Short abstract
Objective
PD98059 is a potent and selective inhibitor of mitogen-activated protein kinase. Substantial preclinical evidence has shown an anti-tumor effect of curcumin on various solid tumors. This study aimed to investigate whether curcumin synergistically acts with PD98059 in exerting anti-gastric cancer effects.
Methods
The cell counting kit-8 assay was used to detect cell proliferation of the human gastric cancer MGC-803 cell line. Flow cytometry was performed to detect apoptosis. Western blotting was used to detect phosphatase and tensin homolog (PTEN) and phosphorylated Akt (p-Akt) expression levels. Quantitative reverse transcription-polymerase chain reaction was used to determine microRNA-21 (miR-21).
Results
A dose of 5 to 40 µM curcumin inhibited proliferation of MGC-803 cells in a dose- and time-dependent manner. A high dose of curcumin strongly inhibited p-Akt protein expression. With increasing curcumin levels, PTEN expression increased and miR-21 levels decreased. These results suggest that curcumin negatively modulated the miR-21/PTEN/Akt pathway. Moreover, after pretreatment with PD98059, cell apoptosis induced by curcumin was significantly increased. Additionally, the inhibitory effects of curcumin on the miR-21/PTEN/Akt pathway were significantly enhanced.
Conclusion
PD98059 combined with curcumin may be a potential strategy for managing gastric cancer.
Keywords: Curcumin, microRNA-21 (miR-21), apoptosis, mitogen-activated protein kinase (MAPK) inhibitor, stomach neoplasm, phosphatase and tensin homolog (PTEN)
Introduction
Gastric cancer is one of the most common malignant tumors worldwide. Gastric cancer has a relatively poor prognosis in the early stage and remains a serious threat to human life in the advanced stage. According to the statistics of the International Agency for Research on Cancer in 2012, there were approximately 951,000 new cases of gastric cancer worldwide and approximately 723,000 cases of gastric cancer death, with the ranking of third in the incidence of cancer and fifth for mortality rate.1 Although great progress has been made in treating gastric cancer in recent years, the overall 5-year survival rate of patients with gastric cancer was less than 20% globally.2 Gastric cancer is one of the major life-threatening malignancies. Gastric cancer initiates as a localized disease, but may quickly spread to distant sites through invasion and migration,3 making its prevention and control arduous.
Accumulating data have shown that chemical prevention is an economical and realistic method of preventing gastric cancer in high-risk groups.4 Natural plants and herbs with multiple biological activity have long attracted the attention of pharmacists and oncologists.5 Curcumin, a type of polyphenol that is extracted from the root of turmeric rhizome, has a variety of pharmacological effects, such as anti-tumor,6 anti-inflammation,7 anti-oxidant,8 anti-atherosclerosis,9 blood lipid-lowering,10 and anti-human immunodeficiency virus.11 Several studies have shown that curcumin has chemopreventive and therapeutic effects on a variety of human digestive system tumors.6,12,13 Curcumin can selectively kill tumor cells by interfering with cell cycle progression, proliferation, autophagy, apoptosis, invasion and metastasis, angiogenesis, drug resistance, and natural killer cell activity.14 Among these, inducing tumor cell apoptosis and regulating apoptotic pathways are the most important anti-tumor mechanisms of curcumin, but the mechanism of inducing apoptosis still needs to be further investigated.15
MicroRNA-21 (miR-21) is a type of onco-miRNA, which is upregulated in gastric cancer cells.16 This miRNA targets a panel of target genes involved in carcinogenesis and progression of cancer.17 Phosphatase and tensin homolog (PTEN), which is a tumor suppressor that negatively regulates the Akt/PKB signaling pathway by inhibiting phosphoinositide 3-kinase (PI3K), is a validated target of miR-21 in cancer cells.16,17 The miR-21/PTEN/PI3K/Akt pathway is involved in tumor growth, migration, and invasion.18 Additionally, miRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN.17 Whether curcumin exerts its anti-gastric cancer effect by interfering with the miR-21/PTEN/PI3K/Akt pathway has not been completely determined yet.
