Significance
Platelets are professional hemostatic and immune cells. Integrin αIIbβ3 is required for fibrinogen-dependent platelet aggregation, but its role in immune/inflammatory responses is poorly understood. We identified interactions between αIIbβ3 and SHARPIN, a requisite member of the linear ubiquitin chain assembly complex (LUBAC), which mediates Met1 ubiquitination of signaling proteins. Using human platelets and megakaryocyte-lineage cells derived from human induced pluripotent stem cells, we find that SHARPIN is expressed in platelets and associates either with αIIb to suppress αIIbβ3 activation or with LUBAC to promote Met1 ubiquitination and NF-κB activation. Knockdown of SHARPIN in the megakaryocyte/platelet lineage not only primes αIIbβ3 for fibrinogen binding, but also increases MHC class I presentation and proinflammatory sCD40L release. Thus, SHARPIN functions at the nexus of αIIbβ3 and immune/inflammatory signaling in platelets.
Keywords: integrin, platelet, LUBAC, hemostasis, inflammation
Abstract
Platelets mediate primary hemostasis, and recent work has emphasized platelet participation in immunity and inflammation. The function of the platelet-specific integrin αIIbβ3 as a fibrinogen receptor in hemostasis is well defined, but the roles of αIIbβ3 or integrin-associated proteins in nonhemostatic platelet functions are poorly understood. Here we show that human platelets express the integrin-associated protein SHARPIN with functional consequences. In leukocytes, SHARPIN interacts with integrin α cytoplasmic tails, and it is also an obligate member of the linear ubiquitin chain assembly complex (LUBAC), which mediates Met1 linear ubiquitination of proteins leading to canonical NF-κB activation. SHARPIN interacted with αIIb in pull-down and coimmunoprecipitation assays. SHARPIN was partially localized, as was αIIbβ3, at platelet edges, and thrombin stimulation induced more central SHARPIN localization. SHARPIN also coimmunoprecipitated from platelets with the two other proteins comprising LUBAC, the E3 ligase HOIP and HOIL-1. Platelet stimulation with thrombin or inflammatory agonists, including lipopolysaccharide or soluble CD40 ligand (sCD40L), induced Met1 linear ubiquitination of the NF-κB pathway protein NEMO and serine-536 phosphorylation of the p65 RelA subunit of NF-κB. In human megakaryocytes and/or platelets derived from induced pluripotent stem (iPS) cells, SHARPIN knockdown caused increased basal and agonist-induced fibrinogen binding to αIIbβ3 as well as reduced Met1 ubiquitination and RelA phosphorylation. Moreover, these SHARPIN knockdown cells exhibited increased surface expression of MHC class I molecules and increased release of sCD40L. These results establish that SHARPIN functions in the human megakaryocyte/platelet lineage through protein interactions at the nexus of integrin and immune/inflammatory signaling.
Platelets are primary cellular mediators of hemostasis, and recent work has established their key roles in immune and inflammatory responses (1). Integrin αIIbβ3 and its structural shift to an active conformation on platelet stimulation are essential for normal, fibrinogen-dependent platelet aggregation during hemostasis. αIIbβ3 activation is regulated by interactions of the β3 cytoplasmic tail with adapter proteins, such as talin and kindlin-3 (2). Proteins that may regulate αIIbβ3 activation through interactions with the αIIb cytoplasmic tail have been less well studied (3).
SHANK-Associated RH Domain Interactor (SHARPIN) is a ∼40-kDa protein that binds α1 and α2 integrin tails at conserved membrane-proximal residues (W/yKXGFFKR), thereby inhibiting β1 and β2 integrin activation in leukocytes (4, 5). SHARPIN, together with HOIL-1 and the E3 ubiquitin ligase HOIP, form the linear ubiquitin chain assembly complex (LUBAC) (6), and interactions of SHARPIN with α integrins and HOIP are mutually exclusive (7).
LUBAC is involved in inflammatory and prosurvival signaling in leukocytes, in part by catalyzing Met1-polyubiquitination of NEMO (IKKγ), thereby leading to canonical activation of NF-κB (6, 8). The three proteins composing LUBAC have overlapping and distinct roles, as exemplified by the proinflammatory phenotypes of mouse knockouts (9). Humans with mutations in HOIL-1 or HOIP exhibit deregulation of NF-κB in lymphoma and autoinflammation, respectively (9), and mutations in OTULIN, a specific Met1 deubiquitinase, cause an autoinflammatory syndrome (10).
SHARPIN overexpression in humans is observed in a number of solid tumors (https://www.proteinatlas.org/ENSG00000179526-SHARPIN/pathology), and SHARPIN null mice exhibit severe dermatitis, system-wide organ inflammation, and ineffective secondary lymphoid development (11–13). Although dermatitis is rescued by TNF ablation, lymphocyte-independent mechanisms appear to drive residual systemic inflammation (14). SHARPIN also cooperates with HOIL-1 to support embryogenesis and hematopoiesis (15). Thus, SHARPIN is implicated in mammalian physiology and pathophysiology.
Platelets and their precursor megakaryocytes express many of the proteins involved in canonical NF-κB signaling (16), but neither SHARPIN nor LUBAC has been studied in these cells. Here we establish that human platelets and megakaryocytes express SHARPIN, which interacts with the integrin αIIb subunit to dampen αIIbβ3 activation and with LUBAC to promote Met1 polyubiquitination and NF-κB pathway signaling in activated cells. Furthermore, SHARPIN knockdown in megakaryocytes or platelets derived from human iPS cells promotes αIIbβ3 activation, increased surface expression of MHC class I molecules and release of the cytokine, sCD40L. Consequently, SHARPIN may play a coordinating role in the adhesive and immune/inflammatory functions of cells of the megakaryocyte/platelet lineage.
Results
SHARPIN Associates with Platelet Integrin αIIb and with HOIP/HOIL-1 to Form LUBAC.
SHARPIN expression was detected in human platelets on Western blot analysis (Fig. 1A). The two other essential components of LUBAC, HOIP and HOIL-1, were also detected, as were the Met1-specific deubiquitinase (DUB) OTULIN (17) and SPATA2, which collaborates with the Met1, K63 DUB CYLD (Fig. 1B) (17, 18). Additional components of the canonical pathway to NF-κB activation potentially downstream of LUBAC were expressed as well (Fig. 1C) (16).
