Significance
Suppression of inhibitory interneurons, termed disinhibition, has been suggested as a conserved circuit mechanism crucial for associative learning. In Drosophila, a pair of GABAergic neurons negatively regulates aversive olfactory learning. Here we show that these neurons receive suppressive signals directly from a group of DA neurons through the inhibitory dopamine receptor DD2R. During odor-electric shock (ES) conditioning, ES-induced dopaminergic (DA) signal restrains GABAergic inhibition, thereby allowing efficient learning. Moreover, this inhibitory DA signal is required for learning-induced synaptic modification. Therefore, we propose that the suppressive DA signal provides a new twist on the Drosophila learning circuit, which is essential for accomplishing disinhibition in the learning center and sculpturing a memory trace in the GABAergic inhibitory neurons.
Keywords: Drosophila aversive olfactory learning, DA-to-GABA suppression, DD2R, disinhibition, memory trace
Abstract
The GABAergic system serves as a vital negative modulator in cognitive functions, such as learning and memory, while the mechanisms governing this inhibitory system remain to be elucidated. In Drosophila, the GABAergic anterior paired lateral (APL) neurons mediate a negative feedback essential for odor discrimination; however, their activity is suppressed by learning via unknown mechanisms. In aversive olfactory learning, a group of dopaminergic (DA) neurons is activated on electric shock (ES) and modulates the Kenyon cells (KCs) in the mushroom body, the center of olfactory learning. Here we find that the same group of DA neurons also form functional synaptic connections with the APL neurons, thereby emitting a suppressive signal to the latter through Drosophila dopamine 2-like receptor (DD2R). Knockdown of either DD2R or its downstream molecules in the APL neurons results in impaired olfactory learning at the behavioral level. Results obtained from in vivo functional imaging experiments indicate that this DD2R-dependent DA-to-APL suppression occurs during odor-ES conditioning and discharges the GABAergic inhibition on the KCs specific to the conditioned odor. Moreover, the decrease in odor response of the APL neurons persists to the postconditioning phase, and this change is also absent in DD2R knockdown flies. Taken together, our findings show that DA-to-GABA suppression is essential for restraining the GABAergic inhibition during conditioning, as well as for inducing synaptic modification in this learning circuit. Such circuit mechanisms may play conserved roles in associative learning across species.
GABAergic neurons constitute the major inhibitory system in the central nervous system. The tight regulation of these neurons is critical for cognitive functions, such as learning and memory (1). In rodents, for example, a group of amygdala GABAergic interneurons innervates principle neurons and exerts negative regulation in fear conditioning (2). In addition, suppression of these inhibitory neurons using an optogenetic approach facilitates associative learning (3). It has been reported that these GABAergic neurons are suppressed on dopamine perfusion (4), while an independent study showed that another subgroup of GABAergic interneurons provides the control signal (3). Behavioral studies in Drosophila indicate that the inhibitory Drosophila dopamine 2-like receptor (DD2R) plays an essential role in olfactory learning (5, 6), although the precise circuit mechanisms remain to be elucidated.
In Drosophila, a single pair of GABAergic anterior paired lateral (APL) neurons projects exclusively to the mushroom body (MB) (7), the center of olfactory learning and memory in flies (8). Similar to GABAergic interneurons in mammals, APL neurons also negatively regulate aversive learning by suppressing Kenyon cells (KCs), the intrinsic neurons of the MB (7). It is known that APL neurons are activated by KCs and also send negative feedback to KCs (9). This feedback loop is essential for sparse odor coding in the MB and odor discrimination during learning (7, 9). Intriguingly, the odor response of KCs has been found to increase after conditioning (10, 11), while decreasing significantly in APL neurons (7). These phenomena suggest that other neurons may participate in the regulation of APL neurons during conditioning.
In addition to KCs, dorsal paired medial (DPM) neurons are the only other neurons known to connect with APL neurons (12, 13). However, DPM neurons exhibit a delayed increase in activity starting at 30 min after conditioning (14) and function only during the stage of memory consolidation (12, 15, 16). Thus, we propose that specific synaptic connections of APL neurons have not yet been identified, and that these connections may exert suppressive regulation on these GABAergic neurons during conditioning.
In Drosophila aversive olfactory learning, an electric shock (ES) serves as the unconditioned stimulus, transmitted mainly by a group of TH-Gal4–labeled dopaminergic (DA) neurons, paired posterior lateral 1 (PPL1) neurons (17, 18). These neurons are strongly activated by ES (17). In addition, activation of these neurons promotes olfactory learning (18). Using GFP reconstitution across synaptic partners (GRASP) (19) and functional syb:GRASP (20) approaches, we found synaptic connections between these DA neurons and APL neurons. Using an in vivo calcium imaging approach, we also found that during conditioning, the ES-DA signal exerts suppressive regulation on APL neurons through DD2R, and that this suppression is important for efficient odor-ES conditioning.
Results
Potential Synaptic Connections Between DA Neurons and APL Neurons.
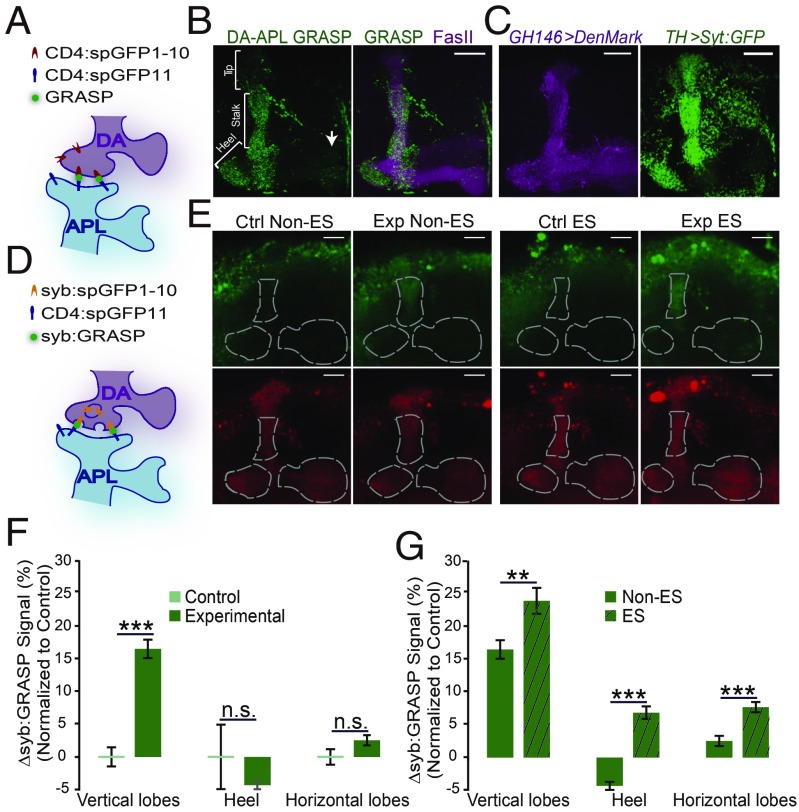
In Drosophila, TH-Gal4–labeled PPL1 DA neurons have been shown to project to the vertical lobes and heel of the MB (18, 21), where APL neurons also project (22, 23) (SI Appendix, Fig. S1). We tested whether these two types of neurons connect with direct synapses through a GRASP experiment. We first generated a QUAS-CD4:spGFP11 transgenic fly strain and constructed QUAS-CD4:spGFP11/cyo;GH146QF,UAS-mCD4:spGFP1-10/TH-Gal4 flies. In these flies, mCD4:spGFP1-10 was expressed in DA neurons, and CD4:spGFP11 was expressed in APL neurons (Fig. 1A and SI Appendix, Fig. S2A). We then examined the brains of these flies and detected the GRASP signal in both MB lobe and calyx regions (SI Appendix, Fig. S2B). Although the overlapping projection regions of PPL1 neurons and APL neurons entirely cover the MB vertical lobes and heels (7, 18, 21), we detected a dense GRASP signal only in the stalks of the vertical lobes and the heels, but only a weak signal in the vertical lobe tip region (Fig. 1B). The reconstituted GFP signal was found in some, but not all, of the overlapping regions, suggesting that this signal represents the possible connection area of the two groups of neurons.