PD98059 is a potent and selective inhibitor of the mitogen-activated protein (MAP) kinase kinases (MAPKKs) MEK1 and MEK2. PD98059 binds to the inactive form of MAPKK and prevents activation by upstream activators, such as c-Raf.19 In recent years, preclinical research has indicated good anti-tumor effects of PD98059 alone and in combination with chemotherapy drugs.20,21 However, whether PD98059 can cooperate with curcumin to show multi-channel blocking effects on gastric cancer has not been reported yet. In this study, we investigated whether PD98059 synergizes with curcumin to induce apoptosis and inhibit the miR-21/PTEN/Akt pathway in human gastric cancer cells.
Materials and methods
Experimental materials
Curcumin (Sigma, St. Louis, MO, USA) and PD98059 (MCE, Monmouth Junction, NJ, USA) were fully dissolved in dimethyl sulfoxide (Sigma), and then diluted with Dulbecco’s modified Eagles medium (Gibco, New York, NY, USA) as stocks and stored at 4°C in the dark. These solutions were taken out and diluted accordingly to the required concentration.
Other materials used included electrophoresis apparatus (PowerPac, San Francisco, CA, USA) and chemiluminescence apparatus (Bio-Rad, Hercules, CA, USA). The primer sequences of hsa-miR-21-5p and internal reference U6 for reverse transcription (RT) and polymerase chain reaction (PCR) were synthesized by RiboBio Co., Ltd. (Guangzhou, China).
Cell culture
The gastric cancer cell line MGC-803 (Guangxi Normal University, Guilin, China) was cultured in Dulbecco’s modified Eagles medium containing 100 mL/L fetal bovine serum (Gemini, Woodland, CA, USA) at 37°C and 5% CO2 in an incubator, where the medium was displaced every day. When the cells are 80% confluent, cells were digested by 0.25% trypsin (Solarbio, Beijing, China), followed by counting and passage. When the cells had stabilized, those in the logarithmic growth phase were selected for experiments. Cells were stored at −80°C or liquid nitrogen with cryopreserve medium.
Cell viability assay
MGC-803 gastric cancer cells of 8 × 103 cells/mL were suspended in culture medium containing different concentrations of curcumin, and then 100 µL of suspension solution was added to each well of a 96-well culture plate. Each experimental group had five replicate wells. Subsequently, cell culture plates were placed in an incubator containing 5% CO2 and indicators were measured at 24, 48, and 72 hours. At 4 hours before each time point, 10 µL of cell counting kit-8 was added to each well, and the plate was kept in the incubator at 37°C in 5% CO2 until the end of the experiments. The absorbance value of each well was measured with an automatic microplate reader (wavelength of 450 nm) (Bio-Rad). The cell growth inhibition rate (GI) was calculated by the following formula: GI (%) = 1 − optical density value of the experimental group/optical density value of the control group. The experiment was repeated three times.
Apoptosis assay
Cells (3 × 105 cells/well) were seeded onto a six-well plate and incubated overnight. Treatment with different concentrations of curcumin (0, 5, 10, 20, and 40 µM) and treatment with PD98059 10 µM or curcumin 20 µM + PD98059 10 µM for 24 hours were performed. Cells were harvested, trypsinized, and washed. The cells were stained with Annexin V and propidium iodide for 30 minutes in the dark using an Annexin V-FITC/PI Apoptosis Detection Kit (Beyotime Institute of Biotechnology, Shanghai, China). Finally, the samples were evaluated by flow cytometry. This experiment was repeated three times.
Quantitative RT-PCR analysis
Total RNA was extracted with Triozol (Gibco/Life Technologies, Grand Island, NY, USA) according to the manufacturer’s protocol. Total miR-21 was reverse transcribed to cDNA with miRNA-specific RT primers (RiboBio) or random primers (RiboBio). Gene expression was measured by PCR using an Applied Biosystems 7300 Fast Sequence Detection System and Bestar® SybrGreen qPCR Mastermix (No: DBI-2043, DBI, Ludwigshafen, Germany) under the following conditions: denaturation at 95°C for 5 minutes, followed by 40 cycles of denaturation at 95°C for 10 seconds and annealing and extension at 60°C for 30 seconds. Specificities were analyzed according to the melting curve and triplicates were performed for each group. Relative miRNA expression levels were normalized to U6 expression.