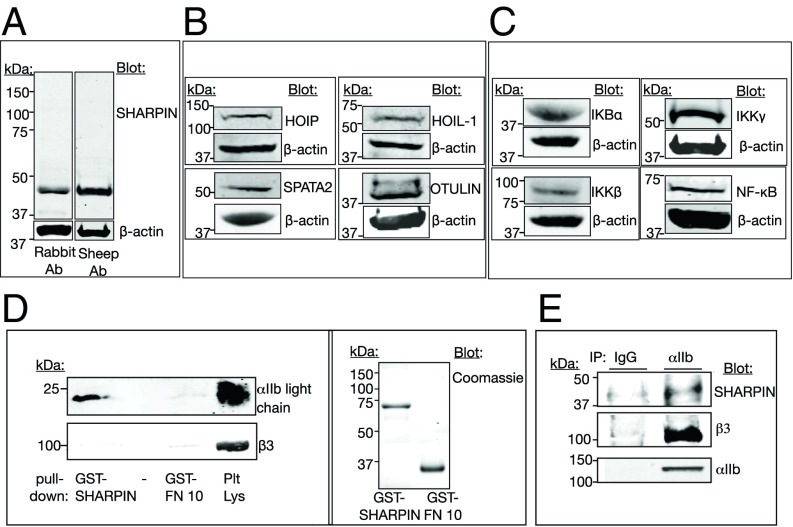
Fig. 1.
SHARPIN, LUBAC, and NF-κB pathway proteins are expressed in human platelets. (A) Lysate from unstimulated peripheral blood platelets was run on SDS-polyacrylamide gels, and protein expression was detected on Western blots. (A) SHARPIN was detected with antibodies raised in either rabbit (Left lane) or sheep (Right lane) hosts. (B) Expression of LUBAC members HOIP and HOIL-1 as well as SPATA2 and OTULIN, two proteins involved in Met1 deubiquitination. (C) Expression of NF-κB pathway proteins. Each protein depicted is of the expected molecular size, and β-actin reprobes from the same gels are shown. (D and E) SHARPIN associates with the integrin αIIb subunit. (D) Pulldowns in RIPA buffer using GST-SHARPIN or an irrelevant fusion protein (GST-FN10) as bait were probed with antibodies against integrin αIIb light chain or β3, under reducing gel conditions (Left), and then 5% of each total pulldown sample was used to assess protein loading on gels stained with Coomassie brilliant blue (Right). (E) SHARPIN coimmunoprecipitates with platelet αIIb. Nonidet P-40 lysate from unstimulated platelets was immunoprecipitated with an antibody to αIIb or with a nonspecific IgG and probed for SHARPIN, β3, and αIIb.
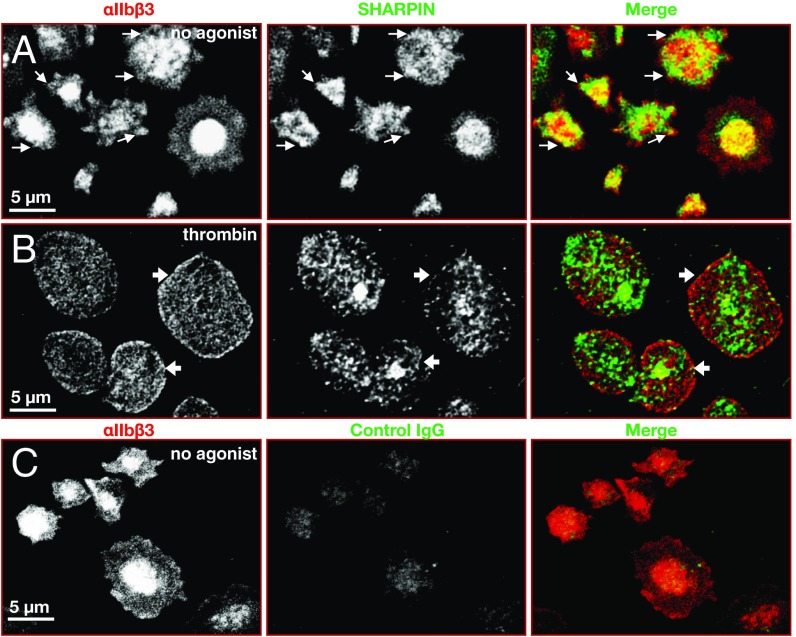
Since SHARPIN interacts with the conserved membrane-proximal region of some integrin α tails (4), SHARPIN interaction with αIIb was tested. GST-SHARPIN specifically pulled down the αIIb light chain, but not β3, from human platelets solubilized in RIPA buffer (Fig. 1D). Furthermore, an antibody specific for the αIIb tail coimmunoprecipitated SHARPIN from platelets solubilized in Nonidet P-40 lysis buffer, conditions under which αIIb remains complexed with β3 (Fig. 1E). SHARPIN expression in platelets was detectable by confocal super-resolution microscopy at a resolution of 120 nm. SHARPIN localization appeared to change on platelet activation. In unstimulated, fibrinogen-adherent platelets, SHARPIN localized in part at platelet edges, as did αIIbβ3 (Fig. 2A, arrows). However, in platelets that had spread after thrombin stimulation, the relative distribution of αIIbβ3 and SHARPIN changed and SHARPIN appeared to adopt a more centralized localization (Fig. 2B). Taken together, these findings indicate that SHARPIN can associate in vitro with αIIbβ3 via αIIb, and a pool of SHARPIN may localize to platelet edges, particularly in unstimulated cells.
Fig. 2.
Localization of αIIbβ3 and SHARPIN in human platelets. Shown are confocal super-resolution immunofluorescence images of human platelets adherent to fibrinogen, at 100× magnification. Unstimulated (A) or 0.5 U/mL thrombin-stimulated (B) platelets were fixed and stained with antibodies against αIIbβ3, SHARPIN, or (C) control IgG as indicated. Edge localization of SHARPIN and αIIbβ3 was apparent in unstimulated platelets (arrows), but edge localization of SHARPIN appeared to be reduced in thrombin-stimulated platelets (arrowheads). Experiments were conducted three times, with similar results.
