Short abstract
Objective
To investigate the relationship between serum high mobility group box-1 protein (HMGB-1) levels and prognosis in patients with community-acquired pneumonia (CAP).
Methods
This prospective study included 35 patients who attended our hospital from January 2016 to December 2016. Pneumonia severity was defined by pneumonia severity index (PSI). Serum levels of C-reactive protein (CRP), cortisol, and HMGB-1 were analyzed in relation to disease severity and clinical outcome.
Results
High HMGB-1 levels were associated with high cortisol levels. High HMGB-1 and high cortisol were both significantly associated with high white blood cell count and high serum CRP, compared with low HMGB-1 and low cortisol, respectively. PSI score and 30-day mortality were also significantly higher in patients with high HMGB-1 or high cortisol levels compared with patients with low HMGB-1 or cortisol levels, respectively. CRP, cortisol, and HMGB-1 levels were all significantly higher in patients who died compared with survivors.
Conclusion
HMGB-1 was associated with clinical outcomes and was an independent risk factor for 30-day mortality in patients with CAP. Serum HMGB-1 levels were also positively correlated with serum levels of cortisol. These results demonstrate a role for HMGB-1 in CAP, and suggest possible new therapeutic targets for patients with CAP.
Keywords: High mobility group box-1 protein, community-acquired pneumonia, prognosis, clinical outcome, cortisol, C-reactive protein
Introduction
Despite widespread use of antibiotics over the last 70 years, community-acquired pneumonia (CAP) remains a serious threat to global health, with an incidence of 3.3 to 46 per 1000 per year in the elderly population.1–3 CAP has high mortality rates of up to 48%, and is the most common cause of death in patients with severe infectious diseases.4,5 Numerous factors have been shown to influence mortality among patients with severe CAP, including age, comorbidities, pneumonia severity, presence of bacteremia, and serum levels of inflammatory factors.6,7
Many inflammatory factors are thought to be associated with development and prognosis of CAP, including interleukin (IL)-1, IL-6, IL-8, IL-10, tumor necrosis factor-α, C-reactive protein (CRP), procalcitonin, and cortisol.8,9 Some of these factors are thought to be correlated with mortality risk in CAP patients, and some factors have been implicated as independent risk factors for death in patients with severe CAP.10–12 The nuclear high mobility group box-1 protein (HMGB-1) has demonstrated important roles in cancer and inflammatory processes such as breast cancer and sepsis.13–15 HMGB-1 was also shown to be elevated in patients with uncomplicated pneumonia and pneumonia with severe sepsis,16 and was associated with Pseudomonas aeruginosa pneumonia in patients with cystic fibrosis.17 However, few studies have examined the relationships of HMGB-1 with prognosis and mortality in patients with CAP.
In the present study, we investigated the relationships between serum levels of HMGB-1 and prognosis in patients with CAP, and between HMGB-1 and cortisol. These results provide clinical evidence of the role of HMGB-1 in CAP, and suggest potential new therapeutic targets for patients with CAP.
Patients and methods
Patients
This prospective study included 35 inpatients who attended the Breath Internal Medicine Department at the First Affiliated Hospital of Guangxi Medical University from January 2016 to December 2016. All patients were diagnosed with CAP according to the criteria of the American Thoracic Society guidelines for pneumonia.18 All patients were over 18 years old, and all had pulmonary infiltration diagnosed by chest X-ray and clinical symptoms including cough, purulent sputum, positive auscultation, or fever. Patients with any serious systemic or respiratory diseases at admission were excluded, including patients with pulmonary tuberculosis, bronchial asthma, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, urinary tract infection, and cancer. The severity of pneumonia was defined according to the pneumonia severity index (PSI) as mild/moderate CAP I–III or severe CAP IV–V, as described previously.19 Informed consent was obtained from all patients within 24 hours after admission. The present study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University.
Data collection and measurement
Demographic data including age, sex, and antibiotic use in the 1 month prior to admission were collected from the patients’ medical records. Symptoms and PSI scores were recorded. White blood cell count (WBC) was determined by routine blood tests. Blood samples were collected within 24 hours after admission and serum levels of CRP, cortisol, and HMGB-1 were determined by enzyme-linked immunosorbent assay using commercial kits (MSKBIO, Wuhan, China) according to the manufacturer’s instructions. In terms of survival, all-cause death during hospitalization was considered and recorded, with a follow-up time of 30 days from the time of admission. Survival time was considered as the time from admission to the time of death or last follow-up.