Western blot analysis
Cells were treated with different concentrations of curcumin and PD98059 for 24 hours, and then harvested and lysed in cell lysis buffer containing Tris-HCl and Triton X-100 to extract total protein. An equal amount of denatured protein was decentralized on a sodium dodecyl sulfate-polyacrylamide gel that was transferred to a nitrocellulose membrane. The membrane was sealed by 5% non-fat milk in TBS containing 0.05% Tween-20. A specific primary antibody (PTEN and p-Akt, 1:500 dilution; beta-actin, 1:1000 dilution) (Cell Signaling, Boston, MA, USA) was added to the membrane and incubated at 4°C overnight, and then washed by TBS containing 0.05% Tween-20. The samples were then incubated with a horseradish peroxidase-conjugated second antibody (goat anti-rabbit IgG, 1:500 dilution) at room temperature for 30 minutes. Protein bands were visualized using enhanced chemiluminescent reagents (7 Sea Biotech, Shanghai, China) and detected by a ChemiDoc XRS+ Imaging System (Bio-Rad). Images were converted to digital images and Image J software (https://imagej.nih.gov/ij/) was used to perform semi-quantitative analysis of protein bands. β-actin was used as a control. The experiment was repeated three times.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA). Measurement data are expressed as mean ± standard deviation. Statistical significance of differences between two or more groups was analyzed by one-way analysis of variance (ANOVA). Statistical significance was set at P < 0.05
Results
Cytotoxic effects of curcumin on gastric carcinoma cells
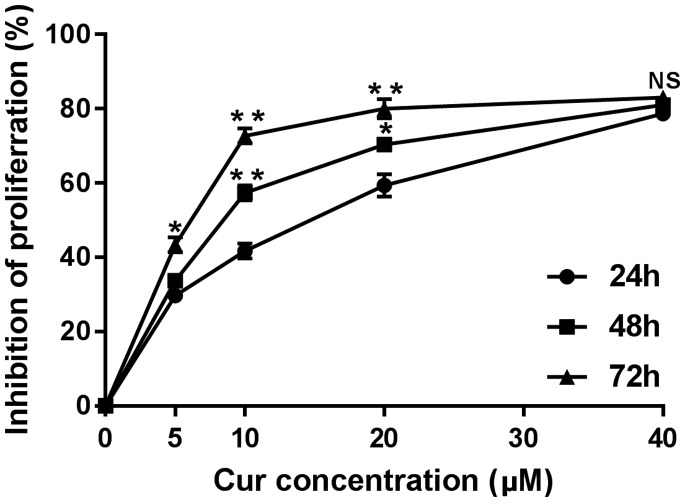
Cell viability was investigated to determine the cytotoxic effects of curcumin on the gastric cancer cell line MGC-803. With an increase in curcumin concentrations, the inhibitory effect of gastric cancer cells gradually increased. With an extension of the treatment time, the inhibitory rate was gradually increased. The inhibitory effect was strongest at each time point when the curcumin concentration was 40 µM, where the cell inhibition rate was more than 80%. The cell inhibition rate was 28.1% ± 2.6% with a curcumin concentration of 5 µM, which was significantly lower than that with 40 µM (82.4% ± 5.4%) at 24 hours (P < 0.01, Figure 1). In the present study, we also observed the inhibitory effect of curcumin on proliferation of SGC-7901 gastric cancer cells and HepG2 hepatoma cells. The inhibition rate was gradually increased with increased curcumin concentration and treatment time (data not shown).
Figure 1.