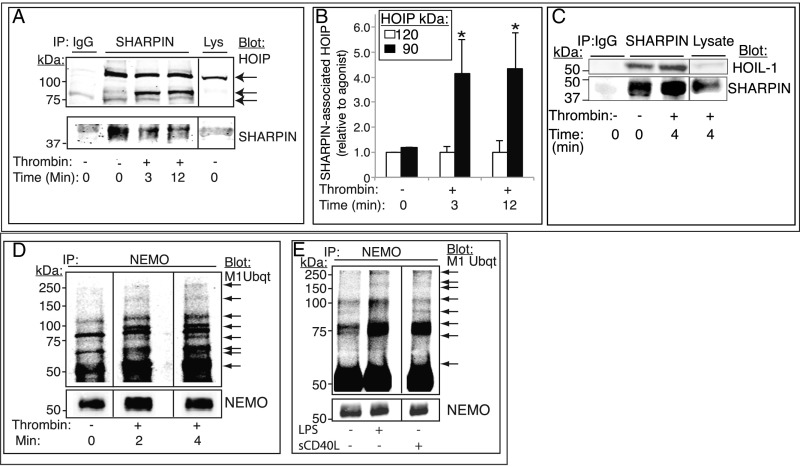
Met1 polyubiquitination of NEMO by LUBAC is important for NF-κB activation in leukocytes (6). To address whether LUBAC assembles in platelets, SHARPIN was immunoprecipitated from platelets, and the immunoprecipitate was probed with an antibody to the HOIP carboxyl-terminal LDD domain. Full-length HOIP (∼123 kDa; Fig. 3 A and B) and HOIL-1 (Fig. 3C) associated with SHARPIN in resting platelets, and thrombin stimulation resulted in increased SHARPIN association with HOIP, including a newly cleaved ∼90-kDa immunoreactive HOIP band (Fig. 3 A and B). Consistent with a functional LUBAC in platelets, thrombin stimulation caused rapid Met1 linear ubiquitination of NEMO, as detected with either of two antibodies specific for linear ubiquitin, and this was reflected by new and/or increased-intensity Met1 ubiquitin-positive bands in NEMO immunoprecipitates (19, 20) (Fig. 3D, arrows). Similar results were obtained when platelets were stimulated by the inflammatory mediators lipopolysaccharide (LPS) or sCD40L instead of thrombin (Fig. 3E). Thus, SHARPIN associates with αIIb or with HOIP/HOIL-1 in human platelets to form LUBAC, with the latter complex mediating Met1 linear ubiquitination during platelet activation in response to hemostatic or inflammatory agonists.
Fig. 3.
LUBAC-mediated Met1 ubiquitination of NEMO and NF-κB pathway signaling are triggered in platelets by hemostatic and inflammatory agonists. (A–C) SHARPIN association with HOIP (A and B) and HOIL-1 (C) was analyzed in SHARPIN immunoprecipitates from human platelets that had been incubated with vehicle or thrombin (1 U/mL). (A) Western blot analysis with an antibody specific for the C-terminal LDD domain of HOIP revealed a full-length 120-kDa band under all incubation conditions and additional 90-kDa cleavage bands in thrombin-stimulated platelets. (B) Extent of HOIP associated with SHARPIN, determined by densitometry and normalized to SHARPIN intensity (n = 4; mean ± SEM; *P < 0.05). (D and E) Met1 linear ubiquitination of NEMO. Platelets were incubated in the absence or presence of 1 U/mL thrombin for 0–4 min (D) or with either 1 μg/mL LPS or 1 μg/mL sCD40L for 4 min (E). Lysates were immunoprecipitated with an anti-NEMO antibody and probed on Western blots for Met1 ubiquitin. Arrows indicate new or more intense Met1 ubiquitin bands of variable molecular sizes following agonist stimulation. All samples within a panel were run on the same gel, and an 8% aliquot of each immunoprecipitation sample was run separately to determine gel loading. The data shown are representative of at least three separate experiments.
SHARPIN Knockdown in Cells of the Human Megakaryocyte/Platelet Lineage.
To begin to assess SHARPIN’s function in the megakaryocyte/platelet lineage, human megakaryocytes and platelets were generated in culture from iPS cells through a genetically tractable progenitor intermediate (21) (Fig. 4A). Differentiated megakaryocytes appeared as characteristically large polyploid cells (Fig. 4 B and C) expressing αIIbβ3 (SI Appendix, Fig. S1) and increased levels of key integrin-regulatory proteins, including Rap1b, talin, and kindlin-3 (2) (Fig. 4D). Extended differentiation in culture yielded platelet-like particles, with surface αIIbβ3 and GPIbα levels comparable to those of human donor platelets (SI Appendix, Fig. S2), and expressing megakaryocyte lineage-restricted β1-tubulin (22) (SI Appendix, Fig. S3).
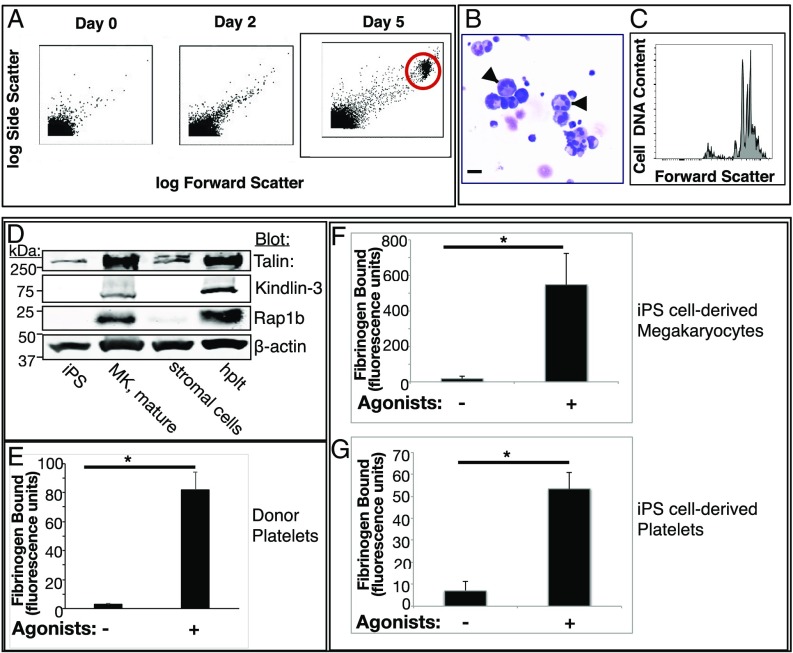
Fig. 4.
Characterization of human iPS cell-derived megakaryocytes and platelets. (A) Human iPS cell-derived hematopoietic progenitors were incubated with doxycycline, stem cell factor, and thrombopoietin to induce differentiation to megakaryocytes (21, 46). Note the progressive appearance with time of a population of cells with increased forward and side light scatter characteristic of megakaryocytes (red circle). (B) Cytospins of maturation day 5/6 megakaryocytes stained with Wright Giemsa. (Original magnification 40×; scale bar: 35 μm.) Note the large cells with multilobed nuclei (arrows). (C) Flow cytometry analysis of DNA ploidy using PI in day 6 mature megakaryocytes indicating populations of cells with variably increased ploidy. (D) Western blots of lysates prepared from human iPS cells, megakaryocytes (MK), stromal cells, or peripheral blood platelets (hplt) were probed for known integrin-regulating proteins. (E–G) Fibrinogen binding to human donor platelets (E) or to iPS cell-derived megakaryocytes (F) and platelets (G). Cells were incubated for 30 min with vehicle or an agonist mixture of 50 μM ADP, 50 μM epinephrine, and 100 μM PAR1 peptide (SFLLRN) in the presence of FITC-fibrinogen. Specific fibrinogen binding was determined by flow cytometry as described in Materials and Methods (n = 4; mean ± SEM; *P < 0.05).