Fig. 1.
Synaptic connections between DA neurons and APL neurons. (A) Schematic diagram of the GRASP experiment. (B) Representative brain image of the MB lobe region of QUAS-mCD4:spGFP11/cyo;GH146-QF,UAS-mCD4:spGFP1-10/TH-Gal4 flies. The GRASP signal (green) was detected in the stalks of MB vertical lobes and the heel region, but with weak signals in the tip of vertical lobes and the distal tip of MB horizontal lobes (white arrow). The MB lobes were labeled by FasII staining (magenta). (C) The postsynaptic sites of APL neurons labeled by DenMark are distributed to the entire MB lobes. The presynaptic sites of TH-Gal4 labeled DA neurons labeled with Syt:GFP localize to the entire vertical lobe, heel, and the tip of horizontal lobe regions of the MB. The genotypes are GH146-Gal4/UAS-DenMark (Left) and TH-Gal4/UAS-Syt:GFP (Right). (D) Schematic diagram of the syb:GRASP experiment. The signals of syb:GRASP were detected only when the transmitters were released from presynaptic DA neurons. (E) Representative images of activity-dependent syb:GRASP signal (Upper) and activity-independent mCherry signal (Lower) in MB regions without or with ES treatment (non-ES and ES groups). Experimental group: GH146-Gal4,TH-LexA/LexAop-syb:spGFP1-10,UAS-CD4:spGFP11;UAS-mCherry/+. Control group: GH146-Gal4/LexAop-syb:spGFP1-10,UAS-CD4-spGFP11;UAS-mCherry/+. (F) Without ES treatment, the syb:GRASP signal was significantly higher in the vertical lobes, but not in the heel and horizontal lobes, in the experimental group compared with the control group. (G) On ES treatment, the syb:GRASP signals were significantly increased in all three MB regions, while the signals were still much lower in the heel and horizontal lobe regions than in the vertical lobe region. In E–G, the syb:GRASP signal was normalized to the mCherry signal, and the difference between the experimental and control groups is shown as ∆syb:GRASP. n = 42 in the control non-ES group, n = 57 in the experimental non-ES group, n = 36 in the control ES group, and n = 75 in the experimental ES group. Data were quantified by the independent-samples t test and are shown as mean ± SEM. n.s. indicates no significant difference. *P < 0.05; **P < 0.01; ***P < 0.001. (Scale bars: 20 μm in all panels.)
TH-Gal4 also labels MB-M3 DA neurons, which project to the distal tip of MB horizontal lobes (24), where we also detected a weak GRASP signal. Because these neurons were previously shown to have a minor function in aversive olfactory learning (24), we did not investigate potential connections further in this study. We did not detect any GRASP signal in the two control groups, which contained all the transgenes except either GH146-QF or TH-Gal4 (SI Appendix, Fig. S2 C and D). Taken together, these results suggest that the projection terminals of the PPL1 DA neurons and APL neurons are morphologically close enough to form synapses, and that the potential connections are located largely in the stalk of the vertical lobes and heels in the MB lobe regions.
Hypothesizing that the PPL1 neurons form synapses to APL neurons, we next examined the synaptic distribution of these two types of neurons. DenMark, a postsynaptic marker, was expressed in APL neurons and revealed extensive postsynaptic sites in the MB region (Fig. 1C), in agreement with previous reports (7, 23, 25). In particular, the postsynaptic signals were reticulate, covering the entire MB lobes. In addition, syt-GFP revealed the presynaptic sites of the PPL1 neurons, which localize mainly in the vertical lobes and heel of the MB (Fig. 1C) (18, 21).
A recent study using electron microscopy reported that the PPL1 neurons form synapses directly onto APL neurons in the MB α lobe (26), providing independent structural evidence. Our observation of large overlapping regions between the presynaptic sites of PPL1 DA neurons and the postsynaptic sites of APL neurons, together with the strong GRASP signal in these regions, raised the possibility that PPL1 DA neurons form functional connections to APL neurons.
Functional Connections Between DA Neurons and APL Neurons.
We examined the presumed synaptic connections between these DA neurons and APL neurons using a newly developed syb:GRASP method (20). We constructed GH146-Gal4,TH-LexA/LexAop-syb:spGFP1-10,UAS-CD4:spGFP11;UAS-mCherry/+ flies (experimental group). In these flies, a vesicle-anchored form of GFP truncated protein, syb:spGFP1-10, was expressed in the DA neurons under the control of TH-LexA, and the rest the GFP protein with a membrane localization tag, CD4:spGFP11, was expressed in the APL neurons driven by GH146-Gal4 (Fig. 1D). The GH146-Gal4/LexAop-syb:spGFP1-10,UAS-CD4:spGFP11;UAS-mCherry/+ flies served as the control group. Compared with the signals in the control group, the syb:GRASP signals were significantly higher in the experimental group within the MB vertical lobes but differed little in the heel and horizontal lobes (Fig. 1 E and F and SI Appendix, Fig. S3). These results suggest that without induction, there is strong basal synaptic activity in the DA-to-APL synapses in the vertical lobe regions.