Effect of curcumin at different concentrations on cell proliferation of gastric cancer cells (MGC-803) at different times (24, 48, 72 hours). *P < 0.05, **P < 0.01 compared with 24 hours
Effect of curcumin and PD98059 on cellular morphology
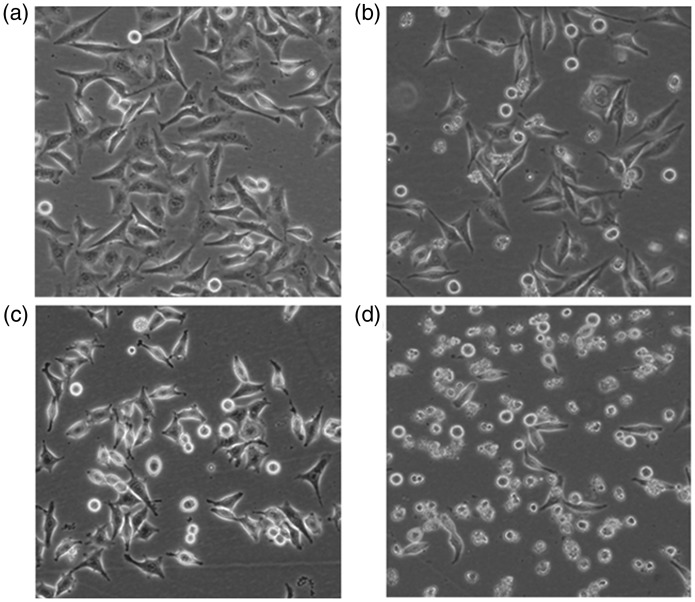
MGC-803 cells in the blank control group grew densely, and displayed a polygonal shape with good growth status. However, growth of cells in the curcumin alone group and PD98059 alone group were loose, and displayed abnormal morphology, such as rounding, shrinking, and detachment, with no adhesion. In the combined treatment group, there were fewer cells than in the other groups, and the cells were round and smaller, and a large number of cells were detached. The number of adherent cells was lower in the combined treatment group than in the other groups. Some cells showed lysis-like changes in the cytoplasm. Morphological changes in each group are shown in Figure 2.
Figure 2.
Morphological changes of cells in different groups (× 100). (A) Blank control group. (B) Curcumin alone group (20 µM). (C) PD98059 alone group (10 µM). (D) Combined drug group (curcumin 20 µM + PD98059 10 µM)
Effect of curcumin and PD98059 on apoptosis of human gastric cancer MGC-803 cells
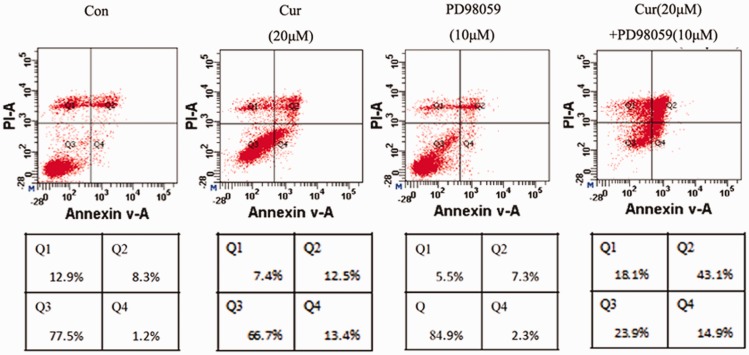
Apoptosis of gastric cancer cells was significantly induced by treatment of curcumin at concentrations of 20 µM and 40 µM. When the curcumin concentration was 20 µM, the number of cells was highest in the Q4 quadrant, which indicated the largest amount of early apoptotic cells. When the curcumin concentration was 40 µM, the number of cells in the Q2 quadrant was increased, which indicated a rapid increase in late apoptotic cells and dead cells. There was no significant difference in the rate of apoptosis between the 5 µM and 10 µM curcumin groups and the blank control group. However, the rate of apoptosis in the 20 µM and 40 µM curcumin groups was significantly higher compared with that in the control group (both P < 0.05). The sum of early and late apoptotic rates of MGC-803 cells peaked at 58.5% ± 4.3% in the PD98059 (10 µM) combined with curcumin (20 µM) group. This rate was significantly higher than that in the 20 µM curcumin group (25.2% ± 4.1%) and 40 µM curcumin group (30.4% ± 3.7%) (both P < 0.05, Figure 3).
Figure 3.