When cells were coincubated with the conventional platelet agonists ADP, epinephrine, and PAR1 receptor activation peptide, they demonstrated a marked increase in specific binding of soluble fibrinogen that was comparable for human donor cell- and iPS cell-derived platelets (Fig. 4 E–G). Thus, megakaryocytes and platelets derived from human iPS cells recapitulate a fundamental platelet hemostatic response: agonist-dependent fibrinogen binding to αIIbβ3.
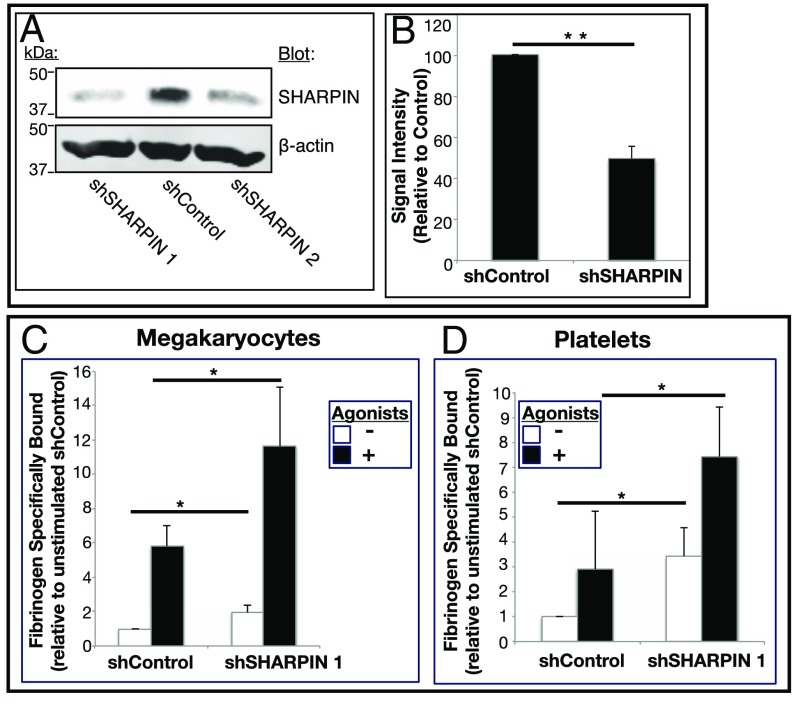
We rationalized that knockdown of SHARPIN might reduce its extent of association with αIIb and impair LUBAC-mediated linear ubiquitination. To test this, two distinct pIRES-GFP-expressing SHARPIN shRNAs were prepared (shSHARPIN 1 and shSHARPIN 2) as well as a scrambled, negative control shRNA (shControl). After lentiviral infection of immature megakaryocytes, none of the shRNAs affected megakaryocyte maturation or αIIbβ3 expression, and 70–90% of the cells were GFP-positive. Western blot analyses of cell lysates indicated reduction of SHARPIN in cells expressing shSHARPIN 1 or 2 shRNA compared with cells expressing shControl RNA (Fig. 5 A and B).
Fig. 5.
shRNA-mediated SHARPIN knockdown in megakaryocytes and platelets leads to increased fibrinogen binding to αIIbβ3. Immature megakaryocytes were transduced with either of two GFP-IRES-shRNA lentiviruses targeting SHARPIN (shSHARPIN) or with a scrambled control shRNA (shControl). (A) Following megakaryocyte maturation, cells were lysed, and Western blots were probed with a SHARPIN antibody or a β-actin antibody as a loading control. (B) Summary of signal intensity of SHARPIN bands from cells, as treated in A (n = 6; mean ± SEM; **P = 0.01). (C and D) Specific Alexa Fluor 647-fibrinogen binding to iPS cell-derived megakaryocytes (C) or platelets (D) incubated in the absence or presence of an agonist mixture (ADP, epinephrine, and PAR1 agonist peptide) as in Fig. 4. Data were collected for GFP-positive live cells and depicted as specific fibrinogen binding relative to unstimulated shControl cells (n = 7; mean ± SEM; *P < 0.05).
SHARPIN Regulates Hemostatic and Immune/Inflammatory Responses of Megakaryocyte/Platelet Lineage Cells.
Previous reports have suggested an inverse correlation between SHARPIN levels and β1 integrin activation in unstimulated prostate cancer cell lines and primary leukocytes (4). To assess the role of SHARPIN in affinity regulation of αIIbβ3, fibrinogen binding to human iPS cell-derived megakaryocytes and platelets was examined. Megakaryocytes and platelets expressing control shRNA showed a low level of specific fibrinogen binding in the absence of platelet agonists and a several-fold increase in binding after agonist stimulation. However, compared with shControl cells, SHARPIN knockdown cells exhibited significant increases in both basal and agonist-induced fibrinogen binding (Fig. 5 C and D). Thus, SHARPIN knockdown may relieve physical constraints on αIIbβ3 activation, potentially allowing greater access of key regulatory proteins, such as talin, to the β3 integrin cytoplasmic tail.