Statistical analysis
Measured data were expressed by mean ±standard deviation when normally distributed, and median (range) in other instances. Rates were compared using χ2 tests, and comparisons between two groups of continuous data were made using Student’s t-test or Mann–Whitney U test. Correlations were determined by Spearman’s analysis and survival analysis was performed using Kaplan–Meier curves. Relationships between serum levels of HMGB-1 and cortisol and 30-day mortality were analyzed using a logistic regression model by a stepwise method. Values of P < 0.05 were considered to be statistically significant. All calculations were made using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
HMGB-1 and cortisol were up-regulated in patients with severe pneumonia
Details of the 35 patients with CAP are shown in Table 1. Nineteen patients were diagnosed with mild/moderate CAP and 16 with severe CAP. There was no significant difference between the groups in terms of age, sex, antibiotic situation, or symptoms. Laboratory evaluation showed that WBC and serum levels of CRP, cortisol, and HMGB-1 were all significantly higher in patients with severe CAP compared with patients with mild/moderate CAP (P < 0.05). The 30-day mortality rate was also significantly higher in patients with severe compared with mild/moderate CAP (P < 0.05).
Table 1.
Basic clinical information for all participants.
| Variable | Mild/moderate CAP (n = 19) | Severe CAP (n = 16) |
|---|---|---|
| Age, years (mean±SD) | 57.5±11.4 | 61.4±9.9 |
| Sex, male:female | 12:7 | 10:6 |
| Antibiotics received before treatment, n (%) | 10 (52.3) | 8 (50) |
| Symptoms, n (%) | ||
| Fever | 9 (47.4) | 9 (56.3) |
| Cough | 7 (36.8) | 8 (50.0) |
| Sputum | 6 (31.6) | 6 (37.5) |
| Shortness of breath | 4 (21.1) | 4 (25.0) |
| Chest pain | 3 (15.8) | 4 (25.0) |
| Laboratory tests | ||
| CRP, mg/L | 60 (23–178) | 122.5 (84–229)* |
| WBC, 109/mL | 9.6 (7.5–13.7) | 12.5 (9.4–16.9)* |
| Cortisol, nmol/L | 528 (295–764) | 907 (638–1309)* |
| HMGB-1, ng/mL | 57 (31–101) | 102.5 (89–151)* |
| Mortality during 30 days follow-up, n (%) | 4 (21.1) | 7 (43.8)* |
CAP: community-acquired pneumonia, SD: standard deviation, CRP: C-reactive protein, WBC: white blood cell count, HMGB-1: high mobility group box-1 protein. *P < 0.05, compared with mild/moderate group.
Relationships of HMGB-1 and cortisol with clinical outcome in patients with CAP
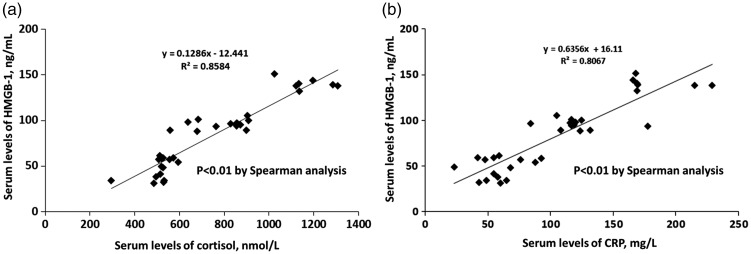
Serum levels of HMGB-1 were significantly correlated with serum levels of CRP and cortisol in all patients (P < 0.05) (Figure 1). We further investigated the relationships of HMGB-1 and cortisol with clinical outcomes of CAP by stratifying patients into high/low HMGB-1 and cortisol groups according to the median values. WBC and serum levels of CRP and cortisol were all significantly higher in the high-HMGB-1 group compared with the low-HMGB-1 group (P < 0.05) (Table 2). PSI stage and 30-day mortality were also significantly higher in the high-HMGB-1 group (P < 0.05). Similar results were found for the high- and low-cortisol groups (Table 3). These results showed that serum levels of both HMGB-1 and cortisol were associated with clinical outcomes in patients with CAP.
