Group membership shapes how we interact with others. Individuals tend to conform more to ingroup members than to outgroup members (1, 2). In PNAS, Lin et al. (3) report an intergroup conformity neural network that tracked the social influence of ingroup over outgroup members. However, we question the researchers’ interpretation of the findings.
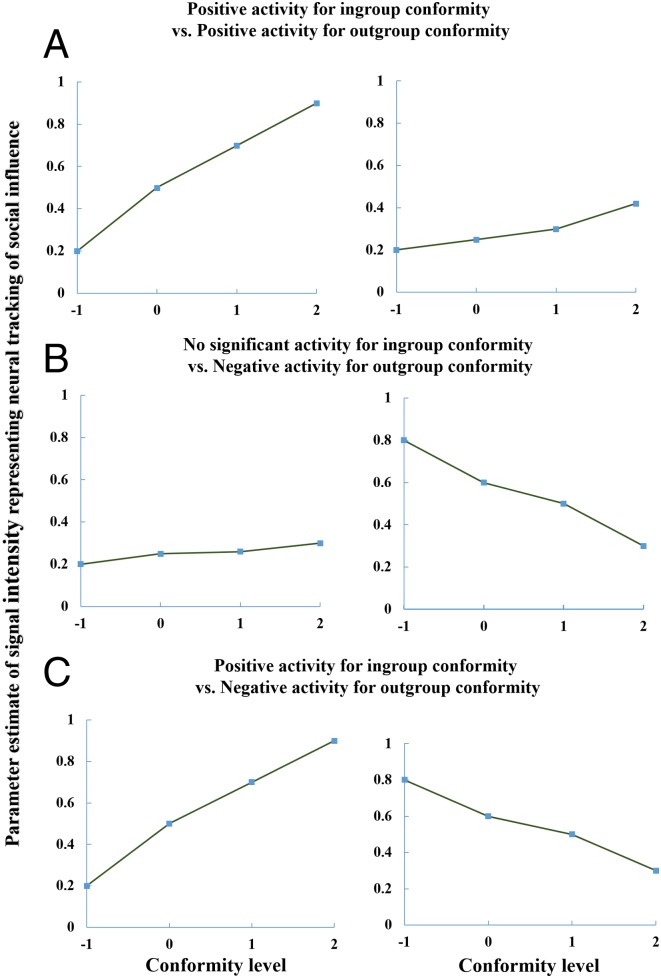
Lin et al. (3) report “ingroup > outgroup conformity” activity, but not neural tracking of ingroup conformity and outgroup activity separately. The parametric design allowed us to reexamine the brain regions that were positively or negatively correlated with ingroup and outgroup conformity scores independently, before contrasting the two conditions. This is an important point because, as shown in our schematic illustration (Fig. 1), even when a region such as the ventral striatum (VS) tracks the degree of outgroup conformity in the opposite direction, the ingroup > outgroup contrast can still result in similar positive activity. The different patterns speak to a critical question regarding intergroup interactions: Is the way we interact with outgroup members quantitatively or qualitatively different from how we react to ingroup members? A quantitative difference would predict similar neural patterns (4), but the degree of activity would differ between ingroup and outgroup (Fig. 1A) (i.e., an ordinal interaction in which both slopes have the same sign but differ significantly in strength). However, a qualitative difference would predict fundamentally different or even opposite neural activity patterns in ingroup and outgroup (Fig. 1 B and C) (5) (i.e., a disordinal interaction in which the slopes have opposite signs).
Fig. 1.
Schematic illustration of different brain activity patterns tracking the amount of social influence in the ingroup and outgroup conditions quantitatively (A) or qualitatively (B and C). All these ordinal and disordinal interaction patterns can result in similar positive activity for the ingroup > outgroup conformity effect contrast (a difference score).