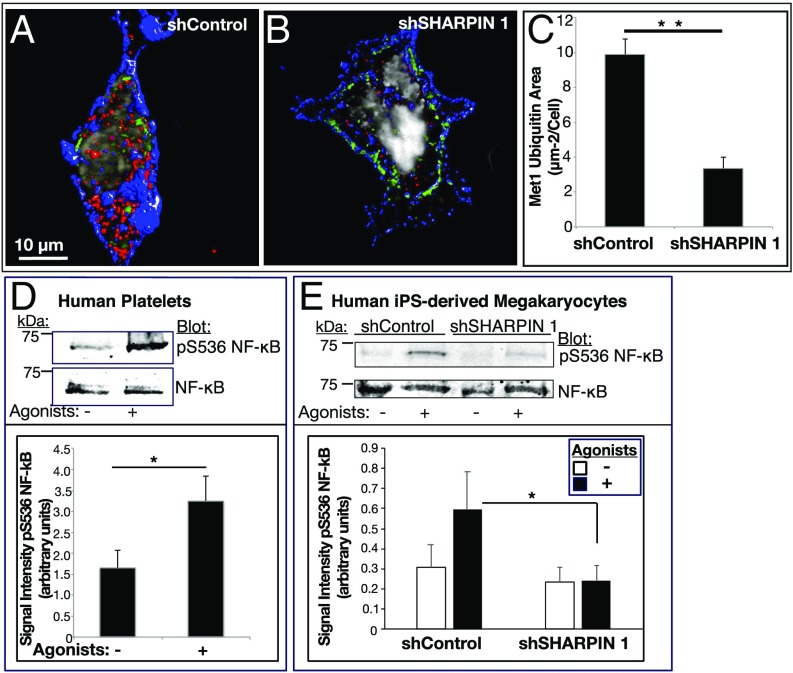
To evaluate the effect of SHARPIN knockdown on LUBAC-mediated Met1 ubiquitination, megakaryocytes plated on fibrinogen were stained with an antibody to Met1 ubiquitin. In cells expressing control shRNA, antibody staining appeared as small punctae or occasionally as larger clusters diffusely in the cytoplasm, cell periphery, and perinuclear area (Fig. 6A). In contrast, SHARPIN knockdown cells showed markedly less staining for Met1 ubiquitin (Fig. 6 B and C). To address whether SHARPIN knockdown and the apparent attendant reduction in LUBAC activity affected downstream NF-κB activation, phosphorylation of serine-536 of the p65 RelA subunit of NF-κB was studied, because this response is closely associated with NF-κB activation and occurs in both nucleated cells (23–25) and agonist-stimulated human platelets (Fig. 6D and SI Appendix, Fig. S4) (26). Indeed, shControl megakaryocytes exhibited increased phosphorylation of RelA serine-536 after agonist stimulation, and this response was reduced in shSHARPIN knockdown cells (Fig. 6E). The substantial decrease in Met1 ubiquitination and NF-κB activation in SHARPIN knockdown cells suggests that NF-κB activation relies substantially on the linear ubiquitination pathway in cells of the human megakaryocyte/platelet lineage.
Fig. 6.
SHARPIN knockdown in megakaryocytes and platelets reduces Met1 ubiquitination and NF-κB pathway signaling. (A–C) Reconstructed immunofluorescence images of cells transduced with shControl RNA (A) or shSHARPIN 1 RNA (B). GFP-positive, shRNA expressing megakaryocytes were plated on fibrinogen and stained for Met1 ubiquitin (red), αIIb (blue), and DNA (gray). Images were acquired at 100× magnification. Met1 and αIIb are represented as isosurfaces. (C) Quantification of Met1 ubiquitination was carried out using Volocity image analysis software and is expressed as total Met1 ubiquitin area per cell. The data were collected from 3D projections of 13 optical slices of 0.2 μm each. A minimum of 20 cells were analyzed in each of three separate experiments (mean ± SEM; **P < 0.01). (D and E) Human platelets (D) or shControl- and shSHARPIN-treated megakaryocytes (E) were incubated for 15 min in the absence or presence of an agonist mixture (ADP, epinephrine and PAR1 agonist peptide as in Fig. 4). Cells were lysed and blots were probed with an antibody to phosphoserine-536 of the p65 (RelA) subunit of NF-κB and reprobed for total RelA. Quantification is represented as phosphoserine-536 RelA signal intensity normalized for total RelA NF-κB. (n = 4 in D; n = 8 in E; mean ± SEM; *P < 0.05).
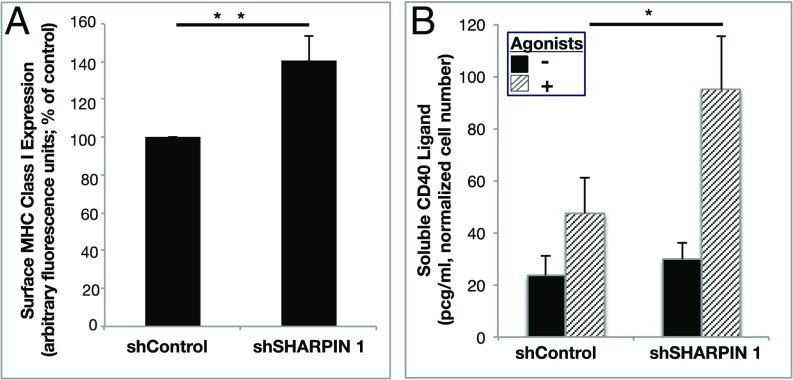
Platelets execute their immune and inflammatory functions through direct and indirect interactions with pathogens and leukocytes (1). Prominent among potential mediators of platelet function in this regard are surface expression of MHC class I molecules for antigen presentation to T lymphocytes (27, 28) and release of sCD40L from platelet granules to promote adaptive immune and inflammatory responses (29). Knockdown of SHARPIN was associated with an increase in basal surface expression of MHC class I molecules in iPS cell-derived human megakaryocytes (Fig. 7A) and an increase in the release of sCD40L in response to platelet agonists (Fig. 7B). Therefore, SHARPIN may serve to restrain not only αIIbβ3 activation, but also certain immune and proinflammatory responses of the megakaryocyte/platelet lineage.
Fig. 7.
SHARPIN knockdown affects potential immune/inflammatory functions of megakaryocyte lineage cells. iPS cell-derived immature megakaryocytes were infected with lentivirus expressing either shSHARPIN 1 or shControl shRNA. (A) Following megakaryocyte maturation, surface expression of MHC class I HLA-ABC was determined by flow cytometry (n = 6; mean ± SEM; **P < 0.01). (B) Mature megakaryocytes were incubated for 20 min with vehicle or agonist mixture as in Fig. 4, and sCD40L was determined in cell supernatants by ELISA. Results are normalized for cell count (n = 5; mean ± SEM; *P < 0.05).
Discussion
In this study, we used human platelets or megakaryocytes and platelets derived from human iPS cells to study the roles of SHARPIN in αIIbβ3 affinity regulation and megakaryocyte/platelet responses implicated in immunity and inflammation. Our major conclusions are that (i) SHARPIN is expressed in megakaryocytes and platelets and is associated in part with the integrin αIIb subunit; (ii) platelets assemble a SHARPIN-dependent functional LUBAC and also express proteins known to function in Met1 linear deubiquitination; (iii) SHARPIN negatively regulates fibrinogen binding to αIIbβ3 and, by implication, platelet hemostatic function; and (iv) SHARPIN may restrain certain megakaryocyte/platelet functions potentially relevant to immunity and inflammation, such as surface expression of MHC class I molecules and release of sCD40L. These results highlight the advantages of this and other iPS cell model systems (30) for studies of signaling and disease modeling in human megakaryocytes and platelets, thus complementing nonhuman experimental systems.