During aversive olfactory learning, the PPL1 DA neurons are responsible for mediating the ES signal (18, 21). Therefore, we treated the flies with ES and examined the syb:GRASP signals. After ES in the vertical lobe, the syb:GRASP signal was significantly increased (Fig. 1G and SI Appendix, Fig. S3). Although the syb:GRASP signals also increased in MB heel and horizontal lobes after ES, signals were relatively low compared with those in the vertical lobes (Fig. 1G). In contrast, signals for all three regions changed little on ES in the control groups (SI Appendix, Fig. S3). Taken together, these data confirm that TH-labeled DA neurons form synaptic connections with APL neurons, and these synapses at the stalks of MB vertical lobes display strong basal activity, which is further elevated on ES.
APL Neurons Directly Receive the DA Signal.
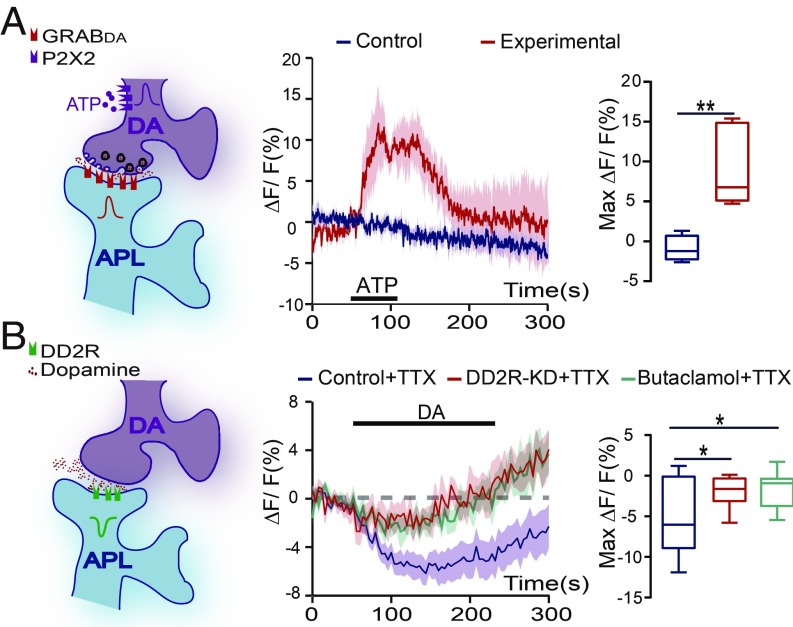
A previous study indicated that DA neurons may also release other neurotransmitters (27). To determine whether this synaptic regulation is dopaminergic, we used a newly developed genetic reporter, G protein-coupled receptor-activation–based DA sensor (GRABDA) (28). In GH146-Gal4,TH-LexA/+;UAS-GRABDA/LexAop-P2X2 flies, P2X2 was expressed in the PPL1 DA neurons, which can be activated by ATP perfusion (SI Appendix, Fig. S4). In addition, the GRABDA sensor was expressed in APL neurons, with the signal recorded at the transverse plane of the lower stalk region of MB vertical lobes (SI Appendix, Fig. S4). As shown in Fig. 2A, on ATP perfusion, the GRABDA signal was significantly increased in APL neurons. Thus, activation of these DA neurons induces release of dopamine in this DA-to-APL synapse, which is received by APL neurons.
Fig. 2.
APL neurons directly receive the DA signal. (A) GRABDA was significantly increased on ATP perfusion in APL neurons. The genotype was GH146-Gal4,TH-LexA/+;UAS-GRABDA/LexAop-P2X2 in the experimental group and GH146-Gal4,TH-LexA/+;UAS-GRABDA/+ in the control group. Images were recorded at the transverse plane of MB vertical lobe region. The ATP-induced signals during the platform period were averaged. n = 5 in each group. (B) Dopamine perfusion induced a calcium signal in APL neurons that was abolished by genetic KD of DD2R or its antagonist butaclamol hydrochloride. The genotype was GH146-Gal4,UAS-GCaMP3/+ in the control (n = 15) and butaclamol (n = 10) groups and GH146-Gal4,UAS-GCaMP3/UAS-DD2R-RNAi in the DD2R-KD group (n = 9). The calcium signals were recorded in the stalk of the vertical lobe and heel regions in the presence of TTX, and the response during dopamine perfusion was averaged. Data were quantified using the independent-samples t test and are presented in the boxplots. *P < 0.05; **P < 0.01.
We next examined whether the dopamine signal induces an excitatory or an inhibitory response in APL neurons using an in vivo calcium imaging approach. The calcium signals were recorded in the entire MB lobe region from the dorsal-anterior side of the fly head, and the dopamine perfusion status was determined by coperfused fluorescent dye (SI Appendix, Fig. S5A). Remarkably, on dopamine application, the calcium signal of APL neurons was significantly decreased in the stalk and heel regions of the vertical lobes (SI Appendix, Fig. S5B). In contrast, the signal in the tip region was significantly increased (SI Appendix, Fig. S5C).
To eliminate the dopamine-induced signals from other neurons, we used the neurotoxin tetrodotoxin (TTX) to block synaptic transmission (29), which was shown to be effective in the odor response experiments (SI Appendix, Fig. S6). After blocking, the increase in calcium signals at the APL tip region was abolished, whereas no difference in calcium signals in the stalk and heel regions was observed between the TTX-treated and untreated groups (SI Appendix, Fig. S5 B and C). We examined the calcium responses in the stalk and heel regions with different dopamine dosages. The results show that dopamine perfusion at concentrations of 100 μM and 1 mM induced significant decreases in calcium signals, while lower concentrations failed to induce any calcium response (SI Appendix, Fig. S5D). Thus, these results indicate that the APL neurons receive a direct suppressive dopamine signal at the stalk and heel regions of the MB.
Dopamine 2-Like Receptor Mediates Suppressive DA Signals in the DA-to-APL Synapse.
Mammalian dopamine 2-like receptor is an inhibitory dopamine receptor that down-regulates neural activity (30). DD2R has been reported to possess a conserved inhibitory function (31). Here we generated a polyclonal antibody against DD2R and detected the immunochemistry signals in APL neurons in both the cell body and projection regions (SI Appendix, Fig. S7). We next tested whether DD2R plays a role in the dopamine response of the APL neurons by knockdown (KD) of DD2R. A previous report (32) showed that mRNA levels of DD2R is reduced to 37%. As shown in SI Appendix, Fig. S8, we obtained similar results, as verified by quantitative PCR. In GH146-Gal4 > UAS-DD2R-RNAi flies, the dopamine response in the stalk and heel regions was significantly lower in APL neurons compared with wild-type flies (Fig. 2B). In agreement with these results, the dopamine response was nearly abolished when DD2R antagonist butaclamol hydrochloride (31) was added to the bath (Fig. 2B).