Figure 1.
Correlations between serum levels of HMGB-1 and cortisol (a) and CRP (b). HMGB-1: high mobility group box-1 protein.
Table 2.
Clinical outcomes in patients with CAP in relation to serum HMGB-1 levels.
| Variable | Low HMGB-1 (n = 18) | High HMGB-1 (n = 17) |
|---|---|---|
| Age, years (mean±SD) | 56.2±11.0 | 62.5±9.8 |
| Sex, male:female | 12:6 | 10:7 |
| Antibiotics received before treatment, n (%) | 9 (50.0) | 9 (52.9) |
| Symptoms, n (%) | ||
| Fever | 8 (44.4) | 10 (58.8) |
| Cough | 7 (38.9) | 8 (47.1) |
| Sputum | 6 (33.3) | 6 (35.3) |
| Shortness of breath | 3 (16.7) | 5 (29.4) |
| Chest pain | 3 (16.7) | 4 (23.5) |
| Laboratory | ||
| CRP, mg/L | 59.5 (23–132) | 125 (84–229)* |
| WBC, 109/mL | 9.3 (7.5–14.6) | 12.0 (9.9–16.9)* |
| Cortisol, nmol/L | 528 (295–899) | 905 (684–1309)* |
| PSI, n (%) | ||
| I–III | 17 (94.4) | 2 (11.8)* |
| IV–V | 1 (5.6) | 15 (88.2)* |
| Mortality during 30 days follow-up, n (%) | 2 (11.1) | 9 (52.9)* |
CAP: community-acquired pneumonia, SD: standard deviation, WBC: white blood cell count, CRP: C-reactive protein, HMGB-1: high mobility group box-1 protein, PSI: pneumonia severity index. *P < 0.05, compared with the low-HMGB-1 group.
Table 3.
Clinical outcomes in patients with CAP in relation to serum cortisol levels.
| Variable | Low cortisol (n = 18) | High cortisol (n = 17) |
|---|---|---|
| Age, years (mean±SD) | 56.1±11.0 | 62.7±9.7 |
| Sex, male:female | 11:7 | 11:6 |
| Antibiotics received before treatment, n (%) | 9 (50.0) | 9 (52.9) |
| Symptoms, n (%) | ||
| Fever | 8 (44.4) | 10 (58.8) |
| Cough | 8 (47.1) | 8 (38.9) |
| Sputum | 6 (33.3) | 6 (35.3) |
| Shortness of breath | 4 (22.2) | 4 (23.5) |
| Chest pain | 3 (16.7) | 4 (23.5) |
| Laboratory | ||
| CRP, mg/L | 59.5 (23–132) | 125 (84–229)* |
| WBC, 109/mL | 9.3 (7.5–14.6) | 12.0 (9.9–16.9)* |
| HMGB-1, ng/mL | 55.5 (31–98) | 101 (89–151)* |
| PSI, n (%) | ||
| I–III | 17 (94.4) | 2 (11.8)* |
| IV–V | 1 (5.6) | 15 (88.2)* |
| Mortality during 30 days follow-up, n (%) | 2 (11.1) | 9 (52.9)* |
CAP: community-acquired pneumonia, SD: standard deviation, WBC: white blood cell count, CRP: C-reactive protein, HMGB-1: high mobility group box-1 protein, PSI: pneumonia severity index. *P < 0.05, compared with the low-cortisol group.