The apoptotic rate of MGC-803 cells in different groups was determined by flow cytometry
Effect of curcumin and PD98059 on miR-21 expression
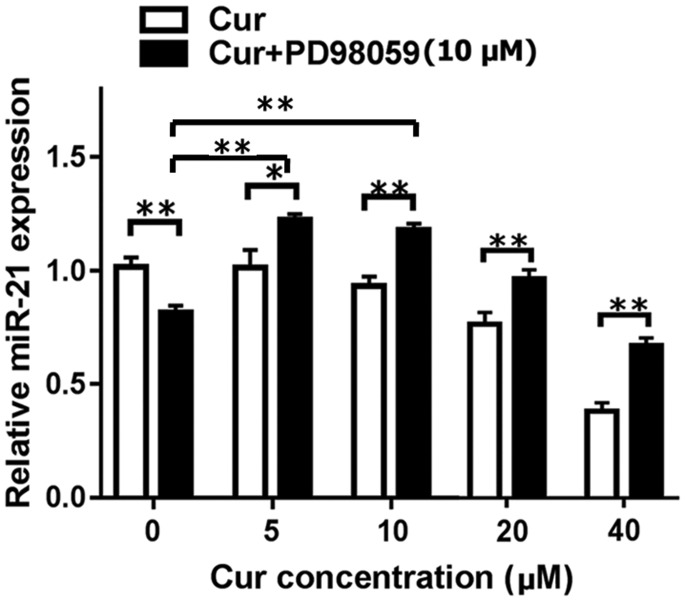
Expression of miR-21 gradually decreased when MGC-803 cells were treated with increased concentrations of curcumin. This decrease was significant after the cells were treated with 40 µM curcumin for 24 hours (P < 0.01, Figure 4). Notably, pretreatment with PD98059 (10 µM) partially upregulated miR-21 expression. We found that miR-21 expression in the combined treatment group was significantly higher than that in the curcumin alone group at all concentrations (all P < 0.05) Expression levels of miR-21 were even higher than those in the no treatment group and PD98059 + 0 µM curcumin group compared with treatment with PD98059 + 5 µM curcumin or PD98059 + 10 µM curcumin.
Figure 4.
Levels of miR-21 in MGC-803 cells after treatment with curcumin at different concentrations (0, 5, 10, 20, 40 µM) alone or PD98059 (10 µM) alone, and with a combination of these drugs were analyzed by quantitative reverse transcription-polymerase chain reaction. *P < 0.05, **P < 0.01. miR-21: microRNA-21
Expression levels of p-Akt and PTEN after curcumin and PD98059 treatment
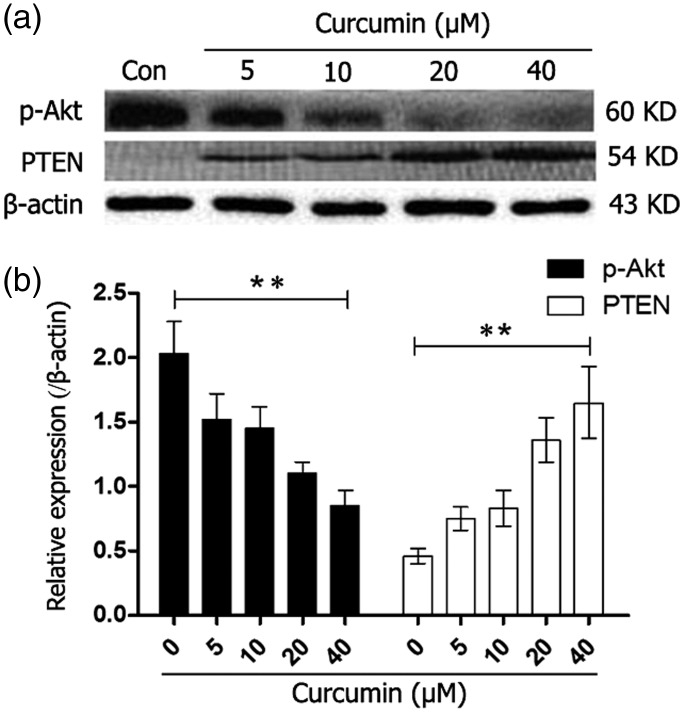
MGC-803 cells were treated for 24 hours with different curcumin concentrations to detect p-Akt expression Western blot analysis showed that curcumin significantly downregulated p-Akt expression in a concentration-dependent manner ranging from 5 to 40 µM (P < 0.01). Phosphorylated Akt showed the lowest expression level at a curcumin concentration of 40 µM (Figure 5a, 5b).
Figure 5.