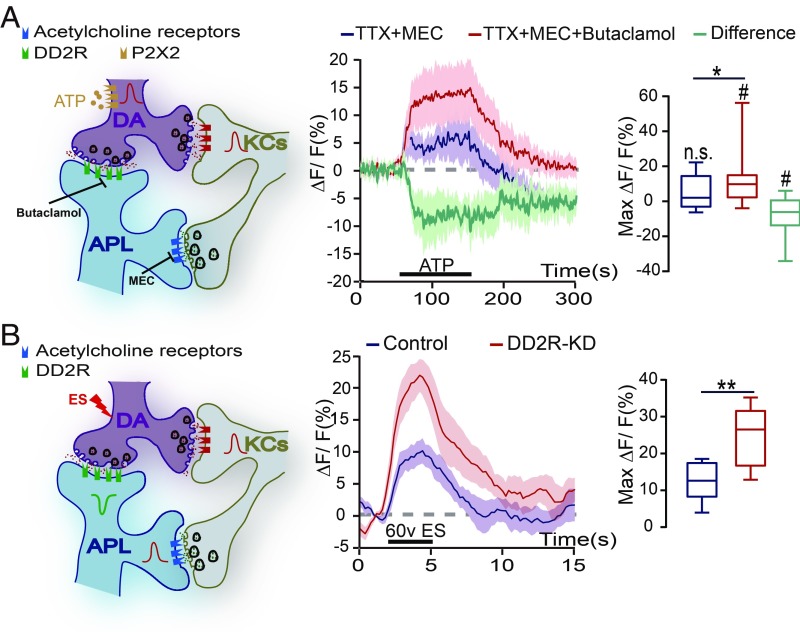
To examine the physiological response of APL neurons, we activated the TH-labeled DA neurons using the P2X2 method and recorded the calcium signals in the transverse plane of the MB lower stalk region (SI Appendix, Fig. S6). PPL1 DA neurons have been shown to have reciprocal connections with the KCs (33), and KCs activate APL neurons mainly through cholinergic signals (9). To block potential indirect effects, TTX and mecamylamide hydrochloride (MEC), a nicotinic acetylcholine receptor antagonist (26, 33), were added to the bath. In the of GH146-Gal4,TH-LexA/UAS-GCaMP6m;UAS-mCherry/LexAop-P2X2 flies, the calcium signal in APL neurons did not change significantly on ATP perfusion; however, this response was significantly elevated when butaclamol was added to block DD2R activity (Fig. 3A). The difference between the TTX+MEC and TTX+MEC+butaclamol groups showed a negative response, representing the dopamine signal mediated by DD2R (Fig. 3A). Together with the suppressive signals recorded in the dopamine perfusion experiments, these results suggest that APL neurons receive an inhibitory signal from DA neurons, which is mediated by DD2R.
Fig. 3.
D2-like receptor mediates a suppressive DA signal in the DA-to-APL synapses. (A) Diagram of the KC-DA-APL circuitry, with P2X2 expressed in the DA neurons. On ATP perfusion, the APL neurons displayed no significant change in the calcium signal (n = 13), while this signal significantly increased after the incubation of DD2R antagonist butaclamol (n = 13). Images were recorded at the transverse plane of MB vertical lobe region. Data were quantified by the Wilcoxon signed-rank test and are presented in the boxplot graphs. The green line indicates the difference in the response to ATP activation. The genotype was GH146-Gal4,TH-LexA/UAS-GCaMP6m;UAS-mCherry/LexAop-P2X2. Shown is the average calcium response during the response platform to ATP treatment. n.s. and # indicate the statistical differences in each group compared with 0 (one-sample t test). (B) On ES stimulation, the calcium signal in APL neurons increased in control flies, and this elevation was significantly higher in DD2R-KD flies. The genotypes were GH146-Gal4,UAS-GCaMP6m/+ in the control group (n = 7) and GH146-Gal4,UAS-GCaMP6m/UAS-DD2R-RNAi in the DD2R-KD group (n = 8). The calcium response of the APL neurons was recorded in the transverse plane of the MB vertical lobe region. The peak response was quantified by the independent-samples t test and presented in the boxplot graphs. n.s. indicates no significant difference. #P < 0.05; *P < 0.05; **P < 0.01.
We further investigated whether APL neurons were restrained by the DA signal on ES. During conditioning, the PPL1-DA signal represents the aversive ES stimulus (17, 18), and APL neurons are activated by ES as well (7). In agreement, we found that ES triggered an increase in the calcium signal in APL neurons (Fig. 3B). According to our hypothesis that DA neurons send a suppressive signal to APL neurons, we speculated that ES could induce a stronger response in APL neurons if DA inhibition were removed. To test this speculation, we examined the ES response in the APL neurons of DD2R-KD flies. Strikingly, we recorded a significant elevation in the calcium signal compared with that in wild-type flies (Fig. 3B). Taken together, our results indicate that the DA signal directly inhibits APL neurons through DD2R. Moreover, loss of this inhibition results in overactivation of this GABAergic neuron on ES stimulus.
DD2R-Go Signaling Is Required in APL Neurons for Olfactory Learning.
We next tested whether DD2R-mediated suppression in APL neurons plays a role in Drosophila aversive olfactory learning. Using the standard odor-ES conditioning paradigm (34), we found that the learning index of GH146-Gal4 > UAS-DD2R-RNAi flies was significantly impaired (Fig. 4B), and we found a similar result when a second, independent UAS-DD2R-RNAi fly line was used (SI Appendix, Fig. S9A). Along with APL neurons, GH146-Gal4 is also expressed in the antenna lobe projection neurons. Thus, we used two additional Gal4 lines (7, 13) to distinguish the function of these two groups of neurons (SI Appendix, Fig. S10). As shown in Fig. 4B, the learning index was decreased significantly when UAS-DD2R-RNAi was expressed with APL-positive NP2631-Gal4 but did not change with APL-negative NP225-Gal4 (35). As controls, the naïve responses to odors and ES of these flies were comparable to those of the parental control flies (SI Appendix, Table. S1).
Fig. 4.
The DD2R-Go signaling is required in APL neurons for efficient olfactory learning. (A) Schematic diagram of DD2R signaling in APL neurons. (B) Knockdown of DD2R in the APL neurons attenuated olfactory learning with APL-positive Gal4 lines of GH146 and NP2631, while no effect was observed with APL-negative Gal4 line NP225. n ≥ 6 for each group. (C) Heat induction of DD2R-KD in the APL neurons occurred specifically in adult-stage attenuated olfactory learning, while the learning index remained intact in the no-induction group. n ≥ 7 in each group. (D) The flies with Go-KD in APL neurons displayed impaired leaning index. Dicer2 was coexpressed with Go-RNAi to enhance knockdown efficiency. n ≥ 8 in each group. (E) Blockage of GABA synthesis by knockdown of GAD rescued the impaired olfactory learning induced by DD2R-KD. n ≥ 8 in each group. Data were quantified by one-way ANOVA followed by the Bonferroni post hoc test and are shown as mean ± SEM. ***P < 0.001.