Relationships of HMGB-1 and cortisol with 30-day mortality in patients with CAP
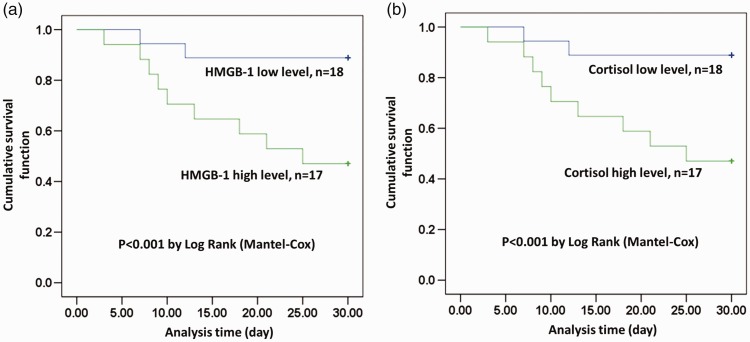
We also analyzed the relationships of HMGB-1 and cortisol with 30-day mortality in patients with CAP. Patients with high serum levels of HMGB-1 or cortisol had higher 30-day mortality (lower survival) than patients with low serum levels of HMGB-1 or cortisol, respectively, according to Kaplan–Meier analysis (P < 0.05) (Figure 2). Further analysis showed that mean age and serum levels of CRP, cortisol, and HMGB-1 were all significantly higher in non-survivors compared with survivors (P < 0.05) (Table 4). Logistic analysis identified both cortisol and HMGB-1 as independent risk factors for 30-day mortality in patients with CAP (Table 5).
Figure 2.
Kaplan–Meier survival analyses of patients with high/low HMGB-1 (a) and high/low cortisol (b). HMGB-1: high mobility group box-1 protein.
Table 4.
Clinical outcomes in surviving and non-surviving patients with CAP.
| Variable | Surviving (n = 24) | Non-surviving (n = 11) |
|---|---|---|
| Age, years (mean±SD) | 54.1±8.7 | 70.6±3.6* |
| Sex, male:female | 15:9 | 7:4 |
| Antibiotics received before treatment, n (%) | 13 (54.2) | 5 (45.5) |
| Symptoms, n (%) | ||
| Fever | 12 (50.0) | 6 (54.5) |
| Cough | 10 (41.7) | 5 (41.7) |
| Sputum | 8 (33.3) | 4 (36.4) |
| Shortness of breath | 5 (20.8) | 3 (27.3) |
| Chest pain | 5 (20.8) | 2 (18.2) |
| Laboratory | ||
| CRP, mg/L | 72 (23–125) | 169 (117–229)* |
| WBC, 109/mL | 8.4 (7.5–11.6) | 14.5 (11.2–16.9)* |
| Cortisol, nmol/L | 545 (295–909) | 1123 (558–1309)* |
| HMGB-1, ng/mL | 58.5 (31–105) | 138 (88–151)* |
| PSI, n (%) | ||
| I–III | 15 (62.5) | 4 (36.4)* |
| IV–V | 9 (37.5) | 7 (63.6)* |
CAP: community-acquired pneumonia, SD: standard deviation, WBC: white blood cell count, CRP: C-reactive protein, HMGB-1: high mobility group box-1 protein, PSI: pneumonia severity index. *P < 0.05, compared with surviving group.
Table 5.
Correlations of serum HMGB-1 and cortisol levels with 30-day mortality in patients with severe CAP according to multivariate logistic regression analysis.
| Wald | Odds ratio | 95% CI | P value | |
|---|---|---|---|---|
| Age | 4.734 | 2.620 | (1.100–6.238) | 0.030 |
| CRP | 2.025 | 1.280 | (0.913–1.796) | 0.152 |
| Cortisol | 9.029 | 1.007 | (1.002–1.011) | 0.003 |
| HMGB-1 | 6.108 | 1.095 | (1.019–1.177) | 0.013 |
| PSI | 0.500 | 1.680 | (0.399–7.075) | 0.479 |
CAP: community-acquired pneumonia, CI: confidence interval, CRP: C-reactive protein, HMGB-1: high mobility group box-1 protein, PSI: pneumonia severity index.
Discussion
More than 5.6 million cases of CAP are reported annually in the United States, accounting for more than 1 million hospitalizations, with hospital mortality rates of 5% to 48%.20 Patients with severe CAP usually require admission to the intensive care unit; however, despite advances in supportive care, severe CAP is still associated with high mortality.21 Serum levels of inflammatory factors have attracted much attention as risk factors for mortality among patients with CAP, and several inflammatory factors have been associated with mortality in these patients. Walters et al.10 showed that failure to reduce CRP levels by ≥50% at ≥4 days increased the probability of death in patients with CAP, and Guertler et al.22 found that procalcitonin was also associated with long-term mortality risk among patients with CAP. In addition to these widely studied factors, HMGB-1 is a newly identified factor associated with the inflammation process, though more clinical evidence is needed to clarify the relationship between HMGB-1 and CAP prognosis. In the present study, we provide the first evidence for an association between HMGB-1 and clinical outcomes in patients with CAP, and as an independent risk factor for 30-day mortality in these patients.