Protein expression levels of p-Akt and PTEN in MGC-803 cells that were incubated with different concentrations (0, 5, 10, 20, 40 µM) of curcumin for 24 hours were determined by western blotting. **P < 0.01. p-Akt: phosphorylated Akt; PTEN: phosphatase and tensin homolog
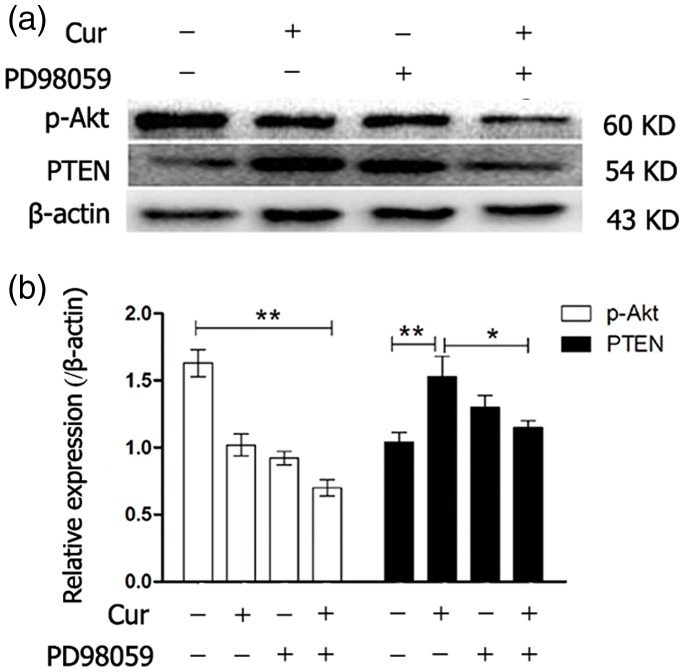
MGC-803 cells were also treated for 24 hours with curcumin (20 µM) alone, PD98059 (10 µM) alone, and curcumin (20 µM) combined with PD98059 (10 µM) to detect p-Akt protein levels. One-way ANOVA of Western blot results showed that p-Akt protein expression levels were significantly lower in the curcumin alone, PD98059 alone, and combined treatment groups (all P < 0.01) compared with the blank control group. Furthermore, p-Akt protein expression levels were significantly lower in the combined treatment group than in other treatment groups (P < 0.01, Figure 6a, 6b).
Figure 6.
Protein expression levels of p-Akt and PTEN in MGC-803 cells that were incubated with curcumin (20 µM) alone, PD98059 (10 µM) alone, or a combination of curcumin and PD98059 for 24 hours were determined by western blotting. *P < 0.05, **P < 0.01. p-Akt: phosphorylated Akt; PTEN: phosphatase and tensin homolog
PTEN expression levels significantly gradually increased with increased curcumin concentrations (P < 0.01, Figure 5a, 5b). PTEN protein expression was observed after treatment of cells with the MEK-specific inhibitor PD98059 combined with curcumin. Furthermore, curcumin alone (P < 0.01) and PD98059 alone upregulated PTEN expression. However, combined treatment slightly downregulated PTEN expression compared with curcumin alone treatment (P < 0.05).
Discussion
Our in vitro study showed that curcumin inhibited proliferation and induced apoptosis of MGC-803 cells in a concentration- and time-dependent manner. We also found that expression of components of the miR-21/PTEN/Akt pathway were disrupted by curcumin (Figures 4 and 5).
PI3K/Akt/mTOR is a classical anti-apoptotic and pro-survival signal transduction pathway, which regulates many physiology and pathophysiology processes, such as cell proliferation, survival, and migration.22 The Akt signaling pathway is frequently activated in gastric cancer and plays an important role in regulating gastric cancer cell proliferation and growth.23 Inhibition of the Akt signaling pathway can significantly promote apoptosis of gastric cancer cells.24
MicroRNA modulates gene expression post-tanscriptionally. Recent studies have shown that miR-21 is frequently upregulated and functions as an oncogene in multiple malignacies.25 PTEN, which is a validated target of miR-21, dephosphorylates phosphatidylinositol-3,4,5-trisphosphate in cells and functions as a tumor suppressor by negatively regulating the Akt/protein kinase B signaling pathway. In gastric cancer, miR-21 is upregulated, and the miR-21/PTEN/Akt molecular pathway plays an essential role in carcinogenesis and progression of gastric cancer.17 Inhibitors of miR-21 markedly suppress proliferation, migration, invasion, and colony formation of gastric cancer cells.26 Our study showed that curcumin inhibited miR-21 and p-Akt expression, while it increased PTEN protein expression in MGC 803 cells. These findings suggested that curcumin effectively inhibited the miR-21/PTEN/Akt molecular pathway. Furthermore, curcumin significantly inhibited proliferation (as shown in Figure 1) and induced apoptosis (Figures 2 and 3) in MGC 803 cells. These results suggest that the anti-cancer effects of curcumin may function through inhibiting the miR-21/PTEN/Akt molecular pathway in gastric cancer.