To rule out developmental effects of DD2R-KD, we knocked down DD2R specifically in the adult stage using the TARGET method (36). In the heat-induction group, the GH146-Gal4/UAS-DD2R-RNAi;tub-Gal80ts/+ flies had a significantly lower learning index compared with their parental controls, while their siblings in the noninduction group performed normally (Fig. 4C). The efficiency of the TARGET method was validated by expressing mCD8::GFP as a reporter (SI Appendix, Fig. S9B). Taken together, our results indicate that loss of DD2R-mediated suppression in the APL neurons impairs olfactory learning physiologically rather than developmentally.
As illustrated in Fig. 4A, DD2R exerts its inhibitory function through coupling with the Gi/o protein in both mammals (30, 37) and Drosophila (31). Therefore, we tested whether Go is required in APL neurons to mediate the suppressive DA signal during learning. Knockdown of Go expression using the APL-positive strains GH146-Gal4 and NP2631-Gal4 led to impaired olfactory learning, while a normal learning index was observed when an APL-negative NP225-Gal4 strain was used (Fig. 4D). In addition, we used pertussis toxin (PTX), which is known to target Go protein specifically in Drosophila (38, 39). Similar to Go-KD, expression of PTX in APL neurons significantly decreased the learning index (SI Appendix, Fig. S9C). These results indicate that the DD2R-Go signaling in APL neurons is required for learning, possibly by reducing the GABAergic activity.
APL neurons negatively regulate olfactory learning due to GABAergic inhibition in KCs (7), and the coexpressed octopaminergic system has been shown to be uninvolved in the aversive olfactory learning (25). We then knocked down the GABA synthetase glutamate acid decarboxylase (GAD) in APL neurons by expressing UAS-GAD-RNAi with GH146-Gal4 or NP2631-Gal4. As shown in Fig. 4E, the reduction in learning on DD2R-KD was abolished when the GABA signal was impaired. Taken together, these behavioral results suggest that during aversive olfactory learning, DD2R-Go signaling in APL neurons is required for efficient conditioning, possibly through restraining the GABAergic inhibition in KCs.
The Odor Response of the APL Neurons Is Suppressed During Odor-ES Conditioning.
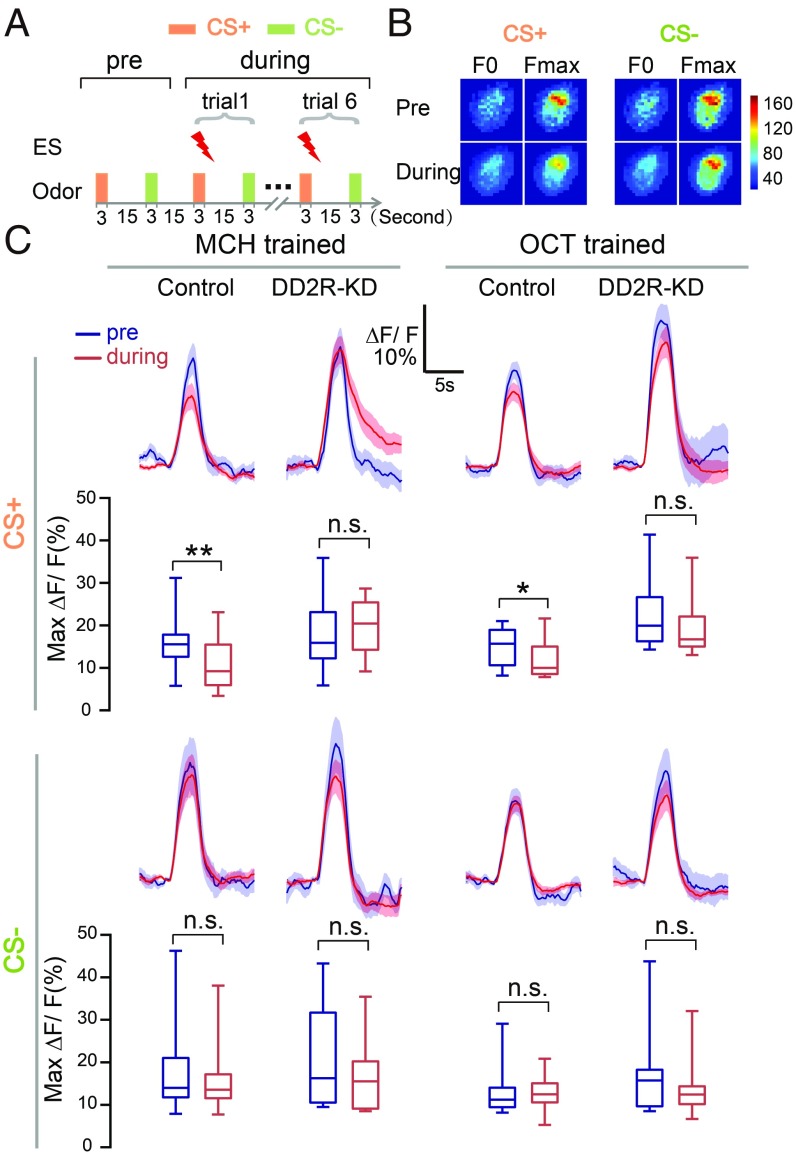
Our finding that the APL neurons receive direct DA suppression (as shown in Figs. 2 and 3) prompted us to test whether this suppression plays a role during conditioning. To monitor neural activities during conditioning, we designed a training paradigm for in vivo calcium imaging, consisting of interlaced 3 s of odor-ES coupling and 3 s of control odor at 15-s intervals, repeated six times (Fig. 5A). With this training paradigm, the wild-type Canton-S flies achieved effective learning in behavioral tests (SI Appendix, Fig. S11A). In the imaging experiments, the flies were dissected at the dorsal-posterior side of the head to ensure that their antennae remained intact, and calcium signals were recorded at the transverse plane of APL neurons in the lower stalk region of the MB vertical lobes (SI Appendix, Fig. S11B and Fig. 5B).
Fig. 5.