The interaction of SHARPIN with integrin α chains is determined by three variable amino acids N-terminal to the highly conserved GFFKR α subunit juxtamembrane sequence. A salt bridge links Arg-995 within this αIIb sequence to β3 Asp-723, and truncation at αIIb 991 results in constitutive activation of αIIbβ3 (31). The transmembrane region of αIIb forms a short helix with the two phenylalanines (F992 and F993) folded against the transmembrane helix (32). This suggests that a pool of platelet SHARPIN may be partially embedded with the αIIb transmembrane domain at this functional hotspot, effectively clamping αIIbβ3 in the resting state. SHARPIN in this subcellular region may also potentially impede the clustering of integrins, an important component of full integrin adhesive activity (2). SHARPIN removal might then prime the integrin for activation by permitting the talin-dependent reorientation of the αIIb and β3 transmembrane domains that lead to the ectodomain conformational changes in αIIbβ3 required for high-affinity fibrinogen binding and platelet aggregation (33, 34). αIIbβ3 must be very tightly regulated, because platelets normally circulate in a fibrinogen-rich plasma milieu in which circulation of aggregated platelets would be detrimental. Assuming that SHARPIN’s interaction with αIIb is reversible, it may serve to prevent circulating platelet thrombi on the one hand and facilitate platelet adhesive function during hemostasis on the other hand.
Polyubiquitin chains may be attached to any of seven lysines or to the N-terminal Met1 of ubiquitin. Ubiquitination of specific proteins through lysine-48 with consequent proteasomal targeting has been a focus of studies in platelets (35), and platelet proteomics have identified several E3 ligases (36). The demonstration here of a functional Met1 linear ubiquitination pathway now extends the known platelet ubiquitome. The canonical NF-κB activation pathway incorporates hybrid K63/Met1 ubiquitin linkages (37), and whether this is the case in platelets remains to be seen. SHARPIN and LUBAC appear to support NF-κB activation in platelets and megakaryocytes since agonist-induced Met1 polyubiquitination of NEMO was observed in platelets (Fig. 3 D and E), and SHARPIN knockdown in megakaryocytes was associated with a parallel reduction in downstream phosphorylation of pS536-RelA (Fig. 6E). Important questions remain as to whether alternative pathways to NF-κB activation exist in megakaryocytes and platelets (38), as well as the precise nongenomic functional roles that NEMO and other effectors of NF-κB signaling might play in anucleate platelets (16, 39).
MHC class I samples degrade proteins in the endoplasmic reticulum, and peptide-loaded MHC molecules traffic to the cell surface for presentation to CD8 T cells (40). Platelets and megakaryocytes express MHC class I molecules (27, 28), and knockdown of SHARPIN in megakaryocytes leads to increased MHC class I surface expression (Fig. 7A). Although the mechanism for this effect remains unknown, platelets can scavenge pathogen-derived peptides as well as self-peptides and load these onto MHC class I for cross-presentation to CD8 and Treg cells (27, 41), thereby impacting immunity. SHARPIN, through its association with cytoskeletal protein Arp2/3 or with proteins involved in endosomal trafficking (42), may affect MHC class I delivery to the platelet plasma membrane or its re-endocytosis.
Platelets are the largest cellular contributors of plasma sCD40L, a cytokine implicated in proinflammatory and prothrombotic conditions, including transfusion-related acute lung injury (29). We found that SHARPIN knockdown increased the release of sCD40L from agonist-stimulated megakaryocytes (Fig. 7B). Although sCD40L is reported to interact with the ectodomain of αIIbβ3 to trigger outside-in αIIbβ3 signaling and to stabilize arterial thrombi (43), whether the increases in αIIbβ3 activation and sCD40L release observed in SHARPIN knockdown cells are mechanistically linked remains to be determined. In conclusion, SHARPIN may play previously unanticipated roles in the hemostatic, immune, and inflammatory functions of platelets (44) (Fig. 8). Therefore, we speculate that the functional changes described here in SHARPIN knockdown cells of the megakaryocyte/ platelet lineage may contribute to the autoinflammatory phenotype of mice (11–13) and, theoretically, of humans with mutated or deficient SHARPIN.
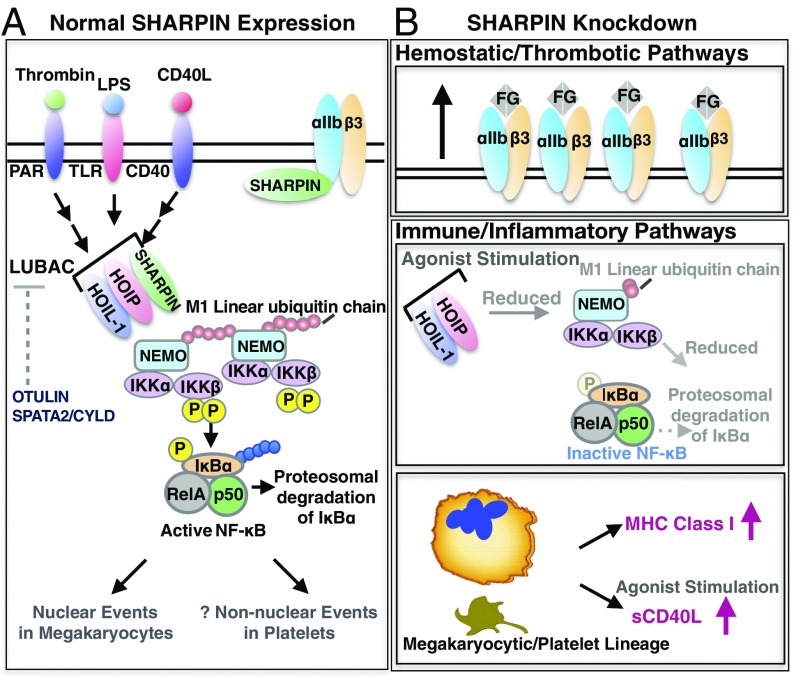
Fig. 8.
Schematic of protein associations and potential functions of SHARPIN in human megakaryocytes and platelets. (A) Normal SHARPIN expression. SHARPIN associates with αIIbβ3, likely predominantly in resting platelets. In addition, HOIP and HOIL-1 are expressed in platelets and, together with SHARPIN, form LUBAC. Also expressed are OTULIN and SPATA2, involved in Met1 deubiquitination. Stimulation of platelets through receptors for thrombin, LPS, or sCD40L activates LUBAC to add Met1 linear ubiquitin chains (red circles) to NEMO, provoking transautophosphorylation of IKKβ and phosphorylation of IκBα. In nucleated cells, Lys-48-ubiquitination (blue circles) results in proteasomal degradation of IκBα, which frees NF-κB for nuclear translocation. Activation of LUBAC and NF-κB pathway components presumably induces nongenomic responses in anucleate platelets (48, 49). (B) Effects of SHARPIN knockdown. (Upper) Reduction of SHARPIN levels associated with αIIb may prime αIIbβ3 for talin-mediated activation and fibrinogen binding. (Middle) Reduction in SHARPIN levels may also destabilize LUBAC, thereby reducing Met1 linear ubiquitination and NF-κB activation. (Lower) SHARPIN reduction in the megakaryocyte/platelet lineage also leads to an increase in surface expression of MHC class I molecules and increased release of sCD40L, potentially contributing to platelet function in immunity and inflammation.