The current results showed that serum levels of HMGB-1 were up-regulated in patients with severe CAP and were associated with clinical outcomes. Previous studies have demonstrated a role for HMGB-1 in pneumonia and other inflammatory processes. Wang et al.23 showed that HMGB-1 was elevated in patients with untreated compared with treated CAP and was associated with PSI stage, while Achouiti et al.24 demonstrated that HMGB-1 was associated with lung injury during Staphylococcus aureus pneumonia. Furthermore, Nosaka et al.25 found that anti-HMGB-1 monoclonal antibody could protect against influenza A virus (H1N1)-induced pneumonia in mice.
We also showed that serum levels of HMGB-1 were positively correlated with serum levels of cortisol, and that both HMGB-1 and cortisol were associated with 30-day mortality among patients with CAP. Previous studies have revealed a role for cortisol in CAP. In a prospective study, Kolditz et al.26 found that serum cortisol levels predicted death and critical disease independently of CRB-65 score in patients with CAP, and Omelyanenko et al.27 demonstrated that cortisol could also be used as a potent prognostic biomarker in patients with severe CAP. In the present study, we demonstrated that serum levels of HMGB-1 were positively correlated with serum levels of cortisol; however, further insights are still needed. We also identified HMGB-1 as an independent risk factor for 30-day mortality in patients with CAP.
The present study had some limitations. First, it was a single-center study with a relatively small study population. Second, further studies are needed to clarify the mechanisms by which HMGB-1 influences CAP and its relationship with cortisol.
In conclusion, we investigated the relationship between serum HMGB-1 levels and prognosis in patients with CAP. HMGB-1 was associated with clinical outcomes and was an independent risk factor for 30-day mortality, and serum levels of HMGB-1 were also positively correlated with serum levels of cortisol in patients with CAP. These results provide clinical evidence for the role of HMGB-1 in CAP, and may thus help to identify novel research targets for CAP treatment.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Talents Highland of Emergency and Medical Rescue of Guangxi Province in China (GXJZ201605), and the Health and Family Planning Commission of Guangxi Zhuang Autonomous Region, self-funded projects (Z20170960).
References
- 1.van Werkhoven CH, Postma DF, Oosterheert JJ, et al. Antibiotic treatment of moderate-severe community-acquired pneumonia: design and rationale of a multicentre cluster-randomised cross-over trial. Neth J Med 2014; 72: 170–178. [PubMed] [Google Scholar]
- 2.Yu H, Rubin J, Dunning S, et al. Clinical and economic burden of community-acquired pneumonia in the Medicare fee-for-service population. J Am Geriatr Soc 2012; 60: 2137–2143. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CP, Ryan M, Chapman JD, et al. Incidence and cost of pneumonia in Medicare beneficiaries. Chest 2012; 142: 973–981. [DOI] [PubMed] [Google Scholar]
- 4.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012; 67: 71–79. [DOI] [PubMed] [Google Scholar]
- 5.Loeb M. Community-acquired pneumonia. N Engl J Med 2015; 372: 331–340.25607427 [Google Scholar]
- 6.Wesemann T, Nüllmann H, Pflug MA, et al. Pneumonia severity, comorbidity and 1-year mortality in predominantly older adults with community-acquired pneumonia: a cohort study. BMC Infect Dis 2015; 15: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viasus D, Del Rio-Pertuz G, Simonetti AF, et al. Biomarkers for predicting short-term mortality in community-acquired pneumonia: a systematic review and meta-analysis. J Infect 2016; 72: 273–282. [DOI] [PubMed] [Google Scholar]
- 8.Lee YL, Chen W, Chen LY, et al. Systemic and bronchoalveolar cytokines as predictors of in-hospital mortality in severe community-acquired pneumonia. J Crit Care 2010; 25: 176.e7. [DOI] [PubMed] [Google Scholar]