The MAPK pathway regulates physiological and pathophysiological processes, including proliferation, gene expression, differentiation, mitosis, cell survival, and apoptosis.27 The MAPK pathway is constitutionally activated in many malignancies, including gastric cancer.28 Several MAPK inhibitors are effective in animal models of disease and have advanced to clinical trials for treating inflammatory diseases and cancer.29 PD98059 is a potent and selective inhibitor of the MAPKKs MEK1 and MEK2. PD98059 induces apoptosis in gastric cancer cells when combined with other drugs.21 In our study,when combined with curcumin, PD98059 drastically increased the apoptosis-inducing effect of curcumin in MGC803 cells (Figure 3). PD98059 also increased the inhibitory effects of curcumin on expression of components in the miR-21/PTEN/Akt molecular pathway (Figure 6). These findings suggest that there was a synergistic effect between curcumin and PD98059 on apoptosis of MGC803 cells. Consistent with our findings, PD98059 can cooperate with curcumin to induce apoptosis of human leukemia HL-60 cells.30
There are multiple levels of cross-talk between the PI3K/AKT/mTOR pathway and MAPK pathway.31 Therefore, blockade of both pathways with combinations of signaling inhibitors might result in a more efficient anti-tumor effect compared with a single agent.32 Our study showed that curcumin combined with PD98059 efficiently induced apoptosis in MGC-803 cells. PD98059 enhanced the inhibitory effects of curcumin on miR-21/PTEN/Akt signaling. However, the underlying mechanism still needs to be determined in detail.
In conclusion, curcumin shows potent anti-cancer effects by inhibiting the miR-21/PTEN/Akt molecular pathway. PD98059 enhances curcumin’s apoptosis-inducing effects and Akt signaling-inhibiting effects in MGC-803 cells. Therefore, PD98059 combined with curcumin may be a potential strategy in cancer therapy.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Guangxi Education Department Middle-aged and Young Teachers’ Basic Ability Promotion Project (grant no. KY2016LX237) and Science and Technology Development Project of Guilin (grant no. 20150206-1-1).
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Nagini S. Carcinoma of the stomach: a review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol 2012; 4: 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmona-Bayonas A, Jimenez-Fonseca P, Lorenzo ML, et al. On the effect of triplet or doublet chemotherapy in advanced gastric cancer: results from a national cancer registry. J Natl Compr Canc Netw 2016; 14: 1379–1388. [DOI] [PubMed] [Google Scholar]
- 4.Chikara S, Nagaprashantha LD, Singhal J, et al. Oxidative stress and dietary phytochemicals: role in cancer chemoprevention and treatment. Cancer Lett 2018; 413: 122–134. [DOI] [PubMed] [Google Scholar]
- 5.Zheng R, Deng Q, Liu Y, et al. Curcumin inhibits gastric carcinoma cell growth and induces apoptosis by suppressing the Wnt/beta-catenin signaling pathway. Med Sci Monit 2017; 23: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haghi A, Azimi H, Rahimi R, et al. A Comprehensive Review on Pharmacotherapeutics of Three Phytochemicals, Curcumin, Quercetin, and Allicin, in the Treatment of Gastric Cancer. J Gastrointest Cancer 2017; 48: 314–320. [DOI] [PubMed]
- 7.Wang X, Jiang Y, Wang YW, et al. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem 2008; 108: 419. [DOI] [PubMed] [Google Scholar]
- 8.Aldebasi YH, Aly SM, Rahmani AH. Therapeutic implications of curcumin in the prevention of diabetic retinopathy via modulation of anti-oxidant activity and genetic pathways. Int J Physiol Pathophysiol Pharmacol 2013; 5: 194–202. [PMC free article] [PubMed] [Google Scholar]
- 9.Yamei LI, Liao D. Effect target predict of nicotinic acid curcumin ester for anti-atherosclerosis by using the Discovery Studio software. China Medical Herald 2013; 10: 16–18. [Google Scholar]