Materials and Methods
Human Platelet Preparation.
Venous blood was obtained from consenting medication-free volunteers, according to a protocol approved by the University of California Human Research Protections Program’s Institutional Review Board, and then anticoagulated with acid-citrate-dextrose. Washed platelets were obtained and diluted into Walsh’s buffer (45).
Differentiation of Human iPS Cells to Megakaryocytes and Platelets.
iPS cells were subjected to stepwise differentiation to produce mature megakaryocytes and platelets as described previously (21). In brief, feeder-free iPS cultures were expanded, differentiated to hematopoietic progenitors, and then processed to immature or mature megakaryocytes. Doxycycline was used to maintain megakaryocytes at an immature stage before final maturation for use in experiments (46). Further details on harvesting and characterization of iPS-derived cells are provided in SI Appendix, Materials and Methods.
Western Blot Analysis and Immunoprecipitation.
Human platelets and human iPS cell-derived megakaryocytes and platelets from unsorted cell cultures were incubated under resting or stimulated conditions as indicated. For coimmunoprecipitation experiments, peripheral blood platelets were lysed with Nonidet P-40 buffer supplemented with inhibitors to serine and cysteine proteases, and GST pulldown experiments were performed in RIPA buffer with inhibitors (details in SI Appendix, Materials and Methods). Linear ubiquitination was tested in samples processed according to the method described by Sasaki and Iwai (6), and Western blots were probed with either of two antibodies specific for Met1 ubiquitin.
Flow Cytometry and ELISA Assay.
To evaluate activation of αIIbβ3, iPS cell-derived megakaryocytes and platelets from unsorted cell cultures were incubated in the presence or absence of indicated agonists, and specific (EDTA-inhibitable) FITC-fibrinogen binding was quantified by flow cytometry (47). Data are presented for live, PI-negative megakaryocytes with characteristic scatter profiles and positive surface expression of αIIb (CD41) and GPIbα (CD42b) (SI Appendix, Fig. S1). Platelets generated in iPS cell cultures were identified by their scatter profiles and by their expression of CD41/CD42b, all similar to those of peripheral blood platelets (SI Appendix, Fig. S2). For shRNA-expressing megakaryocytes and platelets, GFP-positive cells were selected, and Alexa Fluor 647-fibrinogen was used for flow cytometry fibrinogen-binding assays. APC–anti-CD41 antibody staining indicated similar CD41 levels on shControl and shSHARPIN cells. A PE–anti-MHC HLA-ABC or IgG control antibody was used to assess MHC class I surface expression on resting cells, and ELISA was used to detect released sCD40L from resting or agonist-stimulated megakaryocytes, according to the manufacturer’s instructions (R&D Systems). FlowJo software was used to analyze flow cytometry parameters.
Microscopy Image Acquisition.
Resting or stimulated platelets and megakaryocytes in Walsh’s buffer were plated on fibrinogen-coated coverslips for 1 h at 37 °C, fixed, and then stained with the indicated antibodies and Hoechst dye to stain DNA. All cell images within an experiment were acquired and processed under identical conditions. Acquired images were minimally processed using Volocity (PerkinElmer), Image J, and Adobe Photoshop. Further details are provided in SI Appendix, Materials and Methods.
Statistical Analysis.
Analyses were performed using Student’s t test.
Supplementary Material
Acknowledgments
We thank Dr. Johanna Ivaska (University of Turku) for the gift of GFP-SHARPIN cDNA and Drs. Robert Kelley and Marissa Matsumoto (Genentech) for the gift of the 1F11 anti-Met1 ubiquitin antibody. This work was funded by the National Institutes of Health (Grants HL56595 and HL78784, to S.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1819156116/-/DCSupplemental.
References
- 1.Rondina MT, Weyrich AS, Zimmerman GA. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112:1506–1519. doi: 10.1161/CIRCRESAHA.113.300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morse EM, Brahme NN, Calderwood DA. Integrin cytoplasmic tail interactions. Biochemistry. 2014;53:810–820. doi: 10.1021/bi401596q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett JS. Regulation of integrins in platelets. Biopolymers. 2015;104:323–333. doi: 10.1002/bip.22679. [DOI] [PubMed] [Google Scholar]
- 4.Rantala JK, et al. SHARPIN is an endogenous inhibitor of β1-integrin activation. Nat Cell Biol. 2011;13:1315–1324. doi: 10.1038/ncb2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pouwels J, et al. SHARPIN regulates uropod detachment in migrating lymphocytes. Cell Rep. 2013;5:619–628. doi: 10.1016/j.celrep.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki K, Iwai K. Roles of linear ubiquitinylation, a crucial regulator of NF-κB and cell death, in the immune system. Immunol Rev. 2015;266:175–189. doi: 10.1111/imr.12308. [DOI] [PubMed] [Google Scholar]
- 7.De Franceschi N, et al. Mutually exclusive roles of SHARPIN in integrin inactivation and NF-κB signaling. PLoS One. 2015;10:e0143423. doi: 10.1371/journal.pone.0143423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rittinger K, Ikeda F. Linear ubiquitin chains: Enzymes, mechanisms and biology. Open Biol. 2017;7:170026. doi: 10.1098/rsob.170026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrdinka M, Gyrd-Hansen M. The Met1-linked ubiquitin machinery: Emerging themes of (de)regulation. Mol Cell. 2017;68:265–280. doi: 10.1016/j.molcel.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q, et al. Biallelic hypomorphic mutations in a linear deubiquitinase define otulipenia, an early-onset autoinflammatory disease. Proc Natl Acad Sci USA. 2016;113:10127–10132. doi: 10.1073/pnas.1612594113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda F, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature. 2011;471:637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokunaga F, et al. SHARPIN is a component of the NF-κB–activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 13.Gerlach B, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 14.Potter CS, et al. Chronic proliferative dermatitis in Sharpin null mice: Development of an autoinflammatory disease in the absence of B and T lymphocytes and IL4/IL13 signaling. PLoS One. 2014;9:e85666. doi: 10.1371/journal.pone.0085666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peltzer N, et al. LUBAC is essential for embryogenesis by preventing cell death and enabling haematopoiesis. Nature. 2018;557:112–117. doi: 10.1038/s41586-018-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuentes E, Rojas A, Palomo I. NF-κB signaling pathway as target for antiplatelet activity. Blood Rev. 2016;30:309–315. doi: 10.1016/j.blre.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Elliott PR, Komander D. Regulation of Met1-linked polyubiquitin signalling by the deubiquitinase OTULIN. FEBS J. 2016;283:39–53. doi: 10.1111/febs.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott PR, et al. SPATA2 links CYLD to LUBAC, activates CYLD, and controls LUBAC signaling. Mol Cell. 2016;63:990–1005. doi: 10.1016/j.molcel.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki Y, Fujita H, Nakai M, Iwai K. Immunoblot analysis of linear polyubiquitination of NEMO. Methods Mol Biol. 2015;1280:297–309. doi: 10.1007/978-1-4939-2422-6_17. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto ML, et al. Engineering and structural characterization of a linear polyubiquitin-specific antibody. J Mol Biol. 2012;418:134–144. doi: 10.1016/j.jmb.2011.12.053. [DOI] [PubMed] [Google Scholar]