The APL response is reduced during odor-ES coupling in control flies but not in DD2R-KD flies. (A) The olfactory learning paradigm for in vivo calcium imaging. Conditioned odor (CS+), coupled with ES, and unconditioned odor (CS−) were sequentially presented with a 3 s-duration and a 15 s-interval. One trial was recorded in the preconditioning phase without the ES and repeated six times in the during-conditioning phase with odor-ES coupling. (B) Representative pseudocolor images of the peak odor responses in preconditioning and during-conditioning phases. The calcium signals were recorded in the transverse plane of the lower stalk of MB vertical lobes. Baseline fluorescence (F0) was calculated by averaging five frames before odor treatment. Maximum fluorescence (Fmax) was averaged from five frames flanking the maximum odor response. Data from the six trials in the during-conditioning phase were averaged. Pseudocolor images according to the F0 and Fmax are shown on a pixel-by-pixel basis. (C) Compared with that in preconditioning phase, the calcium activity of the APL neurons in response to the conditioned odor decreased during conditioning in control flies, a decrease not observed in DD2R-KD flies. The APL neurons showed an unchanged response to unconditioned odor in both control and DD2R-KD flies. The genotypes were GH146-Gal4,UAS-GCaMP6m/+ in the control group and GH146-Gal4,UAS-GCaMP6m/UAS-DD2R-RNAi in the DD2R-KD group. n = 12 in both control and DD2R-KD groups when MCH was used as the conditioned odor; n = 12 and 10 in control and DD2R-KD groups, respectively when OCT was used as the conditioned odor. The peak odor response was analyzed by the Wilcoxon signed-rank tests and is presented in the boxplots. n.s. indicates no significant difference. *P < 0.05; **P < 0.01.
When the odor 4-methylcyclohexanol (MCH) was coupled with ES treatment during conditioning, the calcium response of APL neurons to this conditioned odor was significantly reduced compared with that in the preconditioning phase (Fig. 5C). In contrast, APL neurons exhibited an unchanged response to the unconditioned odor 3-octanol (OCT). Similar results were obtained in parallel experiments in which OCT was coupled with the ES and MCH served as the control odor (Fig. 5C). Previous studies have shown that after conditioning, APL neurons exhibited a reduced response specifically to the conditioned, and not the unconditioned, odor (7). Our results presented here indicate that this specific modulation occurs during conditioning.
DD2R Is Essential for Suppression of APL Neurons During and After Odor-ES Conditioning.
Our results show that DD2R-KD resulted in an elevated ES response in APL neurons (Fig. 3B), accompanied by an impaired learning index (Fig. 4B). Therefore, we further examined whether DD2R is required for the suppressive regulation of APL neurons during odor-ES conditioning. In the DD2R-KD flies, there were no significant changes the calcium response of APL neurons during conditioning with either MCH or OCT coupled to the ES signal (Fig. 5C). For the nonconditioned odors, the response of APL neurons of the DD2R-KD flies were unchanged during conditioning, similar to that seen in parental control flies (Fig. 5C). Thus, DD2R-mediated DA suppression plays an essential role in the suppressive modulation of APL neurons during conditioning.
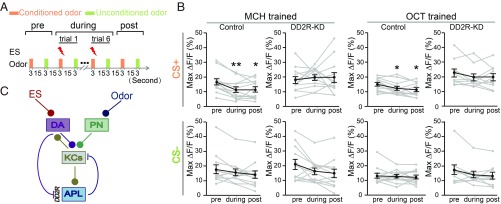
The decreased odor response in APL neurons at the postconditioning phase has been implied as a memory trace, possibly due to synaptic modification (7, 40). Thus, we asked whether DD2R is also required for this postconditioning change. As shown in Fig. 6 and SI Appendix, Fig. S12, the response in APL neurons was significantly reduced in both the during-conditioning and postconditioning phases to the conditioned odor, but not to the control odor. More importantly, compared with the preconditioning phase, the reduction observed in the postconditioning phase was also abolished in the DD2R-KD APL neurons. In both the preconditioning and postconditioning phases, only odor was supplied. Thus, our observation of no significant change in APL odor response in the DD2R-KD flies suggests that when DA-to-APL inhibition is absent, learning-induced synaptic modification is impaired in the circuit of KC-DA-APL. Taken together, our findings reveal that GABAergic APL neurons are regulated by suppressive DA signals, which is an important mechanism for restraining GABAergic inhibition as well as for circuit modulation during conditioning.
Fig. 6.
APL neurons show a decreased response to the conditioned odor in both the during-conditioning and postconditioning phases in control flies but not in DD2R-KD flies. (A) The olfactory learning paradigm for in vivo calcium imaging. ES was not delivered in either the preconditioning or postconditioning phase. The ES-coupled trial was repeated six times in the during-conditioning phase. (B) The odor response of the APL neurons decreased in both the during-conditioning and postconditioning phases when the odor was coupled with ES in the control flies. This suppressive effect was absent in both phases in DD2R-KD flies. Gray lines show odor responses of individual flies, and black lines show the mean responses. The animals used were the same as in Fig. 5. Data were quantified by the Wilcoxon signed-rank test and are shown as mean ± SEM. *P < 0.05; **P < 0.01. No significant difference was found between other groups. (C) Model of KC-DA-APL circuit functioning in odor-ES conditioning. In this circuit, the DA signal exerts a suppressive signal on the GABAergic APL neurons through DD2R, thereby restraining the APL-to-KC inhibition to secure efficient learning.
Discussion
How the neural information of learning and memory is processed is one of the most intriguing questions in neuroscience. The olfactory learning circuitry of Drosophila has been comprehensively investigated, providing an ideal system for studying the complex circuit mechanisms underlying learning and memory. In this study, we identified a synaptic connection from DA neurons to GABAergic APL neurons and characterized an inhibitory DA signal in this synapse mediated by DD2R. This DA inhibition restrains APL neurons during odor-ES conditioning, thereby releasing the GABAergic inhibition on KCs. Moreover, the DA-to-GABA connection is important for synaptic modification within the learning circuit, in which DD2R plays an essential function.
GABAergic neurons are the major inhibitory neurons in the nervous system, and different groups of GABAergic neurons may form connections to achieve disinhibition in associative learning (41). In the Drosophila circuit studied here, the GABAergic APL neurons have been found to be suppressed by learning processes (5); however, how this suppression is achieved remains unknown. Intriguingly, we found that the signal that restrains this GABAergic neuron comes directly from a group of excitatory DA neurons, which have been shown to be activated by ES (21, 42). We further show that the inhibitory DA receptor DD2R in APL neurons is required to mediate this DA inhibition. In mammalian systems, the GABAergic interneurons in the amygdala also have been reported to be suppressed by dopamine perfusion (4, 43). In addition, the inhibitory D2R also has been proposed to play a critical role in learning (4, 43). Our findings provide in vivo evidence of DA-to-GABA direct inhibition, and its function in disinhibition may serve a conserved circuit mechanism in associative learning during evolution.