- 10.Qin S, Huang L, Gong J, et al. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: a meta-analysis of randomized controlled trials. Nutr J 2017; 16: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali A, Banerjea AC. Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci Rep-UK 2016; 6: 27539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell FC, Collett GP. Chemopreventive properties of curcumin. Future Oncol 2005; 1: 405–414. [DOI] [PubMed] [Google Scholar]
- 13.Murray-Stewart T, Dunworth M, Lui Y, et al. Curcumin mediates polyamine metabolism and sensitizes gastrointestinal cancer cells to antitumor polyamine-targeted therapies. PLoS One 2018; 13: 1–23. [DOI] [PMC free article] [PubMed]
- 14.Kasi PD, Tamilselvam R, Skalicka-Wozniak K, et al. Molecular targets of curcumin for cancer therapy: an updated review. Tumour Biol 2016; 37: 13017–13028. [DOI] [PubMed] [Google Scholar]
- 15.Song G, Mao YB, Cai QF, et al. Curcumin induces human HT-29 colon adenocarcinoma cell apoptosis by activating p53 and regulating apoptosis-related protein expression. Braz J Med Biol Res 2005; 38: 1791–1798. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Cai Q, Jiang Z, et al. Prognostic role of microRNA-21 in gastric cancer: a meta-analysis. Med Sci Monit 2014; 20: 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang BG, Li JF, Yu BQ, et al. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep 2012; 27: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy S, Yu Y, Padhye SB, et al. Difluorinated-curcumin (CDF) restores PTEN expression in colon cancer cells by down-regulating miR-21. PloS One 2013; 8: e68543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alessi DR, Cuenda A, Cohen P. PD98059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 1995; 270: 27489–27494. [DOI] [PubMed] [Google Scholar]
- 20.Ge X, Liu J, Shi Z, et al. Inhibition of MAPK signaling pathways enhances cell death induced by 5-Aminolevulinic acid-photodynamic therapy in skin squamous carcinoma cells. Eur J Dermatol 2016; 26: 164–172. [DOI] [PubMed] [Google Scholar]
- 21.Yao J, Qian C, Shu T, et al. Combination treatment of PD98059 and DAPT in gastric cancer through induction of apoptosis and downregulation of WNT/beta-catenin. Cancer Biol Ther 2013; 14: 833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501. [DOI] [PubMed] [Google Scholar]
- 23.Ye Y, Ge YM, Xiao MM, et al. Suppression of SHIP2 contributes to tumorigenesis and proliferation of gastric cancer cells via activation of Akt. J Gastroenterol 2016; 51: 230–240. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Chen Y, Li G, et al. Her3 is associated with poor survival of gastric adenocarcinoma: Her3 promotes proliferation, survival and migration of human gastric cancer mediated by PI3K/AKT signaling pathway. Med Oncol 2014; 31: 903. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Xu T, Chen C. The critical roles of miR-21 in anti-cancer effects of curcumin. Ann Transl Med 2015; 3: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P, Guan Q, Zhou D, et al. miR-21 Inhibitors Modulate Biological Functions of Gastric Cancer Cells via PTEN/PI3K/mTOR Pathway. DNA Cell Biol 2018; 37: 38–45. [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Huang CZ. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J Gastroenterol 2015; 21: 11673–11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshino R, Chatani Y, Yamori T, et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene 1999; 18: 813–822. [DOI] [PubMed] [Google Scholar]
- 29.English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharm Sci 2002; 23: 40–45. [DOI] [PubMed] [Google Scholar]
- 30.Banjerdpongchai R, Wilairat P. Effects of water-soluble antioxidants and MAPKK/MEK inhibitor on curcumin-induced apoptosis in HL-60 human leukemic cells. Asian Pac J Cancer Prev 2005; 6: 282–285. [PubMed] [Google Scholar]
- 31.Wong MH, Xue A, Baxter RC, et al. Upstream and downstream co-inhibition of mitogen-activated protein kinase and PI3K/Akt/mTOR pathways in pancreatic ductal adenocarcinoma. Neoplasia 2016; 18: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Luca A, Maiello MR, D'Alessio A, et al. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets 2012; 16: S17–S27. [DOI] [PubMed] [Google Scholar]