- 21.Takayama N, Eto K. In vitro generation of megakaryocytes and platelets from human embryonic stem cells and induced pluripotent stem cells. Methods Mol Biol. 2012;788:205–217. doi: 10.1007/978-1-61779-307-3_15. [DOI] [PubMed] [Google Scholar]
- 22.Schwer HD, et al. A lineage-restricted and divergent beta-tubulin isoform is essential for the biogenesis, structure and function of blood platelets. Curr Biol. 2001;11:579–586. doi: 10.1016/s0960-9822(01)00153-1. [DOI] [PubMed] [Google Scholar]
- 23.Ma Z, Chalkley RJ, Vosseller K. Hyper-O-GlcNAcylation activates nuclear factor κ light-chain enhancer of activated B cells (NF-κB) signaling through interplay with phosphorylation and acetylation. J Biol Chem. 2017;292:9150–9163. doi: 10.1074/jbc.M116.766568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buss H, et al. Cyclin-dependent kinase 6 phosphorylates NF-κB P65 at serine 536 and contributes to the regulation of inflammatory gene expression. PLoS One. 2012;7:e51847. doi: 10.1371/journal.pone.0051847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Xiang GS, Kourouma F, Umar S. Citrobacter rodentium-induced NF-kappaB activation in hyperproliferating colonic epithelia: Role of p65 (Ser536) phosphorylation. Br J Pharmacol. 2006;148:814–824. doi: 10.1038/sj.bjp.0706784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu WJ, et al. Suppression of NF-κB signaling by andrographolide with a novel mechanism in human platelets: Regulatory roles of the p38 MAPK-hydroxyl radical-ERK2 cascade. Biochem Pharmacol. 2012;84:914–924. doi: 10.1016/j.bcp.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Chapman LM, et al. Platelets present antigen in the context of MHC class I. J Immunol. 2012;189:916–923. doi: 10.4049/jimmunol.1200580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zufferey A, et al. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood Adv. 2017;1:1773–1785. doi: 10.1182/bloodadvances.2017007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aloui C, et al. The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int J Mol Sci. 2014;15:22342–22364. doi: 10.3390/ijms151222342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borst S, Sim X, Poncz M, French DL, Gadue P. Induced pluripotent stem cell-derived megakaryocytes and platelets for disease modeling and future clinical applications. Arterioscler Thromb Vasc Biol. 2017;37:2007–2013. doi: 10.1161/ATVBAHA.117.309197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Toole TE, et al. Modulation of the affinity of integrin alpha IIb beta 3 (GPIIb-IIIa) by the cytoplasmic domain of alpha IIb. Science. 1991;254:845–847. doi: 10.1126/science.1948065. [DOI] [PubMed] [Google Scholar]
- 32.Lau TL, Dua V, Ulmer TS. Structure of the integrin alphaIIb transmembrane segment. J Biol Chem. 2008;283:16162–16168. doi: 10.1074/jbc.M801748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagarrigue F, Kim C, Ginsberg MH. The Rap1-RIAM-talin axis of integrin activation and blood cell function. Blood. 2016;128:479–487. doi: 10.1182/blood-2015-12-638700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagani G, Gohlke H. On the contributing role of the transmembrane domain for subunit-specific sensitivity of integrin activation. Sci Rep. 2018;8:5733. doi: 10.1038/s41598-018-23778-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta N, Li W, McIntyre TM. Deubiquitinases modulate platelet proteome ubiquitination, aggregation, and thrombosis. Arterioscler Thromb Vasc Biol. 2015;35:2657–2666. doi: 10.1161/ATVBAHA.115.306054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck F, et al. Temporal quantitative phosphoproteomics of ADP stimulation reveals novel central nodes in platelet activation and inhibition. Blood. 2017;129:e1–e12. doi: 10.1182/blood-2016-05-714048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emmerich CH, et al. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc Natl Acad Sci USA. 2013;110:15247–15252. doi: 10.1073/pnas.1314715110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat Rev Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 39.Lannan KL, et al. Breaking the mold: Transcription factors in the anucleate platelet and platelet-derived microparticles. Front Immunol. 2015;6:48. doi: 10.3389/fimmu.2015.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blander JM. The comings and goings of MHC class I molecules herald a new dawn in cross-presentation. Immunol Rev. 2016;272:65–79. doi: 10.1111/imr.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a system understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 42.Khan MH, et al. The Sharpin interactome reveals a role for Sharpin in lamellipodium formation via the Arp2/3 complex. J Cell Sci. 2017;130:3094–3107. doi: 10.1242/jcs.200329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad KS, Andre P, Yan Y, Phillips DR. The platelet CD40L/GP IIb-IIIa axis in atherothrombotic disease. Curr Opin Hematol. 2003;10:356–361. doi: 10.1097/00062752-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 45.Leng L, Kashiwagi H, Ren XD, Shattil SJ. RhoA and the function of platelet integrin alphaIIbbeta3. Blood. 1998;91:4206–4215. [PubMed] [Google Scholar]
- 46.Ito Y, et al. Turbulence activates platelet biogenesis to enable clinical scale ex vivo production. Cell. 2018;174:636–648.e18. doi: 10.1016/j.cell.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Shiraga M, et al. Primary megakaryocytes reveal a role for transcription factor NF-E2 in integrin αIIbβ3 signaling. J Cell Biol. 1999;147:1419–1430. doi: 10.1083/jcb.147.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karim ZA, Vemana HP, Khasawneh FT. MALT1-ubiquitination triggers non-genomic NF-κB/IKK signaling upon platelet activation. PLoS One. 2015;10:e0119363. doi: 10.1371/journal.pone.0119363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karim ZA, et al. IκB kinase phosphorylation of SNAP-23 controls platelet secretion. Blood. 2013;121:4567–4574. doi: 10.1182/blood-2012-11-470468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.