In Drosophila, single neurons may use more than one type of neurotransmitter and/or neuropeptide (27). To identify the neurotransmitter used in the DA-to-APL synapse, we used a recently developed DA sensor (28) that allows us to distinguish the DA signal received by APL neurons. Notably, the calcium signal in APL neurons decreased significantly on dopamine perfusion, whereas this decrease was not observed when DA neurons were activated. Nevertheless, when the antagonist of DD2R was added, the DA response was significantly increased, indicating the suppressive DA signal. The different responses in these two experiments may be due to different dopamine concentrations between dopamine perfusion and the physiological activation of DA neurons.
It has been reported that D1R, an excitatory dopamine receptor, is activated in a low concentration of dopamine, whereas D2R responds only at a high concentration (44). A recent study reported that excitatory and inhibitory dopamine receptors are coexpressed in a GABAergic neuron and function oppositely (45). Our findings also suggest that both excitatory and inhibitory signals exist in the DA-to-GABA synapse, likely mediated by different types of dopamine receptors. The molecular dynamics of receptor expression, activation, distribution, and recycling may serve as important mechanisms for synaptic modification, thereby tuning the balance of circuitry.
In Drosophila olfactory learning, the change in odor response after conditioning is considered a sign of memory trace, which is believed to be a result of synaptic modification (7, 40). As shown in Fig. 6C, APL neurons receive input from KCs during conditioning. However, the changes in odor response differ in these two types of neurons, increased in the lobe (10, 11) and remaining unchanged in the calyx (46) of KCs but decreased in APL neurons (10). This difference suggests a modification occurring during learning in the KC-DA-APL circuit. One straightforward guess is that the synaptic strength of the KC-to-APL connection is reduced. As KCs have a reciprocal connection with DA neurons (33), it is also possible that the DA-to-APL synapse or the DA-KC bidirectional synapses are modified. These three types of neurons compose a complex recurrent network; thus, coupled stimuli likely affect every node inside to different degrees. Our findings show that DD2R-mediated DA inhibition in GABAergic neurons is an important regulatory mechanism for circuit modification, which contribute to neural plasticity in associative learning.
Materials and Methods
Fly Strains.
Flies were cultured on standard food (47) at 25 °C and 60% relative humidity and kept on a 12-h light/dark cycle. Mixed sexual flies were collected at eclosion and aged for 2–3 d. In the TARGET experiments (36), flies were raised at 18 °C and shifted to 31 °C after eclosion for heat induction for 3 d before the behavioral experiments. More details on fly strains are provided in SI Appendix, Materials and Methods.
Activity-Dependent syb:GRASP Experiments.
The ES treatment was performed in the training tube of the T-maze used for olfactory learning. Flies were separated at random into two groups, with one group exposed to the ES and the other group loaded into the same tube without the ES. The ES was applied by a series of 1.25-s 90-V pulses at 3.75-s intervals for a total of 5 h. Subsequently, the flies were dissected and imaged within 60 min with Z-stack scanning at 1.0-µm steps. Data were analyzed using ImageJ with the Measure Stack plugin. The mCherry signal served as the reference to mark the region of interest, with both mCherry and GFP signals measured in all slices containing the mCherry signal. The GFP/mCherry ratio was calculated to present the syb:GRASP signal.
Staining and Imaging of Fly Brains.
According to a standard immunohistochemistry protocol (48), brains of adult flies were dissected and subjected to fixation, blocking, and antibody staining. Primary antibodies against GABA, FasII, and DD2R were used in this study (SI Appendix, Materials and Methods). Z-stack scans were collected at 0.4-µm steps.
In Vivo Functional Imaging.
Adult (2–6 d old) female flies were fixed in the hole of a perfusion chamber, with a window opened on the head cuticle (49). For the dopamine perfusion experiments, fluorescence signals of GCaMP3 were recorded from the dorsal-anterior side of the head at a rate of 4 s per frame (Fig. 2B and SI Appendix, Fig. S5). For other functional imaging experiments using GCaMP6m or GRABDA1m as the reporter, images were acquired from the dorsal-posterior side at a rate of 5 frames/s. Dopamine (1 mM) and ATP (1 mM) were applied by perfusion, and TTX (1 μM), butaclamol (100 μM), and MEC (500 μM) were applied by incubation. For imaging during learning, the antennae were kept intact for odor sensing. The ES (60 V) was applied directly to the fly abdomens via two steel wires (50).
Image Analysis.
All images were collected using a Leica SP5 II confocal microscope with a 20× water objective (NA 1.00). In GRASP and staining experiments, images were analyzed using Image J. For functional imaging experiments, Leica LAS-AF software was used for selecting regions of interest and quantifying fluorescence intensity. Imaging traces were smoothed using MATLAB software (MathWorks). The ΔF/F was calculated by dividing the change of fluorescence (ΔF) by the baseline fluorescence (F). Pseudocolor images according to the F0 and Fmax were shown on a pixel-by-pixel basis.
Behavioral Assays.
Olfactory aversive learning and memory experiments were performed according to the standard T-maze paradigm (34). In brief, a group of approximately 100 mixed-sex flies were exposed to odor A accompanied by ES for 1 min, followed after 45 s by exposure to odor B for 1 min, and then tested for 2 min. The learning index was calculated by averaging the preference index of two reciprocally trained groups.
Statistical Analysis.
SPSS 17.0 was used for data analysis. In behavioral experiments, one-way ANOVA was performed, followed by the Bonferroni post hoc test for comparison among multiple groups, and the independent-samples t test was used for comparisons between two groups. For functional imaging experiments, data were tested for normality and then analyzed with a parametric (independent-samples t test) or nonparametric (Wilcoxon signed-rank test for comparison between matched samples) test, as appropriate.
Supplementary Material
Acknowledgments
We thank Drs. J. Hirsh, L. Liu, L. L. Looger, K. Scott, and Z. Wang, as well as the Bloomington Drosophila Stock Center, Vienna Drosophila RNAi Center, and Drosophila Genetic Resource center for fly stocks. We also thank Z. Zhang and Z. Wu for discussions on the manuscript; Z. Lei, J. Hou, and Y. Chen for technical assistance; and language editor T. Juelich (Peking University) for linguistic work on the manuscript. This work was supported by the National Natural Science Foundation of China (Grants 31730045, 91632301, 31571089, and 91132709), the Chinese Academy of Sciences Interdisciplinary Innovation Team, Strategic Priority Research Program (XDB32010100), and Shanghai Municipal Science and Technology Major Project 2018SHZDZX05.
Footnotes
This article is a PNAS Direct Submission. R.L.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812342116/-/DCSupplemental.