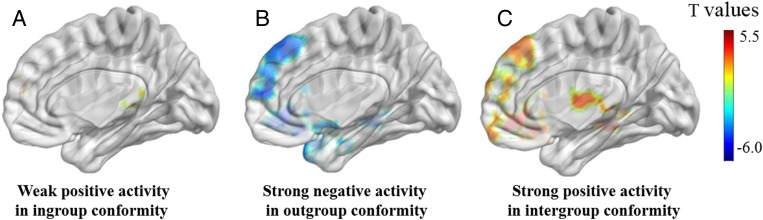
In the Lin et al. (3) paper, degree of ingroup conformity is positively correlated with activity in the dorsolateral prefrontal cortex (Fig. 2A), and outgroup conformity is not positively associated with activity in any brain regions. Surprisingly, degree of outgroup conformity is negatively associated with activity in brain regions that are claimed to be the neural signatures of the ingroup > outgroup conformity effect (Fig. 2 B and C). The explanations regarding the specific functions of brain regions involved in intergroup conformity need substantial revision. For example, VS activity is interpreted as reflecting increased rewarding experience when conforming with others. However, the VS is negatively correlated with outgroup conformity, which contradicts the literature showing that VS is activated by consensus vs. conflicts (6, 7). Such activity pattern may suggest outgroup derogation rather than ingroup favoritism at the neural level. For instance, when deviating more from outgroup opinions, individuals may feel more rewarded, engage higher levels of mentalizing, and be more aroused.
Fig. 2.
Neural regions that tracked ingroup conformity (A), outgroup conformity (B), and ingroup > outgroup conformity effect (C). It is important to note that in Lin et al.’s (3) study, the degree of conformity to outgroups was actually negatively correlated with brain regions implicated in positive valuation, mentalizing, emotion processing, and salience detection. No such negative correlation was found in the ingroup condition. All statistical maps, plotted using Lin et al.’s data on NeuroVault (https://neurovault.org/collections/RHJBYKGH/), are thresholded at P < 0.005 uncorrected with a minimum cluster size of 231 voxels. Data were 3D-rendered on the brain surface of the ICBM-152 T1 template in MNI space, as generated by BrainNet Viewer (https://www.nitrc.org/projects/bnv/).
It is noteworthy that one feature of Lin et al.’s (3) study is that it does not include participants’ initial ratings in the same session as the group ratings. This may cause the social conflicts elicited by the discrepancy between personal attitudes and group attitudes to be less salient than in previous studies (7, 8) and may explain the lack of brain activity that is positively correlated with conformity in both conditions.
Intergroup discrimination can be the result of ingroup favoritism, outgroup derogation, or some combination of both (9). Given the widespread use of difference scores in behavioral and neuroscience research, we emphasize that difference scores can mask important mechanisms. Response patterns in each condition need to be reported and explained (10).
Acknowledgments
We acknowledge the correspondence from Lin et al. (3) that helped to clarify this Letter, including the addition of Fig. 1C. This work was supported by the Singapore Ministry of Education Tier 2 Grant MOE2016-T2-1-015, the Social Science Research Thematic Grant MOE2017-SSRTG-026, and the Singapore National Medical Research Council Grant OFYIRG17may052 (to R.Y.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Huang Y, Kendrick KM, Zheng H, Yu R. Oxytocin enhances implicit social conformity to both in-group and out-group opinions. Psychoneuroendocrinology. 2015;60:114–119. doi: 10.1016/j.psyneuen.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Stallen M, De Dreu CK, Shalvi S, Smidts A, Sanfey AG. The herding hormone: Oxytocin stimulates in-group conformity. Psychol Sci. 2012;23:1288–1292. doi: 10.1177/0956797612446026. [DOI] [PubMed] [Google Scholar]
- 3.Lin LC, Qu Y, Telzer EH. Intergroup social influence on emotion processing in the brain. Proc Natl Acad Sci USA. 2018;115:10630–10635. doi: 10.1073/pnas.1802111115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mobbs D, et al. A key role for similarity in vicarious reward. Science. 2009;324:900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izuma K, Adolphs R. Social manipulation of preference in the human brain. Neuron. 2013;78:563–573. doi: 10.1016/j.neuron.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H, Luo Y, Feng C. Neural signatures of social conformity: A coordinate-based activation likelihood estimation meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev. 2016;71:101–111. doi: 10.1016/j.neubiorev.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 7.Klucharev V, Hytönen K, Rijpkema M, Smidts A, Fernández G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61:140–151. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Kendrick KM, Yu R. Social conflicts elicit an N400-like component. Neuropsychologia. 2014;65:211–220. doi: 10.1016/j.neuropsychologia.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Messick DM, Mackie DM. Intergroup relations. Annu Rev Psychol. 1989;40:45–81. doi: 10.1146/annurev.ps.40.020189.000401. [DOI] [PubMed] [Google Scholar]
- 10.Edwards JR. The fallacy of formative measurement. Organ Res Methods. 2011;14:370–388. [Google Scholar]