- 9.Salluh JIF, Shinotsuka CR, Soares M, et al. Cortisol levels and adrenal response in severe community-acquired pneumonia: a systematic review of the literature. J Crit Care 2010; 25: 541.e1–8. [DOI] [PubMed] [Google Scholar]
- 10.Walters G, Liu HS, Gemza M, et al. Does the serum C-reactive protein (CRP) predict adverse outcomes in patients admitted with community acquired pneumonia? Eur Respir J 2011; 38: 1459. [Google Scholar]
- 11.Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax 2009; 64: 587. [DOI] [PubMed] [Google Scholar]
- 12.Curbelo J, Luquero BS, Galvánromán JM, et al. Inflammation biomarkers in blood as mortality predictors in community-acquired pneumonia admitted patients: importance of comparison with neutrophil count percentage or neutrophil-lymphocyte ratio. Plos One 2017; 12: e0173947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Hu X, Xia D, et al. MicroRNA-181b is downregulated in non-small cell lung cancer and inhibits cell motility by directly targeting HMGB1. Oncol Lett 2016; 12: 4181–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno T, Ikeda T, Yokoyama T, et al. Reduction in circulating level of HMGB-1 following continuous renal replacement therapy in sepsis. Cytokine 2016; 83: 206–209. [DOI] [PubMed] [Google Scholar]
- 15.Stoetzer OJ, Fersching DMI, Salat C, et al. Circulating immunogenic cell death biomarkers HMGB1 and RAGE in breast cancer patients during neoadjuvant chemotherapy. Tumour Biol 2013; 34: 81–90. [DOI] [PubMed] [Google Scholar]
- 16.Angus DC, Yang L, Kong L, et al. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med 2007; 35: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 17.Entezari M, Weiss DJ, Sitapara R, et al. Inhibition of HMGB1 enhances bacterial clearance and protects against P. aeruginosa pneumonia in cystic fibrosis. Mol Med 2012; 18: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sibila O, Meduri GU, Mortensen EM, et al. Improving the 2007 Infectious Disease Society of America/American Thoracic Society severe community-acquired pneumonia criteria to predict intensive care unit admission. J Crit Care 2013; 28: 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336: 243–250. [DOI] [PubMed] [Google Scholar]
- 20.Welte T. Risk factors and severity scores in hospitalized patients with community-acquired pneumonia: prediction of severity and mortality. Eur J Clin Microbiol Infect Dis 2012; 31: 33–47. [DOI] [PubMed] [Google Scholar]
- 21.Sligl WI, Marrie TJ. Severe community-acquired pneumonia. Crit Care Clin 2013; 29: 563–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guertler C, Wirz B, Christ-Crain M, et al. Inflammatory responses predict long-term mortality risk in community-acquired pneumonia. Eur Respir J 2011; 37: 1439–1446. [DOI] [PubMed] [Google Scholar]
- 23.Wang HL, Tsao SM, Yeh CB, et al. Circulating level of high mobility group box-1 predicts the severity of community-acquired pneumonia: regulation of inflammatory responses via the c-Jun N-terminal signaling pathway in macrophages. Mol Med Rep 2017; 16: 2361–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Achouiti A, van der Meer AJ, Florquin S, et al. High-mobility group box 1 and the receptor for advanced glycation end products contribute to lung injury during Staphylococcus aureus pneumonia. Crit Care 2013; 17: R296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nosaka N, Yashiro M, Yamada M, et al. Anti-high mobility group box-1 monoclonal antibody treatment provides protection against influenza A virus (H1N1)-induced pneumonia in mice. Crit Care 2015; 19: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolditz M, Höffken G, Martus P, et al. Serum cortisol predicts death and critical disease independently of CRB-65 score in community-acquired pneumonia: a prospective observational cohort study. BMC Infect Dis 2012; 12: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omelyanenko O, Makarevich A, Amelchenko E, et al. Prognostic value of cortisol and adrenocorticotropic hormone (ACTH) in severe community-acquired pneumonia (SCAP) patients. Eur Respir J 2011; 38: 1473. [Google Scholar]