References
- 1.Ehrlich I, et al. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Shumyatsky GP, et al. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- 3.Wolff SB, et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- 4.Bissière S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- 5.Qi C, Lee D. Pre- and postsynaptic role of dopamine D2 receptor DD2R in Drosophila olfactory associative learning. Biology (Basel) 2014;3:831–845. doi: 10.3390/biology3040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholz-Kornehl S, Schwärzel M. Circuit analysis of a Drosophila dopamine type 2 receptor that supports anesthesia-resistant memory. J Neurosci. 2016;36:7936–7945. doi: 10.1523/JNEUROSCI.4475-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Davis RL. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci. 2009;12:53–59. doi: 10.1038/nn.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 9.Lin AC, Bygrave AM, de Calignon A, Lee T, Miesenböck G. Sparse, decorrelated odor coding in the mushroom body enhances learned odor discrimination. Nat Neurosci. 2014;17:559–568. doi: 10.1038/nn.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Mamiya A, Chiang AS, Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci. 2008;28:4368–4376. doi: 10.1523/JNEUROSCI.2958-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boto T, Louis T, Jindachomthong K, Jalink K, Tomchik SM. Dopaminergic modulation of cAMP drives nonlinear plasticity across the Drosophila mushroom body lobes. Curr Biol. 2014;24:822–831. doi: 10.1016/j.cub.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu CL, et al. Heterotypic gap junctions between two neurons in the Drosophila brain are critical for memory. Curr Biol. 2011;21:848–854. doi: 10.1016/j.cub.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 13.Pitman JL, et al. A pair of inhibitory neurons are required to sustain labile memory in the Drosophila mushroom body. Curr Biol. 2011;21:855–861. doi: 10.1016/j.cub.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 15.Keene AC, Krashes MJ, Leung B, Bernard JA, Waddell S. Drosophila dorsal paired medial neurons provide a general mechanism for memory consolidation. Curr Biol. 2006;16:1524–1530. doi: 10.1016/j.cub.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Keene AC, et al. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron. 2004;44:521–533. doi: 10.1016/j.neuron.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Riemensperger T, Völler T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 18.Claridge-Chang A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinberg EH, et al. GFP reconstitution across synaptic partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Macpherson LJ, et al. Dynamic labelling of neural connections in multiple colours by trans-synaptic fluorescence complementation. Nat Commun. 2015;6:10024. doi: 10.1038/ncomms10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: Anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 23.Masuda-Nakagawa LM, Ito K, Awasaki T, O’Kane CJ. A single GABAergic neuron mediates feedback of odor-evoked signals in the mushroom body of larval Drosophila. Front Neural Circuits. 2014;8:35. doi: 10.3389/fncir.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aso Y, et al. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu CL, Shih MF, Lee PT, Chiang AS. An octopamine-mushroom body circuit modulates the formation of anesthesia-resistant memory in Drosophila. Curr Biol. 2013;23:2346–2354. doi: 10.1016/j.cub.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 26.Takemura SY, et al. A connectome of a learning and memory center in the adult Drosophila brain. eLife. 2017;6:e26975. doi: 10.7554/eLife.26975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trudeau LE, et al. The multilingual nature of dopamine neurons. Prog Brain Res. 2014;211:141–164. doi: 10.1016/B978-0-444-63425-2.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun F, et al. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell. 2018;174:481–496.e19. doi: 10.1016/j.cell.2018.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CH, Ruben PC. Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels. 2008;2:407–412. doi: 10.4161/chan.2.6.7429. [DOI] [PubMed] [Google Scholar]
- 30.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type-specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 31.Hearn MG, et al. A Drosophila dopamine 2-like receptor: Molecular characterization and identification of multiple alternatively spliced variants. Proc Natl Acad Sci USA. 2002;99:14554–14559. doi: 10.1073/pnas.202498299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen B, Liu H, Ren J, Guo A. Mutation of Drosophila dopamine receptor DopR leads to male-male courtship behavior. Biochem Biophys Res Commun. 2012;423:557–563. doi: 10.1016/j.bbrc.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Cervantes-Sandoval I, Phan A, Chakraborty M, Davis RL. Reciprocal synapses between mushroom body and dopamine neurons form a positive feedback loop required for learning. eLife. 2017;6:e23789. doi: 10.7554/eLife.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 35.Thum AS, Jenett A, Ito K, Heisenberg M, Tanimoto H. Multiple memory traces for olfactory reward learning in Drosophila. J Neurosci. 2007;27:11132–11138. doi: 10.1523/JNEUROSCI.2712-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 37.Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc Natl Acad Sci USA. 2001;98:3577–3582. doi: 10.1073/pnas.051632598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thambi NC, Quan F, Wolfgang WJ, Spiegel A, Forte M. Immunological and molecular characterization of Go alpha-like proteins in the Drosophila central nervous system. J Biol Chem. 1989;264:18552–18560. [PubMed] [Google Scholar]
- 39.Yi W, et al. A subset of cholinergic mushroom body neurons requires Go signaling to regulate sleep in Drosophila. Sleep (Basel) 2013;36:1809–1821. doi: 10.5665/sleep.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Letzkus JJ, Wolff SB, Lüthi A. Disinhibition, a circuit mechanism for associative learning and memory. Neuron. 2015;88:264–276. doi: 10.1016/j.neuron.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 42.Cohn R, Morantte I, Ruta V. Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila. Cell. 2015;163:1742–1755. doi: 10.1016/j.cell.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu HY, Ito W, Li J, Morozov A. Target-specific suppression of GABA release from parvalbumin interneurons in the basolateral amygdala by dopamine. J Neurosci. 2012;32:14815–14820. doi: 10.1523/JNEUROSCI.2997-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang J, Marty VN, Mulpuri Y, Olsen RW, Spigelman I. Selective modulation of GABAergic tonic current by dopamine in the nucleus accumbens of alcohol-dependent rats. J Neurophysiol. 2014;112:51–60. doi: 10.1152/jn.00564.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlowsky A, Schor J, Placais PY, Preat T. A GABAergic feedback shapes dopaminergic input on the Drosophila mushroom body to promote appetitive long-term memory. Curr Biol. 2018;28:1783–1793.e4. doi: 10.1016/j.cub.2018.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hige T, Aso Y, Modi MN, Rubin GM, Turner GC. Heterosynaptic plasticity underlies aversive olfactory learning in Drosophila. Neuron. 2015;88:985–998. doi: 10.1016/j.neuron.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo A, et al. Conditioned visual flight orientation in Drosophila: Dependence on age, practice, and diet. Learn Mem. 1996;3:49–59. doi: 10.1101/lm.3.1.49. [DOI] [PubMed] [Google Scholar]
- 48.Wu JS, Luo L. A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat Protoc. 2006;1:2110–2115. doi: 10.1038/nprot.2006.336. [DOI] [PubMed] [Google Scholar]
- 49.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- 50.Zhang S, Roman G. Presynaptic inhibition of gamma lobe neurons is required for olfactory learning in Drosophila. Curr Biol. 2013;23:2519–2527. doi: 10.1016/j.cub.2013.10.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.