tumors - treatment, recurrence, pregnancy and brief review. Gynecol Oncol Rep 2017; 19: 53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blake EA, Sheridan TB, Wang KL, et al. Clinical characteristics and outcomes of uterine tumors resembling ovarian sex-cord tumors (UTROSCT): a systematic review of literature. Eur J Obstet Gynecol Reprod Biol 2014; 181: 163–170. [DOI] [PubMed] [Google Scholar]
- 24.De Franciscis P, Grauso F, Ambrosio D, et al. Conservative resectoscopic surgery, successful delivery, and 60 months of follow-up in a patient with endometrial stromal tumor with sex-cord-like differentiation. Case Rep Obstet Gynecol 2016; 2016: 5736865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pradhan D, Mohanty SK. Uterine tumors resembling ovarian sex cord tumors. Arch Pathol Lab Med 2013; 137: 1832–1836. [DOI] [PubMed] [Google Scholar]
- 26.de Leval L, Lim GS, Waltregny D, et al. Diverse phenotypic profile of uterine tumors resembling ovarian sex cord tumors: an immunohistochemical study of 12 cases. Am J Surg Pathol 2010; 34: 1749–1761. [DOI] [PubMed] [Google Scholar]
- 27.Cao QJ, Jones JG, Li M. Expression of calretinin in human ovary, testis, and ovarian sex cord-stromal tumors. Int J Gynecol Pathol 2001; 20: 346–352. [DOI] [PubMed] [Google Scholar]
- 28.Lugli A, Forster Y, Haas P, et al. Calretinin expression in human normal and neoplastic tissues: a tissue microarray analysis on 5233 tissue samples. Hum Pathol 2003; 34: 994–1000. [DOI] [PubMed] [Google Scholar]
- 29.Deavers MT, Malpica A, Liu J, et al. Ovarian sex cord-stromal tumors: an immunohistochemical study including a comparison of calretinin and inhibin. Mod Pathol 2003; 16: 584–590. [DOI] [PubMed] [Google Scholar]
- 30.McCluggage WG. Value of inhibin staining in gynecological pathology. Int J Gynecol Pathol 2001; 20: 79–85. [DOI] [PubMed] [Google Scholar]
- 31.Movahedi-Lankarani S, Kurman RJ. Calretinin, a more sensitive but less specific marker than alpha-inhibin for ovarian sex cord-stromal neoplasms: an immunohistochemical study of 215 cases. Am J Surg Pathol 2002; 26: 1477–1483. [DOI] [PubMed] [Google Scholar]
- 32.Shah VI, Freites ON, Maxwell P, et al. Inhibin is more specific than calretinin as an immunohistochemical marker for differentiating sarcomatoid granulosa cell tumour of the ovary from other spindle cell neoplasms. J Clin Pathol 2003; 56: 221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillard JB, Malpica A, Ramirez PT. Conservative management of a uterine tumor resembling an ovarian sex cord-stromal tumor. Gynecol Oncol 2004; 92: 347–352. [DOI] [PubMed] [Google Scholar]
- 34.Sutak J, Lazic D, Cullimore JE. Uterine tumour resembling an ovarian sex cord tumour. J Clin Pathol 2005; 58: 888–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mai KT, Teo I, Al Moghrabi H, et al. Calretinin and CD34 immunoreactivity of the endometrial stroma in normal endometrium and change of the immunoreactivity in dysfunctional uterine bleeding with evidence of 'disordered endometrial stroma'. Pathology 2008; 40: 493–499. [DOI] [PubMed] [Google Scholar]
- 36.Hofland J, Zandee WT, de Herder WW. Role of biomarker tests for diagnosis of neuroendocrine tumours. Nat Rev Endocrinol 2018; 14: 656–669. [DOI] [PubMed] [Google Scholar]
- 37.Karpathiou G, Matias-Guiu X, Mobarki M, et al. Ovarian neuroendocrine carcinoma of metastatic origin: clues for diagnosis. Hum Pathol 2018; pii: S0046-8177(18)30330-7. [DOI] [PubMed] [Google Scholar]
- 38.He H, Luthringer DJ, Hui P, et al. Expression of CD56 and WT1 in ovarian stroma and ovarian stromal tumors. Am J Surg Pathol 2008; 32: 884–890. [DOI] [PubMed] [Google Scholar]
- 39.Sills ES, Doan TB, Mock RJ, et al. Immunohistochemical localization patterns for vimentin and other intermediate filaments in calcified ovarian fibrothecoma. Diagn Pathol 2006; 1: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Meara AC, Giger OT, Kurrer M, et al. Case report: recurrence of a uterine tumor resembling ovarian sex-cord tumor. Gynecol Oncol 2009; 114: 140–142. [DOI] [PubMed] [Google Scholar]
- 41.Berretta R, Patrelli TS, Fadda GM, et al. Uterine tumors resembling ovarian sex cord tumors: a case report of conservative management in young women. Int J Gynecol Cancer 2009; 19: 808–810. [DOI] [PubMed] [Google Scholar]
- 42.Anastasakis E, Magos AL, Mould T, et al. Uterine tumor resembling ovarian sex cord tumors treated by hysteroscopy. Int J Gynaecol Obstet 2008; 101: 194–195. [DOI] [PubMed] [Google Scholar]
- 43.Garuti G, Gonfiantini C, Mirra M, et al. Uterine tumor resembling ovarian sex cord tumors treated by resectoscopic surgery. J Minim Invasive Gynecol 2009; 16: 236–240. [DOI] [PubMed] [Google Scholar]
- 44.Umeda S, Tateno M, Miyagi E, et al. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) with metastasis: clinicopathological study of two cases. Int J Clin Exp Pathol 2014; 7: 1051–1059. [PMC free article] [PubMed] [Google Scholar]
- 45.Biermann K, Heukamp LC, Buttner R, et al. Uterine tumor resembling an ovarian sex cord tumor associated with metastasis. Int J Gynecol Pathol 2008; 27: 58–60. [DOI] [PubMed] [Google Scholar]
- 46.Leiser AL, Hamid AM, Blanchard R. Recurrence of prolactin-producing endometrial stromal sarcoma with sex-cord stromal component treated with progestin and aromatase inhibitor. Gynecol Oncol 2004; 94: 567–571. [DOI] [PubMed] [Google Scholar]
- 47.Endo D, Todo Y, Okamoto K, et al. A case of recurrent group II uterine tumor resembling ovarian sex-cord tumors, against which two hormonal agents were ineffective. Taiwan J Obstet Gynecol 2016; 55: 751–753. [DOI] [PubMed] [Google Scholar]